Abstract
Disruption to species-interaction networks caused by irruptions of herbivores and mesopredators following extirpation of apex predators is a global driver of ecosystem reorganization and biodiversity loss. Most studies of apex predators' ecological roles focus on effects arising from their interactions with herbivores or mesopredators in isolation, but rarely consider how the effects of herbivores and mesopredators interact. Here, we provide evidence that multiple cascade pathways induced by lethal control of an apex predator, the dingo, drive unintended shifts in forest ecosystem structure. We compared mammal assemblages and understorey structure at seven sites in southern Australia. Each site comprised an area where dingoes were poisoned and an area without control. The effects of dingo control on mammals scaled with body size. Activity of herbivorous macropods, arboreal mammals and a mesopredator, the red fox, were greater, but understorey vegetation sparser and abundances of small mammals lower, where dingoes were controlled. Structural equation modelling suggested that both predation by foxes and depletion of understorey vegetation by macropods were related to small mammal decline at poisoned sites. Our study suggests that apex predators’ suppressive effects on herbivores and mesopredators occur simultaneously and should be considered in tandem in order to appreciate the extent of apex predators’ indirect effects.
Keywords: trophic cascade, mesopredator release hypothesis, apex predator, regime shift, Canis dingo
1. Introduction
Globally, apex predators play a vital role in the functioning of ecosystems, and their importance has been underestimated because their effects often only become evident after they have been removed from ecosystems [1,2]. Apex predators typically have conspicuous effects on the populations and phenotypes of prey and smaller predators (mesopredators) that arise from direct killing and the fear they instil [3–5]. The disruption to species-interaction networks caused by the irruptions of herbivores and mesopredators that frequently accompanies the loss of apex predators can trigger regime shifts that result in the reorganization of species assemblages [2,6] and has been identified as a key driver of biodiversity loss [1]. Consequently, restoration of apex predator populations and the ecosystem services they provide has been highlighted as a critical imperative for the conservation of biodiversity [7].
While predators’ direct effects are readily observed, they can also propagate a myriad of indirect effects because species that interact with their herbivorous prey and mesopredators are likely to be affected by the removal or introduction of an apex predator [1,8]. Trophic cascade theory predicts that the suppression of apex predators’ effects will result in the irruption of herbivores and subsequent depletion of plant biomass [9]. A related concept, the mesopredator release hypothesis, predicts that the removal of apex predators leads to the irruption of mesopredators with concomitant declines in the abundances of their prey owing to elevated rates of predation by mesopredators [10]. Despite the existence of theory and field studies showing that apex predators can influence ecosystem structure through a multitude of interaction pathways, most studies have considered apex predators’ effects on herbivores and mesopredators and associated ecological cascades in isolation [1]. Few studies have considered how irruptions of herbivores and mesopredators could have interactive effects on other species [11]. Consequently, our appreciation of the magnitude, complexity and extent of apex predators’ effects on ecosystems may not be fully realized.
Although it is widely acknowledged that vertebrate predators in terrestrial ecosystems can regulate populations of their prey [12], debate exists regarding the relative strength and even the existence of their indirect effects [13]. One reason for this debate is that relatively few studies have attempted to quantify the indirect effects of mammalian apex predators [9,14] because the temporal and spatial scales required to conduct controlled experiments on large carnivores are logistically prohibitive [14]. Moreover, in many jurisdictions legal and ethical considerations often prevent manipulations of their abundance. Another reason for the paucity of studies on large predators in terrestrial ecosystems is that they have been extirpated from much of their former ranges [15]. Hence, there are few places where studies can be undertaken to investigate their ecological effects.
One way to advance knowledge of the role of large predators is to use ‘natural experiments’ whereby the abundance of apex predators vary in time or space in otherwise similar landscapes [4,6,16]. If properly conducted, such studies can provide valuable insights into ecological processes at spatial and temporal scales that cannot be achieved through experimentation. In the forested landscapes of southeastern Australia, the existence of long-term eradication programmes that aim to reduce the impacts of Australia's largest terrestrial predator, the dingo (Canis dingo, also known as wild dog; 12–22 kg), on livestock provides the opportunity to conduct a ‘large-scale’ natural experiment to examine the role that apex predators have in structuring ecosystems. In eastern New South Wales, dingo populations are controlled in many but not all conservation reserves by distributing baits impregnated with the toxin sodium fluoroacetate (compound 1080) [17]. This variation in the intensity of dingo control thus permits comparisons to be made of ecosystem attributes in nearby ecosystems where dingoes are common and rare, respectively. In this context, the term ‘dingo’ refers to both dingoes and dingo–domestic dog (Canis familiaris) hybrids [11].
Relatively little is known about the dingo's ecological role in the forests of southeastern Australia, although there is evidence that they can suppress the populations of macropods and red foxes [18,19]. In arid regions, dingoes’ influence on the abundances of mammal species scales with body size. Dingoes suppress the abundances of macropods (more than 15 kg) and the smaller red fox (Vulpes vulpes) (3.5–8 kg) [20]. In turn, where dingoes are common, small mammals (less than 200 g) increase in abundance owing in part to release from predation by foxes [11]. Also, as predicted by trophic cascade theory, the removal of dingoes results in the depletion of pasture biomass owing to an increase in herbivore grazing impact [20]. Theory and results of predator studies from other continents suggest that dingoes’ ecological effects may be weaker or more focused in higher-productivity forest ecosystems than in desert ecosystems [4,21]. This is because the greater complexity of ecosystems that accompanies increases in primary productivity may be expected to diffuse predators' impacts across a greater number of interaction pathways [11,22].
Applying trophic cascade theory, the mesopredator release hypothesis and existing knowledge of dingoes’ effects on other species, we predicted that the effect of dingo suppression on other mammals in forest ecosystems should alternate with trophic group and scale with body size [11]. Our specific predictions were: (i) that abundances of herbivorous macropods (Macropus spp.; Wallabia bicolor; 15–64 kg) and smaller invasive mesopredators, the red fox (3.5–8 kg) and feral cat (Felis catus; 2.5–6.5 kg), should increase in areas where dingo populations are controlled because they would experience less predation or harassment; (ii) smaller ground-dwelling mammals—bandicoots (700–1500 g), rodents (15–200 g) and dasyurid marsupials (20–100 g)—should increase where dingoes were not controlled owing to reduced predation and habitat disturbance from mesopredators and macropods, respectively; (iii) for arboreal mammals, possums (975–2400 g) should increase in baited areas because they are subjected to predation by dingoes, but gliders (120–1300 g) should show little response to dingo control because they occur relatively infrequently in the diets of dingoes and other ground-dwelling predators; and (iv) that the complexity of understorey vegetation structure should decrease in areas subjected to dingo control owing to increased consumption from large herbivores. We tested our predictions by comparing the activity or abundance of all groups and the species composition of the mammal assemblages at seven paired locations in forested conservation reserves in southeastern Australia. Each pair consisted of an area subjected to systematic dingo removal and a control area, with similar environmental attributes, where consistent dingo control was not undertaken. We pooled the results from our paired comparisons using meta-analysis to determine the effects of dingo control on the response variables. We then used structural equation modelling (SEM) to further investigate the hypothesized direct and indirect relationships among the response variables.
2. Material and methods
(a). Study sites
This study was conducted in the Eucalyptus spp.-dominated forest ecosystems of New South Wales, southeastern Australia (figure 1). The main technique used by government authorities to suppress dingo populations is the distribution of poisoned meat baits containing 6 mg of the toxin sodium fluoroacetate (compound 1080) [17]. The baits are typically distributed along unsealed dirt roads or from the air via helicopter or light aeroplane. In some places, baiting is complemented by trapping of dingoes.
Figure 1.
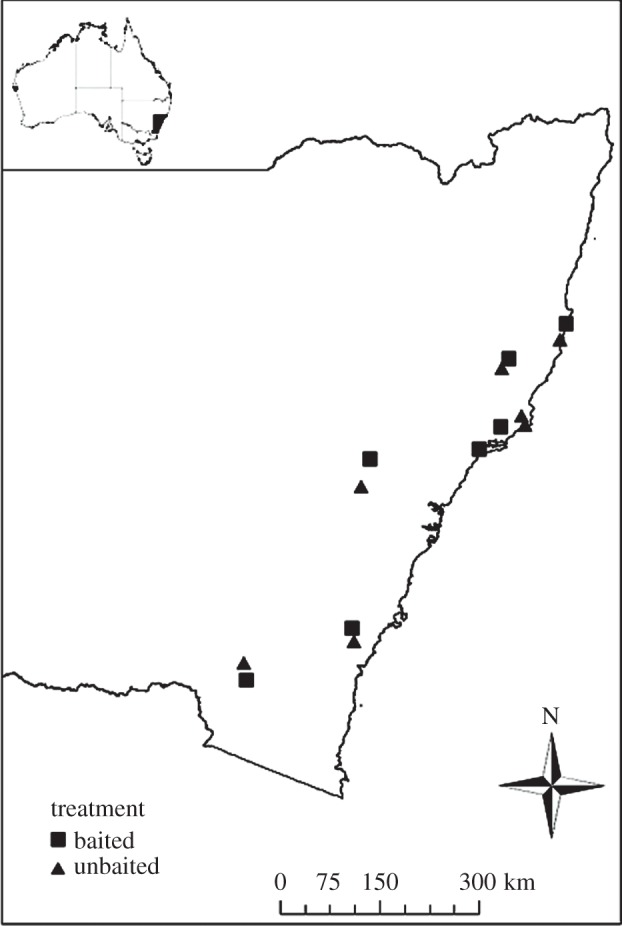
Study site locations in New South Wales, southeastern Australia. Each location consisted of a conservation reserve where dingoes were controlled using poison baiting (squares) and a conservation reserve where dingoes were not subjected to poison baiting (triangles).
Each of our seven study areas consisted of a pair of sub-sites located less than 50 km apart (electronic supplementary material, table S1). Each sub-site pair consisted of a site where dingo control had been undertaken at least once each year for at least 5 years prior to our surveys, and a comparison site that had not been subjected to consistent dingo control. All sites were situated within conservation reserves managed by the New South Wales National Parks and Wildlife Service, with each paired sub-site surveyed within the same two-week period and season. Paired sub-sites were selected on the basis that they shared the same dominant overstorey Eucalyptus species [23] and had similar underlying geology and landforms.
(b). Mammal abundance and vegetation assessments
At each sub-site, we measured the activity of predators (Canis dingo, Vulpes vulpes, Felis catus) and bandicoots using 20 track detection stations, placed at 500 m intervals along unpaved vehicle tracks with washed sand spread across the track at a width of 1 m [24]. To determine whether rain had potentially erased tracks during the course of the evening, an intentional mark was made in the left-hand corner each afternoon. Plots were determined to be unreadable if the unique mark was obscured when the plot was examined the following morning. Animal footprints were identified for three nights. Owing to the difficulty in identification between bandicoot species (Perameles nasuta/Isoodon macrourus), these tracks were recorded as bandicoot in accordance with Catling & Burt [25]. An index of activity for each species at each site was expressed as the percentage of plots on which the tracks were detected during the three-night tracking session [20].
We assessed the abundances of macropods by counting the number of kangaroos (Macropus giganteus) and wallabies (Macropus rufogriseus and Wallabia bicolor) sighted during two to four transect surveys conducted along single lane dirt tracks within each sub-site [26]. During surveys, two observers seated in a four-wheel drive vehicle visually scanned the habitat while moving at a speed of 15 km h−1. Two to four replicate surveys were performed on a different track at a distance of 5–15 km [26]. The surveys were conducted in the hour preceding dusk. An index of macropod abundance on each survey transect was calculated as the number macropods sighted per kilometre of survey.
The abundance of arboreal mammals—possums (Trichosurus vulpecula and Pseudocheirus peregrines) and gliders (Petaurus breviceps and Petauroides volans)—was assessed using two to four 3–16 km spotlight transects at each sub-site. The surveys were conducted at night from the back of a four-wheel-drive utility vehicle along single-lane dirt tracks using a 100-watt spotlight. The vehicle was driven at a speed of 10 km h−1. An index of abundance for each survey was calculated as the number of animals observed per kilometre of survey [27].
Small mammal abundance was assessed over three consecutive nights on seven to eight 1 ha trapping grids within each sub-site. Because time since last fire can influence the abundance of small mammals, we did not place study grids in areas that had been burnt less than 3 years previously, as informed by records provided by the Rural Fire Service of New South Wales. On each grid, we placed 24 Type A Elliott traps (Elliott Scientific Equipment, Upwey, Australia), baited with a mixture of peanut butter, oats and honey, 20 m apart. We identified the small mammals to species level and temporarily marked them to identify recaptures. Indices of rodent abundance (Pseudomys novaehollandiae, Mus musculus, Rattus fuscipes, R. lutreolus, Mastacomys fuscus and R. rattus) and dasyurid (Antechinus stuartii, A. swainsonii and A. agilis) abundance at each sub-site were calculated as the mean number of unique individuals per 100 trap nights. For SEM, we calculated the abundance of all small mammals as the sum of rodent and dasyurid abundance on each trapping grid.
The intensity of herbivory by macropods on each trapping grid was estimated by scoring the presence of groups of recent macropod dung on two 1 × 100 m belt transects on each study grid [26,28,29]. An index of macropod grazing intensity was calculated for each grid as the mean number of macropod scats per grid.
We assessed the complexity of the understorey vegetation of each trapping grid by sampling within four 5 × 5 m quadrats. Within each quadrat, we recorded the percentage of a 20 × 50 cm chequered coverboard obscured by vegetation within five strata (0–20, 20–50, 50–100, 100–150 and 150–200 cm) above ground level [30]. For meta-analyses, we calculated two variables for analysis by summing our observations in the strata between 0–100 and 100–200 cm. For the SEM, we calculated a single vegetation structure variable by summing the observations for the entire 0–200 cm strata.
Because fire and recent rainfall are known to influence the structure of understorey vegetation [31], we obtained data on the average cumulative rainfall received at each sub-site for 2 years prior to trapping from the Australian Bureau of Meteorology, and the fire history of each trapping grid from the New South Wales Rural Fire Service. These variables were used as predictor variables in SEM described later.
(c). Statistical analyses: meta-analysis
As the dominant vegetation communities of the sites differed and each was sampled at a different time, we treated each site as an independent comparison of the effect of systematic dingo population control and pooled the results of these comparisons using a meta-analytic approach [20]. Specifically, we used a random-effects meta-analysis to test our a priori hypotheses regarding the effects of dingo control on the measured response variables. This approach allowed us to determine whether the biological effects of dingo control were consistent among sites and that the mean effect of dingo removal differed significantly from zero [20,32]. A random-effects model was used because we expected the effects of dingo control to vary among sites owing to differences in the intensity of poison baiting and the longevity of the baiting programme (electronic supplementary material, table S1). We used the log response ratio as the metric of effect size [33]. To avoid the problems of taking logs of zero or dividing by zero, comparisons were made on In[(Ncontrol + 0.01)/(Ntreatment + 0.01)] [33,34]. Tests for homogeneity of the effect sizes were conducted using the Q-statistic. The mean effect size was considered statistically significant if the bias-corrected bootstrapped 95% CIs calculated from 999 simulations excluded zero [32]. Analyses were undertaken using METAWIN v. 2 [35].
(d). Structural equation modelling
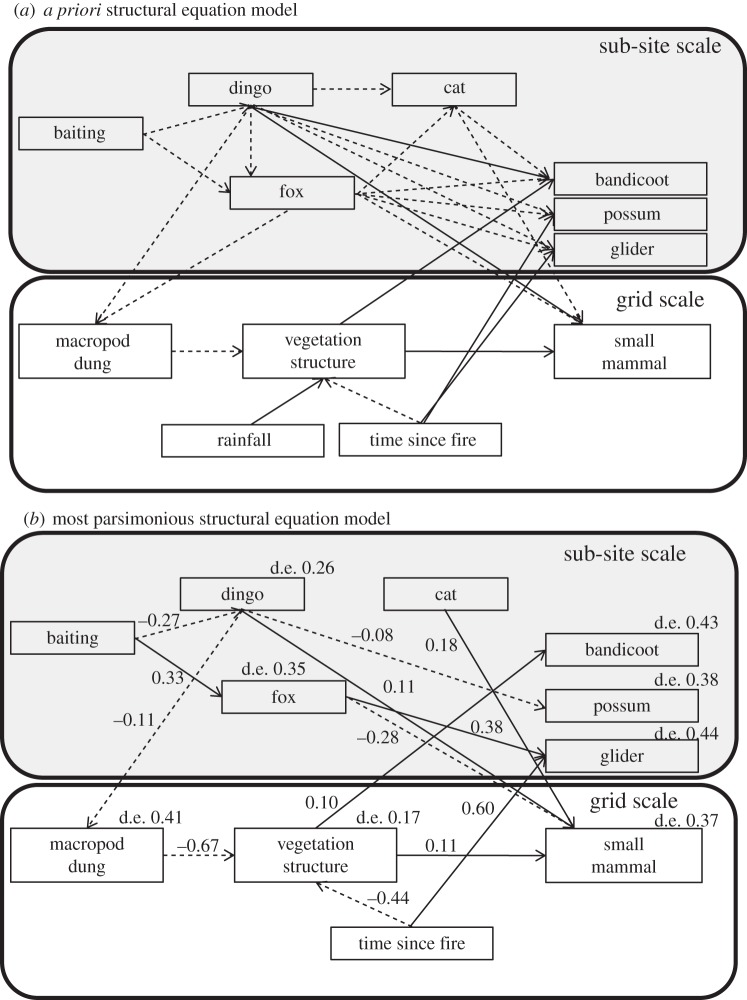
SEM can be used to investigate the direct and indirect relationships between variables in trophic networks based on a priori knowledge of interactions theorized to occur between species [36]. We used piecewise SEM based on information theoretic principles to test hypotheses to explain the inter-relationships between the response variables, fire history and rainfall (figure 3a; electronic supplementary material, tables S1 and S2). We constructed our a priori SEM model based on trophic cascade theory, the mesopredator release hypothesis, and prior knowledge of the factors influencing vegetation structure and the abundances of forest mammals (see Model justification). Unlike classic SEM, which uses covariance matrices, piecewise SEM uses localized estimates to infer direct and indirect effect pathways [37,38]. Piecewise methods allow for the modelling of data that struggle to meet the assumptions of classic SEM analysis, or for the incorporation of exogenous factors into models such as spatial dependence [38]. All localized estimates within our SEM were fitted using generalized linear mixed-effects models with a Poisson or negative binomial distribution, except for the vegetation complexity model, in which case we used a linear mixed-effects model with a Gaussian distribution. To account for biogeographic and temporal variation between sites, site was treated as a random factor in all models. Our initial model was populated with mean values obtained for each sub-site for data using track plots and spotlight surveys (e.g. baiting, dingo activity, fox activity, cat activity, arboreal mammal activity, bandicoot activity; n = 14) and with values obtained for each trapping grid for the variables macropod grazing activity, vegetation structure, small mammal abundance and average rainfall over 2 years and time since fire; n = 111). We used a backwards step-wise elimination process for model simplification, whereby non-significant pathways were sequentially deleted from models until only significant interaction remained [38]. Standardized path coefficients and deviance explained were then calculated for each model [37].
(e). Model justification
The interaction pathways between variables were determined by a priori knowledge and included the following hypothesized pathways (figure 3a). Dingo baiting should negatively affect both dingo and fox activity as even though the control programmes targeted dingoes, it is possible that both species consume baits impregnated with 1080 poison and both species have been observed to decline following baiting programmes [39]. Dingo activity should negatively affect fox activity owing to direct killing or competitive exclusion [19,40]. Cat activity was hypothesized to be affected negatively by dingo and fox activity but not by baiting because cats rarely take baits [41,42]. Dingo and fox activity were hypothesized to negatively affect macropod grazing intensity by suppressing macropod abundance through direct predation [18,43]. Macropod abundance determined by driving surveys was omitted from the SEM because dung count is a proxy measure of abundance [26]. Fox and cat activity were hypothesized to negatively affect small mammal and bandicoot abundance and activity, respectively, owing to predation [44]. Dingo activity and fox activity were hypothesized to negatively affect possums owing to predation but to have little effect on glider because they rarely occur in the diets of terrestrial predators [39,44]. Time since fire was hypothesized to positively affect arboreal possums and gliders, as a previous study has demonstrated negative effects of fire on arboreal mammals [45]. Defoliation resulting from grazing by macropods was hypothesized to have a negative effect on understorey vegetation structure [46]. Rainfall was hypothesized to positively affect understorey vegetation by promoting plant growth [31]. Time since fire was hypothesized to have a negative effect on understorey vegetation cover at our sites, which were aged more than 3 years post-fire, because a previous study has shown that the density of ground cover vegetation initially increases until about 6 years post-fire before decreasing with time since fire [47]. Vegetation structure was hypothesized to positively affect small mammal abundance and bandicoot activity as previous studies have observed small mammal abundance to increase with increasing understorey complexity [48,49].
Figure 3.
(a) A priori piecewise structural equation model describing small mammal responses to dingo baiting in Eucalyptus spp. forests of eastern Australia. (b) Most parsimonious structural equation model showing direct and indirect interaction pathways influencing small mammal abundance. Path co-efficient estimates are shown alongside arrows and deviance explained (d.e.) is shown for all endogenous variables. Dashed lines represent negative interaction pathways, and solid lines represent positive interaction pathways. Grey polygons show sections of the structural equation model sampled at the sub-site scale (n = 14), and white polygons show sections of the structural equation model sampled at the grid scales (n = 111).
3. Results
(a). Paired site comparisons
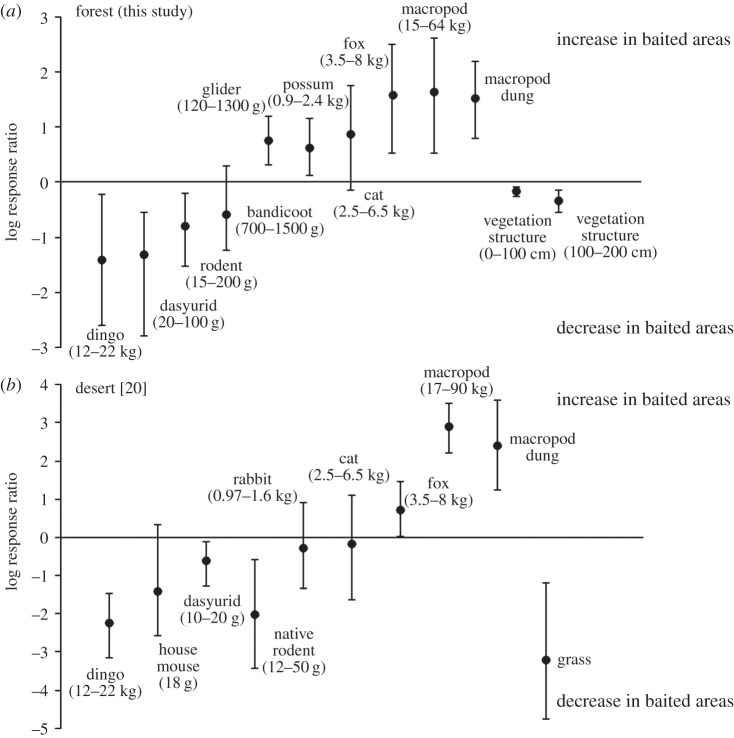
Confirming the effectiveness of poison baiting at reducing dingo populations, dingo activity was on average greater in unbaited than in baited sub-sites (figure 2a; Q = 5.02, d.f. = 6, p = 0.541). Fox activity was consistently lower at unbaited sub-sites (figure 2a; Q = 7.43, d.f. = 5, p = 0.190). Cat activity was unaffected by the dingo baiting (figure 2a; Q = 5.81, d.f. = 6, p = 0.445). Macropod abundance was consistently greater in abundance in baited sub-sites (figure 2a; Q = 3.81, d.f. = 6, p = 0.703). Possums (figure 2a; Q = 5.88, d.f. = 6, p = 0.437) and gliders were detected more frequently at baited sites (figure 2a; Q = 5.65, d.f. = 6, p = 0.463). The activity of bandicoots was unaffected by baiting (figure 2a; Q = 5.32, d.f. = 5, p = 0.378), while abundance of ground-dwelling rodents (figure 2a; Q = 7.67, d.f. = 6, p = 0.263) and dasyurid marsupials (Antechinus spp.; figure 2a; Q = 4.68, d.f. = 6, p = 0.586) was greater in unbaited areas.
Figure 2.
(a) The mean effect size (log response ratio) of dingo control ±95% bias-corrected bootstrapped confidence intervals (CIs) on each of the study variables in this study and (b) from a study of the effects of dingo removal on desert ecosystems in Australia (adapted from [20]). Negative values indicate variables that decreased in the absence of dingoes; positive values indicate variables that increased in the absence of dingoes. The mean effect size was considered statistically significant if the 95% CIs excluded zero. Values in parentheses indicate the body size range of species that contributed to each variable.
Grazing activity of macropods, as estimated by counts of dung, was consistently higher in baited areas (figure 2a; Q = 5.46, d.f. = 6, p = 0.486). The density of understory vegetation between 0–100 cm (figure 2a; Q = 6.70, d.f. = 6, p = 0.350) and 100–200 cm (figure 2a; Q = 4.19, d.f. = 6, p = 0.651) above ground level was on average greater in unbaited than in baited sub-sites.
(b). Structural equation modelling
The variable rainfall was excluded from the final SEM model. Excluded pathways were between dingo activity and fox activity, dingo/fox activity and cat activity, and dingo, fox and cat activity with bandicoot activity. All other variables were included within the final SEM explaining vegetation structure and small mammal abundance (figure 3b). Dingo baiting was correlated negatively with dingo activity, but, counter to our a priori SEM model, was correlated positively with fox activity (Fig. 3b). In accordance with the a priori SEM model, dingo activity was correlated positively with small mammal abundance, and fox activity was correlated negatively with small mammal abundance (figure 3b). Thus, dingo baiting had a negative indirect relationship on small mammal abundance mediated through both dingoes and foxes. Cat activity was unaffected by dingo and fox activity, and, counter to our a priori SEM model, had a positive relationship on small mammal abundance. Dingo activity also as hypothesized had a weak negative correlation with possum activity. Glider activity was positively correlated with fox activity and time since fire.
In line with our a priori SEM model, dingo activity was correlated negatively with macropod grazing activity. In turn, macropod grazing activity and time since fire were correlated negatively with vegetation structural complexity. Also in line with our expectations, vegetation structural complexity was correlated positively with small mammal abundance and bandicoot activity (figure 3b). Thus, dingo baiting had a negative indirect relationship on small mammal abundance and bandicoot activity mediated through dingo activity, macropod grazing activity and vegetation structure.
4. Discussion
(a). Ecological cascades induced by dingo control
Our results demonstrate marked differences in the relative abundances of mammals and the complexity of understorey vegetation between areas with consistent dingo removal compared to those without. These differences accorded well with our a priori predictions generated from trophic cascade theory and the mesopredator release hypothesis. Our results (figure 2a) were also remarkably consistent with previous studies undertaken in desert (figure 2b) and forest biomes in Australia that have found negative relationships between the presence of dingoes and the abundances of macropods and foxes [11,18,19] and positive relationships between the abundances of dingoes and small mammals [50]. In summary, our findings are consistent with the idea that large mammalian carnivores can function as keystone species owing to their direct suppressive effects on herbivores and mesopredators, and that ecological cascades induced by their removal result in the reorganization of ecosystems [5].
In common with previous studies on the effects of mammalian carnivores, our study used a pre-existing land-management framework, the presence or the absence of dingo population control, for the experimental treatment [6,20]. During the design of our study, we matched our paired sub-sites as closely as possible for vegetation type, underlying geology, land use and recent fire history, but without having conducted a manipulative experiment causation remains difficult to attribute as it remains possible that confounding factors could have influenced our results. One potential weakness of our study was that long-term fire regimes of the paired sub-sites were unlikely to have been identical as we could only control for contemporary land use and the occurrence of recent fires. However, given the concordance between the results and our a priori predictions generated from theory, as well as previous studies investigating the effects of dingo control (figure 2a,b), we contend that it is unlikely that any other source of variation, other than the presence/absence of dingo control, could have caused the consistent effects that we observed with respect to trophic group and body size.
Trophic cascade theory, the mesopredator release hypothesis and previous field studies suggest that apex predators can function as ecosystem architects by propagating cascades of direct and indirect effects on species at lower trophic levels. Indirect effects can arise if apex predators moderate the top-down effects of herbivores and mesopredators [5,11]. The most parsimonious structural equation model (figure 3b) provides support for the hypothesis that the negative responses of vegetation structural density, small mammals and bandicoots to dingo control were indirect effects of predator suppression and that these effects occur simultaneously. Specifically, our SEM provided support for the following hypotheses. (i) Predation by dingoes reduced macropod grazing activity, which in turn simplifies the structure of understorey vegetation [46]. The ensuing simplification of vegetation results in lower abundances of ground-dwelling small mammals and bandicoots which require dense vegetation for shelter [48]. (ii) Dingo control reduced dingo activity but increased fox activity, presumably because fox populations increase where dingo control is undertaken (figure 2). Abundant foxes would then have a negative impact on small mammals because of increased levels of predation (figure 3b).
These findings suggest that apex predators’ suppressive effects on herbivores and mesopredators can have interactive effects on other species and should be considered in tandem in order to appreciate the extent of apex predators’ indirect effects. We caution, however, that controlled experiments are required to test these hypotheses.
The absence of a significant correlation between dingo and fox activity obtained in the SEM was unexpected, because previous studies have found evidence for negative correlations between indices of dingo and fox abundance [11,19]. While it is possible that dingoes only have negligible effects on fox abundance/activity or that baiting aimed at dingoes suppressed populations of both dingoes and foxes, our meta-analysis does not support these explanations and showed that, in accordance with our predictions, fox activity was greater at baited sites. The negligible correlation observed in the SEM may have been due to the relatively low power of this test, which was conducted at the scale of sub-site (n = 14). Further studies are recommended to explore the interactions between dingoes and foxes in forest environments.
Although our results are in accordance with the mesopredator release hypothesis and previous studies demonstrating that fox activity was greater in areas where dingoes were subjected to population control [19], dingo control had no effect on cat activity. In addition, contrary to our prediction, cat activity was correlated positively with small mammal abundance. These findings are not inconsistent with those of previous studies, which have reported positive, neutral and negative relationships between dingo activity and cat activity, and positive relationships between cat activity and small mammal abundance [8,42,51]. Taken together, the results of our study and previous studies suggest that cat activity may be influenced by both the abundances of larger predators and the availability of their prey.
Our meta-analysis showed that, as predicted, semi-arboreal possums responded positively to dingo control, but counter to our predictions, strictly arboreal gliders also responded positively to dingo control. The SEM showed that dingoes were negatively associated with possums, which is consistent with dietary studies showing that possums are frequently consumed by dingoes [39]. The positive correlation evident in the SEM between fox and glider lends support to a hypothesized indirect trophic interaction mooted by Dexter [52], whereby suppression of foxes results in an increase in the abundances of species frequently preyed on by large owls. Subsequent increases in owl abundance and predation may then lead to the suppression of gliders. The positive correlation between gliders and time since fire is consistent with the results of previous studies showing that fires can suppress their abundances [45,49].
(b). Unintended effects of dingo control and the management of forest ecosystems
Disturbance by fire is an important factor influencing plant and animal assemblages in the forested landscapes of southeastern Australia [49,53]. Consequently, much research on forest mammals in Australia has focused on how fire, particularly through its effects on vegetation structure, influences the species abundances and community composition [45,47,49,53]. However, there has been growing awareness of the influence that predation by terrestrial predators can have on forest ecosystems through both their direct predatory effects and indirectly by influencing grazing pressure [46,47,54].
Our study has implications for the management of forest ecosystems, because it provides evidence that ecological cascades induced by the lethal control of an apex predator can produce unintended shifts in the composition of species assemblages and vegetation structure. In the forests of southeastern Australia, where this study was undertaken, the control of dingo populations is associated with the reorganization of mammal assemblages whereby relatively large-bodied species, such as macropods and red foxes, and arboreal mammals benefit from dingo control while small-bodied terrestrial mammal species decline in abundance.
Predation by foxes has been identified as one of the major (if not the most important) threatening processes to terrestrial native mammals weighing less than 5 kg and ground nesting birds in Australia [55]. If dingo control releases foxes from top-down control by dingoes it will probably exacerbate the predatory impact of foxes [50]. In addition, increased macropod abundance and subsequently grazing pressure in areas where dingoes are controlled may also have suppressive effects on small mammals by simplifying the structure of understorey vegetation [56]. Such changes could affect small and medium-sized terrestrial mammals by removing their preferred shelter habitats and increasing their exposure to predators.
The broad-scale benefits that dingoes appear to provide for ground-dwelling small and medium-sized mammals provides evidence that dingo control programmes in conservation reserves may be counter-productive from a biodiversity conservation perspective. Indeed, the results of this and other studies suggest that actively seeking to maintain dingo populations or restoring them in areas where they have previously been extirpated has potential to be used as a strategy to mitigate the impacts of herbivores and foxes [11,55]. However, such strategies are likely to be controversial owing to the adverse impacts that dingoes can have on livestock producers. Further research is required to develop management strategies that can allow both for the maintenance of ecologically effective dingo populations while simultaneously minimizing their impacts on livestock producers.
Acknowledgements
We thank Brenton von Takach Dukai, Anna Feit, Nick Tong, Brad Nesbitt, Lynn Baker, Kylie Madden and staff from NSW National Parks and Wildlife Service for their assistance.
Animal census procedures were in accordance with Australian laws under the Animal Research Authority: University of Western Sydney: A9199.
Data accessibility
Data files containing necessary data to perform meta-analysis and structural equation model are included in the electronic supplementary material, S1 and S2.
Funding statement
This work is financially supported by the Australian Research Council.
References
- 1.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306 (doi:10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 2.Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH. 2007. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315, 1846–1850 (doi:10.1126/science.1138657) [DOI] [PubMed] [Google Scholar]
- 3.Creel S, Christianson D. 2008. Relationships between direct predation and risk effects. Trends Ecol. Evol. 23, 194–201 (doi:10.1016/j.tree.2007.12.004) [DOI] [PubMed] [Google Scholar]
- 4.Elmhagen B, Ludwig G, Rushton SP, Helle P, Lindén H. 2010. Top predators, mesopredators and their prey: interference ecosystems along bioclimatic productivity gradients. J. Anim. Ecol. 79, 785–794 (doi:10.1111/j.1365-2656.2010.01678.x) [DOI] [PubMed] [Google Scholar]
- 5.Ripple WJ, et al. 2014. Status and ecological effects of the world's largest carnivores. Science 343, 1241484 (doi:10.1126/science.1241484) [DOI] [PubMed] [Google Scholar]
- 6.Terborgh J, et al. 2001. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926 (doi:10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- 7.Ritchie EG, Elmhagen B, Glen AS, Letnic M, Ludwig G, McDonald RA. 2012. Ecosystem restoration with teeth: what role for predators? Trends Ecol. Evol. 27, 265–271 (doi:10.1016/j.tree.2012.01.001) [DOI] [PubMed] [Google Scholar]
- 8.Hanna E, Cardillo M. In press Island mammal extinctions are determined by interactive effects of life history, island biogeography and mesopredator suppression. Glob. Ecol. Biogeogr. (doi:10.1111/geb.12103) [Google Scholar]
- 9.Schmitz OJ, Hamback PA, Beckerman AP. 2000. Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am. Nat. 155, 141–153 (doi:10.1086/303311) [DOI] [PubMed] [Google Scholar]
- 10.Ritchie EG, Johnson CN. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998 (doi:10.1111/j.1461-0248.2009.01347.x) [DOI] [PubMed] [Google Scholar]
- 11.Letnic M, Ritchie EG, Dickman CR. 2012. Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study. Biol. Rev. 87, 390–413 (doi:10.1111/j.1469-185X.2011.00203.x) [DOI] [PubMed] [Google Scholar]
- 12.Sinclair ARE, Pech RP. 1996. Density dependence, stochasticity, compensation and predator regulation. Oikos 75, 164–173 (doi:10.2307/3546240) [Google Scholar]
- 13.Shurin JB, Gruner DS, Hillebrand H. 2006. All wet or dried up? Real differences between aquatic and terrestrial food webs. Proc. R. Soc. B 273, 1–9 (doi:10.1098/rspb.2005.3377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borer ET, Seabloom EW, Shurin JB, Anderson KE, Blanchette CA, Broitman B, Cooper SD, Halpern BS. 2005. What determines the strength of a trophic cascade? Ecology 86, 528–537 (doi:10.1890/03-0816) [Google Scholar]
- 15.Woodroffe R. 2000. Predators and people: using human densities to interpret declines of large carnivores. Anim. Conserv. 3, 165–173 (doi:10.1111/j.1469-1795.2000.tb00241.x) [Google Scholar]
- 16.Frank KT, Petrie B, Choi JS, Leggett WC. 2005. Trophic cascades in a formerly cod-dominated ecosystem. Science 308, 1621–1623 (doi:10.1126/science.1113075) [DOI] [PubMed] [Google Scholar]
- 17.Fleming PJ, Bomford M, Trust NH. 2001. Managing the impacts of dingoes and other wild dogs. Canberra, Australia: Bureau of Rural Sciences [Google Scholar]
- 18.Robertshaw JD, Harden RH. 1986. The ecology of the dingo in north eastern New South Wales. IV. Prey selection by dingoes, and its effect on the major prey species, the swamp wallaby, Wallabia bicolor (Desmarest). Aust. Wildl. Res. 13, 141–163 (doi:10.1071/WR9860141) [Google Scholar]
- 19.Johnson CN, VanDerWal J. 2009. Evidence that dingoes limit abundance of a mesopredator in eastern Australian forests. J. Appl. Ecol. 46, 641–646 (doi:10.1111/j.1365-2664.2009.01650.x) [Google Scholar]
- 20.Letnic M, Koch F, Gordon C, Crowther MS, Dickman CR. 2009. Keystone effects of an alien top-predator stem extinctions of native mammals. Proc. R. Soc. B 276, 3249–3256 (doi:10.1098/rspb.2009.0574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy JE. 2003. Biodiversity loss, trophic skew and ecosystem functioning. Ecol. Lett. 6, 680–687 (doi:10.1046/j.1461-0248.2003.00494.x) [Google Scholar]
- 22.Finke DL, Denno RF. 2004. Predator diversity dampens trophic cascades. Nature 429, 407–410 (doi:10.1038/nature02554) [DOI] [PubMed] [Google Scholar]
- 23.Keith D. 2004. Ocean shores to desert dunes: the native vegetation of New South Wales and the ACT. Hurstville, Australia: Department of Environment and Climate Change [Google Scholar]
- 24.Catling PC, Burt RJ, Kooyman R. 1997. A comparison of techniques used in a survey of the ground-dwelling and arboreal mammals in forests in north-eastern New South Wales. Wildl. Res. 24, 417–432 (doi:10.1071/WR96073) [Google Scholar]
- 25.Catling PC, Burt RJ. 1994. Studies of the ground-dwelling mammals of eucalypt forests in south-eastern New South Wales—the species, their abundance and distribution. Wildl. Res. 21, 219–239 (doi:10.1071/WR9940219) [Google Scholar]
- 26.Woolnough A. 2005. Comparison of two techniques to survey macropod abundance in an ecologically sensitive habitat. Aust. Mammal. 27, 69–72 (doi:10.1071/AM05069) [Google Scholar]
- 27.Wayne AF, Cowling A, Rooney JF, Ward CG, Wheeler IB, Lindenmayer DB, Donnelly CF. 2005. Factors affecting the detection of possums by spotlighting in Western Australia. Wildl. Res. 32, 689–700 (doi:10.1071/Wr04089) [Google Scholar]
- 28.Johnson CN, Jarman PJ, Southwell CJ. 1987. Macropod studies at Wallaby Creek. 5. Patterns of defecation by eastern gray kangaroos and red-necked wallabies. Wildl. Res. 14, 133–138 (doi:10.1071/WR9870133) [Google Scholar]
- 29.Terpstra JW, Wilson AD. 1989. Grazing distribution of sheep and kangaroos in a semi-arid woodland. Appl. Anim. Behav. Sci. 24, 343–352 (doi:10.1016/0168-1591(89)90061-0) [Google Scholar]
- 30.Fox BJ, Fox MD, Taylor JE, Jackson GP, Simpson J, Higgs P, Rebec L, Avery R. 1996. Comparison of regeneration following burning, clearing or mineral sand mining at Tomago, NSW: I. Structure and growth of the vegetation. Aust. J. Ecol. 21, 184–199 (doi:10.1111/j.1442-9993.1996.tb00599.x) [Google Scholar]
- 31.Austin MP, Meyers JA. 1996. Current approaches to modelling the environmental niche of eucalypts: implication for management of forest biodiversity. For. Ecol. Manag. 85, 95–106 (doi:10.1016/S0378-1127(96)03753-X) [Google Scholar]
- 32.Gurevitch J, Hedges LV. 1999. Statistical issues in ecological meta-analyses. Ecology 80, 1142–1149 (doi:10.1890/0012-9658(1999)080[1142:SIIEMA]2.0.CO;2) [Google Scholar]
- 33.Wootton JT. 1997. Estimates and tests of per capita interaction strength: diet, abundance, and impact of intertidally foraging birds. Ecol. Monogr. 67, 45–64 (doi:10.1890/0012-9615(1997)067[0045:EATOPC]2.0.CO;2) [Google Scholar]
- 34.Quin GP, Keough MJ. 2011. Experimental design and data analysis for biologists. New York, NY: Cambridge University Press [Google Scholar]
- 35.Rosenberg MS, Adams DC, Gurevitch J. 2000. MetaWin: statistical software for meta-analysis. Version 2. Sunderland, MA: Sinauer Associates [Google Scholar]
- 36.Grace JB. 2006. Structural equation modeling and natural systems. Cambridge, UK: Cambridge University Press [Google Scholar]
- 37.Grace JB, Schoolmaster DR, Guntenspergen GR, Little AM, Mitchell BR, Miller KM, Schweiger EW. 2012. Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 3, 73 (doi:10.1890/es12-00048.1) [Google Scholar]
- 38.Pasanen-Mortensen M, Pyykönen M, Elmhagen B. 2013. Where lynx prevail, foxes will fail—limitation of a mesopredator in Eurasia. Glob. Ecol. Biogeogr. 22, 868–877 (doi:10.1111/geb.12051) [Google Scholar]
- 39.Mitchell BD, Banks PB. 2005. Do wild dogs exclude foxes? Evidence for competition from dietary and spatial overlaps. Austral Ecol. 30, 581–591 (doi:10.1111/j.1442-9993.2005.01473.x) [Google Scholar]
- 40.Glen AS, Dickman CR. 2005. Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biol. Rev. 80, 387–401 (doi:10.1017/S1464793105006718) [DOI] [PubMed] [Google Scholar]
- 41.Risbey DA, Calver MC, Short J, Bradley JS, Wright IW. 2000. The impact of cats and foxes on the small vertebrate fauna of Heirisson Prong, Western Australia. II. A field experiment. Wildl. Res. 27, 223–235 (doi:10.1071/WR98092) [Google Scholar]
- 42.Brook LA, Johnson CN, Ritchie EG. 2012. Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression. J. Appl. Ecol. 49, 1278–1286 (doi:10.1111/j.1365-2664.2012.02207.x) [Google Scholar]
- 43.Banks PB. 2001. Predation-sensitive grouping and habitat use by eastern grey kangaroos: a field experiment. Anim. Behav. 61, 1013–1021 (doi:10.1006/anbe.2001.1686) [Google Scholar]
- 44.Dexter N, Meek P, Moore S, Hudson M, Richardson H. 2007. Population responses of small and medium sized mammals to fox control at Jervis Bay, Southeastern Australia. Pac. Conserv. Biol. 13, 283 [Google Scholar]
- 45.Lindenmayer DB, Blanchard W, McBurney L, Blair D, Banks SC, Driscoll D, Smith AL, Gill AM. 2013. Fire severity and landscape context effects on arboreal marsupials. Biol. Conserv. 167, 137–148 (doi:10.1016/j.biocon.2013.07.028) [Google Scholar]
- 46.Dexter N, Hudson M, James S, MacGregor C, Lindenmayer DB. 2013. Unintended consequences of invasive predator control in an Australian forest: overabundant wallabies and vegetation change. PLoS ONE 8, e69087 (doi:10.1371/journal.pone.0069087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catling PC, Coops N, Burt RJ. 2001. The distribution and abundance of ground-dwelling mammals in relation to time since wildfire and vegetation structure in south-eastern Australia. Wildl. Res. 28, 555–565 (doi:10.1071/WR00041) [Google Scholar]
- 48.Cork SJ, Catling PC. 1996. Modelling distribution of arboreal and ground-dwelling mammals in relation to climate, nutrients, plant chemical defences and vegetation structure in the eucalypt forests of south-eastern Australia. For. Ecol. Manage 85, 163–175 (doi:10.1016/S0378-1127(96)03757-7) [Google Scholar]
- 49.Lindenmayer DB, et al. 2008. Contrasting mammal responses to vegetation type and fire. Wildl. Res. 35, 395–408 (doi:10.1071/Wr07156) [Google Scholar]
- 50.Letnic M, Dworjanyn SA. 2011. Does a top predator reduce the predatory impact of an invasive mesopredator on an endangered rodent? Ecography 34, 827–835 (doi:10.1111/j.1600-0587.2010.06516.x) [Google Scholar]
- 51.Kennedy M, Phillips BL, Legge S, Murphy SA, Faulkner RA. 2012. Do dingoes suppress the activity of feral cats in northern Australia? Austral Ecol. 37, 134–139 (doi:10.1111/j.1442-9993.2011.02256.x) [Google Scholar]
- 52.Dexter N, Ramsey DSL, MacGregor C, Lindenmayer D. 2012. Predicting ecosystem wide impacts of wallaby management using a fuzzy cognitive map. Ecosystems 15, 1363–1379 (doi:10.1007/s10021-012-9590-7) [Google Scholar]
- 53.Fox BJ. 1982. Fire and mammalian secondary succession in an Australian coastal heath. Ecology 63, 1332–1341 (doi:10.2307/1938861) [Google Scholar]
- 54.Dexter N, Murray A. 2009. The impact of fox control on the relative abundance of forest mammals in East Gippsland, Victoria. Wildl. Res. 36, 252–261 (doi:10.1071/WR08135) [Google Scholar]
- 55.Johnson C. 2006. Australia's mammal extinctions: a 50 000 year history. Melbourne, Australia: Cambridge University Press [Google Scholar]
- 56.Pedersen S, Andreassen HP, Keith DA, Skarpe C, Dickman CR, Gordon IJ, Crowther MS, McArthur C. In press. Relationships between native small mammals and native and introduced large herbivores. Austral Ecol. (doi:10.1111/aec.1207) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data files containing necessary data to perform meta-analysis and structural equation model are included in the electronic supplementary material, S1 and S2.