Abstract
A huge variety of organisms respond to the presence of predators with inducible defences, each of which is associated with costs. Many genotypes have the potential to respond with more than one defence, and it has been argued that it would be maladaptive to exhibit all possible responses at the same time. Here, we test how a well-known anti-fish defence in Daphnia, life-history changes (LHC), is controlled by light. We show that the kairomone-mediated reduction in size at first reproduction is inversely coupled to the light intensity. A similar effect was found for the kairomone-mediated expression of candidate genes in Daphnia. We argue that the light intensity an individual is exposed to determines the degree of LHC, which allows for plastic adjustment to fluctuating environments and simultaneously minimizes the associated costs of multiple alternately deployable defences. It is hypothesized that this allows for a coupling of multiple defences, i.e. LHC and diel vertical migration.
Keywords: daphnia, fish kairomone, life history, diel vertical migration, coupling
1. Introduction
In a fluctuating environment, an individual's probability of survival depends on its ability to cope with environmental changes; a high degree of phenotypic plasticity enhances the fitness of an individual in such a fluctuating environment. If the modified phenotype exhibits a higher fitness than the unmodified one, then phenotypic plasticity is adaptive [1]. Predators are potent agents of natural selection in biological communities. The introduction of predators has been shown to cause rapid evolution of defensive behaviours in prey [2–5]. Although predation in freshwater systems is fluctuating seasonally in intensity and manner [6–8], it has led to a variety of adaptive phenotypic defences in aquatic prey organisms [9,10]. In particular, Daphnia—an important prey organism for planktivorous fish in standing freshwaters—shows a wide range of anti-predator defences, for example, life-history changes (LHC), morphology and behaviour [11]. These defences have been shown to be inducible by chemical cues released by fish, the so-called kairomones [12]. Fish-produced kairomones (hereafter referred to as ‘fish kairomones’) affect the resource allocation in Daphnia, which leads to LHC, for example, a reduced body size at first reproduction (SFR) [8]. A smaller body size increases the chance of survival owing to the selection for large prey by visually hunting predators such as fish [6]. Furthermore, fish kairomones enhance diel vertical migration (DVM) in Daphnia [13], a widespread adaptive migration behaviour, which reduces the encounter with visually hunting fish: owing to DVM, Daphnia reside in the deep, dark hypolimnion of stratified lakes during the day and spend the night in the epilimnion [14,15]. The induction of DVM depends on a relative change in light intensity [16,17].
Inducible defences allow prey to reap the benefits of defence while avoiding potential costs associated with investment in the defensive strategy when it is not needed. Such phenotypic benefits and costs have been demonstrated for inducible life-history and behavioural defences in Daphnia: the inducible character of LHC and DVM allows Daphnia to adjust the defence strategy only when required, saving substantial costs. LHC lead to an adaptive reduction in SFR [18], which goes along with a lower quality of the eggs [19], and DVM results in demographic costs owing to the daytime residence in the deep and cold water layers of stratified lakes [20,21]. The finding that the amplitude of DVM increased by chemically mediated predator density [21,22] indicates that the extent of the defence is the result of integrating costs and benefits of inducible defences.
A comparison of different clones of Daphnia magna revealed that all genotypes had the potential to respond with more than one plastic trait to the presence of fish kairomones [23]. However, exhibiting multiple defences at the same time may not be adaptive if a single defence is sufficient for protection against fish [24]. De Meester et al. [25] found variability within populations with respect to the extent of LHC and DVM by fish kairomones, which suggests the coexistence of clones that exhibit one or the other defence.
Further support for a coadaptation between size-related life-history traits and DVM comes when different lakes are compared. Under high risk of predation by visually hunting predators, DVM seems to be preferred as a defence over the reduction of SFR [26]. In habitats in which DVM was not possible owing to lake morphometry or hypolimnetic anoxia which prevent migration into the deeper strata, daphnids underwent LHC resulting in an earlier reproduction at a smaller size [27]. Despite this strong evidence for a coadaptation of DVM and size-related LHC, it remained to be tested whether the degree of performing DVM and LHC as defence against fish was fixed for a given genotype, or if genotypes would be plastic with respect to the coupling of these anti-predator responses.
Evidence for a plastic coupling of size-related life-history responses and DVM comes from a population of Daphnia catawba which performed DVM when exposed to fish kairomones; however, when a net barrier prevented migration into deeper strata, a decrease in SFR was observed [28]. These findings demonstrated that DVM and LHC were not strictly coupled; instead, the animals were plastic with respect to the degree of responding with either defence.
Here, we have investigated how LHC are suppressed under low light levels. We hypothesize that the light level a given Daphnia genotype is exposed to during a large part of the day determines the degree of LHC. Accordingly, low light levels would lead to no or very low LHC, and high light levels would lead to stronger LHC in response to kairomones from fish. We therefore performed life-history experiments in the presence and absence of fish kairomone extracts under different light conditions and used the size at first reproduction as parameter for the LHC. We further explored the relevance of light for kairomone-induced changes in Daphnia by analysing effects on gene expression of actin, cyclophilin and HSPs, which are all known to play a role in the response of Daphnia to fish [29–31].
2. Material and methods
(a). Test species and cultures
Daphnia magna clone B from Lake Binnensee, Germany [32] was cultured at 20°C in aged, membrane-filtered (pore size: 0.45 µm) tap water under dim light. It has been shown previously that D. magna clone B responds to fish kairomones with LHC [8] and with DVM behaviour in indoor experiments [21,33] similar to more pelagic Daphnia species [34]. Twelve animals per litre were kept under non-limiting food concentrations: 2 mg Cpart l−1 of the green algae Chlamydomonas klinobasis, strain 56, culture collection of the Limnological Institute at the University of Konstanz. C. klinobasis was grown in 5 l semi-continuous batch cultures (20°C; illumination: 120 µmol m−2 s−1) by replacing 20% of the culture with fresh, sterile Cyano medium [35] every other day. The test animals originated from mothers that had been raised under control conditions (saturating concentrations of Chlamydomonas sp.) for at least five generations.
(b). Experimental set-up
(i). Fish kairomone extract
Three Perca fluviatilis (body size: 10–12 cm) were pre-conditioned for 24 h without food and then kept for 24 h in 8 l of aged tap water at 18°C without feeding. The water containing fish kairomones was filtered through membrane filters (pore size: 0.45 µm). For bulk enrichment of the kairomones, a C18 solid-phase cartridge (10 g of sorbent, volume 60 ml, end-capped, Varian Mega Bond Elut, Agilent Technologies) was pre-conditioned with 50 ml methanol and 50 ml ultrapure water prior to adding the sample. Methanol was added to the filtered incubation water containing fish kairomones to obtain a 1% concentration, and 2 l of sample was passed through the cartridge. The loaded cartridge was washed with 50 ml of ultrapure water and then eluted with 50 ml of methanol. The eluates originating from 10 l of fish incubation water were pooled and evaporated to dryness using a rotary evaporator, re-dissolved in 1 ml of absolute ethanol and tested for biological activity. Water without fish was used for the production of a control extract. The same standardized extracts of control water and fish incubation water in the concentration equivalent of three fish in 8 l of water were used for all experiments.
(ii). Life-history experiments
Test animals originated from the third clutch and had been released within 12 h. The neonates were maintained at 24 ind. l−1 and were transferred daily to new water supplemented with algal food (2 mg C l−1) and kept under permanent dim light at 20°C until day three, at which time the cohort was divided and kept either under dim light (0.48 µmol s−1 m−2) or dark (less than 0.1 µmol s−1 m−2) conditions. On the fifth day, five animals were exposed to either control water extract or fish water extract in 250 ml in the presence (0.48 µmol s−1 m−2) and absence (less than 0.1 µmol s−1 m−2) of light without a photoperiod until the first clutch was visible. The pre-conditioned animals from the dim light cohort were used for the light treatment, and the animals from the dark cohort were used for the dark treatment in the experiment. All treatments were run in triplicate, and D. magna were fed daily. The size at first reproduction (SFR) was measured for each egg-bearing individual (from the top of the eye to the base of the tailspine) with the aid of a dissecting microscope equipped with a digital camera (Imaging Source) and image analysis software. A mean SFR was calculated for each replicate; these mean values were used to calculate the respective mean value and the variance for the treatment. Somatic growth rates were calculated according to Wacker & Von Elert [36]:
where dw is the body dry weight of a subsample of the animals at the beginning (dw0) and end (dwt) of the experiment and d is length of the experiment in days. Mean individual dry weights were mean values of three individuals (dw0 is the same for all treatments, and dwt is specific for each replicate).
(c). RNA extraction and reverse transcription
The test animals were kept as described above. On day five, 10 animals were transferred into 200 ml containing either fish water or control water extract in the presence or absence of light. After 1, 2 and 4 h, subsamples of three animals were used for RNA extraction. The experiment was run in triplicate. RNA was extracted and purified using the NucleoSpin RNA II kit (Macherey and Nagel). The integrity of each RNA sample was verified using the 2200 Tape Station (Agilent Technologies) on a high sensitivity screen tape, and the RIN values ranged from 7.5 to 8.5. The RNA concentration was determined using the Nanodrop ND-1000 v. 3.7 (Thermo Scientific). RNA (1 µg) was reverse-transcribed using the high-capacity cDNA reverse transcription kit (Applied Biosystems). The cDNA was stored at −20°C.
(d). Quantitative PCR
28S ribosomal RNA (28S), alpha-tubulin (α-tubulin), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and TATA-box binding protein (TBP) were used as endogenous control genes of constitutive expression in D. magna [29,37]. Cyclophilin was used as a candidate gene in our qPCR analysis. To discover the other candidate genes, the D. magna sequences for actin (accession no. AJ292554) and HSPs (accession no. EU514494, DQ845268) were used as queries for sequence similarity searches using BLASTn against the D. magna assembly v. 2.4 in wFleaBase. All gene sequences with a significant hit were aligned with Geneious v. 6.0.3 (Biomatters Ltd.). Primers were designed with Primer 3 v. 2.3.5 and the quality checked with NetPrimer (Premier Biosoft; table 1). Melting curve analyses confirmed specific amplification without primer dimer formation. The data acquisition for the relative expression was performed on a 7300 qPCR system (Applied Biosystems). Each reaction contained 5 ng of cDNA template, 10 µl SYBR green PCR master mix and 2.5 µM of each primer in a final volume of 20 µl. Cycling parameters were 95°C for 10 min for the initial start of the DNA polymerase, followed by 40 cycles of 95°C for 15 s, 55°C for 30 s, 68°C for 30 s, and a final dissociation step with 95°C for 15 s, 55°C for 30 s, 68°C for 30 s and 95°C for 15 s. The baseline and threshold for the cycle threshold (Ct) was set automatically, and the each gene was tested in triplicate. Amplification efficiencies for every primer pair of each candidate gene were determined.
Table 1.
Gene IDs and primers for the qPCR analysis
| gene ID | wFleaBase D. magna assembly v. 2.4 | GenBank accession no. | forward primer (5′–3′) | reverse primer (5′–3′) |
|---|---|---|---|---|
| actin 1 | WFes0011739 | AJ292554 | CCTCCTCTCCCCCTTTCATA | GATGTGGATCTCCAAACAGGA |
| actin 2 | WFes0005512 | GAATTCATGTCACTTCCAAGTCC | TTAATTGGCCGTTCTCTTGA | |
| actin 3 | WFes0009949 | GGGAGCTTCAGTCAGGAGAA | CCCAGTCCAAACGTGGTATT | |
| actin 4 | WFes0003283 | CGATCCATACGGAGTACTTGC | AGGATCTGTACGCCAACACC | |
| cyclophilin | WFes0012034 | GACTTTCCACCAGTGCCATT | AACTTTCCATCGCATCATCC | |
| HSP 10 | WFes0002064 | CATGGTTGTTGCAGTTGGAC | AGTGCCACCATACTCAGGAA | |
| HSP 70 | WFes0008791 | EU514494 | AAGATGAAGGAGACGGCTGA | CTGCATCCTTTGTTGCTTGA |
| HSP 90 | WFes0106938 | DQ845268 | CAAGGCTGATTTGGTCAATAAC | GCAACCAGGTAAGCCGAGTA |
| alpha-tubulin | WFes0007807 | TGGAGGTGGTGACGACT | CCAAGTCGACAAAGACAGCA | |
| GADPH | AJ292555 | GGCAAGCTAGTTGTCAATGG | TATTCAGCTCCAGCAGTTCC | |
| TBP | WFes0002485 | GCAGGGAAGTTTAGTTTCTGGA | TGGTATGCACAGGAGCAAAG | |
| 28S | AF532883 | GAGGCGCAATGAAAGTGAAG | TGTTCGAGACGGGATCA |
(e). Data analysis and statistics
After qPCR, raw data were analysed with qBasePLUS v. 2.0 (Biogazelle) based on qBase [38] and geNorm [39]. The relative gene expression of candidate genes was normalized with the generated normalization factor, and standard errors were calculated. A repeated-measurement analysis of variances (RM-ANOVA) for the qPCR data of each candidate gene generated with the qBasePLUS software was conducted to analyse the effects of the factors ‘treatment’ and ‘time’ on the mean relative expression (table 2). The dependent variable was checked for homogeneity of variances (Levene test). A single analysis of variance (one-way ANOVA) was carried out for the qPCR data generated with the qBasePLUS software of each gene after 2 h of exposure to extract of fish incubation water in the absence and presence of light. The dependent variable was checked for homogeneity of variances (Shapiro–Wilk). A two-way analysis of variance (two-way ANOVA) was conducted for the life-history experiments. The dependent variables were checked for homogeneity of variances (Shapiro–Wilk). The effect of single treatments was tested by post hoc tests (Tukey's HSD multiple comparison test) at the same probability level as the respective analysis of variance. A significance level of p = 0.05 was applied to all statistical analyses. All statistics were performed with SigmaPlot v. 11.0 (Systat Software) and Statistica v. 6.0 (Starsoft Inc.).
Table 2.
Results of repeated-measurement analyses of variances (RM-ANOVA) of the mean relative candidate gene expression in D. magna. All analyses compared the treatment (exposure of animals to extract of either control water or to fish incubation water) to a time series (1, 2 and 4 h).
| SS | d.f. | F | p | ||
|---|---|---|---|---|---|
| actin 2 | |||||
| treatment | 3.484 | 1 | 5.729 | 0.096 | n.s. |
| error | 1.825 | 3 | |||
| time | 11.946 | 2 | 7.730 | 0.022 | * |
| time × treatment | 2.866 | 2 | 1.854 | 0.236 | n.s. |
| error | 4.636 | 6 | |||
| actin 3 | |||||
| treatment | 0.461 | 1 | 25.450 | 0.015 | * |
| error | 0.054 | 3 | |||
| time | 0.999 | 2 | 15.839 | 0.004 | ** |
| time × treatment | 0.951 | 2 | 15.079 | 0.005 | ** |
| error | 0.189 | 6 | |||
| actin 4 | |||||
| treatment | 0.006 | 1 | 0.334 | 0.604 | n.s. |
| error | 0.056 | 3 | |||
| time | 0.080 | 2 | 1.740 | 0.254 | n.s. |
| time × treatment | 0.077 | 2 | 1.675 | 0.264 | n.s. |
| error | 0.139 | 6 | |||
| cyclophilin | |||||
| treatment | 0.338 | 1 | 19.908 | 0.021 | * |
| error | 0.051 | 3 | |||
| time | 1.435 | 2 | 17.352 | 0.003 | ** |
| time × treatment | 1.451 | 2 | 17.544 | 0.003 | ** |
| error | 0.248 | 6 | |||
Asterisks indicate significant differences (*p < 0.05; **p < 0.01).
3. Results
(a). Life-history and growth experiments
In full factorial life-history experiments, D. magna was grown in the presence of control water extract or extract of fish incubation water (P. fluviatilis) in the presence or absence of light. Size at first reproduction (SFR) decreased to 2.48 from 2.59 mm in the presence of fish kairomones and light (figure 1a), but did not in the control extract. This kairomone-mediated reduction in SFR was not observed in the absence of light (figure 1a). SFR was affected by the factors ‘light’ (two-way ANOVA, factor light: F1,11: 5.805, p < 0.05) and ‘fish water extract’ (two-way ANOVA, factor extract: F1,11: 8.883, p < 0.05), and both factors together (two-way ANOVA: F1,11: 6.087, p < 0.05). Juvenile somatic growth rates of D. magna were neither affected by light (two-way ANOVA, factor light: F1,11: 0.956, p = 0.357; figure 1b) nor by fish water extract (two-way ANOVA, factor extract: F1,11: 0.376, p = 0.557; figure 1b). The daphnids were exposed either to control water extract or fish incubation water extract on day five of the experiment, and one moult per individual was observed in all treatments prior to deposition of eggs to the brood pouch.
Figure 1.
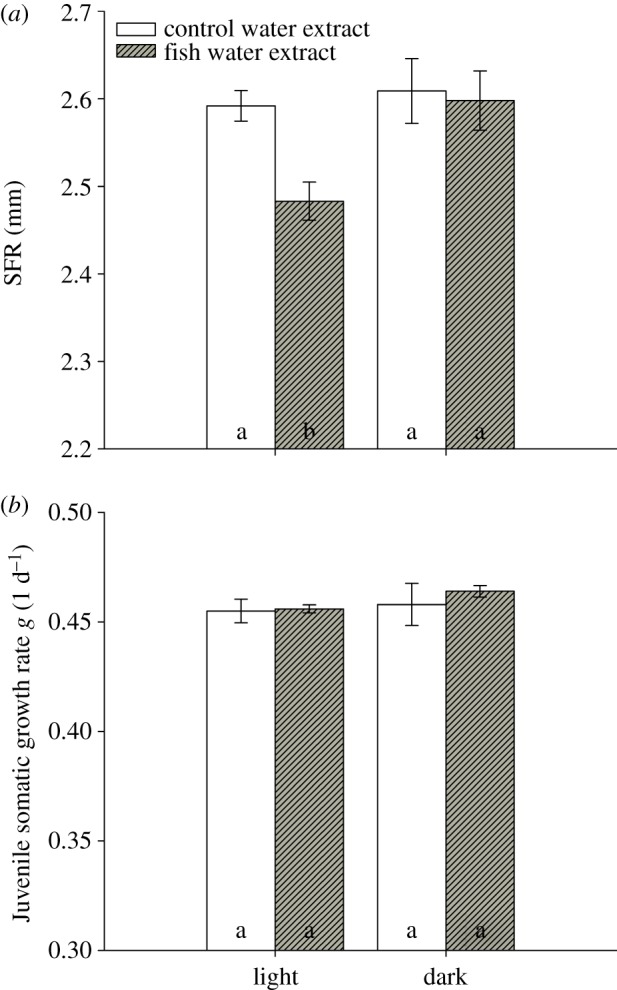
Size at first reproduction (SFR) and juvenile somatic growth rate g of Daphnia magna grown in the presence or absence of fish kairomones. Daphnia were exposed to control water extract (open bars) or to fish incubation water extract (filled bars) in light or dark. (a) Size at first reproduction (SFR; n = 3, mean ± s.e.), (b) Juvenile somatic growth rate g (n = 3, mean ± s.e.). Different letters denote a significant difference (p < 0.05) between the treatments. (Online version in colour.)
(b). Relative expression of candidate genes
The relative expression of the candidate genes actin 1–4 and cyclophilin in 5-day-old D. magna was monitored 1, 2 and 4 h after exposure to control water extract or extract of fish incubation water. The genes 28S, TBP, α-tubulin and GAPDH served as endogenous control genes in all qPCR analyses and were used for calculation of the normalization factor. The qPCR analysis revealed no significant difference in expression levels of candidate genes after 1 h of exposure to fish water extract compared with control water extract. After 2 h of exposure, a significant increase in expression in response to kairomones was found for the genes actin 3 (RM ANOVA: F1,6 = 25.450, p < 0.01; figure 2) and cyclophilin (RM ANOVA: F1,6 = 19.908, p < 0.05; figure 2). The gene actin 1 was not tested, because the assumption of variance homogeneity was not fulfilled. After 4 h of exposure to fish incubation water extract, a significant decrease in expression for cyclophilin compared with the control water extract was observed (RM ANOVA: F1,6 = 19.908, p < 0.05; figure 2). Expression of the HSP genes (HSP 10, HSP 70, HSP 90) which served as proxy genes for a more general stress response [40], was not differently affected by exposure to extract of fish incubation water or control water after 1, 2 or 4 h (data not shown). The results of this time series indicated that effects of fish kairomones on gene expression were most likely to be observed after 2 h of exposure.
Figure 2.
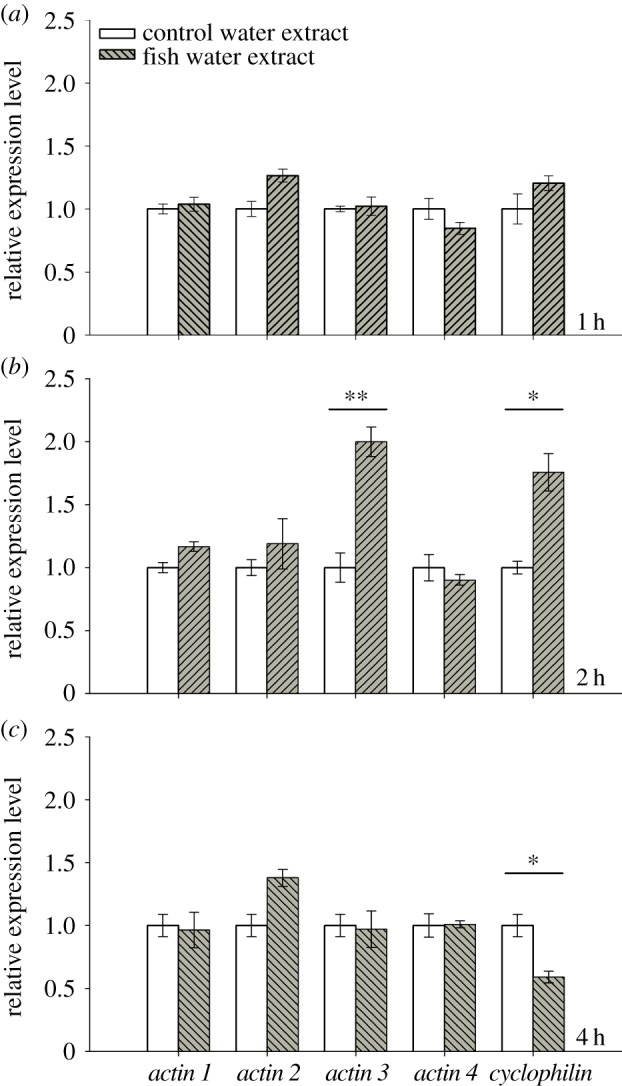
Mean relative expression of candidate genes in Daphnia magna. The expression pattern of the candidate genes actin 1–4 and cyclophilin (n = 3, mean ± s.e.) was determined 1, 2 and 4 h after exposure to extract of control water (open bars) or to fish incubation water (filled bars). Asterisks indicate a significant difference between control and kairomones (*p < 0.05; **p < 0.01). (Online version in colour.)
Building on the results of the time series described above, the effect of light on the kairomone-mediated increase of gene expression in D. magna was quantified after 2 h of exposure to fish incubation water extract in the presence and absence of light. The genes actin 2 (one-way ANOVA: F1,5 = 45.230, p < 0.01), actin 3 (one-way ANOVA: F1,5 = 21.305, p < 0.05) and cyclophilin (one-way ANOVA: F1,5 = 23.673, p < 0.01) showed significantly increased expression in the presence of light compared with the dark treatment (figure 3). Similar to these significant effects, mean expression values for actin 1 and actin 4 were higher in the presence of light, although this difference was not significant. These results revealed that the kairomone-mediated effects on gene expression in D. magna were affected by light.
Figure 3.
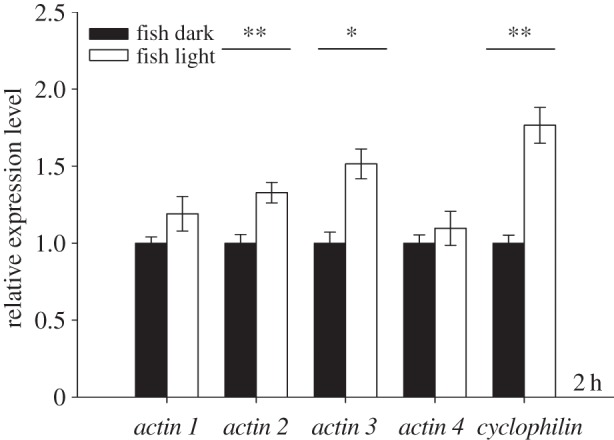
Mean relative expression (n = 3, ±s.e.) of candidate genes in Daphnia magna after 2 h of exposure to extract of fish incubation water in the absence (filled bars) or presence (open bars) of light. Asterisks indicate a significant difference between the control and kairomone treatment (*p < 0.05; **p < 0.01).
4. Discussion
Despite the well-known relevance of light for the induction of DVM, investigations of the induction of LHC have been confined to predator kairomones only [8,18,41]. It has been demonstrated that fish kairomones do not affect carbon assimilation in Daphnia [42] but lead to earlier allocation of assimilated resources into vitellogenin [43]. This earlier onset of vitellogenin synthesis happens at the expense of allocation to somatic growth and thus results in smaller SFR. The role of light as an environmental factor for LHC in response to predator kairomones might easily have been overlooked in the aforementioned studies, as all these investigations were performed in the presence of light.
Here, we show that the absence/presence of light has no immediate effect on somatic growth and SFR, a fact which indicates that resource allocation is not affected by the light intensities that Daphnia individuals are exposed to. Similarly, fish kairomones affected neither growth nor resource allocation in the absence of light; only in the presence of both light and kairomones was a reduction of SFR observed, demonstrating the interactive effect of the two cues. The significant reduction of SFR in the presence of light and kairomones clearly points towards changes in resource allocation as a cause for the smaller SFR observed. Despite the effect on the SFR no effect on the clutch size was found. Similar to our findings, fish kairomones did not affect clutch size but other life-history parameters (e.g. SFR, size of neonates) in several clones of D. magna [23], which demonstrates that clones differ in their responsive traits to fish kairomones.
Our result that LHC in D. magna (figure 1) occurred only in the presence of light suggests that the coupling between fish kairomone and LHC is modulated by light. Plasticity with respect to DVM [44,45] and LHC [46,47] caused by, for example, food conditions and different predation threats has been described. When Daphnia clones that are capable of using both defences were prevented from migration, they showed a decrease in SFR [28], indicating that a higher light intensity leads to a higher degree of life-history shifts. However, in this study, we studied the effect of presence and absence of light. The vertical migration in Daphnia is initiated by a relative rather than by an absolute change in light intensity [16], and the velocity of upward or downward migration in DVM is linearly related to a decrease or increase of this relative rate of change in light intensity [16,48,49]. If fish are present, then the kairomones are dispersed over the epilimnion all the time indicating predator presence, and thus provide neither a cue for the timing nor for the direction of migration. However, it was shown that the presence of kairomones released by fish leads to an increase in vertical displacement velocity [50,51] and thus alters the amplitude of DVM [48,49]. In the absence of changes in light intensity (i.e. permanent darkness), the presence of kairomones did not induce DVM [34], indicating that the pattern of DVM is not triggered by an endogenous rhythm in Daphnia but is rather caused by the diel changes in ambient light. Thus, the fish kairomone only does not cause DVM in Daphnia, but it is enhancing the amplitude of migration caused by changes in the relative light intensity.
Based on our findings, we hypothesize that the light intensity an individual Daphnia is exposed to results from its daytime residence depth, i.e. its DVM amplitude, which thereby determines the degree of LHC. Taking into account earlier observations that DVM is preferred over LHC [26,27], this coupling of DVM and life-history changes nicely explains the observations that Daphnia clones that were collected at daytime from the epilimnion showed a smaller SFR [52]. It remains to be seen if Daphnia genotypes differ with respect to light intensity thresholds for the induction of life history. However, in this study the experimental animals were exposed to constant light conditions to observe the putatively strongest effects, whereas in the field animals are subjected to alternating periods of light and dark under non-migrating conditions.
We conducted our approach for the fish kairomone effects on the gene expression of Daphnia with genes that were shown to be involved in the LHC response. These genes were announced to be candidate genes and expected to show a specific response in our experiments. Pijanowska & Kloc [30] found that Daphnia exposed to fish kairomones show a strong decrease in actin protein concentration. As a dynamic component of the cytoskeleton, actin could play a major role in the decrease of SFR (LHC) [8]. Here, we assumed that differences in actin protein level result from differences in gene expression, and we therefore investigated effects on the expression of actin in a time series after exposure to fish kairomones. For one actin gene, a significant increase in expression in the presence of fish kairomones was shown [29]. Here, we tested for effects of fish kairomones on expression of four actin paralogues in D. magna. The qPCR results revealed a strong response to fish kairomones and light on candidate gene expression in Daphnia after 2 h of exposure (figure 2) for actin 3, but not for the other three actin paralogues, indicating that the different paralogues are differently affected by kairomones from fish and for cyclophilin. It has been shown that the mRNA level of HSP genes in the arthropod Tribolium exposed to UV radiation was strongly affected after 2 h and the mRNA level decreased after 3.5 h [53], supporting our observed gene response over time in Daphnia. The significant increase of expression of one actin gene observed here and the decrease of overall actin protein [30] may be due to the different methods that were used or to the involvement of different actin paralogues: we were able to design specific primers for four of the 10 actin genes present in the D. magna genome, and a different actin paralogue could be responsible for the effect observed on the protein level. Cyclophilins are highly conserved and have been described as peptidyl–prolyl cis–trans isomerases. Acting as chaperones, they are involved in protein folding during protein biosynthesis [54]. Interestingly, in Drosophila the gene ninaA encodes a cyclophilin that resides in the endoplasmic reticulum and is associated with secretory vesicles, where it co-localizes stably with the photoreceptor Rh1 [55]. In addition, ninaA belongs to a group of genes (Nina group) that is involved in the biogenesis of G protein-coupled receptors (GPCRs) in Drosophila influencing photoconversion and transduction in the optical system of flies [56]. These well-known functions of cyclophilin in Drosophila, especially the role in the signal transduction of the optical system, might indicate the involvement of cyclophilin in the response of Daphnia to fish kairomone. We have shown that the presence of light is crucial for the induction of LHC, and therefore the response of a gene that is putatively involved in the perception of the light intensity differences in the optical system of Daphnia deserves further investigation.
HSPs are known to protect organisms against a wide array of environmental stressors and also to be involved in the response of Daphnia to fish [30,31]. We used three HSP genes (HSP 10, HSP 70, HSP 90) in our candidate gene approach to examine the effects on the expression of HSP genes. Surprisingly, none of these genes was affected by the presence of fish kairomones after 1, 2 or 4 h exposure (data not shown). Reports of effects of fish kairomones on HSP levels in Daphnia are confined to the protein level [30,31], and it remains to be tested whether or not the effects of fish kairomones on these three HSP genes investigated here occur after more than four hours of exposure.
We conducted life-history experiments with Daphnia using standardized extracts of either control water or fish incubation water. Effects of fish kairomones on life-history (SFR) and the expression of candidate genes, which are involved in the life-history response of Daphnia, were observed only in the presence of light, whereas somatic growth was not affected. Our observations point to a plastic coupling of anti-predator defences in Daphnia modulated by the factor ‘light’, as our experimental treatments ‘light’ and ‘darkness’ were meant to simulate weak migrators with a daytime residence in the epilimnion (‘light’) and strong migrators with a daytime residence in the hypolimnion (‘darkness’). We assume that in a scenario in which Daphnia are forced to descend deep under heavy fish pressure the amplitude of DVM alters the light level that a given genotype is exposed to. Such a proposed inverse coupling of LHC and DVM would be especially adaptive in habitats that allow for DVM only during parts of the season, i.e. spring; later in the season, when a deep-water refuge is no longer available owing to hypolimnetic anoxia, alternative defences such as the decrease in the SFR (as is frequently observed in eutrophic shallow systems) may be required. The potential to deploy more than one defence is widespread in Daphnia [23,28], and the mechanistic explanation for a coupling of multiple defences suggested here describes a new mode of possible adaptation of multiple alternately deployable defences.
Acknowledgements
We thank C. J. Küster for his excellent help in conducting the experiments, P. Fink and T. Sadler for their helpful comments on the manuscript, A. Schwarzenberger for providing the primers (HSP 10 and HSP 70) and Hanne Krisch for technical assistance.
References
- 1.Stearns SC. 1992. The evolution of life histories, p. 249 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Seeley RH. 1986. Intense natural-selection caused a rapid morphological transition in a living marine snail. Proc. Natl Acad. Sci. USA 83, 6897–6901 (doi:10.1073/pnas.83.18.6897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magurran AE, Seghers BH, Carvalho GR, Shaw PW. 1992. Behavioral consequences of an artificial introduction of guppies (Poecilia reticulata) in N. Trinidad: evidence for the evolution of antipredator behavior in the wild. Proc. R. Soc. Lond. B 248, 117–122 (doi:10.1098/rspb.1992.0050) [Google Scholar]
- 4.Gliwicz ZM. 1986. Predation and the evolution of vertical migration in zooplankton. Nature 320, 746–748 (doi:10.1038/320746a0) [Google Scholar]
- 5.Cousyn C, De Meester L, Colbourne JK, Brendonck L, Verschuren D, Volckaert F. 2001. Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc. Natl Acad. Sci. USA 98, 6256–6260 (doi:10.1073/pnas.111606798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks JL, Dodson SI. 1965. Predation, body-size and composition of plankton. Science 150, 28–35 (doi:10.1126/science.150.3692.28) [DOI] [PubMed] [Google Scholar]
- 7.Ringelberg J, Flik BJG, Lindenaar D, Royackers K. 1991. Diel vertical migration of Daphnia hyalina (sensu latiori) in Lake Maarsseveen: Part 1. Aspects of seasonal and daily timing. Arch. Hydrobiol. 121, 129–145 [Google Scholar]
- 8.Weider LJ, Pijanowska J. 1993. Plasticity of Daphnia life histories in response to chemical cues from predators. Oikos 67, 385–392 (doi:10.2307/3545351) [Google Scholar]
- 9.Dodson SI. 1989. Predator-induced reaction norms. Bioscience 39, 447–452 (doi:10.2307/1311136) [Google Scholar]
- 10.Harvell CD. 1990. The ecology and evolution of inducible defenses. Q. Rev. Biol. 65, 323–340 (doi:10.1086/416841) [DOI] [PubMed] [Google Scholar]
- 11.Lampert W. 1994. Chemische Induktion von Verteidigungsmechanismen bei Süsswassertieren. Naurwissenschaften 81, 375–382 (doi:10.1007/BF01132689) [Google Scholar]
- 12.Larsson P, Dodson SI. 1993. Invited review: chemical communication in planktonic animals. Arch. Hydrobiol. 129, 129–155 [Google Scholar]
- 13.Loose CJ. 1993. Daphnia diel vertical migration behavior: response to vertebrate predator abundance. Arch. Hydrobiol. Beihefte 39, 29–36 [Google Scholar]
- 14.Stich HB, Lampert W. 1981. Predator evasion as an explanation of diurnal vertical migration by zooplankton. Nature 293, 396–398 (doi:10.1038/293396a0) [Google Scholar]
- 15.Lampert W. 1989. The adaptive significance of diel vertical migration of zooplankton. Funct. Ecol. 3, 21–27 (doi:10.2307/2389671) [Google Scholar]
- 16.Ringelberg J. 1991. Enhancement of the phototactic reaction in Daphnia hyalina by a chemical mediated by juvenile perch (Perca fluviatilis). J. Plankton Res. 13, 17–25 (doi:10.1093/plankt/13.1.17) [Google Scholar]
- 17.van Gool E, Ringelberg J. 1995. Swimming of Daphnia-galeata x hyalina in response to changing light intensities: influence of food availability and predator kairomone. Mar. Freshwater Behav. Physiol. 26, 259–265 (doi:10.1080/10236249509378944) [Google Scholar]
- 18.Stibor H, Lüning J. 1994. Predator-induced phenotypic variability in the pattern of growth and reproduction in Daphnia hyalina (Crustacea: Cladocera). Funct. Ecol. 8, 97–101 (doi:10.2307/2390117) [Google Scholar]
- 19.Stibor H, Navarra DM. 2000. Constraints on the plasticity of Daphnia magna influenced by fish-kairomones. Funct. Ecol. 14, 455–459 (doi:10.1046/j.1365-2435.2000.00441.x) [Google Scholar]
- 20.Dawidowicz P, Loose CJ. 1992. Metabolic costs during predator-induced diel vertical migration of Daphnia. Limnol. Oceanogr. 37, 1589–1595 (doi:10.4319/lo.1992.37.8.1589) [Google Scholar]
- 21.Loose CJ, Dawidowicz P. 1994. Trade-offs in diel vertical migration by zooplankton: the costs of predator avoidance. Ecology 75, 2255–2263 (doi:10.2307/1940881) [Google Scholar]
- 22.Von Elert E, Pohnert G. 2000. Predator specificity of kairomones in diel vertical migration of Daphnia: a chemical approach. Oikos 88, 119–128 (doi:10.1034/j.1600-0706.2000.880114.x) [Google Scholar]
- 23.Boersma M, Spaak P, De Meester L. 1998. Predator-mediated plasticity in morphology, life history, and behavior of Daphnia: the uncoupling of responses. Am. Nat. 152, 237–248 (doi:10.1086/286164) [DOI] [PubMed] [Google Scholar]
- 24.De Meester L, Pijanowska J. 1996. On the trait-specificity of the response of Daphnia genotypes to the chemical presence of a predator. In Zooplankton: sensory ecology and physiology (eds Lenz PH, Hartline DK, Purcell JE, Macmillan DL.), pp. 407–417 Amsterdam, The Netherlands: Gordon & Breach [Google Scholar]
- 25.De Meester L, Weider LJ, Tollrian R. 1995. Alternative antipredator defences and genetic polymorphism in a pelagic predator–prey system. Nature 378, 483–485 (doi:10.1038/378483a0) [Google Scholar]
- 26.Vos M, Flik BJG, Vijverberg J, Ringelberg J, Mooij WM. 2002. From inducible defences to population dynamics: modelling refuge use and life history changes in Daphnia. Oikos 99, 386–396 (doi:10.1034/j.1600-0706.2002.990221.x) [Google Scholar]
- 27.Sakwinska O, Dawidowicz P. 2005. Life history strategy and depth selection behavior as alternative antipredator defenses among natural Daphnia hyalina populations. Limnol. Oceanogr. 50, 1284–1289 (doi:10.4319/lo.2005.50.4.1284) [Google Scholar]
- 28.Slusarczyk M, Pinel-Alloul B. 2010. Depth selection and life history strategies as mutually exclusive responses to risk of fish predation in Daphnia. Hydrobiologia 643, 33–41 (doi:10.1007/s10750-010-0133-y) [Google Scholar]
- 29.Schwarzenberger A, Courts C, Von Elert E. 2009. Target gene approaches: gene expression in Daphnia magna exposed to predator-borne kairomones or to microcystin-producing and microcystin-free Microcystis aeruginos. BMC Genomics 10, 527 (doi:10.1186/1471-2164-10-527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pijanowska J, Kloc M. 2004. Daphnia response to predation threat involves heat-shock proteins and the actin and tubulin cytoskeleton. Genesis: J. Genet. Dev. 38, 81–86 [DOI] [PubMed] [Google Scholar]
- 31.Pauwels K, Stoks R, De Meester L. 2005. Coping with predator stress: interclonal differences in induction of heat-shock proteins in the water flea Daphnia magna. J. Evol. Biol. 18, 867–872 (doi:10.1111/j.1420-9101.2005.00890.x) [DOI] [PubMed] [Google Scholar]
- 32.Lampert W, Rothhaupt KO. 1991. Alternating dynamics of rotifers and Daphnia magna in a shallow lake. Arch. Hydrobiol. 120, 447–456 [Google Scholar]
- 33.Bentkowski P, Markowska M, Pijanowska J. 2010. Role of melatonin in the control of depth distribution of Daphnia magna. Hydrobiologia 643, 43–50 (doi:10.1007/s10750-010-0134-x) [Google Scholar]
- 34.Loose CJ. 1993. Lack of endogenous rhythmicity in Daphnia diel vertical migration. Limnol. Oceanogr. 38, 1837–1841 (doi:10.4319/lo.1993.38.8.1837) [Google Scholar]
- 35.Von Elert E, Jüttner F. 1997. Phosphorus limitation not light controls the exudation of allelopathic compounds by Trichormus doliolum. Limnol. Oceanogr. 42, 1796–1802 (doi:10.4319/lo.1997.42.8.1796) [Google Scholar]
- 36.Wacker A, Von Elert E. 2001. Polyunsaturated fatty acids: Evidence for non-substitutable biochemical resources in Daphnia galeata. Ecology 82, 2507–2520 [Google Scholar]
- 37.Heckmann LH, Connon R, Hutchinson TH, Maund SJ, Sibly RM, Callaghan A. 2006. Expression of target and reference genes in Daphnia magna exposed to ibuprofen. BMC Genomics 7, 175 (doi:10.1186/1471-2164-7-175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19 (doi:10.1186/gb-2007-8-2-r19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, research0034-research0034.11. (doi:10.1186/gb-2002-3-7-research0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorensen JG, Kristensen TN, Loeschcke V. 2003. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025–1037 (doi:10.1046/j.1461-0248.2003.00528.x) [Google Scholar]
- 41.Von Elert E, Stibor H. 2006. Predator-mediated life history shifts in Daphnia: enrichment and preliminary chemical characterisation of a kairomone exuded by fish. Arch. Hydrobiol. 167, 21–35 (doi:10.1127/0003-9136/2006/0167-0021) [Google Scholar]
- 42.Stibor H, Machacek J. 1998. The influence of fish-exuded chemical signals on the carbon budget of Daphnia. Limnol. Oceanogr. 43, 997–1000 (doi:10.4319/lo.1998.43.5.0997) [Google Scholar]
- 43.Stibor H. 2002. The role of yolk protein dynamics and predator kairomones for the life history of Daphnia magna. Ecology 83, 362–369 [Google Scholar]
- 44.Gliwicz MZ, Pijanowska J. 1988. Effect of predation and resource depth distribution on vertical migration of zooplankton. Bull. Mar. Sci. 43, 695–709 [Google Scholar]
- 45.Leibold MA. 1991. Trophic interactions and habitat segregation between competing Daphnia species. Oecologia 86, 510–520 (doi:10.1007/BF00318317) [DOI] [PubMed] [Google Scholar]
- 46.Gliwicz ZM, Boavida MJ. 1996. Clutch size and body size at first reproduction in Daphnia pulicaria at different levels of food and predation. J. Plankton Res. 18, 863–880 (doi:10.1093/plankt/18.6.863) [Google Scholar]
- 47.Leibold M, Tessier AJ. 1991. Contrasting patterns of body size for Daphnia species that segregate by habitat. Oecologia 86, 342–348 (doi:10.1007/BF00317599) [DOI] [PubMed] [Google Scholar]
- 48.van Gool E, Ringelberg J. 1997. The effect of accelerations in light increase on the phototactic downward swimming of Daphnia and the relevance to diel vertical migration. J. Plankton Res. 19, 2041–2050 (doi:10.1093/plankt/19.12.2041) [Google Scholar]
- 49.van Gool E, Ringelberg J. 2003. What goes down must come up: symmetry in light-induced migration behaviour of Daphnia. Hydrobiologia 491, 301–307 (doi:10.1023/A:1024406324317) [Google Scholar]
- 50.van Gool E, Ringelberg J. 1998. Light-induced migration behaviour of Daphnia modified by food and predator kairamones. Anim. Behav. 56, 741–747 (doi:10.1006/anbe.1998.0821) [DOI] [PubMed] [Google Scholar]
- 51.van Gool E, Ringelberg J. 1998. Quantitative effects of fish kairomones and successive light stimuli on downward swimming responses of Daphnia. Aquat. Ecol. 32, 291–296 (doi:10.1023/A:1009917929959) [Google Scholar]
- 52.De Meester L, Weider LJ. 1999. Depth selection behavior, fish kairomones, and the life histories of Daphnia hyalina X galeata hybrid clones. Limnol. Oceanogr. 44, 1248–1258 (doi:10.4319/lo.1999.44.5.1248) [Google Scholar]
- 53.Sang W, Ma WH, Qiu L, Zhu ZH, Lei CL. 2012. The involvement of heat shock protein and cytochrome P450 genes in response to UV-A exposure in the beetle Tribolium castaneum. J. Insect Physiol. 58, 830–836 (doi:10.1016/j.jinsphys.2012.03.007) [DOI] [PubMed] [Google Scholar]
- 54.Schiene-Fischer C, Aumuller T, Fischer G. 2013. Peptide bond cis/trans isomerases: a biocatalysis perspective of conformational dynamics in proteins. Top. Curr. Chem. 328, 35–67 [DOI] [PubMed] [Google Scholar]
- 55.Colley NJ, Baker EK, Stamnes MA, Zuker CS. 1991. The cyclophilin homolog Ninaa is required in the secretory pathway. Cell 67, 255–263 (doi:10.1016/0092-8674(91)90177-Z) [DOI] [PubMed] [Google Scholar]
- 56.Ferreira PA, Orry A. 2012. From Drosophila to humans: reflections on the roles of the prolyl isomerases and chaperones, cyclophilins, in cell function and disease. J. Neurogenet. 26, 132–143 (doi:10.3109/01677063.2011.647143) [DOI] [PMC free article] [PubMed] [Google Scholar]


