Abstract
Understanding individual differences in cognitive performance is a major challenge to animal behaviour and cognition studies. We used the Eastern water skink (Eulamprus quoyii) to examine associations between exploration, boldness and individual variability in spatial learning, a dimension of lizard cognition with important bearing on fitness. We show that males perform better than females in a biologically relevant spatial learning task. This is the first evidence for sex differences in learning in a reptile, and we argue that it is probably owing to sex-specific selective pressures that may be widespread in lizards. Across the sexes, we found a clear association between boldness after a simulated predatory attack and the probability of learning the spatial task. In contrast to previous studies, we found a nonlinear association between boldness and learning: both ‘bold’ and ‘shy’ behavioural types were more successful learners than intermediate males. Our results do not fit with recent predictions suggesting that individual differences in learning may be linked with behavioural types via high–low-risk/reward trade-offs. We suggest the possibility that differences in spatial cognitive performance may arise in lizards as a consequence of the distinct environmental variability and complexity experienced by individuals as a result of their sex and social tactics.
Keywords: cognition, spatial learning, behavioural syndromes, alternative reproductive strategies, social specialization, lizards
1. Introduction
A fundamental aim in cognitive studies is to understand the factors that might explain the extraordinary levels of individual variability in cognitive performance observed in almost every animal species thus far studied [1–3]. Recent research has made some progress in this respect, but we are only just beginning to understand how cognitive performance relates to development and selection at the intraspecific level [4,5]. In sharp contrast, the study of intraspecific variation in non-cognitive-behavioural traits is a thriving area of research. The study of behavioural types or personality traits (i.e. consistent behavioural tendencies across time and context) has driven our understanding of intraspecific behavioural differences during the past decade, generating several hypotheses about the evolution of adaptive behavioural variation at the individual level [6–11]. Interestingly, recent hypotheses have proposed that learning and non-cognitive-behavioural traits may covary as part of the same suite of correlated traits. Furthermore, they can both determine the environment that is to be experienced by different individuals, generating feedback loops that may equally lead to cognitive–behavioural syndromes [4,5,8,9,12–15].
Sih & Del Giudice [4] recently proposed that variation in cognition and personality may be functionally related through the existence of a shared risk–reward trade-off between fast–slow behavioural traits and speed–accuracy cognitive styles [4]. Many behavioural traits can be classified into a fast–slow axis (e.g. bold versus shy, proactive versus reactive, fast versus slow exploration), and this variation can be associated with variation in a risk–reward axis because bolder, more aggressive, exploratory and/or proactive individuals have a greater potential to gather resources, but take more risks in doing so [4,15–17]. Similarly, speed–accuracy trade-offs are bound to affect cognitive styles, because animals that learn fast do so at the expense of acquiring inaccurate information [18]. The speed–accuracy trade-off is also essentially related to variation in the risk–reward axis because fast learning is inherently risky (i.e. based on inaccurate information) but will tend to draw more resources in the short term [4]. The overarching idea of the risk–reward hypothesis is that selection for factors leading to the adoption of a more risk-prone lifestyle will result in correlated selection for both faster behavioural traits and faster but less accurate and flexible learning, and vice versa [19]. For example, risk-prone individuals may be selected for in stable local habitats, where fast exploration would give them a competitive advantage and the evolution and/or development of learning abilities would aid in the quick formation of routines, whereas risk-averse individuals with learning abilities that are more flexible and sensitive to environmental change may develop or be selected for in more variable local habitats [20]. This hypothesis has found some support in a few bird and fish species, in which proactive individuals tend to be quicker than reactive individuals at operant learning tasks and in avoidance learning tasks fundamentally guided by external environmental cues [4,21–23], but slower in reversal learning tasks [20,24]. In short, recent advances suggest that an obvious avenue for understanding adaptive individual variation in learning is to study the existence of covariation between cognition and non-cognitive–behavioural traits, ideally in an ecologically relevant context where learning may directly impinge on individual fitness.
Sex is an equally important factor in understanding individual variation in learning and in associated behavioural types. The sexes will frequently experience different environmental complexity and/or variability as a consequence of their different reproductive strategies, which are likely to drive differences in both cognitive and non-cognitive traits, and in the way they covary [24–26]. However, while sex differences in learning have been well documented in some taxa [3], they have been completely neglected in others, such as lizards (and reptiles at large). Even less information is available about sex differences in personality traits, which have been documented in only a handful of fish and bird species [26–29]. Finally, scarcely any attention at all has been paid to studying sexual differences in the existence and form of cognitive–behavioural types [24,30], despite the fact that there are sound theoretical reasons to expect them. In the context of the risk–reward hypothesis, for example, males may frequently be forced to adopt more risky reproductive strategies than females because of their different sexual roles, and this could lead to general sex differences in learning, personality traits and their covariation [31].
Spatial learning is a cognitive dimension likely to be of utmost importance to lizards [32]. It is believed to be under strong selection given its importance in foraging, territorial and anti-predatory behaviour, which often require quick and flexible learning of territorial boundaries, suitable escape routes and refuges [33,34]. Not surprisingly, lizards have been found to be capable of quick and flexible spatial learning when tested under a biologically realistic learning paradigm [34]. Furthermore, males and females of many lizard species are generally subjected to different spatial demands because of differences in reproductive tactics and behaviour during the reproductive season. These sex-specific tactics and behaviours may have given rise to widespread sexual differences in spatial learning abilities, and to sex-specific associations between spatial learning and other behavioural traits [24,25]. An additional dimension in many lizard systems is that exploratory and boldness traits may covary with alternative reproductive tactics (hereafter ARTs) [35]. Because lizard ARTs are closely associated with territorial behaviour, these traits represent ecologically significant behavioural variation in a context in which spatial learning is important for lizards [36].
Here, we used an Australian lizard, the Eastern Water Skink (Eulamprus quoyii), to explore associations between individual variability in spatial learning performance, sex and exploratory and boldness traits that have been previously identified as important covariates of ARTs in E. quoyii and Eulamprus heatwolei, a closely related species [37–41]. Our main aims in this study were: (i) to examine the existence of sexual differences in learning performance and (ii) to explore the existence, form and potential sex differences in associations between spatial learning, exploration and boldness. In order to do so, we assayed behavioural and cognitive traits in four successive experiments in which we quantified exploratory behaviour, boldness in two different contexts (neophobia towards novel prey and boldness after a predatory attack) and performance in a simple spatial task.
2. Material and methods
(a). Study species
The Eastern water skink (E. quoyii) is a large (90–122 mm snout–vent length (SVL)), viviparous lizard species that is widely distributed across Eastern Australia, from South Australia and Victoria through to New South Wales and into Queensland. It frequently inhabits rocky water edges in suburban areas and can reach high densities. We collected 216 water skinks in August and September 2010 from five separate sites throughout the Sydney region as part of a separate natural mating experiment that took place during the breeding season (see [42] for details). After the breeding season, all lizards were transferred to large outdoor bins (3.2 m diameter) containing bark mulch substrate, logs and roofing tiles for refuges. Lizards had constant access to water and were fed crickets every second day.
We used 64 of these lizards (32 males and 32 females) in March 2011 in our experiments. Experiments commenced immediately after transferring the lizards to an indoor facility for 32 lizards (16 males and 16 females), while the remaining 32 lizards (16 males, 16 females) were temporarily held in small holding bins until we finished the first batch of lizards; owing to logistical and space constraints we were only able to process 32 lizards at a time. All lizards were provided with a middle refuge and had constant access to water and UV lighting during the experiments. Heat cord (30°C) was used to create a thermal gradient in each enclosure so that lizards had ample opportunity to thermoregulate. Crickets were fed ad libitum every second day except during the first 6 days of experiments because we did not want lizards to be satiated during feeding trials with novel prey (details in §2(d)i below).
(b). Behavioural assays
All behavioural trials described below were conducted in the lizards' own holding enclosures, recorded using a mounted security camera (Swann security system), and later scored in JWatcher (http://www.jwatcher.ucla.edu/). Video footage was scored ‘blind’ to whether individuals had learnt the spatial test, and subsets of data (e.g. trials within each experiment) were scored by the same individual to avoid inter-observer bias [43]. Full details of all behavioural assays can be found in the electronic supplementary material.
(c). Exploratory behaviour (day 1)
Lizards were introduced into a novel enclosure (683 (L) × 483 (W) × 385 (H) mm) with two weighted black refuge-boxes (170 × 65 × 120 mm). Each refuge had three entrances (front and sides) and was placed at each end of the enclosure (electronic supplementary material, figure S1). After 3 min acclimation in the central refuge-box, the refuge was lifted and trials were run for 30 min. We scored the following behaviours: (i) time in locomotion (TL) and (ii) time taken to enter the two refuges in the enclosure (T2ER). Lizards that did not visit both refuges within 30 min were assigned a latency of 1800 s. We included the latency to visit both refuges as a measure of quickness to explore in a novel environment, and locomotion as a measure of the amount of exploration.
(d). Measures of boldness
Boldness is most often interpreted as the tendency to take risks, especially in novel situations, and is usually measured experimentally in relation to anti-predatory behaviours or individual responses to novel cues [44]. In this study, we used two experiments to assay boldness separately in an anti-predatory and in a neophobia context.
(i). Assay I: neophilia (days 2–7)
We quantified variation in neophobia/neophilia by examining individual lizard responses when presented with a novel food item (i.e. a dead silkworm pupa). We presented the pupa by gently dangling it in front of the lizard (or at the entrance of the refuge it was in) for 3 min (once per day for five consecutive days). On day 6, we presented each lizard with a pupa left hanging ca 1–2 cm from the centre of the tub for 1 h, and in the absence of observers. Lizards were divided in two categories (NEO): neophilic (lizards that ate the novel prey at some point during trials) and neophobic (lizards that did not eat the novel prey at all).
(ii). Assay II: anti-predatory trial (day 8)
Assays began by gently chasing each lizard into the middle refuge and randomly designating one of the two lateral refuges as the ‘hot’ refuge (by suspending a 60/100 W incandescent bulb ca 25 cm above this refuge) and the other as the ‘cold’ refuge (by packing a box with ice and placing it beneath the refuge, under the tub). We then removed the central refuge, left lizards to acclimate for 5 min, switched the basking light over the ‘hot’ refuge on and allowed lizards 15 min to reach the basking platform and initiate basking. We finally simulated a predatory attack by chasing the lizard off the basking refuge until it entered into the ‘cold’ refuge at the opposite end of the tub. After the simulated attack, we measured the time it took lizards to return to their basking sites (LATB; 45 min maximum).
(e). Spatial learning trials (days 9–28)
To measure spatial learning, we set up a simple spatial learning assay using an anti-predatory paradigm that has been used successfully in previous studies [32,34]. We initiated trials by re-introducing the two side refuges in the same positions as in the anti-predatory trial (the ‘cold’ refuge in the anti-predatory trial was selected to act as the ‘safe’ refuge across spatial learning trials) and removing the middle refuge, after which lizards were given a variable amount of exploration time (30–45 min). After this time, we scared lizards around the enclosure until they entered the ‘safe’ refuge. If the lizard entered the ‘unsafe’ refuge, then we lifted the refuge and resumed chasing the lizard until it entered the ‘safe’ refuge. Each lizard was tested once a day for an overall period of 20 days (i.e. 20 trials). We measured whether it chose the correct ‘safe’ refuge or not (‘choice’), the number of extra incorrect choices (i.e. number of choices after the first incorrect choice) needed to choose the correct refuge (‘incorrect choices’), and the overall latency to enter the ‘safe’ refuge (‘latency’). The rationale for choosing these variables was: (i) to keep the variable ‘incorrect choices’ independent of the variable ‘choice’ and (ii) because ‘rule-of-thumb’ learning of the type ‘run into a refuge and if chased again run straight into the other one’ could explain a significant drop in both ‘incorrect choices’ and ‘latency’ across trials without the need to invoke spatial learning. Hence, we analysed learning curves for these three variables. Owing to variation in the time of the day in which trials were conducted, which was balanced within individuals every four days (see the electronic supplementary material for details), results were blocked every four days (i.e. four trials). Lizards were categorized as learners or non-learners according to their cumulative tally of correct/incorrect choices across the 20 trials. A lizard was considered to have chosen correctly when it was already found inside the ‘safe’ refuge at scaring time or when the first refuge it ran into in response to the simulated predatory attack was the ‘safe’ refuge. We considered a lizard to have learnt when: (i) following at least four consecutive correct choices it accumulated a significant correct/incorrect tally according to a binomial distribution (e.g. 5/5, 7/8, etc.) and (ii) when, from this point on, its overall correct/incorrect tally until the end of trials remained significant (see the electronic supplementary material for full details about behavioural trials and data on correct/incorrect tallies for all lizards across the 20 trials).
(f). Statistical analyses
We were able to obtain learning data from 64 lizards (but conservatively excluded two ambiguous non-learners from formal analyses because they exhibited a higher proportion of wrong choices than expected by chance), and full behavioural, learning and morphological data from 56 lizards (owing to missing data; see the electronic supplementary material for details). We used generalized estimating equations (GEEs) to analyse learning curves for our ‘choice’, ‘incorrect choices’ and ‘latency’ variables (n = 31 per sex). We rounded latency to the nearest whole number and modelled both latency and number of incorrect choices using a Poisson error distribution, and lizard choices using a binomial error distribution. We included lizard ID as the grouping variable and used an autoregressive 1 correlation structure (AR1). The GEEs estimate a scale parameter and account for over-dispersion in the models. We compared models using Wald tests to test for significant block, sex and sex × block effects as well as significant effects of learning, block and learning × block effects.
To analyse the relationship between behavioural traits and learning, we used generalized linear models (GLMs) with a binomial error distribution and ‘logit’ link function, in R v. 2.14.0 [45]. Learning (binary: ‘learn’ or ‘no learn’) was modelled as a function of sex and body condition, exploratory behaviour (i.e. time to explore two refuges (T2ER) and time moving in novel environment (TL)) and boldness (i.e. latency to bask after simulated predatory attack (LATB), and whether an individual ate a novel prey item (1 = ate; 0 = did not eat) (NEO)). Body condition was calculated as the scaled mass index [46], but note that using the residuals from a linear regression between log mass and log body size (SVL) [47] yielded equivalent results. Using the latter independent variables and sex and condition as covariates, we generated a series of candidate models based on a priori hypotheses about the role that behavioural types play in affecting the probability of learning based on previous results from birds and fish [12–14,48]. Graphical inspection of variables suggested that LATB was not necessarily linearly related to the probability of learning so we included a quadratic parameter in models with LATB. Given our sample size, we limited the number of parameters to a maximum of five and did not include both measures of exploration in the same model to avoid possible autocorrelation. Prior to analysis, we standardized our independent (input) variables (mean = 0, s.d. = 2) to allow interpretation of main effects in the presence of higher order parameters and to ease comparisons among model estimates.
Alternative models were evaluated using the second-order information criterion, AICC, owing to a parameters-to-sample-size ratio of less than 40 [49]. As there was no clear ‘best’ model in our candidate set (i.e. Akaike model weight greater than 90%), we adopted a model averaging approach in addition to presenting our top-ranked model [50]. We chose to present model-averaged coefficients because we made predictions about the direction of individual parameter estimates and because the hypothesized role of behavioural types on learning is still in its infancy, and hence we feel that it is important to present effect sizes for all hypothesized parameters to guide future research [50]. We also used our top model and the estimated parameters to make predictions about the probability of learning given that the coefficients in this top model explain the greatest amount of variation in our data. We did not exclude models in the candidate set that were more complex versions of reduced models during model averaging [49] to avoid exclusion of biologically relevant effects. Owing to our limited set of models and hypothesized relationships between learning and our behavioural traits, we chose natural model averaging using the models that had a cumulative model weight of 95% [49]. We inspected the fit of all top models (2 ΔIC) by looking for influential points (Cook's distance and hat values) and testing for colinearity using variance inflation factors. We found weak evidence for over-dispersion (residual deviance/residual degrees of freedom less than or equal to 1.3). The model with the highest dispersion was the top model; however, re-fitting this model with a quasi-binomial error distribution, where a dispersion parameter is estimated, did not affect the results.
3. Results
(a). Sex differences in spatial learning
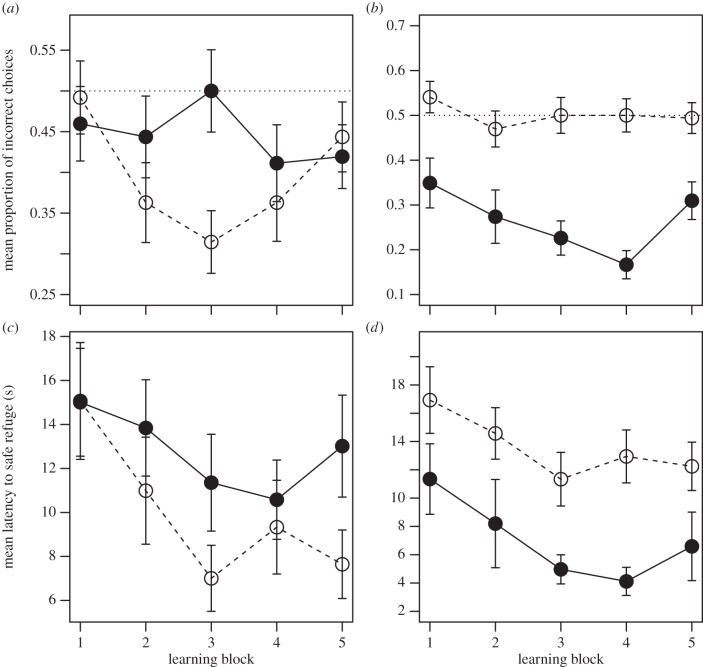
Twice as many males learnt the spatial task within 20 trials compared to females (14 of 31 (45.1%) males and 7 of 31 (22.6%) females). The analysis of learning curves revealed a significant sex × block interaction in the probability of choosing the correct refuge (χ2 = 11.1, p = 0.026). Males where significantly more likely to choose the correct refuge by Learning Block 3 (estimate = −0.90 ± 0.33, Z = 7.32, p = 0.007). This difference disappeared in blocks 4 and 5, by which time females were already performing below chance (i.e. had learnt the task; figure 1), so our results strongly suggest that males were quicker than females in learning the spatial task. The number of extra incorrect choices needed to find the safe refuge decreased significantly across blocks (Wald χ2 = 17.3, p = 0.002; figure 1a), but females did not show a clear tendency to make a higher number of incorrect choices than males (Wald χ2 = 2.24, p = 0.13; figure 1a), with no evidence for a significant sex × block interaction (Wald χ2 = 2.63, p = 0.62). Similarly, males and females did not differ significantly in their latency to enter the safe refuge (LAT) across blocks (sex × block: Wald χ2 = 3.4, p = 0.49; sex: Wald χ2 = 0.312, p = 0.58; block: Wald χ2 = 4.54, p = 0.34; figure 1c), which shows that sex-differences in learning cannot be attributed to differences in rule-of-thumb learning. Males exhibited a notable upward swing in the probability of choosing the incorrect refuge in the last learning block. This could be due to overtraining effects on attention [51] or, perhaps more likely, to gradual extinction of the efficacy of the negative reinforcement across trials due to lizards habituating to human presence/scaring.
Figure 1.
Learning curves for males (white circles in a,c; n = 31) versus females (black circles in a,c; n = 31); and learner (black circles in b,d; n = 21) versus non-learner lizards (white circles in b,d; n = 41). The dashed line in (b,c) marks the probability of entering into the correct refuge by chance given the experimental set-up. Note that instances in which lizards were already encountered in the ‘safe’ refuge at the time of the simulated attack (see Material and methods) were not considered for the analysis of latencies.
(b). Individual behavioural type and the probability of spatial learning
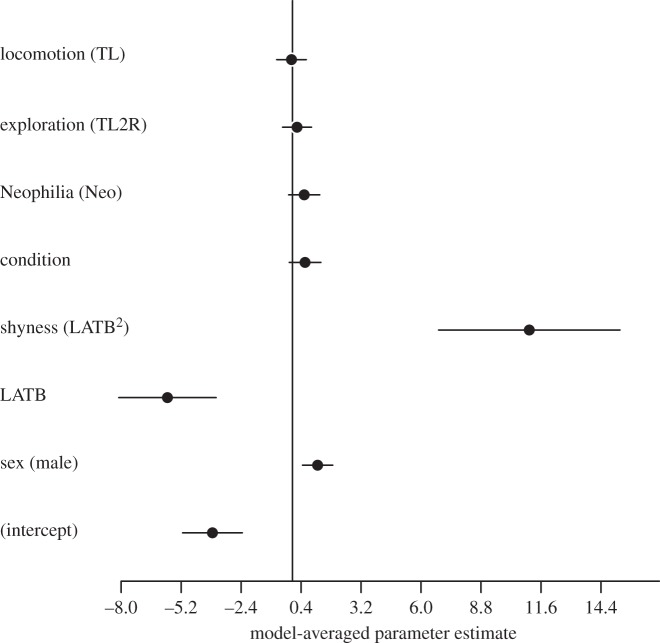
Our two measures of exploratory behaviour, TL and T2ER, explained little variation in the probability of learning, with all models containing these two variables being more than 2 ΔAICC units from the top model (electronic supplementary material, table S1; Model 11 and Model 12). This was also evident in the model-averaged estimates with these two variables having small effect sizes; TL2R had a positive estimate while TL had a slightly negative estimate (figure 2).
Figure 2.
Parameter estimates after natural model averaging of models with a cumulative model weight of 95% (see Material and methods).
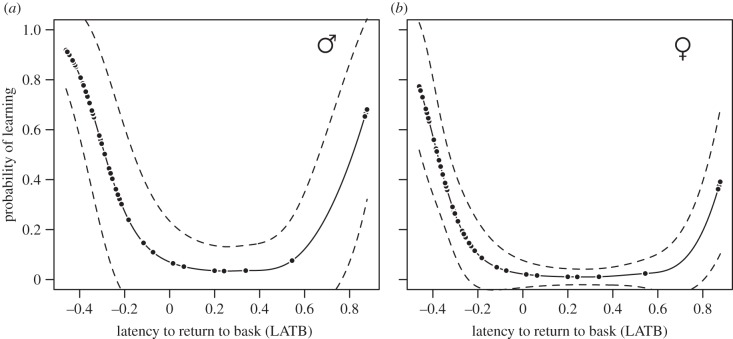
Models that included whether a lizard ate a novel food item or not (NEO) approached 2 ΔAICC units from the top model (Model 10; electronic supplementary material, table S1) and the model-averaged estimate showed a small positive effect on the probability of learning (figure 2). Models containing only the main effect of the latency to return to the basking refuge after a predatory attack (LATB) poorly explained variation in the probability of learning (electronic supplementary material, table S1; ΔAICC > 10); however, there was evidence that the relationship between the probability of learning and LATB was nonlinear (table 1 and electronic supplementary material, table S1). The best-supported model contained sex, LATB and LATB2 (electronic supplementary material, table S1) and confirmed that LATB and LATB2 had significant effects on the probability of learning (table 1 and figure 2). The predicted probabilities of learning showed that there were two groups of individuals with a high probability of learning located at the extremes of this distribution (figure 3). Individuals with short LATB (‘bold’) had a high probability of learning the task and there was a sharp decline in this probability of learning to individuals with intermediate latencies (figure 3). The probability of learning the spatial task increased again for individuals taking a long time to return to the basking refuge (‘shy’). ‘Bold’ males (i.e. individuals at –0.4 units from the mean) were predicted to have an 82% probability of learning, while ‘bold’ females had a 57% probability of learning the spatial task (figure 3a,b). By contrast, ‘shy’ males (individuals at 0.8 units from the mean) were predicted to have a 45% probability of learning the spatial task while ‘shy’ females were predicted to have a 20% probability of learning (figure 3a,b).
Table 1.
Parameter estimates and 95% CI around estimates for the top-supported model (Model 8; electronic supplementary material, table S1). Note that coefficients are standardized 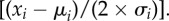
| coefficient | estimate | lower 95% CI | upper 95% CI |
|---|---|---|---|
| intercept | –3.79 | –6.70 | –1.66 |
| sex (male) | 1.20 | –0.09 | 2.63 |
| LATB | –5.82 | –10.71 | –2.07 |
| LATB2 | 10.98 | 4.02 | 20.18 |
Figure 3.
The predicted probability of learning a spatial task for (a) males and (b) females as a function of the latency to return to a basking refuge after a simulated predatory attack (LATB). Predicted probabilities are based on our top-supported model (Model 8; electronic supplementary material, table S1) using standardized input variables 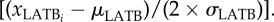 We used our standardized input LATB variable to predict probabilities and fit a smoothed cubic spline function to the data. Dashed lines above and below fitted lines are the 95% CIs (predicted (p) ± 1.96 × s.e. of fit and smoothed with a cubic spline).
We used our standardized input LATB variable to predict probabilities and fit a smoothed cubic spline function to the data. Dashed lines above and below fitted lines are the 95% CIs (predicted (p) ± 1.96 × s.e. of fit and smoothed with a cubic spline).
4. Discussion
Our results strongly suggest that male E. quoyii performed significantly better and faster at our spatial learning task than females. We also found significant variation among individuals in boldness (latency to exit a refuge and return to a basking platform after a simulated attack), and a significant association between variation in boldness and the probability of learning a spatial task: both ‘bold’ and ‘shy’ behavioural types were more likely to learn the spatial task than individuals with intermediate behaviour.
(a). Sex differences in spatial cognition
Sex differences in cognitive ability in mammals and other taxa are most commonly documented for spatial cognition, where males typically perform better than females [1,52–54]. Sex differences in spatial cognition have been hypothesized to arise from differential selective pressures in relation to sex-specific dispersal, mobility during reproduction, intrasexual competition, female choice and differences in home range size (i.e. the range-size hypothesis [31]). Although the latter hypothesis seems to have the most support [25,52], most hypotheses actually link spatial ability to space use and differ only in their explanations as to why the sexes differ in their use of space.
In many lizard species, reproduction seems to pose higher spatial challenges to males compared with females. In particular, males generally possess larger home ranges and/or need to process more complex spatial information than females in order to achieve copulations (e.g. home-range boundaries, location of rivals, location of females within their home range [25,35,36]). As predicted by such differences between male and female social roles [55], we found that male E. quoyii were better at our spatial learning task than females. More males successfully learnt the spatial learning task than females (45.1% male learners versus only 22.6% female learners), and our results also suggest that males were quicker at learning the spatial task than females. To the best of our knowledge, sex differences in learning have never been reported in a reptile, which is particularly striking in the context of lizard spatial cognition because sexual differences in spatial demands seem widespread in lizards [55]. Our study is therefore the first evidence to date for sexually dimorphic cognitive performance in a reptile. Although we used only one learning task to gauge spatial learning, and hence our results cannot be extrapolated to spatial learning in other contexts, we suggest that the home range size hypothesis put forward to explain sexual differences in spatial cognition in mammals may similarly apply to lizard species in other spatial learning contexts [25]. Future studies should address this hypothesis, and the generality of the findings reported here.
(b). Boldness, spatial learning and alternative reproductive tactics in Eulamprus quoyii
We found considerable variation in individual latency to return to basking after a simulated predatory attack, and a clear association between variation in this boldness measure and variation in individual spatial learning performance. ‘Bold’ individuals (quick to return to bask after attack) had the highest probability of learning the spatial task. However, extremely ‘shy’ behavioural types also learnt the spatial task with a higher probability than individuals with intermediate behavioural types. This is the first evidence linking cognition and behavioural types in a reptile, and it is inconsistent with the risk–reward hypothesis and with previous studies [4,21,56,57], where the relationship between boldness and learning was always reported to be linear.
We suggest a tentative but interesting possibility that both extremely ‘bold’ and ‘shy’ behavioural types may have enhanced spatial learning because of the ARTs they adopt. ARTs in Eulamprus are associated with divergent selection for different behavioural types that are likely part of a territorial-floater behavioural syndrome relating to boldness, activity and exploratory behaviour, as has been shown in this genus and in other lizards [35,40,41]. Typically, territorial lizards actively defend against other males core areas that tend to overlap the home range of several resident females, while ‘floater’ males instead navigate their way over larger areas, traversing several different territories in their search for copulations with females. In Eulamprus, territorial males are bolder, more active and explore less than floater males, which matches the behaviour predicted by their ART but does not conform to the typical fast–slow, high–low, risk–reward trade-off behavioural axis [39–41]. Interestingly, the fitness of each of these ARTs probably depends greatly on spatial cognition. Territorial males need to process and memorize detailed spatial information (e.g. the position of females within a territory, territory boundaries with neighbouring males [36,58]). Similarly, floater males need to navigate their way over large home ranges consisting of varied habitat, and where the locations of refuge sites, rival male territories and potential mates are important for both reduced conflict and reproduction, while also aiding survival [35,39]. Under this scenario, divergent behavioural types may have given rise (via divergent correlational selection or developmental pathways) to enhanced spatial learning for both of these male reproductive tactics. ARTs have also been suggested in female Eulamprus in relation to territory residency, anti-predatory behaviour and exploration [41], although to date there is no direct evidence of a clear link between these behavioural traits in E. quoyii females. Alternatively, strong selection for divergent cognitive–behavioural types in males may also have led to correlated selection in females [59]. Indeed, there does appear to be strong sexual selection on the behavioural traits associated with each of the ARTs [39].
As a word of caution, and while the above is certainly compelling, our results cannot be taken as direct support for this hypothesis. First, while ARTs have been documented in Eulamprus, we did not directly assess the ARTs of the individuals used in our study. Second, we did not find any evidence that exploration and/or boldness in the neophilia experiment were significantly associated with spatial learning, which is something that we would have expected if ARTs were shaping cognitive–behavioural syndromes. In particular, we would have expected neophilia/boldness and slow exploration (i.e. characteristic of territorial males) and neophobia/shyness and fast exploration (i.e. characteristic of floater males) to be positively associated with spatial learning in male lizards. Plotting these two variables separately for male learners versus non-learners (post hoc) does hint at such a relationship in males (electronic supplementary material, figure S2), but this was not picked up in our analysis (perhaps owing to the small sample sizes). We suggest that future studies should measure ARTs (i.e. territorial behaviour) directly in the wild, and then relate this to spatial learning as measured in the laboratory or, ideally, in the field.
5. Conclusion
To conclude, we provide the first evidence of sex-dependent spatial learning in a reptile. We suggest that sexual dimorphism in spatial learning may be linked to the different social roles (and ensuing spatial demands) experienced by males and females in territorial lizards and that consequently this phenomenon may be more widespread than previously suspected. We also show that both ‘bold’ and ‘shy’ behavioural types have enhanced spatial learning and propose that this may be because of their association with ARTs in E. quoyii. This is also the first evidence that behavioural traits such as boldness are associated with learning in a reptile and, along with recent studies in birds, highlights the importance of considering cognitive traits in the study of behavioural syndromes, and vice versa. We suggest that future studies consider different social roles and tactics as an important factor in the evolution and/or development of specific behavioural–cognitive syndromes. In lizards, a first step to test this hypothesis would be to examine the link between territorial behaviour (i.e. ARTs), spatial learning and fitness. Characterizing whether spatial learning is under strong selection and, if so, examining its relative strength across different social roles and tactics are bound to provide crucial insight into our understanding of how intraspecific variability in spatial cognition arises.
Acknowledgements
We thank Yee Wah Lau for her help with trials and Ella Cole, Gordon Burghardt and Robert Heathcote for comments on earlier versions.
Data accessibility
Behavioural data deposited in dryad: doi:10.5061/dryad.j83c0.
Funding statement
P.C. was supported by an Endeavour Award from the Australian Department of Education, Employment and Workplace Relations (DEEWR), and by an FP7 IEF Marie Curie fellowship (PIEF-GA-2010-273010) from the European Commission. D.W.A.N. was supported by an NSERC postgraduate scholarship (Canada). The enclosures, facilities and equipment used in this study were funded by an internal Macquarie University grant to M.J.W.
References
- 1.Healy SD, Bacon IE, Haggis O, Harris AP, Kelley LA. 2008. Explanations for variation in cognitive ability: behavioural ecology meets comparative cognition. Behav. Process. 80, 288–294 (doi:10.1016/j.beproc.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 2.Raine NE, Chittka L. 2008. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. B 275, 803–808 (doi:10.1098/rspb.2007.1652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shettleworth S. 2010. Cognition, evolution and behavior. New York, NY: Oxford University Press [Google Scholar]
- 4.Sih A, Del Guidice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762–2772 (doi:10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton A, Lukas D. 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil. Trans. R. Soc. B 367, 2773–2783 (doi:10.1098/rstb.2012.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmüller R, Taborsky M. 2010. Animal personality due to social niche specialization. Trends Ecol. Evol. 25, 504–511 (doi:10.1016/j.tree.2010.06.012) [DOI] [PubMed] [Google Scholar]
- 7.Dall SR, Bell AM, Bolnick DI, Ratnieks FL. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198 (doi:10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dingemanse NJ, Wolf M. 2012. Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958 (doi:10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sih A, Bell AM. 2008. Insights for behavioural ecology from behavioural syndromes. Adv. Study Anim. Behav. 38, 227–281 (doi:10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamps JA, Groothuis GG. 2010. The development of animal personality: relevance, concepts and perspectives. Biol. Rev. (Cambridge) 85, 301–325 (doi:10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- 11.Wolf M, Weissing FJ. 2010. An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968 (doi:10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbilly M, Motro U, Feldman MW, Lotem A. 2010. Co-evolution of learning complexity and social foraging strategies. J. Theoret. Biol. 267, 573–581 (doi:10.1016/j.jtbi.2010.09.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carere C, Locurto C. 2011. Interaction between animal personality and animal cognition. Curr. Zool. 57, 491–498 [Google Scholar]
- 14.Cole EF, Quinn JL. 2012. Personality and problem-solving performance explain competitive ability in the wild. Proc. R. Soc. B 279, 1168–1175 (doi:10.1098/rspb.2011.1539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf M, Sander van Doorn G, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 16.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 17.Réale D, Reader SM, Sol D, McDougall P, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. (Cambridge) 82, 291–318 (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 18.Chittka L, Skorupski P, Raine NE. 2009. Speed–accuracy tradeoffs in animal decision making. Trends Ecol. Evol. 24, 400–407 (doi:10.1016/j.tree.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 19.Burns JG. 2005. Impulsive bees forage better: the advantage of quick, sometimes inaccurate foraging decisions. Anim. Behav. 70, e1–e5 (doi:10.1016/j.anbehav.2005.06.002) [Google Scholar]
- 20.Guillette LM, Reddon AR, Hoeschele M, Sturdy CB. 2011. Sometimes slower is better: slow-exploring birds are more sensitive to changes in a vocal discrimination task. Proc. R. Soc. B 278, 767–773 (doi:10.1098/rspb.2010.1669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boogert NJ, Reader SM, Laland KN. 2006. The relation between social rank, neophobia, and individual learning in starlings. Anim. Behav. 72, 1229–1239 (doi:10.1016/j.anbehav.2006.02.021) [Google Scholar]
- 22.Exnerová A, Svádová KH, Fucíková E, Drent P, Stys P. 2010. Personality matters: individual variation in reactions of naive bird predators to aposematic prey. Proc. R. Soc. B 277, 723–728 (doi:10.1098/rspb.2009.1673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillette LM, Reddon AR, Hurd PL, Sturdy CB. 2009. Exploration of a novel space is associated with individual differences in learning speed in black- capped chickadees, Poecile atricapillus. Behav. Process. 82, 265–270 (doi:10.1016/j.beproc.2009.07.005) [DOI] [PubMed] [Google Scholar]
- 24.Titulaeur M, van Oers K, Naguib M. 2012. Personality affects learning performance in difficult tasks in a sex-dependent way. Anim. Behav. 83, 723–730 (doi:10.1016/j.anbehav.2011.12.020) [Google Scholar]
- 25.Jones CM, Braithwaite VA, Healy SD. 2003. The evolution of sex differences in spatial ability. Behav. Neurosci. 117, 403–411 (doi:10.1037/0735-7044.117.3.403) [DOI] [PubMed] [Google Scholar]
- 26.Schuett W, Dall SRX. 2009. Sex differences, social context and personality in zebra finches, Taeniopygia guttata. Anim. Behav. 77, 1041–1050 (doi:10.1016/j.anbehav.2008.12.024) [Google Scholar]
- 27.Johnsson JI, Sernland E, Blixt M. 2001. Sex-specific aggression and antipredator behaviour in young brown trout. Ethology 107, 587–599 (doi:10.1046/j.1439-0310.2001.00682.x) [Google Scholar]
- 28.Oliveira RF, Taborsky M, Brockmann HJ. 2008. Alternative reproductive tactics: an integrative approach. Cambridge, UK: Cambridge University Press [Google Scholar]
- 29.Piyapong C, Krause J, Chapman BB, Ramnarine IW, Louca V, Croft DP. 2009. Sex matters: a social context to boldness in guppies (Poecilia reticulata). Behav. Ecol. 21, 3–8 (doi:10.1093/beheco/arp142) [Google Scholar]
- 30.Zwoinska MK, Kolm N, Maklakov AA. 2013. Sex differences in cognitive ageing: testing predictions derived from life-history theory in a dioecious nematode. Exp. Gerontol. 48, 1469–1472 (doi:10.1016/j.exger.2013.09.008). [DOI] [PubMed] [Google Scholar]
- 31.Gaulin SJC, Fitzgerald RW. 1989. Sexual selection for spatial learning ability. Anim. Behav. 37, 322–331 (doi:10.1016/0003-3472(89)90121-8) [Google Scholar]
- 32.Burghardt GM. 1977. Learning processes in reptiles. In Biology of the reptilia (eds Gans C, Tinkle TW.), pp. 555–681 New York, NY: Academic Press [Google Scholar]
- 33.Cooper WEJ, Wilson DS. 2007. Beyond optimal escape theory: microhabitats as well as predation risk affect escape and refuge use by the phrynosomatid lizard Sceloporus virgatus. Behaviour 144, 1235–1254 (doi:10.1163/156853907781890940) [Google Scholar]
- 34.Noble DWA, Carazo P, Whiting MJ. 2012. Learning outdoors: male lizards show flexible spatial learning under semi-natural conditions. Biol. Lett. 8, 946–948 (doi:10.1098/rsbl.2012.0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calsbeek R, Sinervo B. 2008. Alternative reproductive tactics in reptiles. In Alternative reproductive tactics (eds Oliveira RF, Taborsky M, Brockmann HJ.), pp. 332–342 Cambridge, UK: Cambridge University Press [Google Scholar]
- 36.Carazo P, Font E, Desfilis E. 2008. Beyond ‘nasty neighbours’ and ‘dear enemies’? Individual recognition by scent marks in a lizard (Podarcis hispanica). Anim. Behav. 76, 1953–1963 (doi:10.1016/j.anbehav.2008.08.018) [Google Scholar]
- 37.Keogh JS, Noble DWA, Wilson EE, Whiting MJ. 2012. Male activity predicts male reproductive success in a polygynous lizard. PLoS ONE 7, 1–5 (doi:10.1371/journal.pone.0038856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keogh JS, Umbers KDL, Wilson E, Stapley J, Whiting MJ. 2013. Influence of alternate reproductive tactics and pre- and postcopulatory sexual selection on paternity and offspring performance in a lizard. Behav. Ecol. Sociobiol. 67, 629–638 (doi:10.1007/s00265-013-1482-0) [Google Scholar]
- 39.Noble DWA, Wechmann K, Keogh JS, Whiting MJ. 2013. Behavioral and morphological traits interact to promote the evolution of alternative reproductive tactics in a lizard. Am. Nat. 182, 726–742 (doi:10.1086/673535) [DOI] [PubMed] [Google Scholar]
- 40.Stapley J, Keogh JS. 2004. Exploratory and antipredator behaviours differ between territorial and nonterritorial male lizards. Anim. Behav. 68, 841–846 (doi:10.1016/j.anbehav.2004.02.008) [Google Scholar]
- 41.Stapley J, Keogh JS. 2005. Behavioral syndromes influence mating systems: floater pairs of a lizard have heavier offspring. Behav. Ecol. 16, 514–520 (doi:10.1093/beheco/ari019) [Google Scholar]
- 42.Noble DWA, Keogh JS, Whiting MJ. 2013. Multiple mating in a lizard increases fecundity but provides no evidence for genetic benefits. Behav. Ecol. 24, 1128–1137 (doi:10.1093/beheco/art040) [Google Scholar]
- 43.Burghardt GM, Bartmess-LeVasseur JN, Browning SA, Morrison KE, Stec CL, Zachau CE, Freeberg TM. 2012. Perspectives—minimizing observer bias in behavioral studies: a review and recommendations. Ethology 118, 511–517 (doi:10.1111/j.1439-0310.2012.02040.x) [Google Scholar]
- 44.Carter AJ, Marshall HM, Heinsohn R, Cowlishaw G. 2012. How not to measure boldness: novel object and antipredator responses are not the same in wild baboons. Anim. Behav. 84, 603–609 (doi:10.1016/j.anbehav.2012.06.015) [Google Scholar]
- 45.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org. [Google Scholar]
- 46.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891 (doi:10.1111/j.1600-0706.2009.17643.x) [Google Scholar]
- 47.Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ. 2005. Restitution of mass-size residuals: validating body condition indices. Ecology 86, 155–163 (doi:10.1890/04-0232) [Google Scholar]
- 48.Cole EF, Morand-Ferron J, Hinks AE, Quinn JL. 2012. Cognitive ability influences reproductive life history variation in the wild. Curr. Biol. 22, 1808–1812 (doi:10.1016/j.cub.2012.07.051) [DOI] [PubMed] [Google Scholar]
- 49.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference, 2nd edn New York, NY: Springer [Google Scholar]
- 50.Symonds MRE, Moussalli A. 2011. A brief guide to model selection, multimodal inference and model averaging in behavioural ecology using Akaike's information criterion. Behav. Ecol. Sociobiol. 65, 13–21 (doi:10.1007/s00265-010-1037-6) [Google Scholar]
- 51.Ishida M, Papini MR. 1997. Massed-trial overtraining effects on extinction and reversal performance in turtles (Geoclemys reevesii). Q. J. Exp. Psychol. 50B, 1–16 (doi:10.1080/027249997393619) [Google Scholar]
- 52.Geary DC. 1995. Sexual selection and sex differences in spatial cognition. Learn. Individual Diff. 7, 289–301 (doi:10.1016/1041-6080(95)90003-9) [Google Scholar]
- 53.Healy S, Rowe C. 2010. Information processing: the ecology and evolution of cognitive abilities. In Evolutionary behavioral ecology (eds Westneat D, Fox CW.), pp. 162–174 Oxford, UK: Oxford University Press [Google Scholar]
- 54.Jozet-Alves C, Moderán J, Dickel L. 2008. Sex differences in spatial cognition in an invertebrate: the cuttlefish. Proc. R. Soc. B 275, 2049–2054 (doi:10.1098/rspb.2008.0501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamps JA. 1977. Social behavior and spacing patterns in lizards. In Biology of the reptilia. Ecology and behavior A, vol. 7 (eds Gans C, Tinkle DW.), pp. 149–171 New York, NY: Academic Press [Google Scholar]
- 56.Dugatkin LA, Alfieri MS. 2003. Boldness, behavioural inhibition and learning. Ethol. Ecol. Evol. 15, 43–49 (doi:10.1080/08927014.2003.9522689) [Google Scholar]
- 57.Overli O, Sorensen C, Nilsson GE. 2006. Behavioral indicators of stress-coping style in rainbow trout: do males and females react differently to novelty? Physiol. Behav. 87, 506–512 (doi:10.1016/j.physbeh.2005.11.012) [DOI] [PubMed] [Google Scholar]
- 58.Stamps JA, Krishnan VV. 1998. Territory acquisition in lizards. IV. Obtaining high status and exclusive home ranges. Anim. Behav. 55, 461–472 (doi:10.1006/anbe.1997.0612) [DOI] [PubMed] [Google Scholar]
- 59.Fortsmeier W, Martin K, Bolund E, Schielzeth H, Kempenaers B. 2011. Female extrapair mating behavior can evolve via indirect selection on males. Proc. Natl Acad. Sci. USA 108, 10 608–10 613 (doi:10.1073/pnas.1103195108) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Behavioural data deposited in dryad: doi:10.5061/dryad.j83c0.