Abstract
We present a formal model of Janzen's influential theory that competition for resources between microbes and vertebrates causes microbes to be selected to make these resources unpalatable to vertebrates. That is, fruit rots, seeds mould and meat spoils, in part, because microbes gain a selective advantage if they can alter the properties of these resources to avoid losing the resources to vertebrate consumers. A previous model had failed to find circumstances in which such a costly spoilage trait could flourish; here, we present a simple analytic model of a general situation where costly microbial spoilage is selected and persists. We argue that the key difference between the two models lies in their treatments of microbial dispersal. If microbial dispersal is sufficiently spatially constrained that different resource items can have differing microbial communities, then spoilage will be selected; however, if microbial dispersal has a strong homogenizing effect on the microbial community then spoilage will not be selected. We suspect that both regimes will exist in the natural world, and suggest how future empirical studies could explore the influence of microbial dispersal on spoilage.
Keywords: metapopulations, fungus, yeast, carrion, frugivory, competition
1. Introduction
Studying plant–animal interactions has been critical for developing both ecological and evolutionary theory, although microorganisms have, in general, received less attention by most ecologists. Competition has long been seen as a major example of such interactions, and it is often assumed that more closely related species are more likely to be in competition for resources than more distantly related ones and so evolve to minimize this competition [1,2].
At first glance, fleshy fruits provide a puzzle. They are meant to be eaten by seed dispersers, yet they all contain secondary compounds, many of which are deterrent to would-be fruit consumers. Plants use secondary compounds as a line of defence against microbes, and other fruit antagonists that consume fruits, but do not disperse seeds [3]. Yet, microbes are not a passive partner in this evolutionary triad. They can actively engineer their environment by altering the quality of the fruit they are living in through fermentation [4] and/or by the production of toxic compounds. Janzen [5,6] was perhaps the first to realize their active role through chemical defence of the resource they are exploiting. He developed a much-cited theory that is based on the fact that many vertebrates, including humans, are very sensitive to microbial spoilage of potential foods and find such spoilage highly aversive [7]. For example, cedar waxwings (Bombycilla cedrorum) display a strong preference for uninfected fruits; dropping more microbially infected fruits during their foraging from trees [8]. Janzen's argument is if an animal consumes a microbe and the resource it is currently exploiting, then this will be deleterious to most microbes. Hence, microbes will be selected to make any resource they are exploiting unpalatable to larger animals. Thus, the way in which fruits rot, seeds mould and meat spoils is particularly effective at discouraging animal consumers such as ourselves, because there is competition between microbes and larger animals for the food value of these resources. Because of their excellent dispersal mechanisms, microbes can reach those resources before larger animals, and those microbes that can make these resources rapidly unpalatable to larger animals will be selected. If true, then this is an example of competition between very distantly related organisms from different kingdoms (or even, if prokaryotes are involved, different domains).
Sherratt et al. [9] argued that although these ideas seem very plausible when expressed verbally, there had been no formal exploration of the circumstances under which microbes that make their resource unattractive to larger animals (hereafter spoilers) would be selected over other microbes (hereafter non-spoilers). Sherratt et al. [9] presented a mathematical model that they interrogated to derive these conditions. Importantly, they found that if the spoiling tactic carries a cost, then there are no circumstances where this strategy is not outcompeted by non-spoilers. Hence, Sherratt et al. questioned the real-world relevance of Janzen's theory as an important factor in explaining the nature of microbial food spoilage. Their paper ends as follows: ‘at the very least we hope this work will stimulate those who advocate Janzen's fascinating theory to show how such a hypothesis could be justified’. Almost a decade on, the authors of this paper (including a subset of the authors of [9]) have taken up this challenge.
We would like to explore the generality of the conclusions drawn by Sherratt et al. [9] to changes in the underlying biology of the theoretical system under exploration. They assumed a fixed number of resource patches (hereafter fruit). These fruit can be colonized by both spoiling and non-spoiling microbes, and can be consumed by frugivorous vertebrates. When a vertebrate consumes a fruit, it is assumed that all the microbes on that fruit perish. An important but implicit assumption of the work of Sherratt et al. [9] is that the dispersal rates of the microbial populations are sufficiently high that fruit are always inoculated with at least a small population of microbes. One consequence of this high dispersal is the fruits are relatively homogeneous in the relative frequencies of spoiling and non-spoiling organisms present (or rapidly become so); and providing that both types were present in the system, as a generality, both types would be present in all fruit within the system. This reduces the potential for spoilers to flourish, because any advantage they gain from enhanced fruit longevity will also be shared by non-spoilers that share the same fruit. Homogenizing dispersal is not an implausible assumption, but it may also be that there are biological circumstances where dispersal is sufficiently low and/or localized that even when spoilers are relatively uncommon at a whole-system level they can be dominant on, or even have exclusive access to, a fruit for at least some time prior to colonization by non-spoilers. We will allow this possibility in our model and explore the predicted consequences for selection for the spoiling trait.
2. The model
Many species exist as a metapopulation across a fragmented habitat, facing inevitable extinction in any occupied patch but persisting regionally by dispersal into unoccupied patches. Such a metapopulation seems an appropriate description of the population dynamics of the microbes that cause fruit to rot, seeds to mould and meat to spoil. Further, it is well established in theoretical ecology that two species can coexist as metapopulations even if one is competitively superior within any given patch, provided the inferior competitor is more effective at dispersal or less vulnerable to local patch extinction [10]. This suggests that fruit-spoiling microbes may be able to coexist with non-spoiling ones, even if there is a cost to spoilage (expressed as reduced local competitive ability), providing (for example) colonies on spoiled fruit survive longer, because spoilage reduces the propensity of vertebrates to consume the fruit. We explore this using a variant of the metapopulation model of Nee & May [11].
For the sake of simplicity, we make the assumption that there are only two types of microbe spoilers (S) and non-spoilers (N)—these can be considered as two species or communities of multiple species all sharing the ‘spoil’ or ‘non-spoil’ characteristic. We assume a cost to spoiling, such that S individuals are competitively inferior to N individuals. We make the extreme assumptions that S individuals are unable to invade a fruit already occupied by N individuals, but that N individuals can invade a fruit occupied by S individuals, and in doing so extinguish all the S individuals. Note that this is a very conservative approach, because microbes typically use chemical defences not only to defend themselves against consumption by vertebrates, but also against consumption by (and competition with) other microbes [4,12]. We deprive the spoilers of this potential added benefit. As such, we assume a very substantial cost to being a spoiler.
We define e, n and s as the numbers of fruit that are respectively not colonized (empty), occupied by N individuals and occupied by S individuals. These three are the only possible states for a fruit to be in. We assume that new fruit (empty of microbes) are added to our system at some constant rate Q. Such empty patches are colonized by N individuals at rate cNne, and by S individuals at rate cSse, where cN and cS are constants. Patches switch from being colonized by S individuals to N individuals at rate cNns.
Extinction of n patches occurs at rate eNn, and s patches at rate eSs, where eN and eS are constants.
These assumptions can be summarized in the differential equations
| 2.1 |
| 2.2 |
| 2.3 |
The only structural difference between the general metapopulation model of Nee & May [11] and our model is in the term for the generation of new empty patches. They assumed that when the competitors on a patch became extinct, then the patch returned to being empty. This is appropriate when the patch is a geological island, and the populations are those of butterflies say. However, in our case, the mechanism that extinguishes the microbes (vertebrate frugivory) also destroys the patch that they were living on (the fruit).
We now look for steady-state solutions to these equations (i.e. populations that remain constant through time), ignoring non-sensical solutions where any of e, n or s are negative or infinitely large. Some algebraic manipulation shows that there is a single such steady state given by e = E*, n = N* and s = S*, where
| 2.4 |
| 2.5 |
| 2.6 |
This solution always exists, providing we satisfy two conditions.
| 2.7 |
| 2.8 |
The first of these is the expected condition that the spoilers must have an advantage (either in colonization ability or colony longevity) to compensate for their competitive disadvantage. The second condition is simply a restriction on the rate at which new fruits are added to the system; if this is too high, then there is no steady-state solution, because the total number of fruit simply increases without limit; if it is too low, then the number of fruit in the system dwindles to zero. Provided we meet this restriction (which is biologically plausible during all times except for the beginning of a ripening season), the rate of new fruits added is balanced by fruit removals (equivalent to colony extinctions).
Thus, our model predictions suggest that even in the extreme case where there is a very high cost to spoilage in terms of competitive ability relative to non-spoilers, spoiling microbes can be sustained providing their spoilage is sufficiently aversive to frugivores, and frugivores are a sufficient factor in fruit destruction that, on average, fruit colonized by spoilers last longer than fruit colonized by non-spoilers.
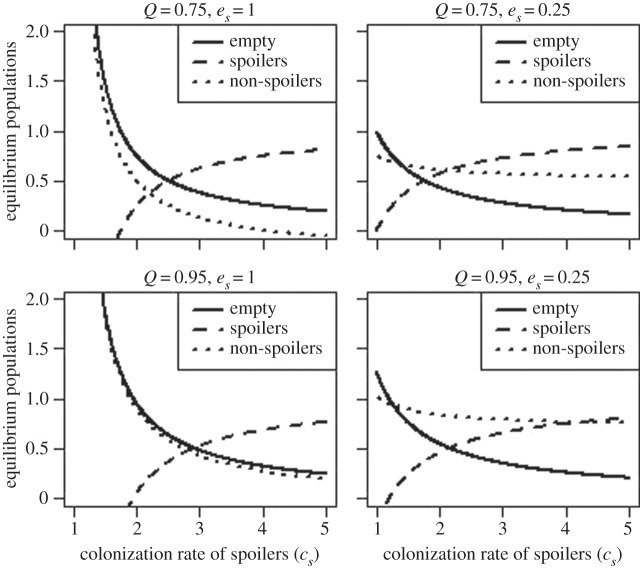
We explore the effects of varying the values given to different parameters on model predictions in figure 1. We can see that increasing the ease (relative to non-spoilers) with which spoilers can colonize empty patches (increasing cs) causes an increase in the number of patches occupied by spoilers and a decrease in the number of empty patches and the number of patches occupied by non-spoilers. Decreasing the rate at which fruit-containing spoilers are consumed (i.e. increasing the aversiveness of spoiler-populated fruit by decreasing the parameter eS) decreases the number of empty patches and increases the number of patches with a population of spoilers, because, if patches with spoilers last longer, then they can send out more colonists to invade pristine fruit. The number of patches with non-spoilers also increases, because, if patches with spoilers last longer, this provides more opportunity for those spoiling populations to be usurped by non-spoilers. The longer a fruit lasts, the more likely it is to end up being populated by non-spoilers. Increasing the rate at which new empty patches are introduced (increasing Q), increases the number of empty patches in the system, and increases the number of patches with non-spoiling populations, although the number of patches with spoiling populations declines. Thus, spoiling will be particularly prevalent if the density of potential colonization opportunities in virgin patches is modest. Here, the superior colonization ability or longevity of spoilers is most advantageous.
Figure 1.
The equilibrium numbers of fruits containing no microbial organisms (empty, E), or a colony of spoiling microorganisms (S) or a colony of non-spoilers (N), as a function of the parameter controlling the rate at which empty patches can be colonized by spoilers (cS). The parameters cN and eN governing non-spoilers are both held at unity throughout. The four panels explore two levels of the remaining two parameters (the rate at which empty patches are introduced to the system Q, and the extinction rate of patches with spoilers on them eS) in a factorial arrangement.
3. Discussion
In contrast to the only previous theoretical work, our model provides strong support for Janzen's verbal model that microbes might be selected to make their food unpalatable to large animals. Specifically, we find that a spoilage strategy involving making food more unpalatable to vertebrates can flourish, even if microbes adopting this strategy must pay a substantial cost in terms of reduced ability to compete with non-spoilers. Our analysis suggests that this result should hold widely provided (i) dispersal is sufficiently limited that spoilers can find temporary refuge in fruits entirely free from non-spoilers, (ii) competition takes place on shorter timescales than extinction, so that fruits containing both spoilers and non-spoilers do not occur and (iii) spoilage is sufficiently aversive to vertebrates that are otherwise willing to eat food resources, and such vertebrates are a sufficiently important factor in food item destruction, that on average food colonized by spoilers last longer than that colonized by non-spoilers. Although we have characterized this in terms of fruit, it may be more applicable to carrion, animal carcasses are often characterized as being relatively rare and isolated within the environment.
The key difference in our predictions from those of Sherratt et al. [9] is that we assume that microbial dispersal mechanisms are sufficiently localized that it is possible for heterogeneity of microbial communities to occur between fruits, so that in at least some fruits spoiling microbes can gain the benefits of their spoilage without those benefits necessarily being shared by non-spoilers on the same fruit. Hence, it would be very valuable for future empirical studies to explore the generality of this requirement. There is evidence for strong regional delineation of yeast populations within and between New Zealand vineyards [13], but more studies would be valuable. We would expect that in some systems, where there is strong spatial proximity of resource items, the previous model will be more realistic than ours and microbes will not be selected to modify their environment because of the effect that this has on vertebrates. However, where this does not apply, we would expect our model assumptions to be more relevant, and selection for a certain degree of spoilage would be more likely. As a generality, where fruiting plants produce many fruits per plant and grow at high density, we would not expect spoilage to be selected, but where fruits occur at a lower spatial density, we would expect selection for spoilage. A general prediction of our model framework is that there will be spatial polymorphism with a fraction of fruit that are never microbially spoiled and a fraction that are spoiled. This polymorphism may be difficult to detect empirically when 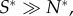 that is when almost all fruit that are colonized by microbes are colonized by a spoiling strain. This will occur when spoilers are much superior dispersers (
that is when almost all fruit that are colonized by microbes are colonized by a spoiling strain. This will occur when spoilers are much superior dispersers (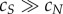 ).
).
In formulating our model, we were at pains to make the structure of the model as simple as possible, both to not only allow us to solve the model analytically and also to allow unpicking the mechanisms underlying model predictions easier. As such our model should be seen as a proof of concept: we show that a set of plausible biological assumptions can yield selection for a spoiling trait in microbes even if spoilage incurs a cost. However, a number of model elaborations would be interesting to explore the generality of our qualitative conclusions. One obvious elaboration would be to model the cost of spoilage directly as being expressed explicitly in the growth rate of the microbial strain (rather than the more derived trait of competitive ability considered here). In addition, our model assumes that predators do not respond flexibly to the fraction of available fruits that are spoiled. This is reasonable for situations where the focal fruit type makes up a small part of the diet of local vertebrate frugivores. However, the consequences of allowing frugivores to modify their diet choice decisions in the light of the types of fruit available would be another useful model elaboration.
While it has long been realized that organisms can engineer their environments [14,15], there is uncertainty on the impact of such engineering on the evolution of traits and of species interactions [16]. Importantly, our model predicts that such an impact is likely, but does not prove it. It only demonstrates that competition with vertebrates can be a relevant factor shaping the way in which microbes affect the properties of the foods they live on. In general, competition for food resources is not limited to the one between microbes and vertebrates. As Sherratt et al. [9] discuss, microbes’ influence on their food resource (what we term spoilage) may be shaped by competition between microbes or simply be a by-product of selection for metabolic activities (such as extracellular digestion). For example, Saccharomyces cerevisiae outcompetes other yeasts by modifying the fruit environment in which they live through the coupled effects of heat and ethanol production [4]. These traits provide a 7% fitness advantage over other members of the microbial community. Obviously, selection pressures to reduce competition with vertebrates and with other microbes are not necessarily exclusive and particularly effective compounds may screen out both types of competitors—a point also raised by Janzen in his original seminal paper [5]. Our contribution is to provide support to Janzen's suggestion that competition with vertebrates could contribute (at least in part) to the selective regime that shapes the way microbes interact with their food substrates. However, the challenge now is to explore empirically how important this selection pressure is in different systems, and whether we can identify taxonomic or evolutionary generality of the relative importance of this mechanism.
To this point, we have used fruit as a short-hand term for a variety of substrates that might be of value to both microbes and vertebrates. Janzen [5] focused on three general classes of substrate: fleshy fruits, seeds and carrion. While our analysis should be relevant to all these systems, there is an interesting difference between these three groups. For fruits, the fitness of the plant that produced them is likely often to be influenced by whether the fruit or seed is consumed by microorganisms or vertebrates. This is obvious in the case of seeds that require passage through the gut of a vertebrate in order to enhance their likelihood of germination. For seeds, consumption by vertebrates should generally be as detrimental as consumption by microbes, but many seed predators such as squirrels or jays cache seeds and thereby contribute to seed dispersal, because not all seeds are later recovered and eaten [17]. By contrast, the fitness of a dead animal is utterly unaffected by the fate of its flesh. Thus, an evolutionary triad occurs only between plants, their mutualists and antagonists. By contrast, the interactions for the exploitation of carrion are simpler as they are only based upon competition among the many species eating this food resource. This makes carrion a somewhat simpler system, and therefore it is not surprising that previous effective experimental tests of Janzen's idea use carrion. In a series of experiments, Burkepile et al. [18] showed that microbes made fish carrion less attractive to a range of marine scavengers. Rozen et al. [19] explored the parental behaviour of the burying beetle Nicrophorus vespilloides, a species that obligately breeds on carcasses of small vertebrates. They found strong detrimental effects of microbial competition on beetle reproductive success and larval growth. They also found that parents respond to this by preferentially selecting fresher carcasses where possible and increasing their parental care when obliged to use a carcass with greater microbial development. These responses were found to ameliorate the detrimental effects of microbial competition.
It is clear that plants can influence rates of microbial spoilage. For example, high sugar levels in nectar reduce bacterial growth (this is exploited also in human jam-making), and, in some plants, with low sugar content in their nectar (famously rhododendron), the plant uses antibiotic compounds instead. Interestingly, to the best of our knowledge whether plants use counterstrategies to reduce the ability of vertebrates to detect any spoilage by microbes that does occur has not been investigated. Plants might be selected to introduce seed traits that resist spoilage itself and also reduce the ease with which vertebrates can detect any spoilage that occurs. That is, in such a situation, the plant may be selected to make it more difficult for seed dispersers to feed selectively on the basis of microbial spoilage, such as cedar waxwings do if they drop significantly more microbially infested fruits. Dropping fruits is one way vertebrates can interact with microbes and plants and it is akin to human horticulturalists weeding-out infected plants which are then selected to reduce or conceal infections. A few vertebrates have evolved tolerance towards chemical defence by microbes. For example, alcohol is a common microbially generated feeding deterrent, but pen-tailed treeshrews (Ptilocercus lowii) are pollinators that frequently consume nectar high in alcohol that is produced by a fermenting yeast community [20]. Their alcohol dosages would intoxicate humans, yet these animals show no signs of intoxication. This example demonstrates that chemical defence by microbes is not always effective. Similarly, alcohols acted as a feeding stimulus in the tropical, fruit-feeding butterfly, Bicyclus anynana. This butterfly uses microbially generated alcohols as a cue to locate fruit resources [21]. This demonstrates that microbes might be selected to conceal biochemical by-products of their metabolism in some situations, depending on the broad effectiveness of their chemical defences. In this case, because the butterfly can damage fruit, but not disperse seeds, then the plant (as well as the microbe) might benefit from suppressing or masking the alcohol cues. Thus, various strategies are feasible within the evolutionary triad of plants, their mutualists and antagonists.
Plants can also be selected to have a relationship with a frugivore or a pollinator extending over several fruit-consumption events. That is, when a monkey, for example, visits a fruiting tree, it may consume many fruits during that visit. In this case, it may be that the plant benefits from helping the vertebrate to selectively avoid microbially infested fruit, if this causes the vertebrate to extend its visit and consume more fruits in total than if it had the aversive experience of mistakenly consuming a spoiled fruit. Here, we have argued that we should expect competition between microbes and vertebrates to be an important selective pressure explaining how fruit rots, seeds mould and meat spoils. However, in the first two of these cases, there will also be interaction between the plant that produced the seed or fruit and the two different consumer classes. It is an outstanding but important challenge to understand how this web of interactions will influence selection of traits in the evolutionary triad among plants, mutualists and antagonists which will vary spatially and temporarily [22]. It may sometimes be advantageous for a microbe to be eaten by a vertebrate. For example, many species of water plant and aquatic invertebrates can be dispersed by birds as they survive passage through their guts [23,24]. The same could be the case for some microbes. It may be that some microorganisms produce chemicals making it more likely that their substrate is eaten by an appropriate dispersal agent. For example, there is evidence that yeasts that colonize fruit can manipulate the odours produced by that the fruit in a way than increases attractiveness to the fruitflies that act as dispersal agents for the yeasts [25].
Acknowledgement
We thank Tim Cooper and two anonymous referees for very helpful comments on a previous version.
References
- 1.Lack D. 1971. Ecological isolation in birds. Oxford, UK: Blackwell [Google Scholar]
- 2.Krebs CJ. 2009. Ecology, 6th edn San Francisco, CA: Benjamin Cummins [Google Scholar]
- 3.Herrera CM. 1982. Defense of ripe fruit from pests: its significance in relation to plant–disperser interactions. Am. Nat. 120, 218–241 (doi:10.1086/283984) [Google Scholar]
- 4.Goddard MR. 2008. Quantifying the complexities of Saccharomyces cerevisiae’s ecosystem engineering via fermentation. Ecology 89, 2077–2082 (doi:10.1890/07-2060.1) [DOI] [PubMed] [Google Scholar]
- 5.Janzen DH. 1977. Why fruits rot, seeds mould and meat spoils. Am. Nat. 111, 691–713 (doi:10.1086/283200) [Google Scholar]
- 6.Janzen DH. 1979. Why food rots. Nat. Hist. Mag. 88, 60–64 [Google Scholar]
- 7.Cipollini ML, Stiles EW. 1993. Fruit rot, antifungal defence and palatability of fleshy fruits for frugivorous birds. Ecology 74, 751–762 (doi:10.2307/1940803) [Google Scholar]
- 8.Buchholz R, Levey DJ. 1990. The evolutionary triad of microbes, fruits, and seed dispersers: an experiment in fruit choice by cedar waxwings, Bombycilla cedrorum. Oikos 59, 200–204 (doi:10.2307/3545535) [Google Scholar]
- 9.Sherratt TN, Wilkinson DM, Bain RS. 2006. Why fruits rot, seeds mould and meat spoils: a reappraisal. Ecol. Model. 192, 618–626 (doi:10.1016/j.ecolmodel.2005.07.030) [Google Scholar]
- 10.Hanski I. 1983. Coexistence of competitors in a patchy environment. Ecology 64, 493–500 (doi:10.2307/1939969) [Google Scholar]
- 11.Nee S, May RM. 1992. Dynamics of metapopulations: habitat destruction and competitive coexistence. J. Anim. Ecol. 61, 37–40 (doi:10.2307/5506) [Google Scholar]
- 12.Wiener P. 2000. Antibiotic production in a spatially structured environment. Ecol. Lett. 3, 122–130 (doi:10.1046/j.1461-0248.2000.00129.x) [Google Scholar]
- 13.Gayevskiy V, Goddard MR. 2012. Geographical delineations of yeast communities and populations associated with vines and wines in New Zealand . ISME J. 6, 1281–1290 (doi:10.1038/ismej.2011.195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margulis L, Lovelock JE. 1974. Biological modulation of the Earth's atmosphere. Icarus 21, 471–489 (doi:10.1016/0019-1035(74)90150-X) [Google Scholar]
- 15.Jones CG, Lawton JH, Shachak M. 1994. Organisms as ecosystem engineers. Oikos 69, 373–386 (doi:10.2307/3545850) [Google Scholar]
- 16.Laland KN, Odling-Smee J, Feldman MW. 2004. Causing a commotion. Nature 429, 609 (doi:10.1038/429609a) [DOI] [PubMed] [Google Scholar]
- 17.Vanderwall SB. 1990. Food hoarding in animals. Chicago, IL: University of Chicago Press [Google Scholar]
- 18.Burkepile DE, Parker JD, Woodson CB, Mills HJ, Kubanek J, Sobecky PA, Hay ME. 2006. Chemically mediated competition between microbes and animals: microbes as consumers in food webs. Ecology 87, 2821–2831 (doi:10.1890/0012-9658(2006)87[2821:CMCBMA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 19.Rozen DE, Engelmoer DJP, Smiseth PT. 2008. Antimicrobial strategies in burying beetles breeding in carrion. Proc. Natl Acad. Sci. USA 105, 17 890–17 895 (doi:10.1073/pnas.0805403105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiens F, Zitzmann A, Lachance M-A, Yegles M, Pragst F, Wurst FM, von Holst D, Guan SL, Spanagel R. 2008. Chronic intake of fermented floral nectar by wild treeshrews. Proc. Natl Acad. Sci. USA 105, 10 426–10 431 (doi:10.1073/pnas.0801628105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dierks A, Fischer K. 2008. Feeding responses and food preferences in the tropical, fruit-feeding butterfly Bicyclus anynana. J. Insect Physiol. 54, 1363–1370 (doi:10.1016/j.jinsphys.2008.07.008) [DOI] [PubMed] [Google Scholar]
- 22.Schaefer HM, Ruxton GD. 2011. Plant animal communication. Oxford, UK: Oxford University Press [Google Scholar]
- 23.Brochet AL, Guillemain M, Fritz H, Gauthier-Clerc M, Green AJ. 2010. Plant dispersal by teal (Anas crecca) in the Camargue: duck guts are more important than their feet. Freshw. Biol. 55, 1262–1273 (doi:10.1111/j.1365-2427.2009.02350.x) [Google Scholar]
- 24.Brochet AL, Gauthier-Clerc M, Guillemain M, Fritz H, Waterkeyn A, Baltanás A, Green AJ. 2010. Field evidence of dispersal of branchiopods, ostracods and bryozoans by teal (Anas crecca) in the Camarge (southern France). Hydrobiologica 637, 255–261 (doi:10.1007/s10750-009-9975-6) [Google Scholar]
- 25.Palanca L, Gaskett AC, Günther CS, Newcomb RD, Goddard MR. 2013. Quantifying variation in the ability of yeasts to attract Drosophila melanogaster. PLoS ONE 8, e75332 (doi:10.1371/journal.pone.0075332) [DOI] [PMC free article] [PubMed] [Google Scholar]