Abstract
Uncovering the mechanisms underlying the evolution of novel traits is a central challenge in biology. The lanterns of fireflies are complex traits that lack even remote homology to structures outside luminescent beetle families. Representing unambiguous novelties by the strictest definition, their developmental underpinnings may provide clues to their origin and offer insights into the mechanisms of innovation in developmental evolution. Lanterns develop within the context of abdominal Hox expression domains, and we hypothesized that lantern formation may be instructed in part by these highly conserved transcription factors. We show that transcript depletion of Abdominal-B in Photuris fireflies results in extensive disruption of the adult lantern, suggesting that the evolution of adult lanterns involved the acquisition of a novel regulatory role for this Hox gene. Using the same approach, we show that the Hox gene abdominal-A may control important secondary aspects of lantern development. Lastly, we hypothesized that lantern evolution may have involved the recruitment of dormant abdominal appendage-patterning domains; however, transcript depletion of two genes, Distal-less and dachshund, suggests that they do not contribute to lantern development. Our results suggest that complex novelties can arise within the confines of ancestral regulatory landscapes through acquisition of novel targets without compromising ancestral functions.
Keywords: Lampyridae, evo devo, Abdominal-B, photic organ
1. Introduction
Determining the mechanisms by which novel complex traits come into being is a fundamental challenge in evolutionary biology [1–3]. Unlike quantitatively varying traits whose evolutionary modifications can be more easily understood as a reflection of quantitative variation in developmental properties, novelties represent qualitative departures from ancestral states which lack obvious homology to traits in related taxa. It is therefore far more difficult to imagine how an ancestral developmental programme can be modified by random mutation to give rise to a new feature that endows its bearer with fundamentally new adaptive potential. Little is known, for instance, about how and at what level developmental programmes are modified to account for novel traits or if indeed the processes that account for them are truly distinct from those that generate quantitative variants. Addressing these issues may be enabled by investigating the developmental-genetic basis of a genuinely novel, complex trait and exploring the ways in which it has been accommodated by an ancestral genetic architecture. Here, we report the first characterization of the developmental genetics underlying the formation of a spectacular novel trait—the luminescent organ of the firefly.
Fireflies or lightning bugs are—despite their common names—beetles, all belonging to a single family, the Lampyridae. The family consists of roughly 2000 described species in 85 genera, all of which produce light in some stage of development [4]. Fireflies luminesce autonomously through light reactions catalysed by the enzyme luciferase which is thought to have arisen via duplication and neofunctionalization of a fatty acyl-CoA synthetase gene [5,6]. These reactions occur in discrete organs located on the ventral abdomen known as lanterns or photic organs (POs). POs are complex, integrated structures (figure 1) that play a well-known role in courtship rituals among conspecific adult fireflies and may have aposematic function in larvae [10]. Most significantly, outside of the POs of a few closely related luminescent beetle families, lanterns lack homology to any known structure in other insect or arthropod groups and therefore can be considered evolutionary novelties by even the strictest definition [1]. Presently, we possess only a modest understanding of the histological basis of lantern formation [7], and nothing is known about the genetic regulation of lantern development. Investigating the genetic basis of these structures thus presents a promising opportunity to further our understanding of how novel complex traits might evolve.
Figure 1.
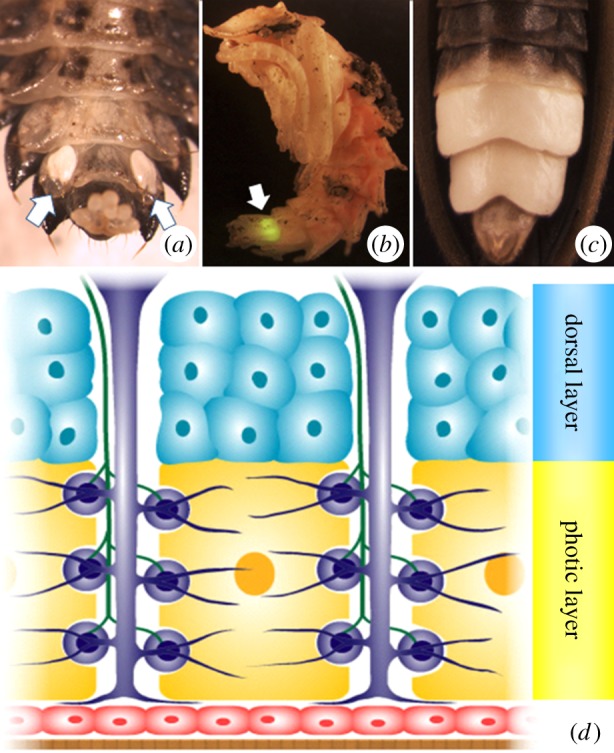
(a) Ventral abdomen of a Photuris larva. Note the paired photic organs (POs) on the eighth abdominal segment (indicated by arrows). (b) Photuris pupa. The larval PO (indicated by arrow) remains functional throughout pupation and glows when the pupa is disturbed. (c) Close-up of the adult Photuris PO. (d) Diagrammatic cross section of adult photurid lantern. Note photic (yellow) and dorsal (blue) layers which are derived from fat body precursors [7]. Also shown are tracheae and tracheal end-cell complexes (purple), nerve axons (green), epidermis (red) and transparent cuticle (brown). Based on Chapman [8], after Smith [9].
All known lampyrids are luminescent in the larval stage, although many lineages are non-luminescent as adults [11]. The placement of lanterns along the ventral abdomen varies as a function of species, sex and developmental stage. In the most common North American genera, adult males luminesce from a large organ that spans the entire ventral surface of the sixth and seventh abdominal segments (figure 1). The organs of adult females are more diverse and vary by genus and/or species from lanterns that occupy the same segments as the male but are withdrawn somewhat from the segment margins (Photuris pennsylvanica), some that are laterally paired (Pyractomena angulata), and some that are restricted entirely to the sixth abdominal segment as a single medial spot (Photinus pyralis). Because adult lanterns are nearly always restricted to one or two abdominal segments, our first main hypothesis posits that the origin of lanterns was enabled by the evolution of a novel regulatory role for the abdominal Hox genes, abdominal-A (abd-A) and Abdominal-B (Abd-B).
The lanterns of larvae, however, differ in important ways from their adult counterparts. Specifically, larval lanterns form on the eighth abdominal segment and are arranged in a paired, ventrolateral position (figure 1). This arrangement is believed to represent the ancestral state of lantern development from which adult lanterns were secondarily derived [11]. The spatial arrangement of larval lanterns and, as detailed below, the proximodistal organization inherent in lantern structure led us to our second main hypothesis, that PO formation is mediated by the action of patterning genes originally involved in appendage formation. Both hypotheses are further detailed next.
(a). Do abdominal Hox genes regulate lantern development in fireflies?
Hox genes encode transcription factors that regulate segment identity along the anterior–posterior axis of the insect body plan [12]. In insects, the abd-A protein is broadly expressed in the developing abdomen, its anterior domain generally beginning in the posterior compartment of A1, and its more variable posterior margin extending through A10 in most insects studied [13]. Among its most well-characterized functions in Tribolium and Drosophila is the abdomen-specific repression of appendage development [14,15]. Abd-B, by contrast, is not known to specify traits anterior to A7 outside of drosophilid insects. Its best understood role is the specification of genitalia in Drosophila, Tribolium and other arthropods [13,16]. As the adult photurid lantern occupies abdominal segments 6 and 7 with associated cuticular effects extending into A5, our initial expectation was therefore that its regulation would be likely to be controlled by abd-A rather than Abd-B.
In addition to providing positional information in PO development, we hypothesized that abdominal Hox genes may also regulate other aspects of PO development, consistent with known abd-A/B functions in other organisms. For instance, Abd-B is known to function in patterning abdominal cuticle pigmentation in Drosophila [17]. Lantern-associated cuticle lacks pigmentation, allowing light generated below to pass largely unimpeded through a translucent cuticle. The occurrence of cuticular depigmentation and the presence of underlying photic tissue are generally very tightly linked in many adult fireflies (figure 1), suggesting that both may rely on shared patterning signals. Furthermore, during Drosophila embryogenesis abd-A is known to specify gonadal mesoderm from precursors that would otherwise differentiate into fat body cells [18]. In this case, abd-A behaves as an indirect activator of a unique tissue fate by repressing genes specifying alternative cell fates. This process shows intriguing similarities to what little we do know of lantern development: histological studies conducted over 90 years ago showed that the photic and dorsal cells of the lantern appear to differentiate from fat body precursors during larval and adult development [7]. A recent study indicates that it is specifically the fat body trophocytes that give rise to PO tissue [19]. In addition, abd-A is a well-known repressor of appendage development in the abdomen [14,15] via repression of appendage-patterning genes, the focus of our second main hypothesis.
(b). Do appendage-patterning genes instruct lantern formation?
The most widespread, and probably most ancient, PO layout is a paired, ventrolaterally positioned organ that, while usually restricted to a single segment in larvae, is sometimes serially arranged on multiple segments in some adult species [11,20]. The adult PO consists of two primary cell layers: the ‘photic layer’ and ‘dorsal layer’. The columnar cells of the photic layer—the photocytes—lie just dorsal to the epidermis and are arranged in repeating rosette patterns around central cylinders which serve as conduits for tracheae and nerves [9]. Covering the photic layer's inner surface are the cells of the dorsal layer (aka ‘reflective layer’), which contain urate crystal granules thought to help reflect the light produced in the photocytes [9]. This arrangement of the photic and dorsal layers gives the lantern a very basic proximodistal organization. These observations suggest the existence of a patterning mechanism able to establish a proximodistal axis during PO development. A particularly well-studied mechanism in arthropods that may be able to achieve this involves a subset of appendage-patterning genes also known as the leg gap genes, the same genes whose function is normally repressed in the abdomen of extant insects through abd-A.
Traditional insect appendage growth is initiated and regulated in the head and thoracic segments by a largely conserved set of core patterning genes. During the embryonic development of Tribolium and Drosophila, the segment-polarity gene wingless (wg), in conjunction with intersecting dorsal–ventral signals, initiates the proximodistal axis of leg formation by activating the appendage-patterning gene Distal-less (Dll) [21,22]. The resultant developing appendage is further specified by a suite of conserved leg gap genes that includes Dll (distal identity), dachshund (dac: medial identity) and homothorax (hth: proximal identity) [23,24]. The initiating signals governing limb induction are present in a ventrolateral location on each thoracic and abdominal segment, but the expression of Dll is inhibited in the abdomen owing to the repressive effect of the Hox protein abdominal-A (abd-A), resulting in the normal absence of abdominal appendages [15]. However, experimental downregulation of abd-A in Tribolium and Drosophila results in the formation of ectopic leg primordia in the abdominal segments [14,25], demonstrating that the inductive potential of the appendage-patterning network is maintained in the normally limbless abdomen. Abd-B, by contrast, is not known to inhibit appendage formation per se, but instead specifies genitalic identity to the most terminal appendages, which in its absence transform into legs [16].
Here, we examine the potential role of the abdominal Hox genes, Abd-B and abd-a, as well as the leg gap genes, Dll and dac, in the formation of the adult Photuris lantern via larval RNA interference-mediated transcript depletion.
2. Material and methods
(a). Larval collection and rearing
Last-instar photurid larvae were collected from stream and pond banks in forested areas around Southern Indiana and maintained in the laboratory largely following McLean et al. [26]. To induce pupation, larvae were placed individually into wells (Falcon 6-well culture plates) containing moistened native soil and incubated at 27°C in a 16 L : 8 D cycle. After 0.5–2 weeks, larvae respond by building a subterranean chamber from small soil pellets within which they pupate a few days later.
(b). Gene cloning
mRNA was harvested from photurid pupae using the RNeasy minikit (Qiagen), and reverse transcribed to cDNA with SuperScript III (Invitrogen). All Photuris orthologues were amplified from cDNA using nested, degenerate PCR with primers designed from protein alignments in MEGA5 [27].
abd-A—Putative conserved regions were identified from protein alignments of Tribolium castaneum, Harmonia axyridis and Onthophagus taurus. A 521 bp segment of the Photuris abd-A gene positioned upstream of the homeodomain was amplified using the following nesting strategy [CONSERVED AA SEQUENCE (DEGENERATE PRIMER)]: primary (outer) primer pair—Fwd: PKYHQQF (5′ CCN AAR TAY CAY CAR CAR TT 3′), and Rev: WMSITDWM (5′-CAT CCA RTC NGT DAT NSW CAT CCA-3′); secondary (interior) pair—Fwd: PKYHQQF (5′ CCN AAR TAY CAY CAR CAR TT 3′), and Rev: QFYHQAA (5′-GC NGD YTG RTG RTA RAA YTG-3′).
Abd-B—Putative conserved regions were identified from protein alignments of Tribolium castaneum, Onthophagus taurus, Pediculus humanus corporis and Apis mellifera. A 417 bp segment of the Photuris Abd-B gene positioned upstream of the homeodomain was amplified using the following nesting strategy: primary (outer) primer pair—Fwd: HIPAKR (5′ CAY ATH CCN GCN AAR MG 3′), and Rev: EWTGQV (5′-AC YTG NCC NGT CCA YTC-3′); secondary (interior) pair—Fwd: HIPAKR (5′ CAY ATH CCN GCN AAR MG 3′), and Rev: RHHVDH (5′-TG RTC NAC RTG RTG NC-3′).
Dll—Putative conserved regions were identified from protein alignments of Tribolium castaneum, Harmonia axyridis and Onthophagus taurus. A 206 bp segment of Photuris Dll positioned upstream of the homeodomain was amplified using the following nesting strategy: primary (outer) primer pair—Fwd: FIELQQH (5′-TTY ATH GAR YTN CAR CAR CA-3′), and Rev: KKMMKAAQ (5′-TG NGC NGC YTT CAT CAT YTT YTT-3′); secondary (interior) pair—Fwd: QQHGYGP (5′-CAR CAR CAY GGN TAY GGN CC-3′), and Rev: YAANCPP (5′-GG NGG RCA RTT NGC NGC RTA-3′).
dac—Putative conserved regions were identified in part from protein alignments of Tribolium castaneum, Onthophagus taurus and Pediculus humanus corporis. A 275 bp segment of Photuris dac positioned downstream of the Ski/Sno domain was amplified using the following nesting strategy: primary (outer) primer pair—Fwd: DNGDYPYE (5′-GAY AAY GGN GAY TAY CCN TAY G-3′), and Rev: KVAADNA (5′-C RTT RTC NGC NGC NAC YTT-3′); secondary (interior) pair—Fwd: ENGHMGD (5′-GAR AAY GGN CAY ATG GGN GA-3′), and Rev: YEDIVKHL (5′-AR RTG YTT NAC DAT RTC YTC RTA-3′).
Sequence identities were confirmed by maximum-likelihood phylogenetic analysis in MEGA5 [27]. All products were ligated into a pSC-A-amp/kan cloning vector using the StrataClone PCR cloning kit (Stratagene).
(c). RNA interference
521bp-abd-A, 417bp-Abd-B, 206bp-Dll and 275bp-dac fragments were amplified from pSC-A cloning vector using M13 primers. Sense and antisense RNA strands were generated by in vitro transcription with Megascript T3 and T7 High Yield Transcription kits (Ambion). Vector-only control injections were first carried out. dsRNA was produced by mixing complementary stands in equimolar amounts, heating to 80°C in a water bath which was cooled to room temperature over 3–4 h. Last-instar larvae were injected laterally just posterior to the first abdominal segment with 0.5–3 µg of dsRNA in a buffer containing 1 mM sodium phosphate (pH 6.8–6.9) and 5 mM KCl (total injection volume = 3 µl). Injections were delivered via a gas-tight 1801 Hamilton syringe equipped with a 31-guage removable needle. Larvae were placed in wells containing moist, native soil for pupation.
(d). Fixation/sectioning/staining/microscopy
Control-injected and Abd-BRNAi adults were fixed in 2% paraformaldehyde at 4°C for 2 h under gentle agitation. Samples were rinsed three times in PBS and then subjected to an 80, 60 and 40% ethanol series. After a final PBS rinse, samples were equilibrated at 4°C in a 30% sucrose/PBS solution, and then imbedded/frozen in O.C.T. compound (VWR). Samples were sectioned to 5 μm on a Microm HM550 OMV cryotome and stained with haematoxylin and eosin. Images were obtained using a Carl Zeiss Axioplan microscope equipped with Qimaging Micropublisher digital camera and associated Qcapture software.
3. Results
(a). cDNA cloning and sequence analysis
We cloned partial cDNAs from the Photuris orthologues of abd-A (521-bp fragment), Abd-B (417-bp), Distal-less (206-bp) and dachshund (275-bp). As each gene's function was to be subsequently analysed in RNA interference (RNAi)-mediated transcript knockdown experiments, care was taken to exclude highly conserved non-gene-specific domains from the cloned product. Thus, the fragments were cloned from regions fully outside of each gene's respective DNA-binding domain (homeobox in abd-A, Abd-B and Dll; Ski/Sno domain in dac). The identity of the fragments was confirmed by phylogenetic analysis. Translated sequence comparisons show varying degrees of conservation among the insect orthologues (electronic supplementary material, figure S1).
(b). Function of abdominal Hox genes and appendage-patterning genes in photic organ development
We used larval RNA interference to determine whether the inhibition of the Photuris orthologues of Abd-B, abd-A, Dll or dac perturbs normal development of the adult lantern.
(i). Control injection
Control-injected animals were indistinguishable from uninjected individuals in each case (n = 9; figure 2a). POs were fully developed and emitted light during the adult stage.
Figure 2.
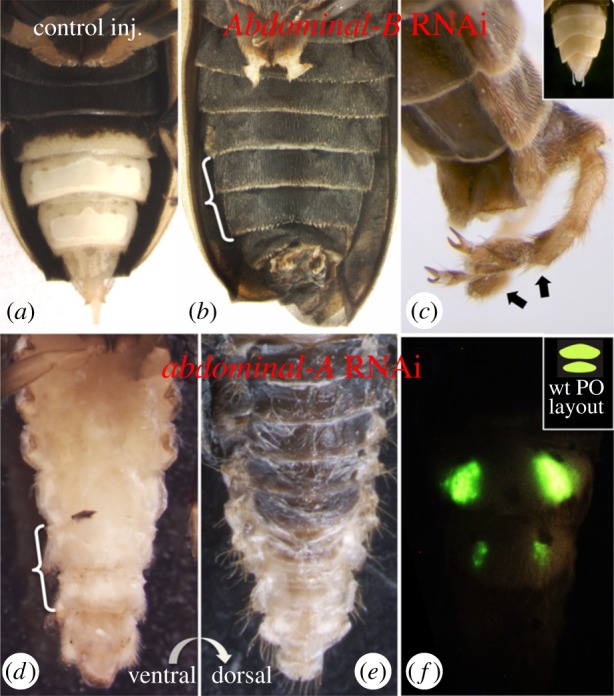
Effects of abdominal Hox gene depletion in the Photuris abdomen. (a) Control-injected individuals exhibit normal PO formation. Shown is a female exhibiting normal adult PO on A6/7 with cuticle depigmentation spilling into A5. Larval lanterns have been resorbed. (b) Larval Abd-B knockdown results in profoundly disrupted lantern development and ectopically pigmented abdominal cuticle. Normal PO-bearing segments, A6/7, are indicated by bracket. Also present, though not clearly visible in photo, are ectopic legs (projecting perpendicular to photographic plane) from a segment that normally bears genitalia. Metathoracic legs have been clipped to improve visibility. (c) Dorsolateral view of posterior terminus of an Abd-B RNAi adult displaying genitalia → leg transformation (indicated by arrows), matching phenotypes observed in Drosophila Abd-B mutants [16]. Inset shows wild-type male genitalia (female pictured in panel (a)). (d) Larval abd-A injection results in varying degrees of ectopic ventral cuticle depigmentation. The most extreme phenotype observed in two cases is shown (again, brackets indicate PO-bearing segments, A6/7). The individual shown displays a translucent cuticle on every abdominal ventrite, in addition to a general narrowing of the abdomen. This raises the possibility that abd-A may function in anterior repression of ventral cuticle depigmentation. Furthermore, the dorsal abdominal cuticle of abd-A knockdown animals exhibited wild-type pigmentation in all cases (e), suggesting a ventral-specific effect as opposed to a general disruption of pigmentation patterning. (f) On occasion, PO function was observable in abd-A knockdown animals. Here, luminescence was restricted to serially arranged pairs of lateral pockets on the normal lantern-bearing segments, suggesting that abd-A is necessary for proper PO formation and function. Inset diagrams wild-type (wt) female PO layout.
(ii). Abdominal-B RNAi massively disrupts lantern development
Abd-B knockdown resulted in the profound disruption of lantern formation from an otherwise almost entirely wild-type-like abdomen (figure 2b). Sectioning confirmed the lack of tissue differentiation into the characteristic dual-layered lantern inside the ectopically pigmented cuticle as well as the complete absence of normal columnar cellular organization or associated tracheal infiltration (electronic supplementary material, figure S2). In addition, we observed a transformation of the genitalia into legs (figure 2c), paralleling Abd-B mutant phenotypes in Drosophila [16] and further corroborating that Abd-B transcript knockdown is responsible for the observed loss of PO differentiation. This phenotype was remarkably consistent and associated with low mortality in the first round of injections. Of 12 injected animals, 11 survived to adulthood. Of these, eight showed complete ectopic abdominal pigmentation associated with the most extreme disruption of lantern development, while the remaining three exhibited a range of moderate to severe lantern disruption. None of the 11 animals that survived to adulthood generated light. Unexpectedly, a second round of injections carried out several months later to increase sample size and generate histological data yielded much lower survivorship, and did so in RNAi as well as control-injected and uninjected control individuals in similar proportions, suggestive of a novel source of mortality unrelated to the experimental procedure. Nevertheless, the single surviving Abd-B-depleted individual (out of 42 dsRNA-injected larvae) again exhibited profound disruption of lantern formation and complete ectopic pigmentation of the abdominal cuticle.
(iii). abdominal-A RNAi causes variable abdominal phenotypes
The effects of abd-A knockdown were more variable and associated with much higher mortality. Of 48 injected animals, only nine survived to adulthood. Of these, all exhibited some degree of abdominal narrowing and varying degrees of retention of pupal abdominal characteristics. While some degree of PO disorganization was noted in all individuals, no individual lacked its lantern entirely. However, six individuals exhibited, to varying degrees, a conspicuous, ectopic de-pigmentation of the ventral cuticle. Whereas a wild-type photurid adult would normally exhibit cuticular transparency limited to the sixth and seventh ventral segments and extending partially into A5, abd-A knockdown resulted in varying degrees of ectopic ventral depigmentation of the anterior abdomen including two individuals that lacked pigmentation over the entire length of the ventral abdomen (figure 2d). By contrast, dorsal pigmentation and the anterior body was wild-type in all cases (figure 2e). The remaining individuals showed no pigmentation effect in two cases (but see below), and highly disrupted abdominal development in another. Most of these animals died too soon after eclosion to observe PO light emission. However, in two cases, including one with mild pigmentation effects and another with normal ventral pigmentation, individuals remained alive long enough for us to observe that luminescence was restricted to a pair of lateral pockets on A6/A7, rather than spanning the entire ventral surface as observed in wild-type or control-injected animals (figure 2f).
(iv). Dll and dac RNAi has no effect on adult photic organ formation
Dll RNAi resulted in the shortening, fusion or omission of distal leg regions, antennae and maxillary palps, consistent with a role of Dll in appendage-patterning and distal appendage identity in other insects [28,29]. Furthermore, there was a clear dosage response as the magnitude of appendage disruption was positively associated with the amount of dsRNA injected. Unambiguous appendage phenotypes were observed in 75% of pupae (n = 15/20) and 80% of surviving adults (n = 8/10). At the same time, none of the individuals with moderate to severe appendage phenotypes exhibited any obvious disruption of PO appearance or function (figure 3d).
Figure 3.
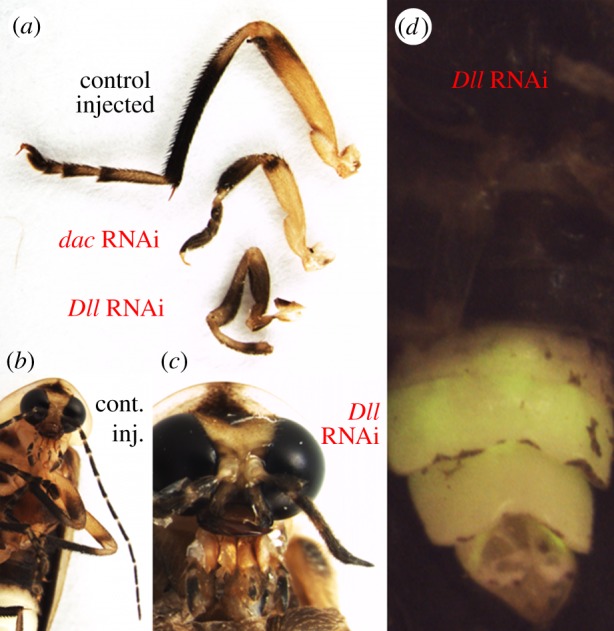
Dll and dac exhibit conserved function in appendage formation but do not function in Photuris lantern development. (a) Metathoracic leg phenotypes in Dll and dac knockdown animals, as well as a control-injected individual. Downregulation of dac results in dramatic shortening of the tibia, and deletion or fusion of tarsi. Dll knockdown, in addition to an overall shortening of the leg, results in the deletion of distal limb elements and abnormal formation of the tibial–tarsal joint. (b) Wild-type photurid adult showing normal antenna and maxillary palps. (c) Dll RNAi results in severe truncation of the antennae, as well as shortening, fusion or loss of maxillary palp segments. (d) No lantern-associated effects were observed in any Dll (or dac) knockdown animal.
Similarly, dac RNAi resulted in a dosage-dependent, severe to moderate shortening of the medial aspects of the leg, and fusion or loss of maxillary palp segments [30] (figure 3a) in 67% of pupae (n = 8/12) and 63% of surviving adults (n = 5/8). However, as with the Dll RNAi, no perturbations of PO development were noted in any individual.
(v). Dll/dac co-knockdown does not affect photic organ formation
We co-injected a subset of individuals with both Dll and dac dsRNAs. These animals exhibited profound disruption of appendage formation well above what we achieved by either treatment alone. Again, however, no individual displayed any obvious indications of lantern abnormality.
4. Discussion
We investigated the function of two classes of candidate genes to begin to characterize the developmental-genetic underpinnings of a striking evolutionary novelty, the firefly lantern. Below we discuss the most important implications of our results.
(a). Abdominal-B is required for normal photic organ development
We have shown, through larval RNA interference, that the normal expression of the photurid Abd-B orthologue is necessary for the formation of the PO. Knockdown of the Abd-B transcript caused extensive defects in tissue differentiation and tracheal formation and resulted in ectopic pigmentation of the posterior cuticle. This suggests that Abd-B may function as an activator of PO formation, initiating PO-specific gene expression in A6/7 while overseeing the ventral repression of pigmentation pathways through A5. This demonstrates a surprisingly anterior role for Abd-B compared to other insects studied, such as silkworm proleg suppression in A7–A10 [31], or scent gland suppression (A7) and pigment induction in A10–A11 in Oncopeltus [32]. In the most closely related insect studied to date, embryos of the flour beetle Tribolium castaneum express Abd-B transcripts no further than the posterior compartment of A8 [33]. Taken together, this suggests that the origin of the adult lantern may have been associated with an anterior expansion of Abd-B function in the firefly lineage.
Intriguingly, POs are thought to have arisen first in larvae, which exhibit a pair of laterally positioned POs on A8. Adult lanterns, which in most species occupy A6/A7 (with associated cuticular depigmentation extending midway through A5), evolved later [11]. This raises the possibility that the first lanterns arose within, and were accommodated by, the ancestral regulatory domain of Abd-B. The origin of the adult organs on A6/A7 may then have been facilitated by a novel expansion of Abd-B—which, if correct, would have come already equipped to instruct PO development in more anterior abdominal segments. Clearly, comparisons of embryonic and pupal Abd-B expression in Photuris and closely related beetles are necessary to further evaluate the merits of this hypothesis.
(b). abdominal-A may be required for secondary aspects of photic organ formation and function
RNAi-mediated transcript depletion of abd-A resulted in cuticular depigmentation and abnormal luminescence. These phenotypes suggest that abd-A function may be necessary for several secondary aspects of PO formation. For example, the most common phenotype in the abd-A-injected animals was a variable degree of ectopic depigmentation of the ventral cuticle while dorsal pigmentation was unaffected. This result suggests that abd-A may regulate the anterior extent of ventral cuticular pigmentation, a crucial component to proper PO function. Alternatively, we cannot exclude the possibility that this phenotype may be the result of partially arrested abdominal development. Wild-type adult photurids emerge from their puparia with the dorsal cuticle fully pigmented, while in the ventral abdominal cuticle the process is somewhat delayed, requiring several hours post-eclosion to darken fully. In conjunction with the observation that the adult abdomen of abd-A knockdown individuals retained at least some pupal characteristics, it is possible that the treated individuals experienced a cessation of abdominal development that could possibly have prevented the normal, delayed tanning of the ventral abdomen.
Two of nine abd-A-knockdown animals that survived to adulthood lived long enough for us to determine with confidence that they lacked luminescent function in the medial portion of the lantern, restricting the functional PO to serially arranged pairs of lateral pockets. This observation was intriguing as this pattern mimics the presumed ancestral PO arrangement as well as the lantern layout in females of a related genus (Pyractomena), raising the possibility that abd-A may have played a role in the transition from a paired structure to the single, ventrite-spanning organ displayed by many adults. Alternatively, we cannot exclude the possibility that our treatment may have simply disrupted tracheal patterning, restricting essential oxygen flow from the laterally oriented spiracles to the medial portion of the organ. Combined, these data support the notion that abd-A may be necessary for aspects of proper photurid lantern development, but detailed histological studies will be necessary to determine the precise loci of its effects.
(c). The appendage-patterning gene circuit has not been recruited for photo organ development
In their review of cis-regulatory evolution in the pigmentation patterns of fruitfly wings, Prud'homme et al. [34] articulated a model of opportunistic evolution bound by pre-existing expression patterns. In short, they proposed that underlying domains of regulatory gene expression represent a developmental scaffold that might bias the potential spatial positioning of emerging novelties. Given the parallel between the regulatory pattern established by the wg → appendage network and the paired, ventrolaterally positioned nature of the PO in larvae (often serially repeated in adults), we hypothesized that PO evolution involved the recruitment of the abdominal appendage-patterning network as a developmental template.
Dll and dac RNAi resulted in profound defects in appendage development but left lantern formation completely unaffected, suggesting that the appendage-patterning circuit has not been recruited in the evolution of adult photurid organs. An alternative hypothesis is that the ventrolateral placement of POs in larvae and some adults may instead rely on a combination of activating and repressive signals in the genes that pattern dorsoventral identity, but this remains to be evaluated.
5. Conclusion
Our results provide the first insights into the developmental-genetic regulation of firefly lanterns, a spectacular, novel trait that in many ways has shaped the ecology and evolution of lampyrid beetles. We have shown that the conserved posterior Hox network has evolved to accommodate the lampyrid lantern, including the elaboration of multiple tissues into an integrated, complex, three-dimensional organ. We speculate that this accommodation may have occurred in at least two steps, first via the evolution of larval lanterns within pre-existing domains of Hox gene expression, followed by a secondary anterior expansion of Abd-B expression, which enabled the development of more anterior lanterns including those found in extant adult fireflies. While the downstream targets and effectors that underlie specific aspects of lantern formation remain to be identified, our results thus provide a first hypothetical developmental-genetic framework from which we can begin to investigate the development and evolution of one of the most enigmatic of insect traits.
Acknowledgements
We thank Harald Parzer for discussion and assistance in larval collection, Teiya Kijimoto for assistance in larval and pupal rearing, Andrea Grantham for cryosectioning and staining, and Lisa Nagy, Ryan Pace and two anonymous reviewers for helpful comments on earlier drafts.
Funding statement
This research was carried out while M.S.S. was supported through NIH Genetics Training grant (2T32 GMOO7757-29), NSF-IGERT grant (DGE-0504627) and NIH PERT Training grant (NIH K12 GM000708).
References
- 1.Müller GB, Wagner GP. 1991. Novelty in evolution: restructuring the concept. Annu. Rev. Ecol. Syst. 22, 229–256 (doi:10.1146/annurev.es.22.110191.001305) [Google Scholar]
- 2.Moczek AP. 2008. On the origin of novelty in development and evolution. BioEssays 5, 432–447 (doi:10.1002/bies.20754) [DOI] [PubMed] [Google Scholar]
- 3.Shubin N, Tabin C, Carroll S. 2009. Deep homology and the origins of evolutionary novelty. Nature 457, 818–823 (doi:10.1038/nature07891) [DOI] [PubMed] [Google Scholar]
- 4.Lloyd JE. 2002. Lampyridae (Latreille 1817). In American beetles, volume 2: polyphaga (eds Arnett RH, Thomas MC, Skelley PE, Frank JH.), pp. 187–196 Boca Raton, FL: CRC Press [Google Scholar]
- 5.Oba Y, Ojika M, Inouye S. 2003. Firefly luciferase is a bifunctional enzyme: ATP-dependent monooxygenase and a long chain fatty acyl-CoA synthetase. FEBS Lett. 540, 251–254 (doi:10.1016/S0014-5793(03)00272-2) [DOI] [PubMed] [Google Scholar]
- 6.Oba Y, Iida K, Inouye S. 2009. Functional conversion of fatty acyl-CoA synthetase to firefly luciferase by site-directed mutagenesis: a key substitution responsible for luminescence activity. FEBS Lett. 583, 2004–2008 (doi:10.1016/j.febslet.2009.05.018) [DOI] [PubMed] [Google Scholar]
- 7.Hess WN. 1922. Origin and development of the light-organs of Photuris pennsylvanica de Geer. J. Morphol. 36, 244–277 (doi:10.1002/jmor.1050360206) [Google Scholar]
- 8.Chapman RF. 1998. The insects: structure and function, 4th edn Cambridge, UK: Cambridge University Press [Google Scholar]
- 9.Smith DS. 1963. The organization and innervation of the luminescent organ in a firefly Photuris pennsylvanica (Coleoptera). J. Cell Biol. 16, 323–359 (doi:10.1083/jcb.16.2.323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Underwood TJ, Tallamy DW, Pesek JD. 1997. Bioluminescence in firefly larvae: a test of the aposematic display hypothesis (Coleoptera: Lampyridae). J. Insect Behav. 10, 365–370 (doi:10.1007/BF02765604) [Google Scholar]
- 11.Branham MA, Wenzel JW. 2003. The origin of photic behavior and the evolution of sexual communication in fireflies (Coleoptera: Lampyridae). Cladistics 19, 1–22 (doi:10.1111/j.1096-0031.2003.tb00404.x) [DOI] [PubMed] [Google Scholar]
- 12.Lewis EB. 1978. Gene complex controlling segmentation in Drosophila. Nature 276, 565–570 (doi:10.1038/276565a0) [DOI] [PubMed] [Google Scholar]
- 13.Hughes CL, Kaufman TC. 2002. Hox genes and the evolution of the arthropod body plan. Evol. Dev. 4, 459–499 (doi:10.1046/j.1525-142X.2002.02034.x) [DOI] [PubMed] [Google Scholar]
- 14.Lewis DL, DeCamillis M, Bennett RL. 2000. Distinct roles of the homeotic genes Ubx and Abd-A in beetle embryonic abdominal appendage development. Proc. Natl Acad. Sci. USA 97, 4504–4509 (doi:10.1073/pnas.97.9.4504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vachon G, Cohen B, Pfeifle C, McGuffin ME, Botas J, Cohen SM. 1992. Homeotic genes of the bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell 71, 437–450 (doi:10.1016/0092-8674(92)90513-C) [DOI] [PubMed] [Google Scholar]
- 16.Estrada B, Sánchez-Herrero E. 2001. The Hox gene Abdominal-B antagonizes appendage development in the genital disc of Drosophila. Development 128, 331–339 [DOI] [PubMed] [Google Scholar]
- 17.Jeong S, Rokas A, Carroll SB. 2006. Regulation of body pigmentation by the Abdominal-B Hox protein and its gain and loss in Drosophila evolution. Cell 125, 1387–1399 (doi:10.1016/j.cell.2006.04.043) [DOI] [PubMed] [Google Scholar]
- 18.Moore LA, Broihier HT, Doren MV, Lehmann R. 1998. Gonadal mesoderm and fat body initially follow a common developmental path in Drosophila. Development 125, 837–844 [DOI] [PubMed] [Google Scholar]
- 19.Tonolli PN, Okawachi FM, Abdalla FC, Viviani VR. 2011. Bioluminescent fat body of larval Aspisoma lineatum (Coleoptera: Lampyridae) firefly: ontogenic precursor of lantern's photogenic tissue. Ann. Entomol. Soc. Am. 104, 761–767 (doi:10.1603/AN10143) [Google Scholar]
- 20.Branham MA, Wenzel JW. 2001. The evolution of bioluminescence in cantharoids (Coleoptera: Elateroidea). Fla. Entomol. 84, 565–586 (doi:10.2307/3496389) [Google Scholar]
- 21.Ober KA, Jockusch EL. 2006. The roles of wingless and decapentaplegic in axis and appendage development in the red flour beetle, Tribolium castaneum. Dev. Biol. 294, 391–405 (doi:10.1016/j.ydbio.2006.02.053) [DOI] [PubMed] [Google Scholar]
- 22.Cohen B, Simcox AA, Cohen SM. 1993. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development 117, 597–608 [DOI] [PubMed] [Google Scholar]
- 23.Angelini DR, Smith FW, Jockusch EL. 2012. Extent with modification: leg patterning in the beetle Tribolium castaneum and the evolution of serial homologs. G3 (Bethesda) 2, 235–248 (doi:10.1534/g3.111.001537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojima T. 2004. The mechanism of Drosophila leg development along the proximodistal axis. Dev. Growth Differ. 46, 115–129 (doi:10.1111/j.1440-169X.2004.00735.x) [DOI] [PubMed] [Google Scholar]
- 25.Simcox AA, Hersperger E, Shearn A, Whittle RS, Cohen SM. 1991. Establishment of imaginal discs and histoblast nests in Drosophila. Mech. Dev. 34, 11–20 (doi:10.1016/0925-4773(91)90087-M) [DOI] [PubMed] [Google Scholar]
- 26.McLean M, Buck J, Hanson F. 1972. Culture and larval behavior of photurid fireflies. Am. Midl. Nat. 87, 133–145 (doi:10.2307/2423887) [Google Scholar]
- 27.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (doi:10.1093/molbev/msr121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen SM, Brönner G, Küttner F, Jürgens G, Jäckle H. 1989. Distal-less encodes a homoeodomain protein required for limb development in Drosophila . Nature 338, 432–434 (doi:10.1038/338432a0) [DOI] [PubMed] [Google Scholar]
- 29.Angelini DR, Kaufman TC. 2004. Functional analysis of appendage-patterning genes in the hemipteran Oncopeltus fasciatus reveals conserved and derived aspects of limb patterning in insects. Dev. Biol. 271, 306–321 (doi:10.1016/j.ydbio.2004.04.005) [DOI] [PubMed] [Google Scholar]
- 30.Prpic NM, Wigand B, Damen WG, Klingler M. 2001. Expression of dachshund in wild-type and Distal-less mutant Tribolium corroborates serial homologies in insect appendages. Dev. Genes Evol. 211, 467–477 (doi:10.1007/s004270100178) [DOI] [PubMed] [Google Scholar]
- 31.Tomita S, Kikuchi A. 2009. Abd-B suppresses lepidopteran proleg development in posterior abdomen. Dev. Biol. 328, 403–409 (doi:10.1016/j.ydbio.2009.01.040) [DOI] [PubMed] [Google Scholar]
- 32.Angelini DR, Liu PZ, Hughes CL, Kaufman TC. 2005. Hox gene function and interaction in the milkweed bug Oncopeltus fasciatus (Hemiptera). Dev. Biol. 287, 440–455 (doi:10.1016/j.ydbio.2005.08.010) [DOI] [PubMed] [Google Scholar]
- 33.He J. 1996. Molecular and genetic analysis of the Abdominal-B homolog of the beetle Tribolium castaneum. PhD dissertation, Kansas State University, Manhattan, KS, USA [Google Scholar]
- 34.Prud'homme B, Gompel N, Carroll SB. 2007. Emerging principles of regulatory evolution. Proc. Natl Acad. Sci. USA 104, 8605–8612 (doi:10.1073/pnas.0700488104) [DOI] [PMC free article] [PubMed] [Google Scholar]


