Abstract
Non-pathological densification (osteosclerosis) and swelling (pachyostosis) of bones are the main modifications affecting the skeleton of land vertebrates (tetrapods) that returned to water. However, a precise temporal calibration of the acquisition of such adaptations is still wanting. Here, we assess the timing of such acquisition using the aquatic sloth Thalassocnus, from the Neogene of the Pisco Formation, Peru. This genus is represented by five species occurring in successive vertebrate-bearing horizons of distinct ages. It yields the most detailed data about the gradual acquisition of aquatic adaptations among tetrapods, in displaying increasing osteosclerosis and pachyostosis through time. Such modifications, reflecting a shift in the habitat from terrestrial to aquatic, occurred over a short geological time span (ca 4 Myr). Otherwise, the bones of terrestrial pilosans (sloths and anteaters) are much more compact than the mean mammalian condition, which suggests that the osteosclerosis of Thalassocnus may represent an exaptation.
Keywords: aquatic adaptation, bone histology, bone microstructure, exaptation, osteosclerosis, Xenarthra
1. Introduction
It is now well established that osteosclerosis, i.e. non-pathological densification of bones, is one of the main specializations of bone microstructure found in the skeleton of land tetrapods adapted to the aquatic realm (review in [1]). It appeared in all major tetrapods taxa (see also review in [2]), and affects to different degrees either a delimited region (e.g. rostrum of ziphiid whales [3,4]) or much broader parts of the skeleton [2,5]. It is often, but not always, associated with pachyostosis, a hyperplasy of the cortical part of bone shafts that creates a massive and swollen aspect and can spectacularly increase skeletal volume [6]. The osteosclerotic and/or pachyostotic conditions are supposed to compensate for enlarged lungs and provide a ballast that contributes to control buoyancy and body trim in water [7,8]. The underlying histogenetic mechanisms responsible for osteosclerosis, as well as the time lapse required to turn a ‘normal’, tubular bone into an amedullary one remain poorly documented. In the case of mammals, palaeontological data suggest that weakly developed osteosclerosis, and to a lesser extent pachyostosis, occurred very early in two major aquatic taxa, the Cetacea and the Sirenia. The earliest forms of these orders, the pakicetids [9,10] and the prorastomids [11,12], respectively, indeed displayed advanced signs of an increase in bone compactness and a loss of the medullary cavity, while their gross external anatomy was still unspecialized. The incipient stages of bone transformation in these groups and the mechanisms involved in this process remain to be documented. The model on which we focus here is the aquatic sloth, Thalassocnus, known in the Pisco Formation (Peru) by five species spanning from Late Miocene to Late Pliocene [13]. Its fossil record provides the most detailed data available about the gradual acquisition of osteosclerosis among tetrapods.
Thalassocnus was indeed interpreted as the only aquatic representative of its superorder, the xenarthrans (sloths, anteaters, armadillos and their extinct relatives) [14]. The initial hypothesis was mainly based on taphonomic arguments, and the macroanatomy of some postcranial elements was also noted as indicative. Subsequent studies further substantiated this interpretation, and suggested an increasing adaptation to feeding on marine vegetation (i.e. specialized grazing) through time between the five species pertaining to this genus, namely T. antiquus, T. natans, T. littoralis, T. carolomartini and T. yaucensis [15], in stratigraphic order. The phylogenetic relationships of these species are consistent with their geological age. Thus, Thalassocnus is ideal to follow the adaptation of bone structure to an aquatic lifestyle. Bone histology and microanatomy of xenarthrans long remained documented only by anecdotal observations [16]. Recently, a broad comparative study on 22 extant and extinct terrestrial forms of this superorder revealed, among other things, the presence of dense Haversian bone and higher compactness values in Pilosa (sloths and anteaters) than in Cingulata (armadillos and extinct relatives) [17]. However, bone structure in the only aquatic xenarthrans, i.e. Thalassocnus, remains undocumented.
2. Material and methods
(a). Systematic framework
The five known species of Thalassocnus, T. antiquus, T. natans, T. littoralis, T. carolomartini and T. yaucensis were sampled. They all come from distinct horizons of the Pisco Formation (Peru). We will consider approximated ages for the four earliest species, respectively, of ca 8, 7, 6 and 5 Myr [13,18–20]. Details on these ages are given in the electronic supplementary material. There is a complete consistency between the intra-generic phylogenetic relationships and the stratigraphic position of each taxon, i.e. the geologically earliest species is the sister-group of all others, and so on. The hypothetical position of T. antiquus as the sister-taxon of all other species, as proposed by Muizon et al. [19], is now confirmed by personal observations on postcranial anatomy [21]. Moreover, the microanatomical data presented herein corroborate a posteriori this hypothesis: T. natans features microanatomical parameters closer to those of the late species than to those of T. antiquus or of the outgroups used in the phylogenetic analysis. The only specimen of the latest species, T. yaucensis, available for measurement (MUSM 37, the holotype) is interpreted as a pregnant female deceased near parturition [13] and is hence not included in the comparisons of compactness. See the electronic supplementary material, tables S1 and S2 for details on this consideration and on other parameters having a possible effect on bone compactness.
The comparative sample comprises five extant and extinct xenarthran species, all considered to be strictly non-aquatic forms: Myrmecophaga (the extant giant anteater), Choloepus (the extant two-toed sloth), Nothrotherium and Nothrotheriops, Quaternary terrestrial representatives of the Nothrotheriidae, the family to which Thalassocnus is attributed and Hapalops, a Miocene form which is, as the nothrotheriids, a megatherioid [22]. See the electronic supplementary material, table S3 for nature of samples and specimen numbers.
(b). Sectioning protocols
We have studied the internal microstructure and histology of the humerus, radius, femur, tibia and ribs. Bone sections were obtained either by ‘conventional’ thin-sectioning or by computed tomography (CT) scanning. See the electronic supplementary material for details on the standard protocols that have been used.
(c). Treatment of sections
After binarization (‘Bitmap’ mode) of the CT-scan slices or photographs of the thin-sections with Adobe Photoshop, the software Bone Profiler [23] was used to measure bone compactness, a dimensionless parameter reflecting the ratio of bone surface to total sectional surface (see the electronic supplementary material, table S3 for available data and table S4 for all compactness values). Cortical development (DC) and compactness profile were calculated following published procedures [11] (see the electronic supplementary material, tables S5 and S6 for calculation of these indices). Trees with mapped compactness values were elaborated with Mesquite [24] and its Stratigraphic Tools [25], using the observed compactness values (electronic supplementary material, table S4, data matrix given as a NEXUS file in the electronic supplementary material). Observed compactness is shown with different colours. Each colour represents an interval of compactness. The number of colours and the boundaries of the intervals were selected in order to display compactness differences between the species of Thalassocnus. Inference models (using respectively the humerus [26], radius [27], tibia [28] and femur [29]) were tentatively applied to our data. Pilosans, which are currently not included in these models, were often incorrectly interpreted as aquatic or amphibious, owing to the high compactness generally displayed by their bones. These irrelevant results are not presented herein; they call for future studies that should include pilosans in inference models.
Histological observations were performed using a photonic microscope (Zeiss Axioskop) in normal or polarized transmitted light.
3. Results and discussion
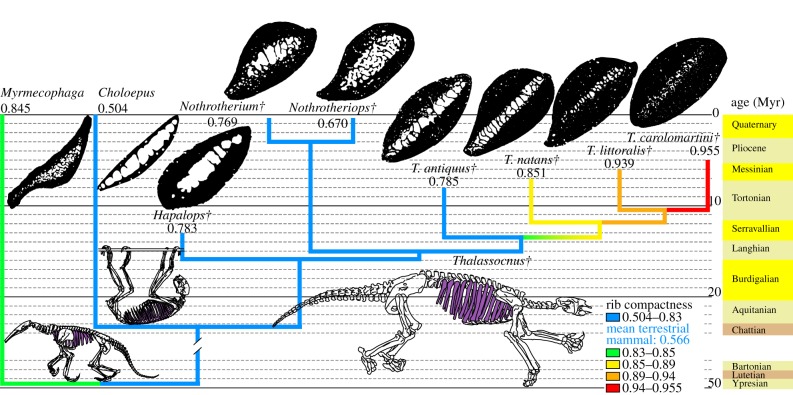
The compactness of the ribs (measured at midlength) of the earliest species of Thalassocnus, T. antiquus, does not greatly differ from that of Hapalops, Nothrotherium or Nothrotheriops (extinct terrestrial sloths closely related to Thalassocnus), which have neither osteosclerotic (electronic supplementary material, table S7) nor amedullar ribs (electronic supplementary material, table S6). Conversely, the four geologically later species of Thalassocnus are both osteosclerotic and amedullar. An increase in rib compactness is observed from T. antiquus to T. carolomartini (no data for T. yaucensis; see Material and methods), with a total gain of 22% of its initial value in ca 3 Myr) (figure 1; see the electronic supplementary material, table S4 and figure S1). Similar values of bone compactness are found in the ribs of the desmostylians Behemotops, Paleoparadoxia and Ashoroa (compactness: 0.859–0.964), sampled by Hayashi et al. [30], who considered that these taxa were adapted to life in water, as evidenced by their osteosclerotic bones.
Figure 1.
Observed compactness in ribs. Cross sections are not to scale. Phylogeny from Muizon et al. [19]. Compactness values are given below the taxon name. The age of the fossils must be read at the tip of the branches. The broken branch marks a break of the stratigraphic scale. Mean value for terrestrial mammals is from Buffrénil et al. [11]. Daggers denote extinct taxa.
Buffrénil et al. [11] defined a DC index to characterize rib pachyostosis. As calculation of this index requires complete ribs, it could not be performed for T. antiquus and T. carolomartini, for which such complete bones are unavailable. Among the three other species, the ribs of T. littoralis and T. yaucensis have an index superior to the threshold defined by Buffrénil et al. [11] and are hence regarded as pachyostotic. Those of T. natans and of the other sloths (electronic supplementary material, table S5), which have a DC below this threshold, are not. The relatively high value found in Myrmecophaga, the giant anteater, which could be viewed as resulting from pachyostosis, is irrelevant because calculation of the index is jeopardized by the large rib expansion (sensu Jenkins [31]), which characterizes this genus. The DC value apparently increases with stratigraphy (from older to younger beds) in the three Thalassocnus species for which it was calculated. Therefore, an increasing tendency towards rib pachyostosis is likely to have occurred, from the earliest to the latest of the five species of Thalassocnus (electronic supplementary material, table S5).
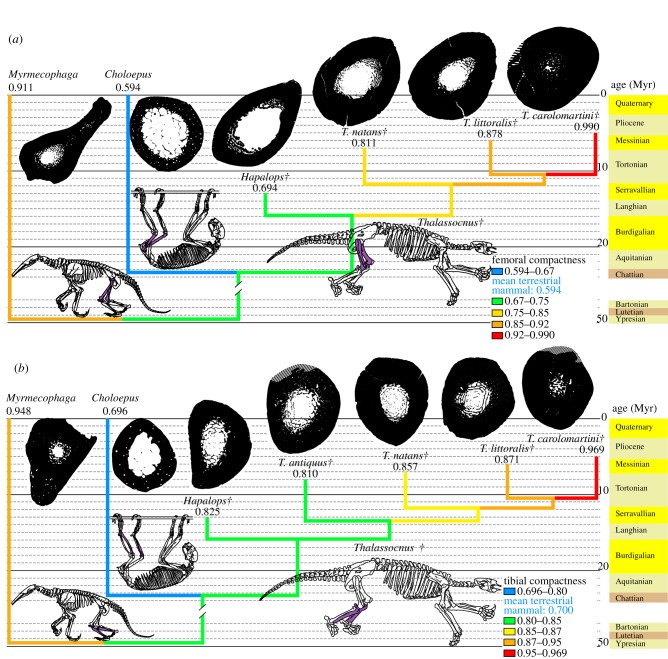
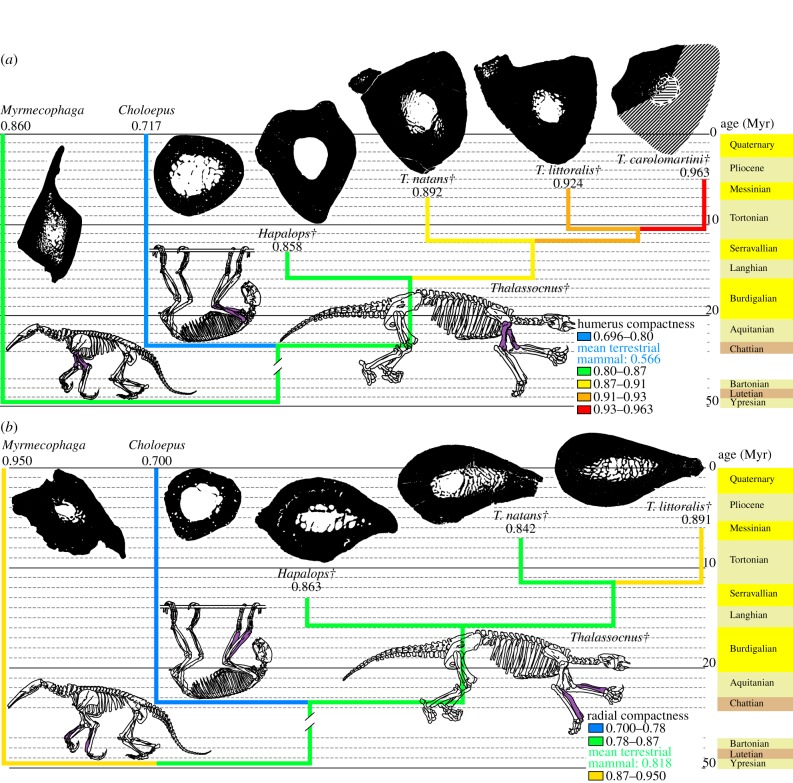
An osteosclerotic structure also affects both fore- and hindlimbs of Thalassocnus, especially in geologically recent species. Femoral compactness increased by 22% of its initial value during ca 2 Myr (figure 2a) and tibial compactness increased by 20% of its initial value during ca 3 Myr (figure 2b; electronic supplementary material, figure S1). In both bones, the greatest compactness increase occurred when T. littoralis is compared with T. carolomartini. Osteosclerosis of these bones is extremely pronounced in the two latest species of Thalassocnus, which lack a free medullary cavity and only exhibit a slight decrease in compactness in their centre. Such advanced osteosclerosis in hindlimbs was previously documented in ‘archaeocetes’ (early cetaceans), but only in taxa in which limbs are conspicuously reduced [32]. The extreme condition found in the latest species of Thalassocnus does not seem to have been reached by desmostylians, since a small medullary cavity persists in the femur of Behemotops [30] (the condition is unknown for Paleoparadoxia, but its extremely compact and amedullar humerus may indicate that the hindlimb is also concerned). The forelimb of Thalassocnus is similarly affected by osteosclerosis, especially in the latest species (figure 3; electronic supplementary material, figure S1). Extensive osteosclerosis hence characterizes Thalassocnus. The return of cetaceans to water is documented by taxa featuring extensive osteosclerosis, such as Indohyus [33,34] and pakicetids [9]. However, even these taxa do not display the nearly solid long bones found in the latest species of Thalassocnus. This difference is understandable, since they did not share the putative ecology of Thalassocnus, a marine grazer requiring passive hydrostatic regulation. As already mentioned by Muizon et al. [15], marine iguanas may be considered as featuring an ecology putatively close to that of Thalassocnus. Such claim is now also supported by bone microstructural data, as marine iguanas also display bones that are relatively more compact than those of their terrestrial close relatives [35].
Figure 2.
Observed compactness in long bones of the hindlimb. Cross sections of all limb bones (including figure 3) are located at the level of maximum cortical thickness and are not to scale (diagonal stripes represent unpreserved parts). Phylogeny from Muizon et al. [19]. Compactness values are given below the taxon name. The age of the fossils must be read at the tip of the branches. The broken branch marks a break of the stratigraphic scale. (a) Femur, mean value for terrestrial mammals is from Quemeneur et al. [29]. (b) Tibia, mean value for terrestrial mammals is from Kriloff et al. [28]. Daggers denote extinct taxa.
Figure 3.
Observed compactness in long bones of the forelimb. For more information, see legend of figure 2. (a) Humerus, mean value for terrestrial mammals is from Canoville & Laurin [26]. (b) Radius, mean value for terrestrial mammals is from Germain & Laurin [27]. Daggers denote extinct taxa.
Because none of the Thalassocnus bones examined contained any remnant of calcified cartilage, endochondral osteogenesis is interpreted as normal: the hypertrophic cartilage produced by the growth plates at the epiphyseal level was entirely eroded by chondroclasts in the metaphysis and replaced by endosteal trabeculae made of lamellar bone tissue. This is the regular mechanism for the growth in length of long bones. This condition differs from that found in sirenians and some early cetaceans, in which an inhibition of clastic cell activity occurred early in life, causing a persistence of abundant remnants of calcified cartilage matrix [11]. Moreover, open erosion bays without traces of secondary reconstruction are numerous in the rib of the juvenile T. littoralis (1.401 bays mm−2 in rib cortices; figure 4a), whereas they are rare in the adults (0.177–0.220 bays mm−2; figure 4b). This observation suggests that Haversian remodelling (which is initiated by the formation of erosion bays) was very active in young individuals of Thalassocnus, and steeply decreased in the adults. The histological result of bone remodelling, i.e. the presence and abundance of secondary osteons (Havers’ systems) depends on the developmental stage reached by an individual; this is why osteons are much more numerous in the adults, despite the steep reduction in the process of bone remodelling (scarcity of resorption bays) that occurred at this late ontogenetic stage. Intense remodelling in juveniles is also shown by the complex assemblages of endosteal lamellar tissue in medullary trabeculae (figure 4c). This process was most probably imbalanced towards re-deposition. Imbalanced remodelling, i.e. excess in secondary (reconstructive) bone deposits, would explain the more compact spongiosa found in the adults. Our results suggest that this process must have been positively selected and increased during Thalassocnus evolution.
Figure 4.
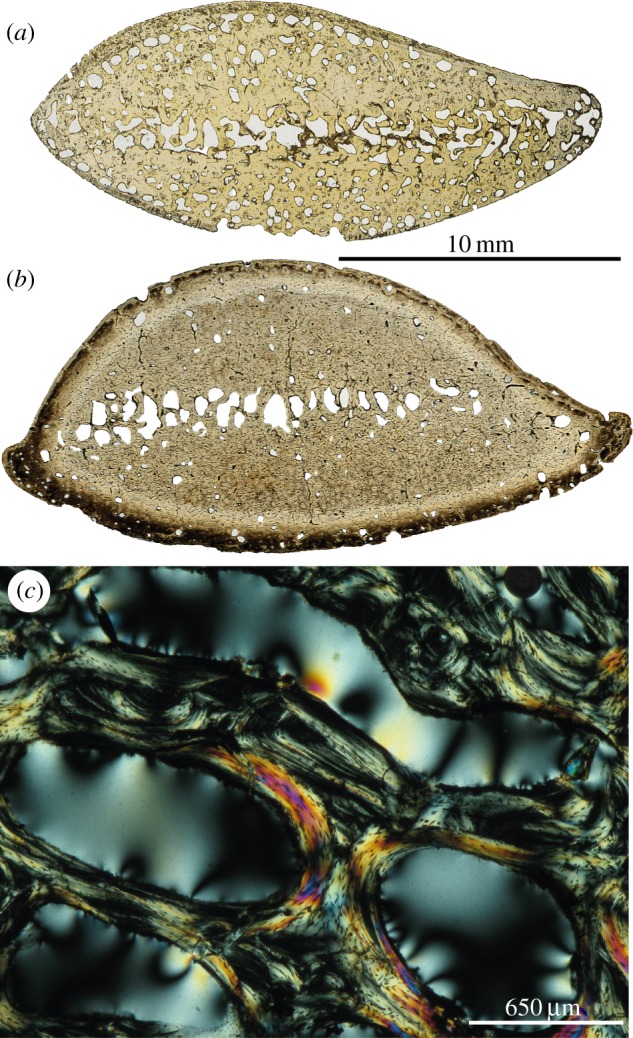
Bone histology of Thalassocnus. (a,b) General views of a rib (cross sections approximately at midlength in natural transmitted light) of (a) juvenile T. littoralis (MNHN.F.SAS 2) and (b) adult T. littoralis (MNHN.F.SAS 53). (c) Remodelled trabeculae in the medulla of the radius of T. littoralis (MNHN.F.SAS 53). (Online version in colour.)
The comparison of bone compactness in various pilosans reveals that several bones of terrestrial species are clearly more compact than the corresponding bones in most other terrestrial mammals. This observation agrees with the data presented by Straehl et al. [17]. Beyond the case of the xenarthrans, our observations converge with the conclusions of previous authors [9–11] who showed that, in cetaceans and sirenians, a modification in bone inner structure is an adaptive trait to life in water that predates gross anatomical transformations of the skeleton. These authors implicitly postulated that pakicetids and prorastomids had actually shifted to aquatic habitats prior to structural and anatomical specialization of their bones, which are consecutive of this environmental change. The case of the Pilosa suggests a different hypothesis. The appearance in a given taxon of moderate osteosclerosis, pachyostosis or a combination of both these conditions, may not necessarily follow the shift to aquatic habits. Incipient osteosclerosis, as evidenced in terrestrial Pilosa, may predate not only the gross anatomical modification of skeletal elements for life in water, but also the occupation of aquatic habitat itself. This would constitute an exaptation, which would suggest that adaptation to life in water, in its earliest stages, might represent an opportunistic exploitation of some pre-existing structural peculiarities of the skeleton. These characteristics would thus constitute the initial cause (or at least a strong inciting factor) of the aquatic adaptation rather than its consequence. In later stages of their evolution, these taxa would have been submitted to a strong selective pressure that would have accentuated their pre-existing osteogenetic peculiarities, and reinforce the ecological specializations of selective value in their new habitat. In the case of Thalassocnus, this exaptation may have facilitated adaptation to feeding on marine vegetation in water forced by the desert nature of the Peruvian coast during the Neogene.
Acknowledgements
The CT-scans were performed at the ‘UMS 2700 outils et méthodes de la systématique intégrative CNRS-MNHN, AST-RX, plate-forme d'accès scientifique à la tomographie à rayons X du Muséum national d'Histoire naturelle (MNHN)’. The authors are indebted to Florent Goussard and Miguel Garcia Sanz (both MNHN) for acquiring the scans and helping in their handling, and Damien Germain (MNHN) who also helped in their handling. We thank Géraldine Veron (MNHN), Castor Cartelle (Museu de Ciencias Naturais da Pontifícia Universidade Católica de Minas Gerais, Belo Horizonte, Brazil), Samuel McLeod and Vanessa Rhue (both from the Natural History Museum of Los Angeles County, Los Angeles, CA, USA), who allowed access to the collections under their care. Rodolfo Salas-Gismondi of the ‘Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Lima, Peru’ receives our gratitude for welcoming E.A. in the MUSM collections, collecting and loaning some Thalassocnus material. Mario Urbina (MUSM) is also thanked for collecting numerous specimens. We are grateful to Philippe Janvier (CNRS) and François Robert (CNRS) for critical review of the manuscript, as well as to the two anonymous reviewers for their comments, which substantially improved the quality of the manuscript. We thank Malcolm Sanders (MNHN) for the line drawings. Thin-sections were produced by Lilian Cazes (CNRS).
Data accessibility
Electronic supplementary material is available on the Dryad repository (http://datadryad.org/; doi:10.5061/dryad.p9c00).
References
- 1.Ricqlès A, Buffrénil V. 2001. Bone histology, heterochronies and the return of tetrapods to life in water: where are we. In Secondary adaptation of tetrapods to life in water (eds Mazin J, Buffrénil V.), pp. 289–310 Munchen, Germany: Verlag Dr Friedrich Pfeil [Google Scholar]
- 2.Houssaye A. 2009. ‘Pachyostosis’ in aquatic amniotes: a review. Integr. Zool. 4, 325–340 (doi:10.1111/j.1749-4877.2009.00146.x) [DOI] [PubMed] [Google Scholar]
- 3.Buffrénil V, Casinos A. 1995. Observations histologiques sur le rostre de Mesoplodon densirostris (Mammalia, Cetacea, Ziphiidae): le tissu osseux le plus dense connu. Ann. des Sci. Nat. Zool. 13ème série 16, 21–32 [Google Scholar]
- 4.Lambert O, Buffrénil V, de Muizon C. 2011. Rostral densification in beaked whales: diverse processes for a similar pattern. C. R. Palevol. 10, 453–468 (doi:10.1016/j.crpv.2011.03.012) [Google Scholar]
- 5.Buffrénil V, Schoevaert D. 1989. Données quantitatives et observations histologiques sur la pachyostose du squelette du dugong, Dugong dugon (Müller) (Sirenia, Dugongidae). Can. J. Zool. 67, 2107–2119 (doi:10.1139/z89-300) [Google Scholar]
- 6.Kaiser HE. 1960. Untersuchungen zur vergleichenden Osteologie der fossilen und rezenten Pachyostosen. Palaeontogr. Abteilung A 114, 113–196 [Google Scholar]
- 7.Domning DP, Buffrénil V. 1991. Hydrostasis in the Sirenia: quantitative data and functional interpretations. Mar. Mammal Sci. 7, 331–368 (doi:10.1111/j.1748-7692.1991.tb00111.x) [Google Scholar]
- 8.Taylor MA. 2000. Functional significance of bone ballastin in the evolution of buoyancy control strategies by aquatic tetrapods. Hist. Biol. 14, 15–31 (doi:10.1080/10292380009380550) [Google Scholar]
- 9.Madar SI. 2007. The postcranial skeleton of early Eocene pakicetid cetaceans. J. Paleontol. 81, 176–200 (doi:10.1666/0022-3360(2007)81[176:TPSOEE]2.0.CO;2) [Google Scholar]
- 10.Gray N-M, Kainec K, Madar SI, Tomko L, Wolfe S. 2007. Sink or swim? Bone density as a mechanism for buoyancy control in early cetaceans. Anat. Rec. 290, 638–653 (doi:10.1002/ar.20533) [DOI] [PubMed] [Google Scholar]
- 11.Buffrénil V, Canoville A, D'Anastasio R, Domning DP. 2010. Evolution of sirenian pachyosteosclerosis, a model-case for the study of bone structure in aquatic tetrapods. J. Mamm. Evol. 17, 101–120 (doi:10.1007/s10914-010-9130-1) [Google Scholar]
- 12.Domning DP. 2001. The earliest known fully quadrupedal sirenian. Nature 413, 625–627 (doi:10.1038/35098072) [DOI] [PubMed] [Google Scholar]
- 13.de Muizon C, McDonald HG, Salas R, Urbina M. 2004. The youngest species of the aquatic sloth Thalassocnus and a reassessment of the relationships of the nothrothere sloths (Mammalia: Xenarthra). J. Vertebr. Paleontol. 24, 287–397 (doi:10.1671/2429a) [Google Scholar]
- 14.de Muizon C, McDonald HG. 1995. An aquatic sloth from the Pliocene of Peru. Nature 375, 224–227 (doi:10.1038/375224a0) [Google Scholar]
- 15.de Muizon C, McDonald HG, Salas R, Urbina M. 2004. The evolution of feeding adaptations of the aquatic sloth Thalassocnus. J. Vertebr. Paleontol. 24, 398–410 (doi:10.1671/2429b) [Google Scholar]
- 16.Ricqlès A, Taquet P, Buffrénil V. 2009. ‘Rediscovery’ of Paul Gervais’ paleohistological collection. Geodiversitas 31, 943–971 (doi:10.5252/g2009n4a943) [Google Scholar]
- 17.Straehl FR, Scheyer TM, Forasiepi AM, MacPhee RD, Sánchez-Villagra MR. 2013. Evolutionary patterns of bone histology and bone compactness in xenarthran mammal long bones. PLoS ONE 8, e69275 (doi:10.1371/journal.pone.0069275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald HG, de Muizon C. 2002. The cranial anatomy of Thalassocnus (Xenarthra, Mammalia), a derived nothrothere from the Neogene of the Pisco Formation (Peru). J. Vertebr. Paleontol. 22, 349–365 (doi:10.1671/0272-4634(2002)022[0349:TCAOTX]2.0.CO;2) [Google Scholar]
- 19.de Muizon C, McDonald HG, Salas R, Urbina M. 2003. A new early species of the aquatic sloth Thalassocnus (Mammalia, Xenarthra) from the Late Miocene of Peru. J. Vertebr. Paleontol. 23, 886–894 (doi:10.1671/2361-13) [Google Scholar]
- 20.Ehret DJ, Macfadden BJ, Jones DS, DeVries TJ, Foster DA, Salas-Gismondi R. 2012. Origin of the white shark Carcharodon (Lamniformes: Lamnidae) based on recalibration of the Upper Neogene Pisco Formation of Peru. Palaeontology 55, 1139–1153 (doi:10.1111/j.1475-4983.2012.01201.x) [Google Scholar]
- 21.Amson E, Argot C, McDonald HG, de Muizon C. In preparation. The forelimb of Thalassocnus (Mammalia, Tardigrada) from the Neogene of Peru. Palaeobiological implications.
- 22.Gaudin TJ. 2004. Phylogenetic relationships among sloths (Mammalia, Xenarthra, Tardigrada): the craniodental evidence. Zool. J. Linn. Soc. 140, 255–305 (doi:10.1111/j.1096-3642.2003.00100.x) [Google Scholar]
- 23.Girondot M, Laurin M. 2003. Bone profiler: a tool to quantify, model, and statistically compare bone-section compactness profiles. J. Vertebr. Paleontol. 23, 458–461 (doi:10.1671/0272-4634(2003)023[0458:BPATTQ]2.0.CO;2) [Google Scholar]
- 24.Maddison WP, Maddison DR. 2011. Mesquite: a modular system for evolutionary analysis. Version 2.75 See http://mesquiteproject.org
- 25.Josse S, Moreau T, Laurin M.2006. Stratigraphic tools for Mesquite. See http://mesquiteproject.org/packages/stratigraphicTools/
- 26.Canoville A, Laurin M. 2010. Evolution of humeral microanatomy and lifestyle in amniotes, and some comments on palaeobiological inferences. Biol. J. Linn. Soc. 100, 384–406 (doi:10.1111/j.1095-8312.2010.01431.x) [Google Scholar]
- 27.Germain D, Laurin M. 2005. Microanatomy of the radius and lifestyle in amniotes (Vertebrata, Tetrapoda). Zool. Scr. 34, 335–350 (doi:10.1111/j.1463-6409.2005.00198.x) [Google Scholar]
- 28.Kriloff A, Germain D, Canoville A, Vincent P, Sache M, Laurin M. 2008. Evolution of bone microanatomy of the tetrapod tibia and its use in palaeobiological inference. J. Evol. Biol. 21, 807–826 (doi:10.1111/j.1420-9101.2008.01512.x) [DOI] [PubMed] [Google Scholar]
- 29.Quemeneur S, Buffrénil V, Laurin M. 2013. Microanatomy of the amniote femur and inference of lifestyle in limbed vertebrates. Biol. J. Linn. Soc. 109, 644–655 (doi:10.1111/bij.12066) [Google Scholar]
- 30.Hayashi S, Houssaye A, Nakajima Y, Chiba K, Ando T, Sawamura H, Inuzuka N, Kaneko N, Osaki T. 2013. Bone inner structure suggests increasing aquatic adaptations in Desmostylia (Mammalia, Afrotheria). PLoS ONE 8, e59146 (doi:10.1371/journal.pone.0059146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins FAJ. 1970. Anatomy and function of expanded ribs in certain edentates and primates. J. Mammal. 51, 288–301 (doi:10.2307/1378479) [PubMed] [Google Scholar]
- 32.Madar SI. 1998. Structural adaptations of early archaeocete long bones. In The emergence of whales, evolutionary patterns in the origin of Cetacea (ed. Thewissen JGM.), pp. 353–375 New York, NY: Plenum Press [Google Scholar]
- 33.Thewissen JGM, Cooper LN, Clementz MT, Bajpai S, Tiwari BN. 2007. Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature 450, 1190–1194 (doi:10.1038/nature06343) [DOI] [PubMed] [Google Scholar]
- 34.Cooper LN, Thewissen JGM, Bajpai S, Tiwari BN. 2012. Postcranial morphology and locomotion of the Eocene raoellid Indohyus (Artiodactyla: Mammalia). Hist. Biol. 24, 279–310 (doi:10.1080/08912963.2011.624184) [Google Scholar]
- 35.Hugi J, Sánchez-Villagra MR. 2012. Life history and skeletal adaptations in the Galapagos marine iguana (Amblyrhynchus cristatus) as reconstructed with bone histological data—a comparative study of iguanines. J. Herpetol. 46, 312–324 (doi:10.1670/11-071) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Electronic supplementary material is available on the Dryad repository (http://datadryad.org/; doi:10.5061/dryad.p9c00).