Abstract
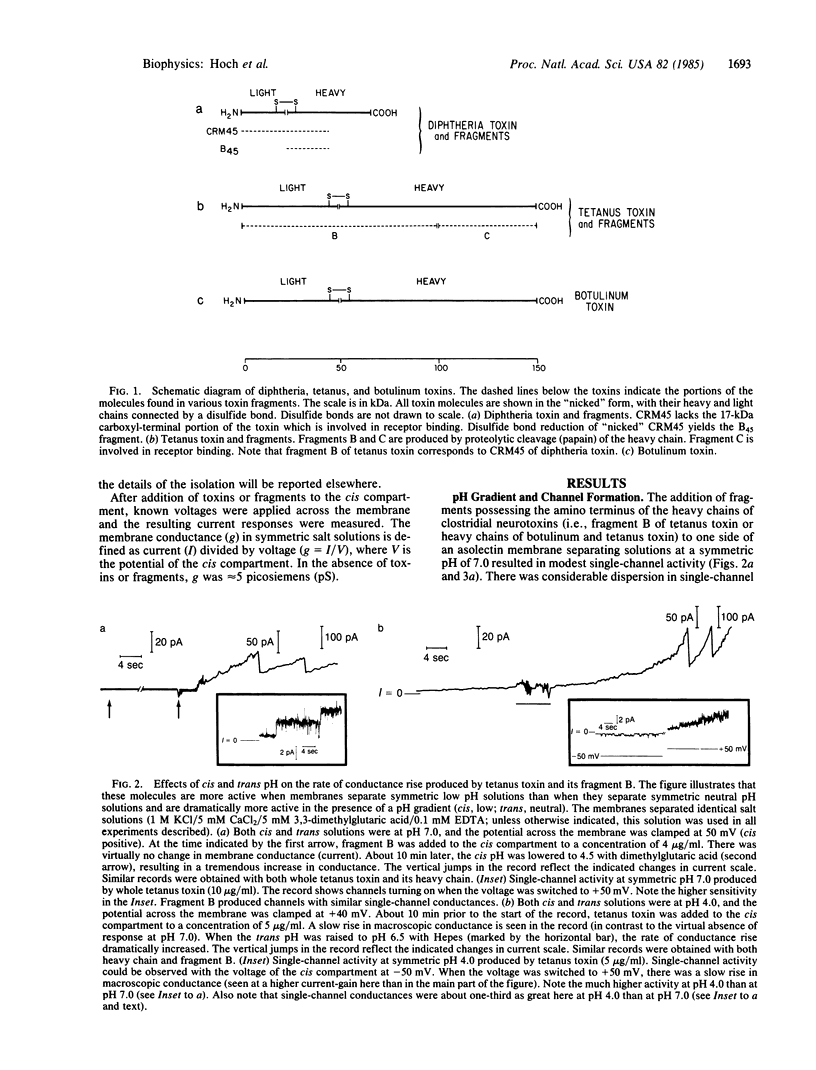
The heavy chains of both botulinum neurotoxin type B and tetanus toxin form channels in planar bilayer membranes. These channels have pH-dependent and voltage-dependent properties that are remarkably similar to those previously described for diphtheria toxin. Selectivity experiments with anions and cations show that the channels formed by the heavy chains of all three toxins are large; thus, these channels could serve as "tunnel proteins" for translocation of active peptide fragments. These findings support the hypothesis that the active fragments of botulinum neurotoxin and tetanus toxin, like that of diphtheria toxin, are translocated across the membranes of acidic vesicles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P., Duflot E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7614–7618. doi: 10.1073/pnas.79.24.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov-Neori H., Yavin E., Montal M. Tetanus toxin forms channels in planar lipid bilayers containing gangliosides. Biophys J. 1984 Jan;45(1):83–85. doi: 10.1016/S0006-3495(84)84117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. The mechanism of ADP-ribosylation of elongation factor 2 catalyzed by fragment A from diphtheria toxin. Biochim Biophys Acta. 1977 Aug 11;483(2):248–257. doi: 10.1016/0005-2744(77)90053-5. [DOI] [PubMed] [Google Scholar]
- Dolly J. O., Black J., Williams R. S., Melling J. Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization. Nature. 1984 Feb 2;307(5950):457–460. doi: 10.1038/307457a0. [DOI] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Falmagne P., Capiau C., Zanen J., Kayser G., Ruysschaert J. M. Structure-activity relationships of the B fragment of diphtheria toxin: the lipid-binding domains. Toxicon. 1982;20(1):243–246. doi: 10.1016/0041-0101(82)90209-4. [DOI] [PubMed] [Google Scholar]
- Goldberg R. L., Costa T., Habig W. H., Kohn L. D., Hardegree M. C. Characterization of fragment C and tetanus toxin binding to rat brain membranes. Mol Pharmacol. 1981 Nov;20(3):565–570. [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer E. J., Muller R. U., Finkelstein A. Inactivation of monazomycin-induced voltage-dependent conductance in thin lipid membranes. I. Inactivation produced by long chain quaternary ammonium ions. J Gen Physiol. 1976 Jun;67(6):703–729. doi: 10.1085/jgp.67.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittelson T. R., Gill D. M. Diphtheria toxin: specific competition for cell receptors. Nature. 1973 Mar 30;242(5396):330–332. doi: 10.1038/242330b0. [DOI] [PubMed] [Google Scholar]
- Kaczorek M., Delpeyroux F., Chenciner N., Streeck R. E., Murphy J. R., Boquet P., Tiollais P. Nucleotide sequence and expression of the diphtheria tox228 gene in Escherichia coli. Science. 1983 Aug 26;221(4613):855–858. doi: 10.1126/science.6348945. [DOI] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S., Dorland R. B., Middlebrook J. L. Inhibition of diphtheria toxin degradation and cytotoxic action by chloroquine. J Biol Chem. 1980 Mar 25;255(6):2247–2250. [PubMed] [Google Scholar]
- Montal M. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 1974;32:545–554. doi: 10.1016/0076-6879(74)32053-8. [DOI] [PubMed] [Google Scholar]
- Morris N. P., Consiglio E., Kohn L. D., Habig W. H., Hardegree M. C., Helting T. B. Interaction of fragments B and C of tetanus toxin with neural and thyroid membranes and with gangliosides. J Biol Chem. 1980 Jul 10;255(13):6071–6076. [PubMed] [Google Scholar]
- Robinson J. P., Hash J. H. A review of the molecular structure of tetanus toxin. Mol Cell Biochem. 1982 Oct 1;48(1):33–44. doi: 10.1007/BF00214820. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. L. Ammonium chloride and methylamine hydrochloride antagonize clostridial neurotoxins. J Pharmacol Exp Ther. 1983 Jun;225(3):546–552. [PubMed] [Google Scholar]
- Simpson L. L. The binding fragment from tetanus toxin antagonizes the neuromuscular blocking actions of botulinum toxin. J Pharmacol Exp Ther. 1984 Apr;229(1):182–187. [PubMed] [Google Scholar]
- Simpson L. L. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981 Sep;33(3):155–188. [PubMed] [Google Scholar]
- VAN HEYNINGEN W. E. The fixation of tetanus toxin, strychnine, serotonin and other substances by ganglioside. J Gen Microbiol. 1963 Jun;31:375–387. doi: 10.1099/00221287-31-3-375. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Wisnieski B. J. Mechanism of insertion of diphtheria toxin: peptide entry and pore size determinations. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3341–3345. doi: 10.1073/pnas.81.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanen J., Muyldermans G., Beugnier N. Competitive antagonists of the action of diphtheria toxin in HeLa cells. FEBS Lett. 1976 Jul 15;66(2):261–263. doi: 10.1016/0014-5793(76)80518-2. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]



