Abstract
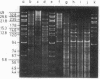
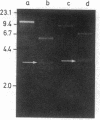
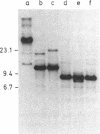
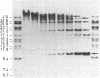
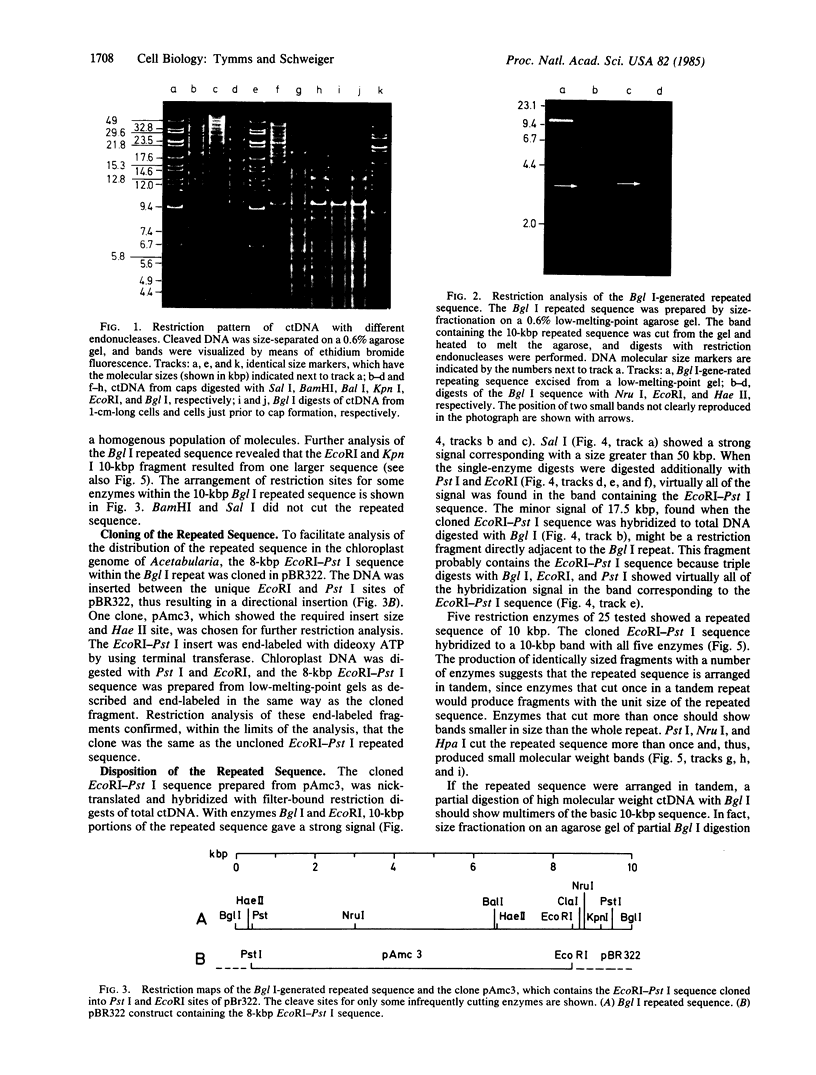
A purified chloroplast fraction was prepared from caps of the giant unicellular green alga Acetabularia mediterranea (strain 17). High molecular weight DNA obtained from these chloroplasts contains at least five copies of a 10-kilobase-pair (kbp) sequence tandemly arranged. This unique sequence is present in DNA from chloroplasts of all stages of the life cycle examined. A chloroplast rDNA clone from mustard hybridized with some restriction fragments from Acetabularia chloroplast DNA but not with the repeated sequence. An 8-kbp EcoRI-Pst I fragment of the repeated sequence was cloned into pBR322 and used as a hybridization probe. No homology was found between the cloned 8-kbp sequence and chloroplast DNA from related species Acetabularia crenulata or chloroplast DNA from spinach.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannwarth H., Ikehara N., Schweiger H. G. Deoxycytidine monophosphate deaminase in Acetabularia: properties and regulation in the early generative phase. Eur J Cell Biol. 1982 Jun;27(2):200–205. [PubMed] [Google Scholar]
- Bannwarth H., Ikehara N., Schweiger H. G. Nucleo-cytoplasmic interactions in the regulation of thymidine phosphorylation in Acetabularia. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):177–190. doi: 10.1098/rspb.1977.0092. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Bogorad L. Endonuclease recognition sites mapped on Zea mays chloroplast DNA. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4309–4313. doi: 10.1073/pnas.73.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Fluhr R., Edelman M. Conservation of sequence arrangement among higher plant chloroplast DNAs: molecular cross hybridization among the Solanaceae and between Nicotiana and Spinacia. Nucleic Acids Res. 1981 Dec 21;9(24):6841–6853. doi: 10.1093/nar/9.24.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Hallick R. B. Physical mapping of the Euglena gracilis chloroplast DNA and ribosomal RNA gene region. Biochemistry. 1978 Jan 24;17(2):284–289. doi: 10.1021/bi00595a015. [DOI] [PubMed] [Google Scholar]
- Green B. R. Protein synthesis by isolated Acetabularia chloroplasts. In vitro synthesis of the apoprotein of the P-700-chlorophyll alpha-protein complex (CP i). Biochim Biophys Acta. 1980 Aug 26;609(1):107–120. doi: 10.1016/0005-2787(80)90205-1. [DOI] [PubMed] [Google Scholar]
- Green B. R. Protein synthesis by isolated Acetabularia chloroplasts. Synthesis of the two minor chlorophyll a complexes in vitro. Eur J Biochem. 1982 Nov 15;128(2-3):543–546. [PubMed] [Google Scholar]
- Green B., Heilporn V., Limbosch S., Boloukhere M., Brachet J. The cytoplasmic DNA's of Acetabularia mediterranea. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1351–1358. doi: 10.1073/pnas.58.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilporn V., Limbosch S. Recherches sur les acides désoxyribonucléiques d'Acetabularia mediterranea. Eur J Biochem. 1971 Oct 26;22(4):573–579. doi: 10.1111/j.1432-1033.1971.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Price C. A. Photosynthetic activity of spinach chloroplasts after isopycnic centrifugation in gradients of silica. Plant Physiol. 1974 Oct;54(4):532–534. doi: 10.1104/pp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan U., Green B. R. The kinetic complexity of Acetabularia chloroplast DNA. Biochim Biophys Acta. 1978 Nov 21;521(1):67–73. doi: 10.1016/0005-2787(78)90249-6. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell. 1982 Jun;29(2):537–550. doi: 10.1016/0092-8674(82)90170-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D. Restriction endonuclease map of the chloroplast DNA of Chlamydomonas reinhardii. J Mol Biol. 1978 Dec 25;126(4):597–617. doi: 10.1016/0022-2836(78)90011-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Schweiger H. G., Berger S. Nucleocytoplasmic interrelationships in Acetabularia and some other dasycladaceae. Int Rev Cytol Suppl. 1979;(9):11–44. doi: 10.1016/s0074-7696(08)60896-7. [DOI] [PubMed] [Google Scholar]
- Shephard D. C., Levin W. B. Biosynthesis in isolated Acetabularia chloroplasts. I. Protein amino acids. J Cell Biol. 1972 Aug;54(2):279–294. doi: 10.1083/jcb.54.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Whitfeld P. R., Herrmann R. G., Bottomley W. Mapping of the ribosomal RNA genes on spinach chloroplast DNA. Nucleic Acids Res. 1978 Jun;5(6):1741–1751. doi: 10.1093/nar/5.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock C. L., Bogorad L. Evidence for variation in the quantity of DNA among plastids of Acetabularia. J Cell Biol. 1970 Feb;44(2):361–375. doi: 10.1083/jcb.44.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot E. J., Schweiger H. G. Thymidylate kinase from Acetabularia. II. Regulation during the life cycle. J Cell Sci. 1983 Nov;64:27–36. doi: 10.1242/jcs.64.1.27. [DOI] [PubMed] [Google Scholar]