Abstract
Migration of HIV infected cells into the CNS is associated with a spectrum of neurological disorders, ranging from milder forms of HIV-associated neurocognitive disorders (HAND) to HIV-associated dementia (HAD). These neuro-psychiatric syndromes are related to the neurodegenerative pathology triggered by the release of HIV proteins and cytokine/chemokines from monocytes/macrophages into the CNS –a condition known as HIV encephalitis (HIVE). As a result of more effective combined anti-retroviral therapy (cART) patients with HIV are living longer and thus the frequency of HAND has increased considerably, resulting in an overlap between the neurodegenerative pathology associated with HIV and that related to aging. In fact, HIV infection is believed to hasten the aging process. The mechanisms through which HIV and aging lead to neurodegeneration include: abnormal calcium flux, excitotoxicity, signaling abnormalities, oxidative stress and autophagy defects. Moreover, recent studies have shown that defects in the processing and transport of neurotrophic factors such as fibroblast growth factors (FGFs), neural growth factor (NGF) and brain-derived growth factor (BDNF) might also play a role. Recent evidence implicates alterations in neurotrophins in the pathogenesis of neurodegeneration associated with HAND in the context of aging. Here, we report FGF overexpression curtails gp120-induced neurotoxicity in a double transgenic mouse model. Furthermore, our data show disparities in brain neurotrophic factor levels may be exacerbated in HIV patients over 50 years of age. In this review, we discuss the most recent findings on neurotrophins and HAND in the context of developing new therapies to combat HIV infection in the aging population.
Introduction
Trafficking HIV infected mononuclear cells into the CNS is associated with a spectrum of neurological disorders, ranging from HIV-associated dementia (HAD) (Fauci, 1988; Portegies et al., 1993) to milder forms of HIV-associated neurocognitive disorders (HAND) (Ellis et al., 2007). These neuro-psychiatric conditions have been related to the neurodegenerative pathology triggered by the migration of productively infected monocytes/macrophages into the CNS –a condition known as HIV encephalitis (HIVE) (Budka et al., 1987; Budka et al., 1991; Gendelman et al., 1994; Gendelman et al., 1997). In recent years, better combined anti-retroviral therapies (cARTs) have helped prolonged the life expectancy of HIV infected patients and have considerably reduced the incidence of HAD and HIVE (Letendre et al., 2007). However, while patients with HIV are living longer (Figure 1A), their quality of life is challenged by the increasing incidence of HAND (Cherner et al., 2004; McArthur et al., 2010; Scott et al., 2011). Thus, as the HIV seropositive population becomes older, interactions with co-morbidities associated with aging present a new variant of neuroAIDS, opening new challenges and needs in clinical therapies (Khanlou et al., 2005; Achim et al., 2009; Khanlou et al., 2009) (Figure 1).
Figure 1. The changing face of HIV epidemic as patients age.

The average age of HIV infected people has increased dramatically. A. The percentage of autopsies performed on HIV-infected individuals over the age of 50 has increased from less than 10% in the 80’s to over 30% at present. B. Mechanisms that lead to HAND in individuals under 50 are exacerbated in individuals over 50 and furthermore after years of chronic neuroinflammation. Neurotoxins, inflammation and oxidative stress combined with normal aging processes may cause increased HAND in aged HIV patients.
Complete eradication of the virus is not possible due to the persistence of latent forms of the virus integrated in the host genome (Bagasra et al., 1996; Jordan et al., 2003). Previous studies have proposed that latent HIV-1 is present in the CNS (Wiley et al., 1999; Jordan et al., 2003; McCrossan et al., 2006). In fact, it has been proposed that due to the low penetration of cART’s into the CNS (Letendre et al., 2008; Varatharajan and Thomas, 2009), latent forms of the virus may represent a reservoir in the CNS (Alexaki et al., 2008; Redel et al., 2010). The host mechanisms involved in suppressing HIV replication and mediating viral latency have therefore gained increased attention recently. In human microglial cells, the transcription factor Bcl11b (a.k.a. CTIP2) was reported to inhibit HIV transcription by the recruitment of a chromatin-modifying complex to the long terminal repeat (LTR) region of the virus, establishing a heterochromatic environment around the site of viral insertion that results in HIV silencing (Marban et al., 2007). We recently reported that latent HIV-1 infection in the CNS was associated with increased levels of chromatin modifiers, including BCL11B that was associated with inflammation, neurodegeneration and neurocognitive impairment (Desplats et al., 2013).
Cognitive impairment in HIV patients is related to the degeneration of selected neuronal populations in the striatum, cortex and limbic system (Masliah et al., 1992b; Masliah et al., 1992a; Masliah et al., 1994; Masliah et al., 1996b). The mechanisms of neurodegeneration in aged individuals with HAND are not completely understood, however HIV activates apoptotic pathways (Kaul et al., 2001), dysregulates calcium homeostasis (Lipton, 1994; Lipton and Rosenberg, 1994; Nath et al., 2000), alters cell cycle signaling pathways such as CDK5 (Figure 2) and promotes oxidative stress (Nath, 2002; Norman et al., 2008). Moreover, recent studies have shown that HIV proteins might interfere with clearance pathways such as autophagy (Alirezaei et al., 2008b; Alirezaei et al., 2008a; Zhou et al., 2011), a pathway necessary for protein quality control and elimination of defective older intracellular organelles (Cuervo, 2004; Cuervo et al., 2005). In aged individuals with HIV, autophagy is down regulated contributing to abnormal protein aggregation and toxicity (Fields et al., 2013) (Figure 1B).
Figure 2. HIV proteins trigger neurodegeneration via CDK5 activation.
HIV proteins bind to receptors on the cell’s surface, and also cross the plasma membrane. Resulting in aberrations in Ca+ homeostasis, activation of calpain 1, p35 cleavage to p25 that hyperactivates CDK5 resulting in aberrant phosphorylation of TAU, CRMP2 and others. The onslaught of HIV infection, neuroinflammation and HIV protein expression culminates in neurodegeneration and ultimately HAND.
In addition to these mechanisms, recent studies have shown that defects in the processing and transport of neurotrophic factors such as fibroblast growth factors (FGFs), neural growth factor (NGF) and brain-derived growth factor (BDNF) might also play a role both in aging and HIV and the mechanisms involved might synergize in older patients with HIV (Mocchetti et al., 2013; Woodbury and Ikezu, 2013) (Figure 1B). Modern treatments with highly active antiretroviral therapy (HAART) regimens result in HIV suppression and immune recovery, however the prevalence of HIV-Associated Neurocognitive Disorders (HAND) and neurodegeneration (Budka et al., 1987; Wiley and Achim, 1994; Gendelman et al., 1997; Cherner et al., 2007; Heaton et al., 2010) has remained the same or increased (Joska et al., 2010; Heaton et al., 2011), in particular among people over the age of 50 (Achim et al., 2009; Fields et al., 2013). Therefore, it is crucial to understand differences in HAND progression among HIV patients above and below the age of 50. In this review, we will discuss some studies implicating alterations in neurotrophins in the pathogenesis of neurodegeneration in HAND in the context of aging.
HIV proteins and neuropathogenesis
During productive HIV replication, permissive host cells including macrophages and microglia in the brain produce viral proteins. These proteins include but are not limited to HIV glycoprotein-120 (gp120), the HIV transactivator of transcription (tat) protein and viral protein R (Vpr). The neurotoxic potential of HIV proteins including gp120, tat, Vpr and others has been addressed by numerous studies (Nath, 2002; Ellis et al., 2007).
Gp120 is required for initial interaction with the CD4 receptor of host cells. Subsequent interaction of gp120 with co-receptors CXCR4 or CCR5 brings the virion and the host cell closer together so that gp41 can insert into the host cell membrane and facilitate virus entry. Tat is an early regulatory protein that significantly enhances viral DNA transcription by binding to the transactivation response (TAR) element in the LTR of the viral promoter. Vpr is incorporated into virions and transports the preintegration complex into the nucleus for viral integration into the host genome. Vpr also activates HIV-1 LTR transcription, and promotes cell-cycle arrest (Kogan and Rappaport, 2011).
Apart from their roles in the HIV life cycle, HIV proteins have secondary effects on neurons (Kovalevich and Langford, 2012). The negative effects of HIV proteins on neurons include disruption of calcium homeostasis, dysregulation of cell-cycle factors, induction of oxidative stress, impaired protein quality control and apoptosis. The effects on neurons are both direct and indirect. For example, the HIV envelope protein gp120 directly enhances neuronal NMDA-evoked calcium flux. Recent studies also show that gp120 contributes to synaptic dysfunction by interfering with NMDA receptor trafficking (Xu et al., 2011). On the other hand, gp120 causes synaptic damage through upregulation of glia-derived inflammatory factors that in turn activates NMDA receptors (Lipton et al., 1991; Ushijima et al., 1993; Corasaniti et al., 1996; Corasaniti et al., 1998; Medina et al., 1999; Geeraerts et al., 2006).
Importantly, even during effective suppression of viral replication by cART, the production of early viral proteins, including tat, continues (Magnuson et al., 1995; Nath, 2002; Chauhan et al., 2003a; Chauhan et al., 2003b; Li et al., 2009). In addition, tat has been shown to induce ryanodine receptor-mediated loss of calcium from the ER followed by the unfolded protein response and leading to pathologic dilatation of the ER in cortical neurons in vitro (Norman et al., 2008). More recently, the protective effects of treating neurons with ketone bodies blocked tat-mediated mitochondrial dysfunction and changes in cellular calcium levels (Hui et al., 2012). Recently, studies have shown that tat-mediated neuronal dysfunction is dependent in part on small noncoding miRNA molecules (Chang et al., 2011).
Some studies have suggested that Vpr is also an early viral protein because it is included in the infecting virion before de novo expression from proviral DNA (Maudet et al., 2013). Studies have shown that Vpr can induce neuronal apoptosis indirectly by stimulating the NFκB –mediated production of inflammatory cytokines (Guha et al., 2012). In this context, HIV proteins also have been shown to contribute to host-cell derived neurotrophic factor production and functioning as described below.
Neurodegeneration in patients with HAND: protective role of FGF’s
The relationship between neurodegeneration, cognitive impairment and HIVE is complex. The brains of patients with HIVE display synapto-dendritic damage, neuronal loss and a spectrum of neuro-inflammatory alterations (Masliah et al., 1992b) (Figure 3). In addition, patients with AIDS show varying degrees of damage to the cerebral microvasculature and white matter. Increased viral load is associated with worsening neuronal damage that correlates with the onset of early cognitive impairment (Masliah et al., 1992b; Everall et al., 1999). Cognitive impairment and neurodegeneration persists in the brains of aged HIV patients, including those with latent HIV in the brain (Desplats et al., 2013). HIVE has shifted from a subacute neuroinflammatory condition to a chronic, more subtle disease process (Langford et al., 2003; Langford et al., 2006) with neurodegeneration particularly involving synapses and dendrites (Langford et al., 2011; Kovalevich and Langford, 2012) (Figure 3).
Figure 3. HIV-induced neuropathology is increased in brains from HIV patients over the age of 50. A.

MAP2 immunostaining shows neuronal atrophy and discontinuous processes during HIVE, and more so in HIVE brains over the age of 50. B. MAP2 signal intensity is decreased in HIVE brains compared to HIV brains in both age groups, but HIVE brains from patients over 50 years of age show the least MAP2 immunostaining. C. Viral load, as estimated by p24 levels, is decreased in brains of HIVE patients over the age of 50. D. CD68 immunostaining, representing activated infiltrating monocytes and microgia, is more robust in brains from HIVE patients and is the most intense in those over the age of 50. E. Astrogliosis is increased in brains of HIVE patients, with the most intense signal in brains from HIVE patients over the age of 50. *p<0.05 by unpaired student T test.
The neurodegenerative process affects primarily the striato-cortical, cortico- cortical, and limbic intrinsic/inhibitory circuitries (Masliah et al., 1996b; Masliah et al., 1996a). It is not known if these circuitries are affected simultaneously or if there is a temporal progression of the neurodegenerative process from one site to another. The neuronal populations most severely affected in these regions include large pyramidal neurons in the neocortex, (Budka et al., 1987; Everall et al., 1991; Wiley et al., 1991a; Wiley et al., 1991b; Masliah et al., 1992b; Weis et al., 1993; Masliah et al., 1997) spiny neurons in the putamen, (Masliah et al., 1992b; Masliah et al., 1996b; Masliah et al., 1996a) medium-sized neurons in the globus pallidus, and interneurons in the hippocampus (Masliah et al., 1992b; Fox et al., 1997). Consistent with these neuropathological studies, analyses of the brains of AIDS patients and observations in animal models show similar alterations in neuronal markers such as decreased N-acetyl-aspartate evidenced by nuclear magnetic resonance spectroscopy (Wilkinson et al., 1997; Marcus et al., 1998; Gonzalez et al., 2006) as well as extensive loss of synapses and dendrites in the hippocampus, neocortex and striatum (Toggas et al., 1994; Lipton et al., 1995; Mucke et al., 1995).
While approximately 70% of HIVE patients show cognitive impairment and neurodegeneration, the remaining 30% are cognitively unimpaired and their neuronal populations are well preserved (Everall et al., 1992; Masliah et al., 1996b; Masliah et al., 1996a; Everall et al., 1999; Cherner et al., 2002; Cherner et al., 2007). Such observations suggest that among the factors responsible for relative sparing of neurons and cognition, these individuals may have the capacity to produce neurotrophic factors capable of protecting neurons against the deleterious effects of HIV. Based on the complex relationship between HAND and HIVE, currently four groups of patients are recognized (Gelman et al., 2012): 1) without HIVE or neurodegeneration (HIVE-ND−); 2) without HIVE, but with neurodegeneration (HIVE-ND+); 3) HIVE, without neuronal degeneration (HIV+ND) and 4) HIVE and with extensive degeneration (HIV+ND+) (Figure 4). Neurodegeneration in the absence of HIVE and/or an inflammatory process in the brain might be observed in cases where cART has reduced viral burden to undetectable levels, but the anti-retroviral agents are also triggering neuronal toxicity. Regarding those patients with HIVE but without neurodegeneration (group 3), it has been proposed that enhanced production of neurotrophic factors might contribute to the protection of the CNS from HIV toxicity (Everall et al., 2001; Langford et al., 2002). Among such factors are the FGF family that includes at least 13 trophic factors important in neurogenesis and angiogenesis (Klint and Claesson-Welsh, 1999; Klint et al., 1999; Reuss and von Bohlen und Halbach, 2003). Of interest in the brain are FGF1 (acidic, aFGF) that is produced by neurons and is primarily neurotrophic, and FGF2 (basic, bFGF) that is produced by glial cells and is angiotrophic (Walicke and Baird, 1988; Walicke, 1989; Eckenstein, 1994; Klint and Claesson-Welsh, 1999). Increased expression of FGF1 ameliorates the neurotoxic effects of HIV proteins (Everall et al., 2001). In cases with HIVE but without degeneration, FGF1 levels were significantly elevated. In contrast, individuals with both HIVE and neurodegeneration showed low levels of neuronal FGF1 immunoreactivity (Everall et al., 2001; Crews et al., 2009). Studies in primary human neuronal cultures treated with the HIV envelope protein showed that FGF1 was protective against gp120 neurotoxicity in a dose-dependent manner (Everall et al., 2001) and FGF1 overexpression ameliorated neurodegeneration in the gp120 tg mice (Crews et al., 2009).
Figure 4. Classification of HIV infected patients according to presence of HIVE.
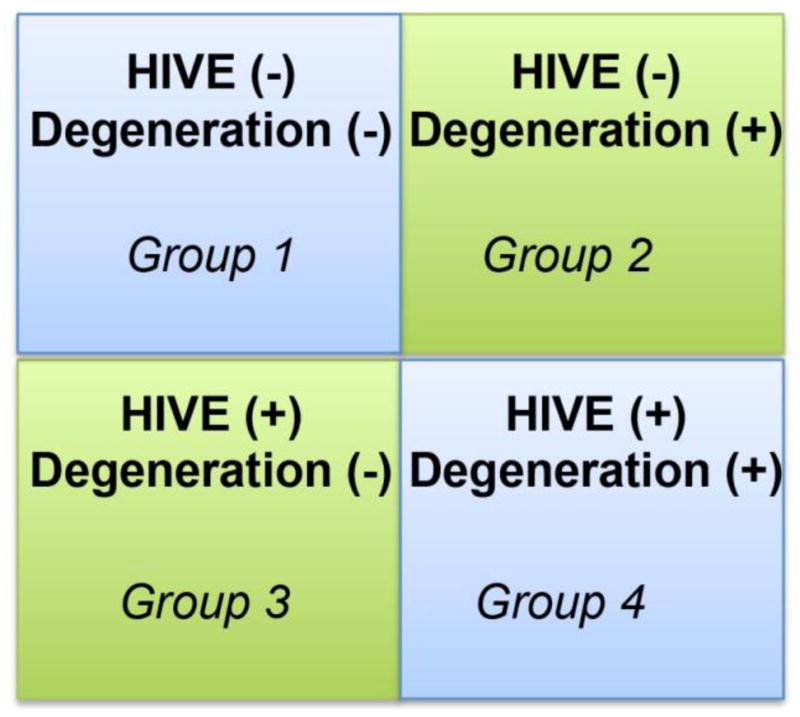
Based on the complex relationship between HAND and HIVE, currently four groups of patients are recognized (Gelman et al., 2012): 1) without HIVE or neurodegeneration (HIVE-ND−); 2) without HIVE, but with neurodegeneration (HIVE-ND+); 3) HIVE, without neuronal degeneration (HIV+ND) and 4) HIVE and with extensive degeneration (HIV+ND+)
In addition to the damage to neurons, the cytotoxic HIV protein, gp120, damages endothelial cells of the blood brain barrier (BBB), thereby compromising its integrity that may lead to migration of HIV-infected cells into the brain (Nakamuta et al., 2008; Yang et al., 2009). FGF2, produced primarily by astrocytes, promotes endothelial cell fitness and angiogenesis. We have previously shown that exposure of endothelial cells to gp120 resulted in dose- and time-dependent cell death; whereas, pretreatment of endothelial cells with FGF2 protected cells from gp120 angiotoxicity (Langford et al., 2005). Using both pharmacological approaches and gene transfer of constitutively active ERK-1, we showed that FGF2-mediated angioprotection against gp120 toxicity is regulated via ERK signaling (Langford et al., 2005).
Aside from damage to synapses, dendrites, endothelial cells and neuronal loss, recent studies have shown that patients with HIVE display defects in adult neurogenesis in the hippocampus (Avraham et al., 2013; Lee et al., 2013). Similar alterations have been observed in HIV-gp120 tg mice (Okamoto et al., 2007). These mice display decreased proliferation and survival of neuronal precursor cells (NPC’s) (Okamoto et al., 2007). Transgenic over-expression of FGF1 (Crews et al., 2009) recovers the neurogenesis defects in gp120 mice as indicated by increased doublecortin expression levels (Langford et al., 2005) (Figure 5).
Figure 5. FGF1 protects gp120 tg mouse model from neurodegeneration.

A. For this experiment GFAP-gp120 tg mice were crossed with PDGF-FGF1 mice. Brain dentate gyrus sections were labeled with the doublecortin for detection of neuroblasts. Non tg and FGF1 tg mice display abundant neurogenesis in the dentate gyrus of the hippomouse. Gp120 tg mice have decreased neurogenesis, but FGF1 overexpression in gp120 mice reverses this pathology. B. Total neuroblast numbers were statistically equivalent in non tg mouse brains from untreated and FGF1 tg mice. Total neuroblast numbers were decreased by 50% in gp120 tg mouse brains, and FGF1 overexpression recovered levels to near that of control. *#p<0.05 by One-way Anova with posthoc Fisher test.
The mechanisms for defective neurogenesis in HIVE might involve neurotoxicity of gp120, vpr and tat to NPC involving neurotrophic factor dysregulation and signaling defects. In HIVE, alterations in GSK3β (Maggirwar et al., 1999; Nguyen et al., 2009a, b; Kehn-Hall et al., 2011) and CDK5 (Patrick et al., 2011; Lee et al., 2013) play important roles (Figure 2). Abnormal activation of CDK5 is associated with neurodegenerative disorders, and recently a critical role for CDK5 in adult neurogenesis was identified (Lee et al., 2013). Abnormal CDK5 activation in an in vitro model of adult neurogenesis resulted in hyperphosphorylation of collapsin-response mediator protein-2 (CRMP2) and impaired neurite outgrowth. Inhibition of CDK5, or expression of a non-phosphorylatable (S522A) CRMP2 construct reduced CRMP2 hyperphosphorylation, and reversed neurite outgrowth deficits. CRMP2 plays a role in microtubule dynamics; therefore we examined the integrity of microtubules in this model using biochemical and electron microscopy techniques. We found that microtubule organization was disrupted under conditions of CDK5 activation. Likewise, CDK5-mediated CRMP2 phosphorylation was significantly increased in the hippocampus of patients with HIVE and in gp120 transgenic mice, and these effects were rescued by genetic down-modulation of CDK5 in the mouse model (Crews et al., 2011). These results reveal a functional mechanism involving microtubule destabilization through which abnormal CDK5 activation and CRMP2 hyperphosphorylation might contribute to defective neurogenesis in neurodegenerative disorders such as HIVE (Crews et al., 2011).
Taken together, these results support the notion that trophic factors, such as FGF1 and FGF2, might protect the CNS from the neurotoxic effects of HIV.
Alterations in neurotrophins in HIV encephalitis
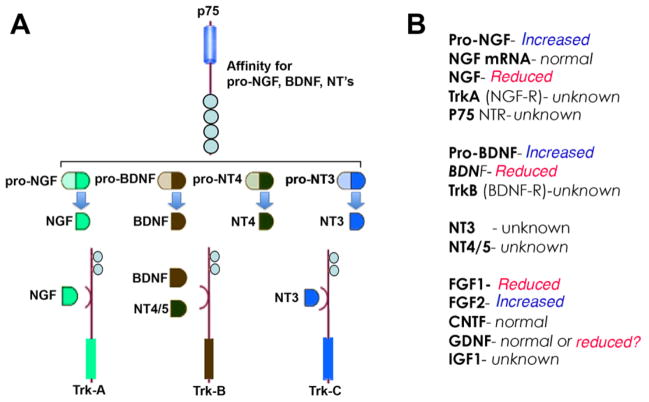
NGF is an original member of the neurotrophin family, and the first to be identified (Levi-Montalcini, 1987). Overall, NGF promotes survival of cholinergic neurons (Liberini et al., 1993), neurogenesis (Frielingsdorf et al., 2007), dendritic complexity and axon growth (Studer et al., 1994; Tuszynski et al., 1996), while preventing neurodegeneration (Tuszynski et al., 1991) (Figure 6). NGF signals by binding to the tyrosine receptor kinase A (TrkA) receptor, which leads to activation of the cytoplasmic adaptor protein and activation of pro-survival pathways, including AKT and MAPK (Kaplan and Miller, 2000) (Figure 6). Previous studies have shown that HIV tat suppresses the NGF-mediated differentiation of PC12 rat pheochromocytoma cells (Zauli et al., 1994), suggesting that HIV proteins may interfere with NGF signaling. NGF stimulation promotes CXCL-12 attraction of monocytes but decreases HIV replication in the attracted population (Samah et al., 2009). Hence, NGF activity may be important for regulating viral replication in non-neural cells during HIV infection. However, a later report showed that NGF reduced apobec3g synthesis and enhanced HIV-1 transcription and replication in human primary macrophages (Souza et al., 2011). It is plausible that NGF has variable effects on different cell types, and its effects on neuronal cells may be supportive or deleterious depending upon direct or indirect interactions. Most recently, it was reported that NGF/TrkA signaling or p75 receptor inhibition protected somatic sensory neurons exposed to Vpr (Webber et al., 2013). Overall, these data suggest NGF is neuroprotective when directly interacting with a neuron, but may indirectly result in neurodegeneration through normal processes involving recruitment of immune cells and enhancement of viral replication.
Figure 6. Nerve growth factor signaling and levels in brains of HIV patients.
A. Pro-growth factors and pro-neurotrophins signal p75, once processed the mature NGF, BDNF or NT signal through the trk (A, B or C) receptors to enact cellular processes. B. Several growth factors and neurotrophins are altered in brains of HIV patiens.
Pro-NGF is the precursor protein of NGF and is the most abundant form of NGF in the brain (Fahnestock et al., 2001; Peng et al., 2004). Pro-NGF can undergo proteolytic cleavage by a family of mammalian processing enzymes called pro-protein convertases (Seidah and Chretien, 1999; Fugere and Day, 2005) (Figure 7A). To date there are nine known convertases (Seidah and Chretien, 1999; Fugere and Day, 2005) with furin being the most throrougly documented. Moreover, the furin recognition site (R118-S-K-R121) has been reported as essential for the proper processing of pro-NGF to the mature form of NGF (Lim et al., 2007). Likewise, pro-BDNF is cleaved by furin to generate mature BDNF (Figure 7B).
Figure 7. HIV proteins inhibit processing of pro-growth factors.
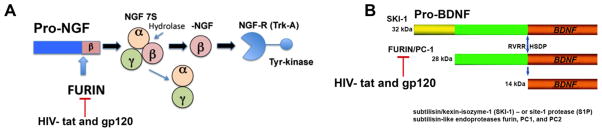
A. Furin is a protease that cleaves pro-NGF to the active form of NGF. B. Furin is a protease that cleaves pro-BDNF to the active form of BDNF. HIV tat and gp120 may prevent furin processing of pro-NGF and pro-BDNF thereby preventing pro growth properties and initiating pro apoptotic properties in neurons.
Unlike the neurotrophic effects associated with the mature form of NGF and BDNF, pro-NGF has been reported to have pro-apoptotic effects mediated by the p75 neurotrophin receptor (Harrington et al., 2004; Pedraza et al., 2005; Podlesniy et al., 2006). A recent report suggested that pro-NGF observed in AD brains has undergone oxidative modifications by advanced glycation and lipid peroxidation that inhibit the processing of pro-NGF to its mature form, thereby resulting in an imbalance between the precursor and mature forms of the protein (Kichev et al., 2009). This altered processing favors the pro-apoptotic cascade mediated by enhanced levels of pro-NGF and is also consistent with the reduced levels of NGF and cholinergic degeneration observed in models of AD (Cuello and Bruno, 2007; Cuello et al., 2007; Cuello and Canneva, 2008; Cuello et al., 2010). Like pro-NGF, pro-BDNF induces apoptosis in peripheral neurons, and helps with long-term depression as opposed to long-term potentiation induced by mature BDNF in the hippocampus (Teng et al., 2005; Woo et al., 2005).
BDNF is a member of the neurotrophin family that includes NGF and the neurotrophins 3 and 4/5 (Figure 6). Like NGF, BDNF supports neural survival, growth and differentiation in several parts of the CNS and peripheral nervous system (PNS), but in contrast to NGF, BDNF acts through binding to the TrkB receptor (Chao, 2003; Chao et al., 2006) (Figure 6). Recent evidence has shown that BDNF is neuroprotective against gp120 both in vivo and in vitro (Bachis et al., 2003). Studies suggest that BDNF exerts its protective effects by regulating neuronal expression of the HIV co-receptor, CXCR4. (Bachis et al., 2003). BDNF binds to the tyrosine kinase receptor, TrkB, and induces its dimerization (Chao, 2003; Chao et al., 2006). In turn, phosphorylation of ERK decreases the expression of CXCR4, by inhibiting caspase-3 (Nosheny et al., 2007). Following the binding of HIV-gp120 to the CD4 receptor, secondary interactions with CXCR4 facilitate viral entry into permissive cells. In neurons expressing CXCR4, binding of gp120 induces the caspase cascade resulting in neuronal apoptosis (Bachis et al., 2003). In vitro and in vivo studies indicate that BDNF induces CXCR4 internalization and blocks caspase-3 (Bachis et al., 2003; Mocchetti and Bachis, 2004; Mocchetti et al., 2007; Mocchetti et al., 2008). Interestingly, CXCR4 internalization is also induced by FGF1 (Sanders et al., 2000), suggesting that neuroprotective properties of some neurotrophic factors may share common mechanisms (Figure 6B).
Downregulation of BDNF may be responsible for neurodegeneration associated with HIV infection. gp120 and TNF-α, a proinflammatroy cytokine common in HIVE, reduce the anterograde transport of BDNF, reduce intracellular stores and stimulate the NMDA receptor, leading to reduced neuronal survival (Bachis et al., 2003; Nosheny et al., 2004) (Figure 6B). HIV also prevents BDNF activity by a more indirect route through stimulating TNF-α expression. TNF-α prevents glucocorticoid receptor (GR) activity and thereby reduces hippocampal neurogenesis. Specifically, TNF-α increases expression of inactive GR-β and decreases expression of GR-α, which is the active form (Miller et al., 2009; Hasler, 2010; Raedler, 2011). Hence, HIV-induced neuroinflammation indirectly leads to neurodegeneration by silencing GR activity. GR regulation of BDNF is important for hippocampus campus structure and functional plasticity (Suri and Vaidya, 2013). Furthermore, BDNF reduces CXCR4 expression, effectively reducing infection of microglia and astrocytes in the CNS (Nosheny et al., 2004). This pro-survival effect may be minimized as HIV also decreases levels of BDNF in lymphocytes, which leads to T-cell apoptosis and possibly neurodegenerative processes (Avdoshina et al., 2011). More recent data suggests that gp120 reduces pro-BDNF processing in neurons by reducing furin levels and effectively altering the balance of anti-apoptotic and pro-apoptotic neurotrophins (Bachis et al., 2012) (Figure 7B). These data were recently corroborated by reports showing reduced neurite out growth and impaired neurogenesis is reversed by exercise via BDNF production in a gp120 mouse model (Lee et al., 2013). Together this evidence suggests that therapies directing BDNF to neurons may be effective in treating neurodegenerative disorders (Figure 6 and 7).
Findings in earlier studies suggest BDNF and NGF could be elevated in brain tissues of HIV patients (Boven et al., 1999). In HIV patients with HAND, levels of FGF2 and BDNF were lower CSF than levels in control patients; whereas, levels of NGF were elevated in HIV patients with HAND compared to control (Albrecht et al., 2006). Differences in neurotrophin levels in the brain among recent studies could be attributed to collection of the specimens, measurement techniques, genetics or a number of other variables. Interestingly, another study showed that gp120 cooperates with BDNF to enhance somatostatin neurotransmission in HIVE, which otherwise is severely impaired in disease (Barnea et al., 1999). In addition, NGF and BDNF play important roles in neuronal survival in HIV infection by activating the NF-κB, thereby inducing expression of the anti-apoptotic Bcl-2 gene to protect neurons from the pro-apoptotic effects of HIV-tat (Ramirez et al., 2001). On the other hand, gp120 has been shown to affect the expression of both NGF and nitric oxide synthase in rodent models of HIV disease (Bagetta et al., 1996; Corasaniti et al., 1998). Also, the tat has been shown to cooperate with p35 signaling in neurons to dysregulate the NGF pathway, thereby disrupting neuronal survival and differentiation (Peruzzi et al., 2002; Darbinian et al., 2008).
During aging and in neurodegenerative disorders such as Alzheimer’s Disease (AD) both NGF (Gage et al., 1989; Perry, 1990; Gage et al., 1991; Kerwin et al., 1991; Kerwin et al., 1992) and BDNF (Mufson et al., 1989; Mufson and Kordower, 1992; Poon et al., 2013) transport and metabolism (Scott et al., 1995; Peng et al., 2004; Schindowski et al., 2008; Ye et al., 2012) are affected resulting in degeneration of selected neuronal populations in the basal nucleus of Meynert and hippocampus, respectively. For this reason, recent experimental therapies for AD have been directed at developing strategies to increase the expression of neurotrophic factors (Tuszynski et al., 1991; Tuszynski et al., 1996; Tuszynski et al., 1998; Tuszynski, 2003; Tuszynski et al., 2005; Blurton-Jones et al., 2009; Nagahara et al., 2009).
Given the contribution of aging to HAND, it is important to understand how aging and HIV interact to affect neurotrophins. To better understand the contributions of aging to HAND, we recently characterized a group of HIV and HIVE patients below and above 50 years of age (Fields et al., 2013). The young (<50) and aged (>50) HIVE patients had higher viral loads, increased neuroinflammation and neurodegeneration as characterized by astrogliosis, monocytic infliltration and discontinuous MAP2 immunostaining; however, aged HIVE postmortem brain tissues showed the most severe neurodegenerative pathology. Interestingly, young HIVE patients displayed increased beclin-1, cathepsin-D and LC3, but these autophagy markers were reduced in older HIVE patients compared to age-matched HIV+ controls. Similar alterations in autophagy markers were observed in aged gp120 transgenic (tg) mice (Fields et al., 2013). These data indicate differential alterations in the autophagy pathway in young versus older HIVE patients, and that autophagy reactivation may ameliorate the neurodegenerative phenotype in these patients. We have now investigated the levels of various growth factors in the neocortex of young and old patients with HIVE. We observed that in older patients with HIV or with HIVE there was a decrease in BDNF and an increase in pro-BDNF. The levels of NGF were decreased in older HIV patients as well as in young and old patients with HIVE, with a concomitant increase in pro-NGF (Figure 6B and 8). In contrast, levels of glial derived neurotrophic factor (GDNF) and ciliary neurotrophic factor (CNTF) were not affected (Figure 8).
Figure 8. Growth factor levels in brains of HIV and HIVE patients below and above 50 years of age.

A. Immunoblot analysis of growth factors in the neocortex of HIV and HIVE patients below and above the age of 50 show NGF and BDNF signal is more intense in brains from patients below 50 years of age. In contrast, pro-NGF and pro-BDNF signal is increased in brains from patients above 50 years of age. GDNF and CNTF levels appear to be unchanged. Densitometry analysis shows: B. BDNF levels are decreased by 70% in brains from patients above 50 years of age for HIV and HIVE C. pro-BDNF levels are increased 4- and 3-fold in brains of patients above 50 years of age diagnosed as HIV and HIVE, respectively D. NGF levels are decreased by 75% in brains of HIV patients above the age of 50, but NGF levels are equivalent in HIVE brains below or above 50 years of age. E. Conversely, pro-NGF levels are increased by 2-fold in brains from HIV patients above the age of 50 and were elevated in HIVE brains from patients above and below 50. These data suggest HIV may manipulate growth factor processing in a way that promotes neurodegeneration. *#p<0.05 by One-way Anova with posthoc Fisher test.
Overall, these results are consistent with the concept that during aging and in HIVE there is a defect in the maturation and transport of NGF or BDNF that could play a role in neurodegeneration and might be a target for improved therapeutics (Figure 6).
Alterations in other trophic factors in the brains of patients with HIV
GDNF is produced by glial cells and promotes survival of many types of neurons including dopaminergic and motor neurons (Lin et al., 1993). Similar to effects on BDNF, gp120 reduces GDNF expression and causes apoptosis in the substantia nigra (Nosheny et al., 2006). Conversely, tat-GDNF fusion proteins rescue dying neurons after optic nerve transection and protect mice after focal cerebral ischemia (Kilic et al., 2004; Yulug et al., 2004). Aside from neurotrophic factors, HIV interaction with other growth factors may indirectly affect neurodegeneration. HIV-1 enhances hepatitis–C virus infection by increasing expression of transforming growth factor-β (TGFβ)-related genes while decreasing expression of interferon (IFN)-related genes (Zhang et al., 2012: Lin, 2008 #9885). These data illustrate that HIV interacts with many growth factor pathways, which results in variable effects depending upon cell type and cellular processes involved.
Platelet-derived growth factor (PDGF) mediated neuroprotection against both gp120 and tat was reported in studies with SHSY-5Y neuroblastoma cells (Peng et al., 2008b; Peng et al., 2008a; Zhu et al., 2009). Pathways through which PDGF protects differentiated neurons from gp120 and tat both involve blocking apoptosis, however, the signaling cascades differ. For example, PDGF protects neurons from gp120-mediated toxicity via the PI3K/AKT/GSK3β pathway (Peng et al., 2008b; Peng et al., 2008a). On the other hand, neurons are protected from tat by PDGF regulation of extracellular glutamate and intracellular calcium levels (Zhu et al., 2009). Further studies showed that intra-striatal delivery of PDGF in mice protected dopaminergic neurons in the substantia nigra from tat toxicity via transient receptor potential canonical channel signaling (Yao et al., 2009). Through molecular mimicry, the virus or viral proteins can exploit the host cell machinery by binding to host cell receptors to disrupt normal host cell signaling (Asensio and Campbell, 1999; Langford, 2002). For example, the HIV-encoded angiogenic tat peptide mimics signaling properties of vascular endothelial cell growth factor (VEGF) by binding to the VEGF receptor on cerebral endothelial cells to activate MAP kinase activity and angiogenesis (Scheidegger et al., 2001). Studies also indicate that tat mimicry of VEGF correlates with increased microvessel density in AIDS-related diffuse large B-cell and Burkitt lymphomas (Nyagol et al., 2008). Molecular mimicry by HIV proteins also includes exploitation of chemokine receptors by both tat and gp120 to promote viral infection of host cells (Berger et al., 1999a; Berger et al., 1999b; Murphy, 2001). Numerous examples of viral mimicry exist and include homologues of cytokines, chemokines, neurotrophic growth factors or their receptors (Lalani and McFadden, 1999; Alcami and Koszinowski, 2000; Murphy, 2001) leading to the dysregulation of host-mediated immune responses. In conclusion, trophic factors and viral proteins convergence to modulate host response to neurodegenerative infectious diseases. Increasing evidence supports roles for neuro-regulatory interactions of trophic factors with HIV in the CNS, providing potential targets for the development of new therapeutic approaches.
Materials and Methods
Study population
Briefly, as previously described (Fields et al., 2013), for the present study we included a total of 12 HIV+ cases, of which 6 are below 50 years (yr) and 6 are above 50 yr from the HIV Neurobehavioral Research Center and California Neuro acquired immunodeficiency syndrome (AIDS) Tissue Network at the University of California, San Diego. Cases had neuromedical and neuropsychological examinations within a median of 12 months before death. The diagnosis of HIV encephalitis was based on the presence of microglial nodules, astrogliosis, HIV p24–positive cells, and myelin pallor.
Generation of FGF-1 tg Mice
To determine the effects of FGF1 on HIV-induced neuropathogenesis an animal model of HIV-protein mediated neurotoxicity, gp120 tg expressing high levels of gp120 under the control of the glial fibrillary acidic protein (GFAP) promoter (Toggas et al., 1994) was crossed with PDGF-FGF1 tg mice (Crews et al., 2009). A total of 6 mice per group, 4–5 months of age, were utilized for neuropathological analysis that was performed as previously described (Crews et al., 2009; Fields et al., 2013)
Antibodies
For immunohistochemical and immunoblot analysis of growth factor expression and neurodegeneration, polyclonal antibodies against microtubule associated protein (MAP) 2 (MAP378, 1:250, Millipore), p24 (04101, 1:1000, Trinity biotech) CD68 (M0876, 1:1000, DAKO) GFAP (MAB3402, dilution 1:500, Millipore), doublecortin (sc-8066, 1:500, Santa Cruz Biotechnology Inc.), BDNF (BML-SA665, 1:1000, Enzo Life Sciences), pro-BDNF (sc-65514, 1:1000, Santa Cruz Biotechnology Inc.) NGF (N6655, 1:1000, Sigma), pro-NGF (04-1142, 1:1000, Millipore), GDNF (GF030, 1:1000, Millipore), CTNF (MAB338, 1:1000, Millipore) and actin (A5441, 1:1000, Sigma) were obtained.
Immunoblot analysis
Frontal cortex tissues from human brains were homogenized and fractionated using a lysis buffer (1.0 mmol/L HEPES, 5.0 mmol/L benzamidine, 2.0 mmol/L 2-mercaptoethanol, 3.0 mmol/L EDTA, 0.5 mmol/L magnesium sulfate, 0.05% sodium azide; final pH 8.8). In brief, as previously described, (Fields et al., 2013) tissues from human brain samples (0.1 g) were homogenized in 0.7 mL of fractionation buffer containing phosphatase and protease inhibitor cocktails (Calbiochem, San Diego, CA). Samples were precleared by centrifugation at 5000 × g for 5 minutes at room temperature. This supernatant was collected and assayed for protein concentration.
After determination of the protein content of all samples by BCA Protein assay (Thermo Fisher Scientific, Rockford, IL), homogenates were loaded (20 μg total protein/lane), separated on 4–12% Bis-Tris gels and electrophoresed in 5% HEPES running buffer, and blotted onto Immobilon-P 0.45 μm membrane using NuPage transfer buffer. The membranes were blocked in either 5% nonfat milk/1% BSA in phosphate buffered saline (PBS) + 0.05% Tween-20 (PBST) or in 5% BSA in PBST for one hour. Membranes were incubated overnight at 4°C with primary antibodies. Following visualization, blots were stripped and probed with a mouse monoclonal antibody against Actin (1:2000, mab1501, Millipore, Billerica, MA) as a loading control. All blots were then washed in PBST, and then incubated with secondary species-specific antibodies (American Qualex, 1:5000 in BSA-PBST) and visualized with enhanced chemiluminescence reagent (ECL, Perkin-Elmer). Images were obtained and semi-quantitative analysis was performed with the VersaDoc gel imaging system and Quantity One software (Bio-Rad).
Immunohistochemistry, Image Analysis and Laser Scanning Confocal Microscopy
Briefly, as previously described (Fields et al., 2013), free-floating 40 μm thick vibratome sections were washed with Tris buffered saline (TBS, pH 7.4), pre-treated in 3% H2O2, and blocked with 10% serum (Vector Laboratories, Burlingame, CA), 3% bovine serum albumin (Sigma), and 0.2% gelatin in TBS-Tween (TBS-T). For human brains, sections from the midfronal cortex were used; for the mice sagittal sections from the complete brain were studied. Sections were incubated at 4°C overnight with the primary antibodies. Sections were then incubated in secondary antibody (1:75, Vector), followed by Avidin D-horseradish peroxidase (HRP, ABC Elite, Vector) and reacted with diaminobenzidine (DAB, 0.2 mg/ml) in 50 mM Tris (pH 7.4) with 0.001% H2O2. Control experiments consisted of incubation with pre-immune rabbit serum.
Immunostained sections were imaged with a digital Olympus microscope and assessment of levels of p24, CD68, GFAP and doublecortin immunoreactivity was performed utilizing the Image-Pro Plus program (Media Cybernetics, Silver Spring, MD). For each case a total of three sections (10 images per section) were analyzed in order to estimate the average number of immunolabeled cells per unit area (mm2) and the average intensity of the immunostaining (corrected optical density).
Neuronal structural integrity was evaluated as previously described (Rockenstein et al., 2005a; Rockenstein et al., 2005b). For confocal images (MAP2) all sections were processed simultaneously under the same conditions and experiments were performed twice for reproducibility. All sections were processed under the same standardized conditions. The immunolabeled blind-coded sections were serially imaged with a laser scanning confocal microscope (MRC- 1024; Bio-Rad) and analyzed with ImageJ v1.43 software (NIH, Bethesda, MD), as previously described (Crews et al., 2010). For each case, a total of three sections were analyzed; for each section, four fields in the frontal cortex were examined. Results were expressed as percent area of the neuropil occupied by immunoreactive signal. All sections were processed under the same standardized conditions. Immunostained sections were imaged with a digital Olympus microscope and the Image-Pro Plus program (version 4.5.1, Media Cybernetics).
Statistical analysis
All the analyses were conducted on blind-coded samples. After the results were obtained, the code was broken and data were analyzed with the StatView program (SAS Institute, Inc., Cary, NC). Comparisons among groups were performed with one-way ANOVA, unpaired Student’s T test and Chi square analysis. All results were expressed as mean ±SEM. The differences were considered to be significant if p values were <0.05.
Role of the funding source
The funding source had no role in the study design, data collection, data analysis, data interpretation or writing of this report. The corresponding author had full access to all the data and had final responsibility for the decision to submit the paper for publication.
References
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht D, Garcia L, Cartier L, Kettlun AM, Vergara C, Collados L, Valenzuela MA. Trophic factors in cerebrospinal fluid and spinal cord of patients with tropical spastic paraparesis, HIV, and Creutzfeldt-Jakob disease. AIDS Res Hum Retroviruses. 2006;22:248–254. doi: 10.1089/aid.2006.22.248. [DOI] [PubMed] [Google Scholar]
- Alcami A, Koszinowski UH. Viral mechanisms of immune evasion. Immunology Today. 2000;21:447–455. doi: 10.1016/S0167-5699(00)01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Fox HS. Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy. 2008a;4:963–966. doi: 10.4161/auto.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS One. 2008b;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- Avdoshina V, Garzino-Demo A, Bachis A, Monaco MC, Maki PM, Tractenberg RE, Liu C, Young MA, Mocchetti I. HIV-1 decreases the levels of neurotrophins in human lymphocytes. AIDS. 2011;25:1126–1128. doi: 10.1097/QAD.0b013e32834671b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Zvonok A, Masliah E, Avraham S. CB cannabinoid agonist enhanced neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis. Br J Pharmacol. 2013 doi: 10.1111/bph.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci. 2012;32:9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MT, Aloe L, Berliocchi L, Costa N, Finazzi-Agro A, Nistico G. Intracerebral injection of human immunodeficiency virus type 1 coat protein gp120 differentially affects the expression of nerve growth factor and nitric oxide synthase in the hippocampus of rat. Proc Natl Acad Sci U S A. 1996;93:928–933. doi: 10.1073/pnas.93.2.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A, Roberts J, Ho RH. Evidence for a synergistic effect of the HIV-1 envelope protein gp120 and brain-derived neurotrophic factor (BDNF) leading to enhanced expression of somatostatin neurons in aggregate cultures derived from the human fetal cortex. Brain Res. 1999;815:349–357. doi: 10.1016/s0006-8993(98)01098-1. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999a;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Berger O, Gan X, Gujuluva C. CXC and CC chemokine receptors on coronary and brain endothelia. Mol Med. 1999b;5:795–805. [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven LA, Middel J, Portegies P, Verhoef J, Jansen GH, Nottet HSLM. Overexpression of nerve growth factor and basic fibroblast growth factor in AIDS dementia complex. J Neuroimmunol. 1999;97:154–162. doi: 10.1016/s0165-5728(99)00044-2. [DOI] [PubMed] [Google Scholar]
- Budka H, Costanzi G, Cristina S, Lechi A, Parravicini C, Trabattoni R, Vago L. Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical, and electron microscopical study of 100 autopsy cases. Acta Neuropathol (Berl) 1987;75:185–198. doi: 10.1007/BF00687080. [DOI] [PubMed] [Google Scholar]
- Budka H, Wiley C, Kleihues P, Artigas J, Ashbury A, Cho E-S, Cornblath D. HIV-associated disease of the nervous system: Review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Chang JR, Mukerjee R, Bagashev A, Del Valle L, Chabrashvili T, Hawkins BJ, He JJ, Sawaya BE. HIV-1 Tat protein promotes neuronal dysfunction through disruption of microRNAs. J Biol Chem. 2011;286:41125–41134. doi: 10.1074/jbc.M111.268466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, Major E, Nath A. Intracellular human immunodeficency virus tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003a doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, Major EO, Nath A. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003b;278:13512–13519. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, Heaton RK. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59:1563–1567. doi: 10.1212/01.wnl.0000034175.11956.79. [DOI] [PubMed] [Google Scholar]
- Cherner M, Cysique L, Heaton RK, Marcotte TD, Ellis RJ, Masliah E, Grant I. Neuropathologic confirmation of definitional criteria for human immunodeficiency virus-associated neurocognitive disorders. J Neurovirol. 2007;13:23–28. doi: 10.1080/13550280601089175. [DOI] [PubMed] [Google Scholar]
- Cherner M, Ellis RJ, Lazzaretto D, Young C, Mindt MR, Atkinson JH, Grant I, Heaton RK. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS. 2004;18(Suppl 1):S27–34. [PubMed] [Google Scholar]
- Corasaniti MT, Bagetta G, Rotiroti D, Nistico G. The HIV envelope protein gp120 in the nervous system: interactions with nitric oxide, interleukin-1beta and nerve growth factor signalling, with pathological implications in vivo and in vitro. Biochem Pharmacol. 1998;56:153–156. doi: 10.1016/s0006-2952(98)00044-6. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Navarra M, Catani MV, Melino G, Nistico G, Finazzi-Agro A. NMDA and HIV-1 coat protein, GP120, produce necrotic but not apoptotic cell death in human CHP100 neuroblastoma cultures via a mechanism involving calpain. Biochem Biophys Res Commun. 1996;229:299–304. doi: 10.1006/bbrc.1996.1796. [DOI] [PubMed] [Google Scholar]
- Crews L, Patrick C, Achim CL, Everall IP, Masliah E. Molecular Pathology of Neuro-AIDS (CNS-HIV) Int J Mol Sci. 2009;10:1045–1063. doi: 10.3390/ijms10031045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Ruf R, Patrick C, Dumaop W, Trejo-Morales M, Achim CL, Rockenstein E, Masliah E. Phosphorylation of collapsin response mediator protein-2 disrupts neuronal maturation in a model of adult neurogenesis: Implications for neurodegenerative disorders. Mol Neurodegener. 2011;6:67. doi: 10.1186/1750-1326-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, Hansen L, Adame A, Galasko D, Masliah E. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello AC, Bruno MA. The failure in NGF maturation and its increased degradation as the probable cause for the vulnerability of cholinergic neurons in Alzheimer’s disease. Neurochem Res. 2007;32:1041–1045. doi: 10.1007/s11064-006-9270-0. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Canneva F. Impact of intracellular beta-amyloid in transgenic animals and cell models. Neurodegener Dis. 2008;5:146–148. doi: 10.1159/000113686. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Bruno MA, Bell KF. NGF-cholinergic dependency in brain aging, MCI and Alzheimer’s disease. Curr Alzheimer Res. 2007;4:351–358. doi: 10.2174/156720507781788774. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Bruno MA, Allard S, Leon W, Iulita MF. Cholinergic involvement in Alzheimer’s disease. A link with NGF maturation and degradation. J Mol Neurosci. 2010;40:230–235. doi: 10.1007/s12031-009-9238-z. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- Darbinian N, Darbinyan A, Czernik M, Peruzzi F, Khalili K, Reiss K, Gordon J, Amini S. HIV-1 Tat inhibits NGF-induced Egr-1 transcriptional activity and consequent p35 expression in neural cells. J Cell Physiol. 2008;216:128–134. doi: 10.1002/jcp.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, Ellis R, Cherner M, Grant I, Masliah E. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80:1415–1423. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenstein FP. Fibroblast growth factors in the nervous system. J Neurobiol. 1994;25:1467–1480. doi: 10.1002/neu.480251112. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Everall I, Luthert P, Lantos P. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Everall I, Gray F, Barnes H, Durigon M, Luthert P, Lantos P. Neuronal loss in symptom-free HIV infection. Lancet. 1992;340:1413. doi: 10.1016/0140-6736(92)92603-d. [DOI] [PubMed] [Google Scholar]
- Everall IP, Trillo-Pazos G, Bell C, Mallory M, Sanders V, Masliah E. Amelioration of neurotoxic effects of HIV envelope protein gp120 by fibroblast growth factor: a strategy for neuroprotection. J Neuropathol Exp Neurol. 2001;60:293–301. doi: 10.1093/jnen/60.3.293. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E Group THNRC. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Rockenstein E, Mante M, Spencer B, Grant I, Ellis R, Letendre S, Patrick C, Adame A, Masliah E. Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. J Neurovirol. 2013;19:89–101. doi: 10.1007/s13365-012-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L, Mallory M, Achim C, Masliah E. Neurodegeneration od somatostatin-immunoreactive neurons in HIV encephalitis. J Neuropathol Exp Neurol. 1997;56:360–368. doi: 10.1097/00005072-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol Dis. 2007;26:47–55. doi: 10.1016/j.nbd.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Fugere M, Day R. Cutting back on pro-protein convertases: the latest approaches to pharmacological inhibition. Trends Pharmacol Sci. 2005;26:294–301. doi: 10.1016/j.tips.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F, Tuszynski M, Yoshida K, Higgins G. Nerve growth factor expression and function in the CNS. In: Hefti F, Brachet P, Will B, Christen Y, editors. Growth factors and Alzheimer’s disease. Berlin: Springer-Verlag; 1991. pp. 106–116. [Google Scholar]
- Gage F, Batchelor P, Chen K, Chin D, Higins G, Koh S, Deputy S, Rosenberg M, Fischer W, Bjorklund A. NGF receptor reexpression and NGF-mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron. 1989;2:1177–1184. doi: 10.1016/0896-6273(89)90184-0. [DOI] [PubMed] [Google Scholar]
- Geeraerts T, Deiva K, M’Sika I, Salim H, Hery C, Tardieu M. Effects of SDF-1alpha and gp120IIIB on apoptotic pathways in SK-N-SH neuroblastoma cells. Neurosci Lett. 2006;399:115–120. doi: 10.1016/j.neulet.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, Masliah E, Commins DL, Brandt D, Grant I, Singer EJ, Levine AJ, Miller J, Winkler JM, Fox HS, Luxon BA, Morgello S. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One. 2012;7:e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H, Lipton S, Tardieu M, Bukrinsky M, Nottet H. The neuropathogenesis of HIV-1 infection. J Leukocyte Biol. 1994;56:389–398. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of the AIDS dementia complex. Aids. 1997;11 (Suppl A):S35–S45. [PubMed] [Google Scholar]
- Gonzalez RG, Greco JB, He J, Lentz MR, O’Neil S, Pilkenton SJ, Ratai EM, Westmoreland S. New insights into the neuroimmunity of SIV infection by magnetic resonance spectroscopy. J Neuroimmune Pharmacol. 2006;1:152–159. doi: 10.1007/s11481-006-9016-4. [DOI] [PubMed] [Google Scholar]
- Guha D, Nagilla P, Redinger C, Srinivasan A, Schatten GP, Ayyavoo V. Neuronal apoptosis by HIV-1 Vpr: contribution of proinflammatory molecular networks from infected target cells. J Neuroinflammation. 2012;9:138. doi: 10.1186/1742-2094-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry. 2010;9:155–161. doi: 10.1002/j.2051-5545.2010.tb00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Chen X, Bhatt D, Geiger NH, Rosenberger TA, Haughey NJ, Masino SA, Geiger JD. Ketone bodies protection against HIV-1 Tat-induced neurotoxicity. J Neurochem. 2012;122:382–391. doi: 10.1111/j.1471-4159.2012.07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16:101–114. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kehn-Hall K, Guendel I, Carpio L, Skaltsounis L, Meijer L, Al-Harthi L, Steiner JP, Nath A, Kutsch O, Kashanchi F. Inhibition of Tat-mediated HIV-1 replication and neurotoxicity by novel GSK3-beta inhibitors. Virology. 2011;415:56–68. doi: 10.1016/j.virol.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin J, Morris C, Oakley A, Perry R, Perry E. Distribution of nerve growth factor receptor immunoreactivity in the human hippocampus. Neurosci Lett. 1991;121:178–182. doi: 10.1016/0304-3940(91)90679-n. [DOI] [PubMed] [Google Scholar]
- Kerwin JM, Morris CM, Perry RH, Perry EK. Hippocampal nerve growth factor receptor immunoreactivity in patients with Alzheimer’s and Parkinson’s disease. Neurosci Lett. 1992;143:101–104. doi: 10.1016/0304-3940(92)90242-y. [DOI] [PubMed] [Google Scholar]
- Khanlou N, Lazzaretto D, Sadek J, Masliah E, Gelman BB, Morgello S, Singer EJ, Grant I, Everall I. National neuro-AIDS tissue consortium autopsy series: a neuropathological study of 371 HIV infected brains 2005 [Google Scholar]
- Khanlou N, Moore DJ, Chana G, Cherner M, Lazzaretto D, Dawes S, Grant I, Masliah E, Everall IP. Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J Neurovirol. 2009;15:131–138. doi: 10.1080/13550280802578075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichev A, Ilieva EV, Pinol-Ripoll G, Podlesniy P, Ferrer I, Portero-Otin M, Pamplona R, Espinet C. Cell death and learning impairment in mice caused by in vitro modified pro-NGF can be related to its increased oxidative modifications in Alzheimer disease. Am J Pathol. 2009;175:2574–2585. doi: 10.2353/ajpath.2009.090018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Dietz GP, Bahr M. The TAT protein transduction domain enhances the neuroprotective effect of glial-cell-line-derived neurotrophic factor after optic nerve transection. Neurodegener Dis. 2004;1:44–49. doi: 10.1159/000076669. [DOI] [PubMed] [Google Scholar]
- Klint P, Claesson-Welsh L. Signal transduction by fibroblast growth factor receptors. Front Biosci. 1999;4:D165–177. doi: 10.2741/klint. [DOI] [PubMed] [Google Scholar]
- Klint P, Kanda S, Kloog Y, Claesson-Welsh L. Contribution of Src and Ras pathways in FGF-2 induced endothelial cell differentiation. Oncogene. 1999;18:3354–3364. doi: 10.1038/sj.onc.1202680. [DOI] [PubMed] [Google Scholar]
- Kogan M, Rappaport J. HIV-1 accessory protein Vpr: relevance in the pathogenesis of HIV and potential for therapeutic intervention. Retrovirology. 2011;8:25. doi: 10.1186/1742-4690-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Langford D. Neuronal toxicity in HIV CNS disease. Future virology. 2012;7:687–698. doi: 10.2217/fvl.12.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani AS, McFadden G. Evasion and exploitation of chemokines by viruses. Cytokine Growth Factor Rev. 1999;10:219–233. doi: 10.1016/s1359-6101(99)00018-0. [DOI] [PubMed] [Google Scholar]
- Langford D, Sanders VJ, Mallory M, Kaul M, Masliah E. Expression of stromal cell-derived factor 1alpha protein in HIV encephalitis. J Neuroimmunol. 2002;127:115–126. doi: 10.1016/s0165-5728(02)00068-1. [DOI] [PubMed] [Google Scholar]
- Langford D, Hurford R, Hashimoto M, Digicaylioglu M, Masliah E. Signalling crosstalk in FGF2-mediated protection of endothelial cells from HIV-gp120. BMC Neurosci. 2005;6:8. doi: 10.1186/1471-2202-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003;34:467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- Langford D, Marquie-Beck J, de Almeida S, Lazzaretto D, Letendre S, Grant I, McCutchan JA, Masliah E, Ellis RJ. Relationship of antiretroviral treatment to postmortem brain tissue viral load in human immunodeficiency virus-infected patients. J Neurovirol. 2006;12:100–107. doi: 10.1080/13550280600713932. [DOI] [PubMed] [Google Scholar]
- Langford T, Letendre SL, Larrea GJ, Masliah E. Changing Patterns in the Neuropathogenesis of HIV During the HAART Era. Brain Pathol. 2002;11:306–312. doi: 10.1111/j.1750-3639.2003.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford TD, Everall I, Masliah E. Neuronal injury, white matter disease, and neurotrophic factors in HIVE. 2011 In press. [Google Scholar]
- Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, Nath A. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neurovirol. 2013;19:418–431. doi: 10.1007/s13365-013-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Paulino AD, Rockenstein E, Adame A, Crews L, Cherner M, Heaton R, Ellis R, Everall IP, Grant I, Masliah E. Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007;196:361–370. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res. 2009;16:205–220. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- Liberini P, Pioro EP, Maysinger D, Ervin FR, Cuello AC. Long-term protective effects of human recombinant nerve growth factor and monosialoganglioside GM1 treatment on primate nucleus basalis cholinergic neurons after neocortical infarction. Neuroscience. 1993;53:625–637. doi: 10.1016/0306-4522(93)90611-i. [DOI] [PubMed] [Google Scholar]
- Lim KC, Tyler CM, Lim ST, Giuliano R, Federoff HJ. Proteolytic processing of proNGF is necessary for mature NGF regulated secretion from neurons. Biochem Biophys Res Commun. 2007;361:599–604. doi: 10.1016/j.bbrc.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lipton S. HIV-related neuronal injury. Potential therapeutic intervention with calcium channel antagonists and NMDA receptors. Molecular Neurobiology. 1994;8:181–196. doi: 10.1007/BF02780669. [DOI] [PubMed] [Google Scholar]
- Lipton S, Rosenberg P. Mechanisms of disease: Excitatory amino acids as a final common pathway for neurologic disorders. New England JMed. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Brenneman DE, Silverstein FS, Masliah E, Mucke L. gp120 and neurotoxicity in vivo. Trends Pharmacol Sci. 1995;16:122. doi: 10.1016/s0165-6147(00)88998-1. [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- Magnuson D, Knudsen B, Geiger J, Brownstone R, Nath A. Human immunodefciency virus type 1 activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. AnnNeurol. 1995;37:373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- Marban C, Suzanne S, Dequiedt F, de Walque S, Redel L, Van Lint C, Aunis D, Rohr O. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. EMBO J. 2007;26:412–423. doi: 10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus CD, Taylor-Robinson SD, Sargentoni J, Ainsworth JG, Frize G, Easterbrook PJ, Shaunak S, Bryant DJ. 1H MR spectroscopy of the brain in HIV-1-seropositive subjects: evidence for diffuse metabolic abnormalities. Metab Brain Dis. 1998;13:123–136. doi: 10.1023/a:1020609213664. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. CritRevNeurobiol. 1996a;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim C, Hansen L, Wiley C. Selective neuronal vulnerability in HIV encephalitis. J NeuropatholExp Neurol. 1992a;51:585–593. doi: 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- Masliah E, Achim C, DeTeresa R, Ge N, Wiley C. Cellular neuropathology in HIV encephalitis. In: Price R, editor. HIV, vAIDS and the brain. New York: Raven Press; 1994. pp. 119–131. [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim C, DeTeresa R, Wiley C. Patterns of neurodegeneration in HIV encephalitis. NeuroAIDS. 1996b;1:161–173. doi: 10.1300/j128v01n01_08. [DOI] [PubMed] [Google Scholar]
- Masliah E, Achim C, Ge N, DeTeresa R, Terry R, Wiley C. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992b;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Maudet C, Sourisce A, Dragin L, Lahouassa H, Rain JC, Bouaziz S, Ramirez BC, Margottin-Goguet F. HIV-1 Vpr Induces the Degradation of ZIP and sZIP, Adaptors of the NuRD Chromatin Remodeling Complex, by Hijacking DCAF1/VprBP. PLoS One. 2013;8:e77320. doi: 10.1371/journal.pone.0077320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- McCrossan M, Marsden M, Carnie FW, Minnis S, Hansoti B, Anthony IC, Brettle RP, Bell JE, Simmonds P. An immune control model for viral replication in the CNS during presymptomatic HIV infection. Brain. 2006;129:503–516. doi: 10.1093/brain/awh695. [DOI] [PubMed] [Google Scholar]
- Medina I, Ghose S, Ben-Ari Y. Mobilization of intracellular calcium stores participates in the rise of [Ca2+]i and the toxic actions of the HIV coat protein GP120. Eur J Neurosci. 1999;11:1167–1178. doi: 10.1046/j.1460-9568.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A. Brain-derived neurotrophic factor activation of TrkB protects neurons from HIV-1/gp120-induced cell death. Critical reviews in neurobiology. 2004;16:51–57. doi: 10.1615/critrevneurobiol.v16.i12.50. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A, Masliah E. Chemokine receptors and neurotrophic factors: potential therapy against aids dementia? J Neurosci Res. 2008;86:243–255. doi: 10.1002/jnr.21492. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A, Campbell LA, Avdoshina V. Implementing Neuronal Plasticity in NeuroAIDS: the Experience of Brain-derived Neurotrophic Factor and other Neurotrophic Factors. J Neuroimmune Pharmacol. 2013 doi: 10.1007/s11481-013-9488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Nosheny RL, Tanda G, Ren K, Meyer EM. Brain-derived neurotrophic factor prevents human immunodeficiency virus type 1 protein gp120 neurotoxicity in the rat nigrostriatal system. Ann N Y Acad Sci. 2007;1122:144–154. doi: 10.1196/annals.1403.010. [DOI] [PubMed] [Google Scholar]
- Mucke L, Abraham C, Ruppe M, Rockenstein E, Toggas S, Alford M, Masliah E. Protection against HIV-1 gp120-induced brain damage by neuronal overexpression of human amyloid precursor protein (hAPP) J Exp Med. 1995;181:1551–1556. doi: 10.1084/jem.181.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson E, Kordower J. Cortical neurons express nerve growth factor receptors in advanced age and Alzheimer disease. PNAS USA. 1992;89:569–573. doi: 10.1073/pnas.89.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson E, Bothwell M, Hersh L, Kordower J. Nerve growth factor receptor immunoreactive profiles in the normal, aged human basal forebrain: colocalization with cholinergic neurons. J Comp Neurol. 1989;285:196–217. doi: 10.1002/cne.902850204. [DOI] [PubMed] [Google Scholar]
- Murphy PM. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat Immunol. 2001;2:116–122. doi: 10.1038/84214. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186–195. doi: 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47:186–194. [PubMed] [Google Scholar]
- Nguyen TB, Lucero GR, Chana G, Hult BJ, Tatro ET, Masliah E, Grant I, Achim CL, Everall IP. Glycogen synthase kinase-3beta (GSK-3beta) inhibitors AR-A014418 and B6B3O prevent human immunodeficiency virus-mediated neurotoxicity in primary human neurons. J Neurovirol. 2009a:1–5. doi: 10.1080/13550280903168131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TB, Lucero GR, Chana G, Hult BJ, Tatro ET, Masliah E, Grant I, Achim CL, Everall IP. Glycogen synthase kinase-3beta (GSK-3beta) inhibitors AR-A014418 and B6B3O prevent human immunodeficiency virus-mediated neurotoxicity in primary human neurons. J Neurovirol. 2009b;15:434–438. doi: 10.1080/13550280903168131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Reynolds HM, Kiebala M, De Mesy Bentley KL, Trejo M, Volsky DJ, Maggirwar SB, Dewhurst S, Masliah E, Gelbard HA. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS One. 2008;3:e3731. doi: 10.1371/journal.pone.0003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Aden SA, De Bernardi MA, Mocchetti I. Intrastriatal administration of human immunodeficiency virus-1 glycoprotein 120 reduces glial cell-line derived neurotrophic factor levels and causes apoptosis in the substantia nigra. J Neurobiol. 2006;66:1311–1321. doi: 10.1002/neu.20288. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Ahmed F, Yakovlev A, Meyer EM, Ren K, Tessarollo L, Mocchetti I. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–2284. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- Nyagol J, De Falco G, Lazzi S, Luzzi A, Cerino G, Shaheen S, Palummo N, Bellan C, Spina D, Leoncini L. HIV-1 Tat mimetic of VEGF correlates with increased microvessels density in AIDS-related diffuse large B-cell and Burkitt lymphomas. Journal of hematopathology. 2008;1:3–10. doi: 10.1007/s12308-008-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Kang Y-J, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 Decreases Adult Neural Progenitor Cell Proliferation via Checkpoint Kinase-Mediated Cell-Cycle Withdrawal and G1 Arrest. Cell Stem Cell. 2007;1:230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Patrick C, Crews L, Desplats P, Dumaop W, Rockenstein E, Achim CL, Everall IP, Masliah E. Increased CDK5 Expression in HIV Encephalitis Contributes to Neurodegeneration via Tau Phosphorylation and Is Reversed with Roscovitine. Am J Pathol. 2011;178:1646–1661. doi: 10.1016/j.ajpath.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza CE, Podlesniy P, Vidal N, Arevalo JC, Lee R, Hempstead B, Ferrer I, Iglesias M, Espinet C. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. Am J Pathol. 2005;166:533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Dhillon NK, Yao H, Zhu X, Williams R, Buch S. Mechanisms of platelet-derived growth factor-mediated neuroprotection--implications in HIV dementia. Eur J Neurosci. 2008a;28:1255–1264. doi: 10.1111/j.1460-9568.2008.06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Dhillon N, Callen S, Yao H, Bokhari S, Zhu X, Baydoun HH, Buch S. Platelet-derived growth factor protects neurons against gp120-mediated toxicity. J Neurovirol. 2008b;14:62–72. doi: 10.1080/13550280701809084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63:641–649. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- Perry EK. Nerve growth factor and the basal forebrain cholinergic system: a link in the etiopathology of neurodegenerative dementias? Alzheimer Dis Assoc Disord. 1990;4:1–13. doi: 10.1097/00002093-199040100-00001. [DOI] [PubMed] [Google Scholar]
- Peruzzi F, Gordon J, Darbinian N, Amini S. Tat-induced deregulation of neuronal differentiation and survival by nerve growth factor pathway. J Neurovirol. 2002;8(Suppl 2):91–96. doi: 10.1080/13550280290167885. [DOI] [PubMed] [Google Scholar]
- Podlesniy P, Kichev A, Pedraza C, Saurat J, Encinas M, Perez B, Ferrer I, Espinet C. Pro-NGF from Alzheimer’s disease and normal human brain displays distinctive abilities to induce processing and nuclear translocation of intracellular domain of p75NTR and apoptosis. Am J Pathol. 2006;169:119–131. doi: 10.2353/ajpath.2006.050787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon WW, Carlos AJ, Aguilar BL, Berchtold NC, Kawano CK, Zograbyan V, Yaopruke T, Shelanski M, Cotman CW. beta-Amyloid (Abeta) oligomers impair brain-derived neurotrophic factor retrograde trafficking by down-regulating ubiquitin C-terminal hydrolase, UCH-L1. J Biol Chem. 2013;288:16937–16948. doi: 10.1074/jbc.M113.463711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portegies P, Enting RH, de Gans J, Algra PR, Derix MM, Lange JM, Goudsmit J. Presentation and course of AIDS dmentia complex: 10 years of follow-up in Amsterdam, The Netherlands. AIDS. 1993;7:669–675. [PubMed] [Google Scholar]
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Current opinion in psychiatry. 2011;24:519–525. doi: 10.1097/YCO.0b013e32834b9db6. [DOI] [PubMed] [Google Scholar]
- Ramirez SH, Sanchez JF, Dimitri CA, Gelbard HA, Dewhurst S, Maggirwar SB. Neurotrophins prevent HIV Tat-induced neuronal apoptosis via a nuclear factor-kappaB (NF-kappaB)-dependent mechanism. J Neurochem. 2001;78:874–889. doi: 10.1046/j.1471-4159.2001.00467.x. [DOI] [PubMed] [Google Scholar]
- Redel L, Le Douce V, Cherrier T, Marban C, Janossy A, Aunis D, Van Lint C, Rohr O, Schwartz C. HIV-1 regulation of latency in the monocyte-macrophage lineage and in CD4+ T lymphocytes. J Leukoc Biol. 2010;87:575–588. doi: 10.1189/jlb.0409264. [DOI] [PubMed] [Google Scholar]
- Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mante M, Alford M, Adame A, Crews L, Hashimoto M, Esposito L, Mucke L, Masliah E. High beta-secretase activity elicits neurodegeneration in transgenic mice despite reductions in amyloid-beta levels: implications for the treatment of Alzheimer disease. J Biol Chem. 2005a;280:32957–32967. doi: 10.1074/jbc.M507016200. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Schwach G, Ingolic E, Adame A, Crews L, Mante M, Pfragner R, Schreiner E, Windisch M, Masliah E. Lysosomal pathology associated with alpha-synuclein accumulation in transgenic models using an eGFP fusion protein. J Neurosci Res. 2005b;80:247–259. doi: 10.1002/jnr.20446. [DOI] [PubMed] [Google Scholar]
- Samah B, Porcheray F, Dereuddre-Bosquet N, Gras G. Nerve growth factor stimulation promotes CXCL-12 attraction of monocytes but decreases human immunodeficiency virus replication in attracted population. J Neurovirol. 2009;15:71–80. doi: 10.1080/13550280802482575. [DOI] [PubMed] [Google Scholar]
- Sanders V, Everall IP, Johnson RW, Masliah E Group tH. Fibroblast growth factor modulates HIV co-receptor expression by neural cells. J Neurosci Res. 2000;59:671–679. doi: 10.1002/(SICI)1097-4547(20000301)59:5<671::AID-JNR10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Scheidegger P, Weiglhofer W, Suarez S, Console S, Waltenberger J, Pepper MS, Jaussi R, Ballmer-Hofer K. Signalling properties of an HIV-encoded angiogenic peptide mimicking vascular endothelial growth factor activity. Biochem J. 2001;353:569–578. doi: 10.1042/0264-6021:3530569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindowski K, Belarbi K, Buee L. Neurotrophic factors in Alzheimer’s disease: role of axonal transport. Genes Brain Behav. 2008;7(Suppl 1):43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H, Tannenberg A, Dodd P. Variant forms of neuronal glutamate transporter sites in Alzheimer’s disease cerebral cortex. J Neurochem. 1995;64:2193–2202. doi: 10.1046/j.1471-4159.1995.64052193.x. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Carey CL, Weber E, Bondi MW, Grant I. Neurocognitive Consequences of HIV Infection in Older Adults: An Evaluation of the “Cortical” Hypothesis. AIDS Behav. 2011;15:1187–1196. doi: 10.1007/s10461-010-9815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Chretien M. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999;848:45–62. doi: 10.1016/s0006-8993(99)01909-5. [DOI] [PubMed] [Google Scholar]
- Souza TM, Rodrigues DQ, Passaes CP, Barreto-de-Souza V, Aguiar RS, Temerozo JR, Morgado MG, Fontes CF, Araujo EG, Bou-Habib DC. The nerve growth factor reduces APOBEC3G synthesis and enhances HIV-1 transcription and replication in human primary macrophages. Blood. 2011;117:2944–2952. doi: 10.1182/blood-2010-05-287193. [DOI] [PubMed] [Google Scholar]
- Studer L, Spenger C, Luthman J, Seiler R. NGF increases neuritic complexity of cholinergic interneurons in organotypic cultures of neonatal rat striatum. J Comp Neurol. 1994;340:281–296. doi: 10.1002/cne.903400212. [DOI] [PubMed] [Google Scholar]
- Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tuszynski M, Sang U, Yoshida K, Gage F. Recombinant human growth factor infusions prevent cholinergic neural degeneration in the adult primate brain. AnnNeurol. 1991;30:625–636. doi: 10.1002/ana.410300502. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH. Gene therapy for neurological disease. Expert Opin Biol Ther. 2003;3:815–828. doi: 10.1517/14712598.3.5.815. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Smith DE, Roberts J, McKay H, Mufson E. Targeted intraparenchymal delivery of human NGF by gene transfer to the primate basal forebrain for 3 months does not accelerate beta-amyloid plaque deposition. Exp Neurol. 1998;154:573–582. doi: 10.1006/exnr.1998.6956. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Gabriel K, Gage FH, Suhr S, Meyer S, Rosetti A. Nerve growth factor delivery by gene transfer induces differential outgrowth of sensory, motor, and noradrenergic neurites after adult spinal cord injury. Exp Neurol. 1996;137:157–173. doi: 10.1006/exnr.1996.0016. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DP, UHS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Ushijima H, Ando S, Kunisada T, Schroder H, Klocking H-P, Kijoa A, Muller W. HIV-1 gp120 and NMDA induce protein kinase C translocation differentially in rat primary neuronal cultures. J AIDS. 1993;6:339–343. [PubMed] [Google Scholar]
- Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82:A99–109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walicke P. Novel neurotrophic factors, receptors, and oncogenes. Ann Rev Neurosci. 1989;12:103–126. doi: 10.1146/annurev.ne.12.030189.000535. [DOI] [PubMed] [Google Scholar]
- Walicke PA, Baird A. Neurotrophic effects of basic and acidic fibroblast growth factors are not mediated through glial cells. Brain Res. 1988;468:71–79. doi: 10.1016/0165-3806(88)90009-0. [DOI] [PubMed] [Google Scholar]
- Webber CA, Salame J, Luu GL, Acharjee S, Ruangkittisakul A, Martinez JA, Jalali H, Watts R, Ballanyi K, Guo GF, Zochodne DW, Power C. Nerve growth factor acts through the TrkA receptor to protect sensory neurons from the damaging effects of the HIV-1 viral protein, Vpr. Neuroscience. 2013;252C:512–525. doi: 10.1016/j.neuroscience.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Haug H, Budka H. Neuronal damage in the cerebral cortex of AIDS brains: a morphometric study. Acta Neuropathol. 1993;85:185–189. doi: 10.1007/BF00227766. [DOI] [PubMed] [Google Scholar]
- Wiley C, Achim C. HIV encephalitis is the pathologic correlate of dementia in AIDS. Ann Neurol. 1994;36:673–676. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- Wiley C, Ge N, Morey M, DeTeresa R, Terry R, Masliah E. Golgi impregnation studies of dendritic pathology in HIV encepahlitis. J Neuropathol Exp Neurol. 1991a;50:324. [Google Scholar]
- Wiley C, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R. Neocortical damage during HIV infection. AnnNeurol. 1991b;29:651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Achim CL, Christopherson C, Kidane Y, Kwok S, Masliah E, Mellors J, Radhakrishnan L, Wang G, Soontornniyomkij V. HIV mediates a productive infection of the brain. AIDS. 1999;13:2055–2059. doi: 10.1097/00002030-199910220-00007. [DOI] [PubMed] [Google Scholar]
- Wilkinson ID, Lunn S, Miszkiel KA, Miller RF, Paley MN, Williams I, Chinn RJ, Hall-Craggs MA, Newman SP, Kendall BE, Harrison MJ. Proton MRS and quantitative MRI assessment of the short term neurological response to antiretroviral therapy in AIDS. J Neurol Neurosurg Psychiatry. 1997;63:477–482. doi: 10.1136/jnnp.63.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Woodbury ME, Ikezu T. Fibroblast Growth Factor-2 Signaling in Neurogenesis and Neurodegeneration. J Neuroimmune Pharmacol. 2013 doi: 10.1007/s11481-013-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Bae M, Tovar-y-Romo LB, Patel N, Bandaru VV, Pomerantz D, Steiner JP, Haughey NJ. The human immunodeficiency virus coat protein gp120 promotes forward trafficking and surface clustering of NMDA receptors in membrane microdomains. J Neurosci. 2011;31:17074–17090. doi: 10.1523/JNEUROSCI.4072-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Akhter S, Chaudhuri A, Kanmogne GD. HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood-brain barrier: modulatory effects of STAT1 signaling. Microvasc Res. 2009;77:212–219. doi: 10.1016/j.mvr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Peng F, Dhillon N, Callen S, Bokhari S, Stehno-Bittel L, Ahmad SO, Wang JQ, Buch S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29:1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Tai W, Zhang D. The early events of Alzheimer’s disease pathology: from mitochondrial dysfunction to BDNF axonal transport deficits. Neurobiol Aging. 2012;33:1122 e1121–1110. doi: 10.1016/j.neurobiolaging.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Yulug B, Kilic U, Kilic E, Bahr M. Rifampicin attenuates brain damage in focal ischemia. Brain Res. 2004;996:76–80. doi: 10.1016/j.brainres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Zauli G, Marchisio M, Bertagnolo V, Celeghini C, Capitani S. Hiv-1 tat protein suppresses the nerve growth-factor (ngf)-mediated differentiation of PC12 rat pheochromocytoma cell-line. Oncology reports. 1994;1:773–777. doi: 10.3892/or.1.4.773. [DOI] [PubMed] [Google Scholar]
- Zhang X, Daucher M, Baeza J, Kim CW, Russell R, Kottilil S. Human immunodeficiency virus enhances hepatitis C virus replication by differential regulation of IFN and TGF family genes. J Med Virol. 2012;84:1344–1352. doi: 10.1002/jmv.23315. [DOI] [PubMed] [Google Scholar]
- Zhou D, Masliah E, Spector SA. Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. J Infect Dis. 2011;203:1647–1657. doi: 10.1093/infdis/jir163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Yao H, Peng F, Callen S, Buch S. PDGF-mediated protection of SH-SY5Y cells against Tat toxin involves regulation of extracellular glutamate and intracellular calcium. Toxicology and applied pharmacology. 2009;240:286–291. doi: 10.1016/j.taap.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]