Abstract
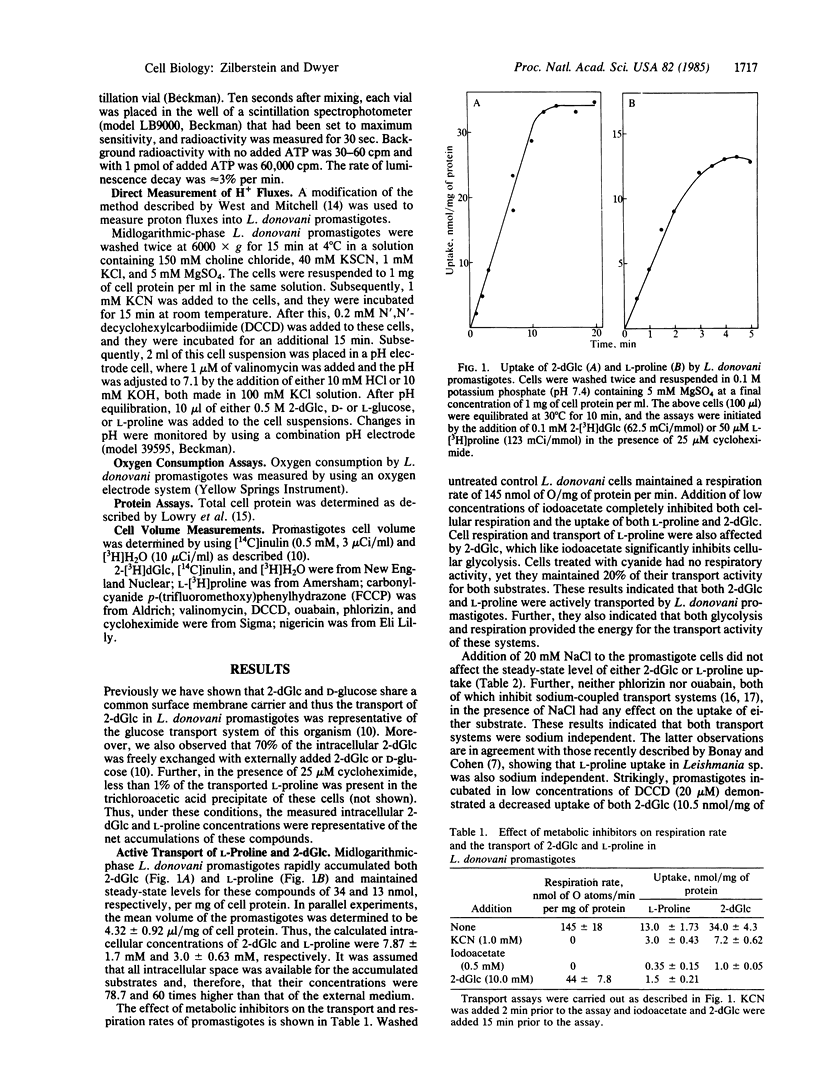
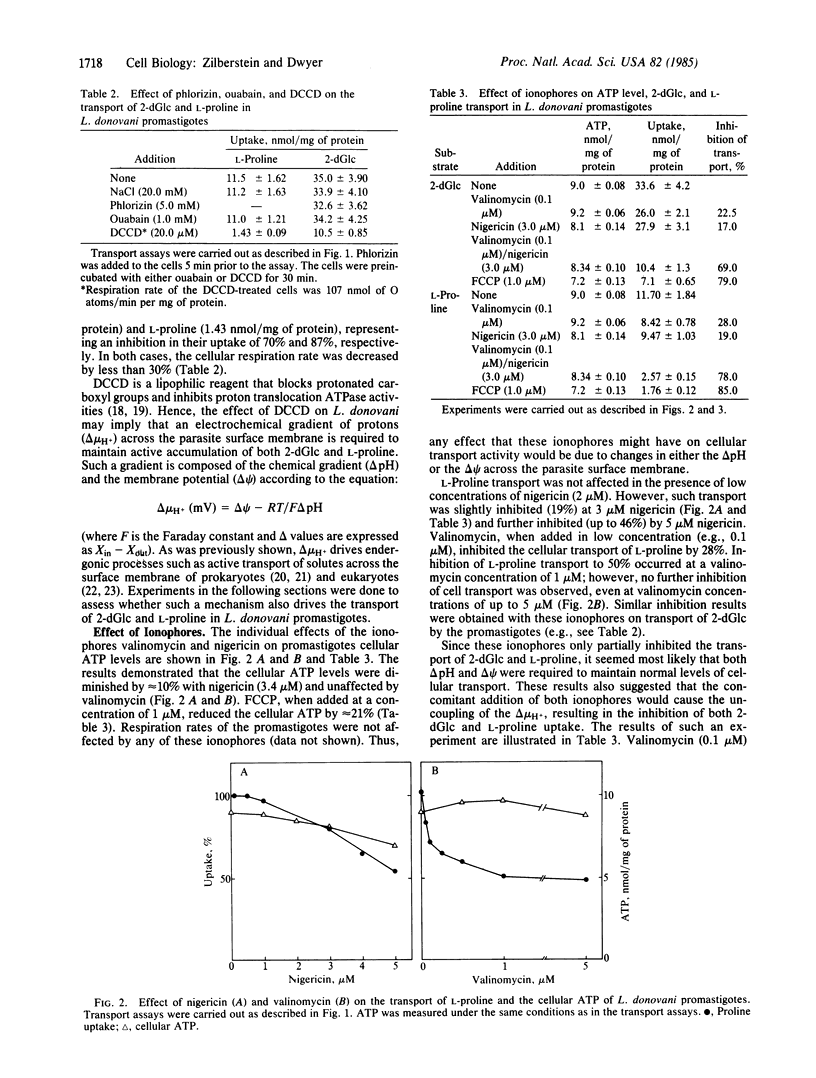
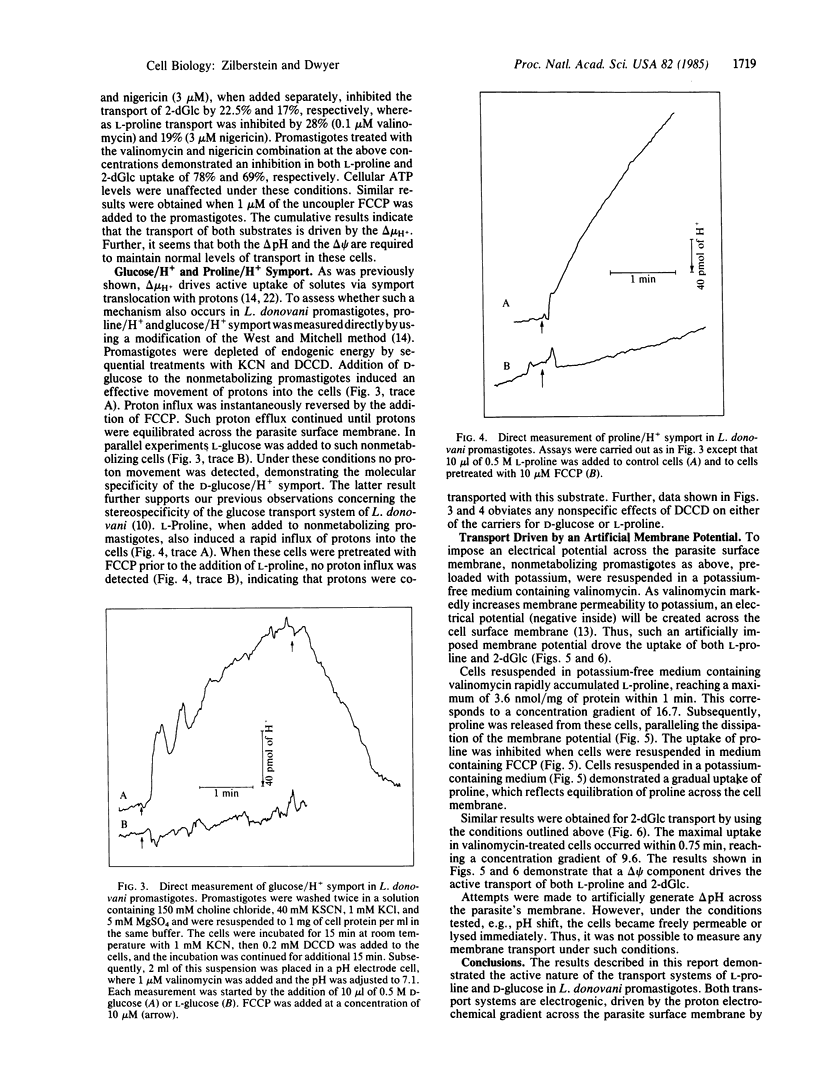
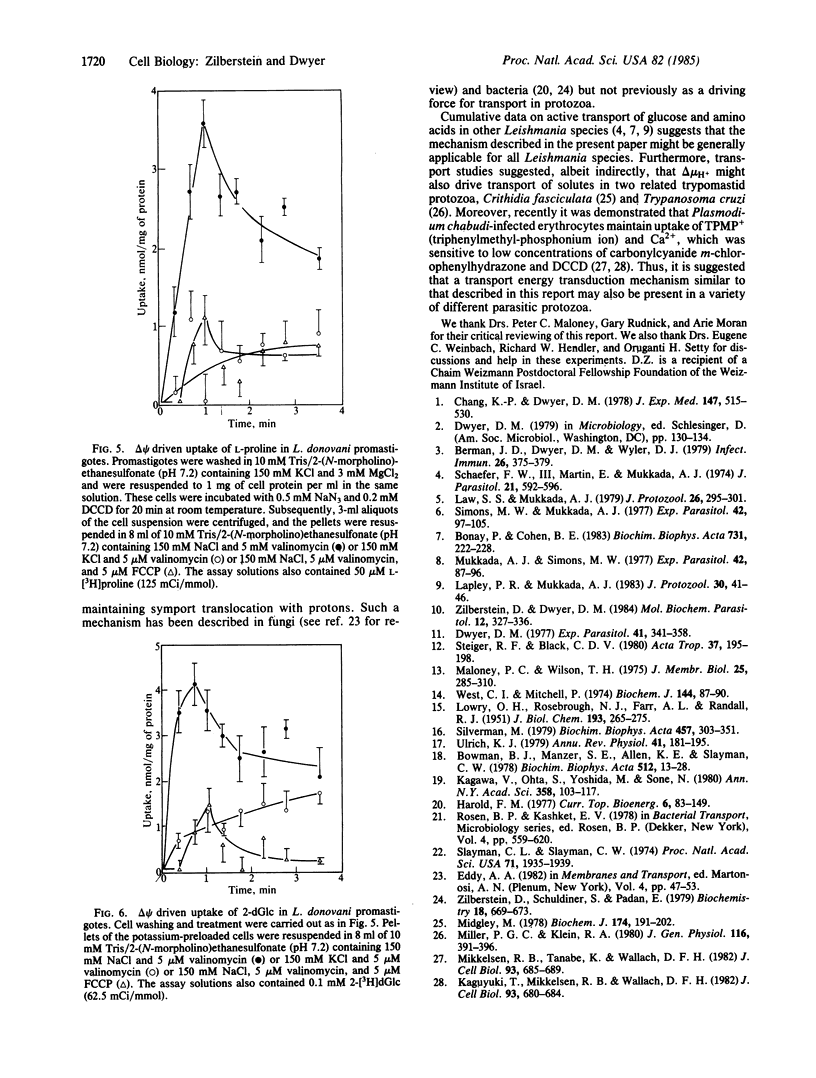
Midlogarithmic phase Leishmania donovani promastigotes accumulate 2-deoxy-D-glucose (2-dGlc) and L-proline, maintaining concentration gradient factors across the surface membrane of 78.7 and 60, respectively. Cyanide (1 mM) and iodoacetate (0.5 mM) inhibited the transport of both substrates. L-proline uptake was also inhibited by 2-dGlc (10 mM). Transport of neither substrate was affected by Na+, phlorizin, or ouabain, indicating the sodium-independent transport of both systems. However, N',N'-dicyclohexylcarbodiimide (DCCD; 20 microM) significantly inhibited the transport of both 2-dGlc and L-proline (70% and 90%, respectively). The ionophores valinomycin (1 microM) and nigericin (5 microM) each partially inhibited the uptake of both substrates. In parallel experiments, nigericin and valinomycin were added concomitantly to promastigotes, each at a concentration that individually inhibited the transport of 2-dGlc and L-proline by less than 30%. Under such conditions, the transport of 2-dGlc and L-proline was inhibited by 69% and 78%, respectively. However, these ionophores had no significant effect on the promastigotes cellular ATP level. Carbonylcyanide p-(trifluoromethoxy)phenylhydrazone (FCCP; 1 microM) inhibited 2-dGlc (79%) and L-proline (85%) transport, whereas ATP levels of such cells were diminished by only 20%. Symport of D-glucose/H+ and L-proline/H+ was measured directly in cells pretreated with KCN and DCCD. Upon addition of D-glucose to such cells, a rapid movement of protons into the organisms occurred and was reversed upon addition of FCCP. Conversely, no proton movement was observed when L-glucose was added to such cells. L-proline, as D-glucose, caused a rapid influx of protons into the promastigotes, indicating that both substrates were cotransported with protons. We conclude that transport of D-glucose and L-proline in L. donovani promastigotes is protonmotive force-driven and is coupled to both delta pH and delta psi.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman J. D., Dwyer D. M., Wyler D. J. Multiplication of Leishmania in human macrophages in vitro. Infect Immun. 1979 Oct;26(1):375–379. doi: 10.1128/iai.26.1.375-379.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonay P., Cohen B. E. Neutral amino acid transport in Leishmania promastigotes. Biochim Biophys Acta. 1983 Jun 10;731(2):222–228. doi: 10.1016/0005-2736(83)90012-3. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Leishmania donovani. Hamster macrophage interactions in vitro: cell entry, intracellular survival, and multiplication of amastigotes. J Exp Med. 1978 Feb 1;147(2):515–530. doi: 10.1084/jem.147.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. M. Leishmania donovani: surface membrane carbohydrates of promastigotes. Exp Parasitol. 1977 Apr;41(2):341–358. doi: 10.1016/0014-4894(77)90107-2. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Ohta S., Yoshida M., Sone N. Functions of subunits of H+-ATPase. Ann N Y Acad Sci. 1980;358:103–117. doi: 10.1111/j.1749-6632.1980.tb15390.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law S. S., Mukkada A. J. Transport of L-proline and its regulation in Leishmania tropica promastigotes. J Protozool. 1979 May;26(2):295–301. doi: 10.1111/j.1550-7408.1979.tb02784.x. [DOI] [PubMed] [Google Scholar]
- Lepley P. R., Mukkada A. J. Characteristics of an uptake system for alpha-aminoisobutyric acid in Leishmania tropica promastigotes. J Protozool. 1983 Feb;30(1):41–46. doi: 10.1111/j.1550-7408.1983.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Wilson T. H. ATP synthesis driven by a protonmotive force in Streptococcus lactis. J Membr Biol. 1975;25(3-4):285–310. doi: 10.1007/BF01868580. [DOI] [PubMed] [Google Scholar]
- Midgley M. The transport of alpha-aminoisobutyrate into Crithidia fasciculata. Biochem J. 1978 Jul 15;174(1):191–202. doi: 10.1042/bj1740191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen R. B., Tanabe K., Wallach D. F. Membrane potential of Plasmodium-infected erythrocytes. J Cell Biol. 1982 Jun;93(3):685–689. doi: 10.1083/jcb.93.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukkada A. J., Simon M. W. Leishmania tropica: uptake of methionine by promastigotes. Exp Parasitol. 1977 Jun;42(1):87–96. doi: 10.1016/0014-4894(77)90065-0. [DOI] [PubMed] [Google Scholar]
- Schaefer F. W., 3rd, Martin E., Mukkada A. J. The glucose transport system in Leishmania tropica promastigotes. J Protozool. 1974 Oct;21(4):592–596. doi: 10.1111/j.1550-7408.1974.tb03708.x. [DOI] [PubMed] [Google Scholar]
- Silverman M. Glucose transport in the kidney. Biochim Biophys Acta. 1976 Dec 14;457(3-4):303–351. doi: 10.1016/0304-4157(76)90003-4. [DOI] [PubMed] [Google Scholar]
- Simon M. W., Mukkada A. J. Leishmania tropica: regulation and specificity of the methionine transport system in promastigotes. Exp Parasitol. 1977 Jun;42(1):97–105. doi: 10.1016/0014-4894(77)90066-2. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Slayman C. W. Depolarization of the plasma membrane of Neurospora during active transport of glucose: evidence for a proton-dependent cotransport system. Proc Natl Acad Sci U S A. 1974 May;71(5):1935–1939. doi: 10.1073/pnas.71.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger R. F., Black C. D. Simplified defined media for cultivating Leishmania donovani promastigotes. Acta Trop. 1980 Jun;37(2):195–198. [PubMed] [Google Scholar]
- Tanabe K., Mikkelsen R. B., Wallach D. F. Calcium transport of Plasmodium chabaudi-infected erythrocytes. J Cell Biol. 1982 Jun;93(3):680–684. doi: 10.1083/jcb.93.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich K. J. Sugar, amino acid, and Na+ cotransport in the proximal tubule. Annu Rev Physiol. 1979;41:181–195. doi: 10.1146/annurev.ph.41.030179.001145. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Proton/sodium ion antiport in Escherichia coli. Biochem J. 1974 Oct;144(1):87–90. doi: 10.1042/bj1440087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Glucose transport in Leishmania donovani promastigotes. Mol Biochem Parasitol. 1984 Jul;12(3):327–336. doi: 10.1016/0166-6851(84)90089-6. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]



