Abstract
OBJECTIVE
To evaluate the efficacy and safety of transdermal nitroglycerin as tocolytic agent in women with preterm labor.
STUDY DESIGN
Systematic review and meta-analysis of randomized controlled trials.
RESULTS
Thirteen studies (1302 women) were included. Two studies evaluated transdermal nitroglycerin versus placebo (N=186), 9 evaluated transdermal nitroglycerin versus β2-adrenergic-receptor agonists (N=1024), and 1 each evaluated transdermal nitroglycerin versus nifedipine (N=50) and transdermal nitroglycerin versus magnesium sulfate (N=42). There were no significant differences between transdermal nitroglycerin and placebo for delivery within 48 hours of initiation of treatment or before 28, 34 or 37 weeks’ gestation, adverse neonatal outcomes, and neurodevelopmental status at 24 months of age. Nevertheless, one study found a marginally significant reduction in the risk of a composite outcome of significant neonatal morbidity and perinatal mortality (3/74 [4.1%] versus 11/79 [13.9%]; relative risk 0.29, 95% confidence interval 0.08–1.00). When compared with β2-adrenergic-receptor agonists, transdermal nitroglycerin was associated with a significant reduction in the risk of preterm birth <34 and <37 weeks’ gestation, admission to the neonatal intensive care unit, use of mechanical ventilation, and maternal side effects. There were no significant differences between transdermal nitroglycerin and nifedipine and magnesium sulfate in delivery within 48 hours of treatment and pregnancy prolongation, respectively. Overall, women receiving transdermal nitroglycerin had a higher risk of headache.
CONCLUSION
Although transdermal nitroglycerin appears to be more effective than β 2-adrenergic-receptor agonists, the current evidence does not support its routine use as tocolytic agent for the treatment of preterm labor. Further additional double-blind placebo-controlled trials are needed.
Keywords: Nitric oxide donors, β2-adrenergic-receptor agonists, nifedipine, magnesium sulfate, tocolytic agents, preterm birth, neonatal morbidity
INTRODUCTION
In 2010, an estimated 15 million babies (range, 12.3–18.1 million) were born preterm, 11.1% of all live births worldwide, ranging from about 5% in several European countries to 18% in some African countries.1 In 2011, the preterm birth rate in the United States was 11.7%2 and currently is one of the ten countries with the highest numbers of preterm births, accounting for 42% of all preterm births in the developed world.1 Preterm birth is now the second most common cause of death in children younger than 5 years after pneumonia and is the leading cause of neonatal death worldwide.3 In addition, preterm birth contributes to long-term growth impairment and substantial long-term morbidity such as cognitive, visual, and learning impairments.4,5
About 40–45% of preterm births follow spontaneous preterm labor.6 Tocolytic therapy continues to be the focus of treatment of preterm labor to allow the administration of antenatal corticosteroids for improving fetal lung maturity and to transfer the mother to a tertiary care facility with a neonatal intensive care unit (NICU). The ideal tocolytic agent should be specific to the common pathway of parturition (activated in the specific patient), easy to administer, effective in preventing preterm birth, and able to improve neonatal outcomes, with few maternal, fetal, and neonatal side effects and without long-term adverse effects.7 A wide variety of agents have been used to suppress uterine contractions, including β2-adrenergic-receptor agonists,8–12 magnesium sulfate,13–16 cyclooxygenase inhibitors,17–22 calcium channel blockers, 7,23–26 oxytocin-receptor antagonists27–31 and nitric oxide donors.32–35 Currently, there is no clear first-line tocolytic agent although a recent meta-analysis suggested that nifedipine appears to meet several characteristics of an ideal tocolytic agent.7
Nitric oxide, a potent relaxant of smooth muscle, is possibly involved in the maintenance of uterine quiescence during pregnancy.36–38 Nitroglycerin, a nitric oxide donor, has been shown to produce a significant decrease in the contractility of human myometrium from pregnant and nonpregnant women in vitro.39–42 In 1994, Lees et al43 reported that transdermal nitroglycerin patches suppressed uterine contractions in all 20 episodes of preterm labor that occurred in 13 consecutive women enrolled in a pilot study and suggested that this nitric oxide donor could be an effective and safe tocolytic agent. Transdermal nitroglycerin has the attraction of its simplicity of administration, potential effectiveness, low cost, and few side effects. However, the use of transdermal nitroglycerin for the management of preterm labor has been and continues being the subject of debate and controversy.44–49 The most recent update of the Cochrane review regarding transdermal nitroglycerin for the treatment of preterm labor included 4 randomized controlled trials involving a total of 436 patients.33 This review concluded that there was insufficient evidence to support the routine administration of nitric oxide donors (mainly transdermal nitroglycerin) in the treatment of preterm labor. However, the literature searches on which this review was based were performed in 2002. Thenceforth, additional randomized controlled trials evaluating transdermal nitroglycerin have been published; consequently, re-assessment of the efficacy and safety of this agent is justified.
The objective of this systematic review and meta-analysis was to evaluate the efficacy and safety of transdermal nitroglycerin as a tocolytic agent in patients with preterm labor.
MATERIALS AND METHODS
This study was conducted following a prospectively prepared protocol and reported using the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines for meta-analysis of randomized controlled trials.50
Literature search
Searches were performed in MEDLINE, EMBASE, CINAHL, and LILACS (all from inception to June 24, 2013), the Cochrane Central Register of Controlled Trials (http://www.mrw.interscience.wiley.com/cochrane/cochrane_clcentral_articles_fs.html) (1960 to June 24, 2013), ISI Web of Science (http://www.isiknowledge.com) (1960 to June 24, 2013), Research Registers of ongoing trials (www.clinicaltrials.gov, www.controlled-trials.com, www.centerwatch.com, www.anzctr.org.au, http://www.nihr.ac.uk, and www.umin.ac.jp/ctr), and Google scholar using a combination of keywords and text words related to nitroglycerin, preterm labor and tocolysis. We also reviewed congress proceedings of international society meetings of maternal-fetal and reproductive medicine and international meetings on preterm birth and tocolysis, and bibliographies of identified studies and review articles to locate additional publications. For studies with multiple publications, the data from the most complete report were used and supplemented if additional information appeared in other publications. The language of publication was not restricted.
Study selection
We included randomized controlled trials in which transdermal nitroglycerin was used for tocolysis in patients with preterm labor compared with placebo, no treatment, or alternative tocolytic agents. Trials were excluded if they were quasi-randomized, if they evaluated nitroglycerin for tocolysis administered intravenously, sublingually or orally, or if they evaluated the use of transdermal nitroglycerin for other obstetric or medical conditions such as improvement of implantation or pregnancy rates in women undergoing assisted reproductive technologies, uterine relaxation in attempted external cephalic version or inverted uterus, acute intrapartum fetal resuscitation, facilitation of fetal extraction of preterm infants during cesarean section, and prevention or management of preeclampsia, intrauterine growth restriction, placental abruption and angina during pregnancy, among others. Published abstracts alone were excluded if additional information on methodological issues and results could not be obtained. All published studies deemed suitable were retrieved and reviewed independently by the two authors to determine inclusion. Disagreements were resolved through discussion. Authors of selected studies were contacted to complement data on trial methods and/or outcomes.
Outcome measures
The prespecified primary outcomes were delivery within 48 hours and 7 days of treatment, delivery before 34 and 37 weeks of gestation, perinatal mortality, neonatal morbidity (respiratory distress syndrome, intraventricular or intracerebral hemorrhage, necrotizing enterocolitis, neonatal sepsis, and admission to neonatal intensive care unit [NICU]), and neurodevelopmental status at ≥12 months of age. Secondary outcomes included interval between trial entry and delivery, gestational age at birth, delivery before 32 and 28 weeks of gestation, recurrent preterm labor, maternal adverse events, discontinuation of treatment because of adverse events, birthweight, fetal bradycardia and tachycardia, other neonatal morbidities, and use of mechanical ventilation.
Assessment of risk of bias in included studies
Study quality assessment was conducted according to a tool recommended by the Cochrane Collaboration,51 which considers 7 items: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other bias. Review authors’ judgments were categorized as “low risk” of bias, “high risk” of bias or “unclear risk” of bias. The risk of bias in each included trial was assessed individually by the two investigators, and discrepancies were resolved through discussion.
Data extraction
Using a standardized data abstraction form, the two authors independently extracted data from each article on study characteristics (randomization procedure, concealment allocation method, blinding of providers, patients and outcome assessors, completeness of outcome data for each outcome, including attrition and exclusions from the analysis, and intention to treat analysis), participants (inclusion and exclusion criteria, definition of preterm labor, cervical dilatation and effacement at trial entry, gestational age at randomization, number of women randomized, baseline characteristics, and country and date of recruitment), details of intervention (aim, loading and maintenance dose, route, duration, retreatment, use of alternative tocolytic therapy, and routine administration of antenatal corticosteroids), and outcomes (number of outcome events and/or mean ± standard deviation [SD] for each outcome). In an attempt to obtain additional data, we contacted five authors by e-mail of whom only one responded. Disagreements regarding extracted data were resolved by discussion among the authors.
Statistical analysis
Statistical analyses were performed according to the guidelines of the Cochrane Collaboration.52 Outcomes were analyzed on an intention-to-treat basis. If this was not clear from the original article then we carried out re-analysis when possible. If we found no evidence of a substantial difference in study populations, interventions, or outcome measurements, we performed a meta-analysis. For dichotomous data, we calculated the summary relative risk (RR) with 95% confidence interval (CI). For continuous data, we used the mean difference (MD) if outcomes were measured in the same way among trials, or standardized mean difference if the same outcome was measured in a variety of ways, with 95% CI.
Four prespecified subgroup analyses were performed to compare transdermal nitroglycerin with placebo, β2-adrenergic-receptor agonists, magnesium sulfate, and nifedipine. The subgroup analyses comparing transdermal nitroglycerin versus cyclooxygenase inhibitors or oxytocin-receptor antagonists were not performed because trials addressing these comparisons were not identified. Additional subgroup analyses were planned to assess primary outcomes according to several characteristics (definition of preterm labor, cervical dilatation at trial entry, dose of transdermal nitroglycerin, membranes status, plurality, gestational age at trial entry, study setting, maintenance therapy, use of alternative tocolytic therapy, and antenatal corticosteroid therapy) but these were not undertaken due to the small number of studies included in each comparison and insufficient data.
Heterogeneity of the results among studies was tested with the quantity I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance.53 A value of 0% indicates no observed heterogeneity whereas I2 values of 50% or more indicate a substantial level of heterogeneity.53 A fixed-effects model was used to pool data across studies if substantial statistical heterogeneity was not present. If I2 values were ≥50%, a random effects model was used to pool data across studies if causes of heterogeneity could not be determined and the average treatment effect was considered clinically meaningful. A sensitivity analysis was planned, by including only trials with adequate concealment allocation and double masked, to explore the impact of study quality on the effect size for the primary outcomes. This analysis was not performed because only 1 included study was truly double masked. However, we undertook a sensitivity analysis for the comparison transdermal nitroglycerin versus β2-adrenergic-receptor agonists by including only studies with adequate concealment of allocation.
The number needed to treat (NNT) for benefit or harm with 95% CI was calculated for the outcomes for which there was a statistically significant reduction or increase in risk difference based on control event rates in the included studies.54 We assessed publication and related biases visually by examining the symmetry of funnel plots and statistically by using the Egger test.55 A probability value of <.1 was considered to indicate significant asymmetry.
Analyses were performed with the Review Manager (RevMan) version 5.1.7 (The Nordic Cochrane Centre, København, Denmark) and StatsDirect version 2.7.9 (StatsDirect Ltd, Cheshire, United Kingdom).
RESULTS
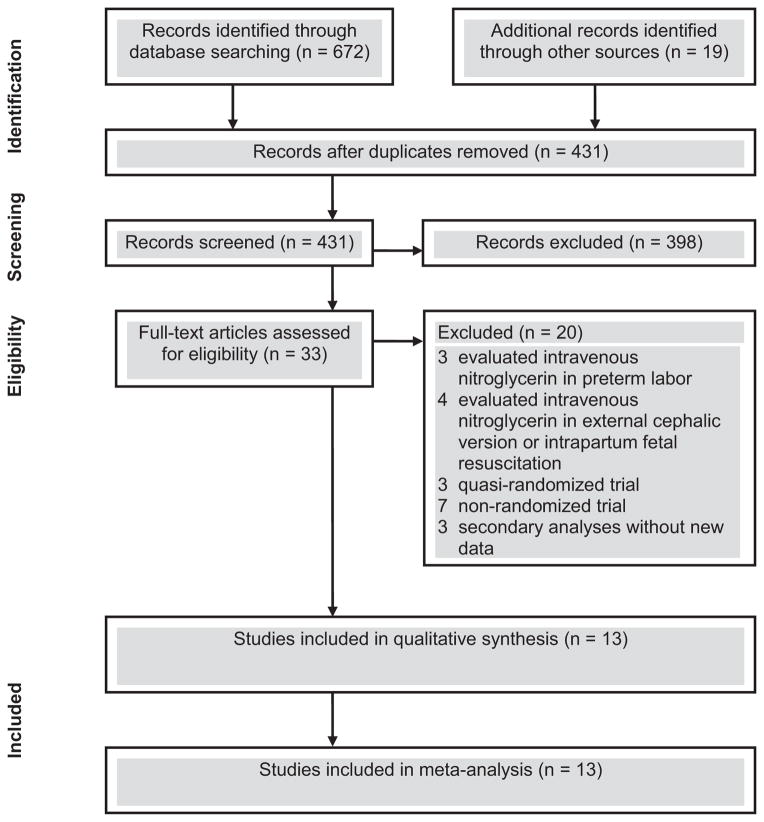
We identified 431 studies in our literature search and considered 33 to be potentially eligible (Figure 1). Thirteen studies,56–68 including 1302 women, met the inclusion criteria of which 2 evaluated transdermal nitroglycerin versus placebo,56,57 9 evaluated transdermal nitroglycerin versus β2-adrenergic-receptor agonists (3 studies using ritodrine,59,63,64 2 studies using fenoterol,60,61 and one each using salbutamol,65 albuterol,58 isoxsuprine,66 and ritodrine or salbutamol62), and 1 each evaluated transdermal nitroglycerin versus nifedipine67 and transdermal nitroglycerin versus magnesium sulfate.68 One study that evaluated primarily transdermal nitroglycerine versus ritodrine also evaluated transdermal nitroglycerine versus placebo in two hospitals that did not use any tocolytics routinely.59 Data on child neurodevelopmental outcomes for 2 trials57,62 were reported in 2 additional publications.69,70 Moreover, one author provided additional unpublished data for one trial.61
Figure 1.
Study selection process
The main characteristics of the studies included in the review are summarized in Table 1. Two studies were conducted in Canada,56,57 2 in European countries,60,61 5 in Asian countries,63–66,68 1 each in Australia58 and Brazil,67 and the remaining 2 were multicenter studies conducted in 9 countries.59,62 The sample size ranged from 2658 to 23662 (median, 60). Preterm labor was defined as the presence of uterine contractions with evidence of cervical changes in 9 trials.56,57,60,61,63,65–68 One study included patients with or without cervical changes,59 2 studies did not include cervical changes in the diagnosis of preterm labor,58,62 and 1 did not report on the definition of preterm labor.64 All but 1 study62 were limited to women with intact membranes. Only 2 trials included women with multiple gestations.56,59 Standard maternal and fetal contraindications to tocolysis were reported as exclusion criteria in the great majority of included studies. The gestational age at inclusion varied from 23 to 36 weeks. The minimum gestational age at trial entry ranged from 23 to 28 weeks, and the maximum ranged from 32 to 36 weeks. Most studies included women between 24 and 34 weeks of gestation.
TABLE 1.
Characteristics of studies included in the systematic review
| First author, year | Location | Inclusion/exclusion criteria | Gestational age (weeks), cervical dilatation/effacement, and frequency of uterine contractions at trial entry | Interventions (sample size) | Alternative tocolytic therapy |
|---|---|---|---|---|---|
| Nitroglycerin compared with placebo | |||||
| Smith,56 1999 | Canada | Inclusion: women with singleton or twin pregnancy in preterm labor (change in the Bishop score over an initial period of saline infusion) and intact membranes. Exclusion: rupture of membranes, any maternal condition such as significant antepartum hemorrhage or fetal condition necessitating immediate delivery, suspicion of fetal anomalies or intrauterine fetal death, multiple gestation greater than twins, cervical dilatation >4 cm, treatment with another tocolytic agent within 24 hours, previous enrollment in the trial, known sensitivity to nitroglycerin, and failure to give consent. |
24–34; mean Bishop score at randomization was 6.8 ± 2.1 and 6.3 ± 2.6 for nitroglycerin and placebo groups, respectively. No data on frequency of uterine contractions at trial entry. | Nitroglycerin (n = 17): one 10 mg/24 h (0.4 mg/h) transdermal patch was applied to the skin of the abdomen. If the uterus was still actively contracting (4 contractions per 20 minutes) after one hour of application of the first patch, or there was evidence of ongoing cervical change, a second patch, in addition to the first, was placed in the same manner. Placebo (n = 16): the impermeable protective backing of the nitroglycerin patch which prevented contact between skin and drug reservoir was not removed, and the patch was placed on the woman’s abdomen. Twenty-four hours after initiation of treatment, the patches were replaced with the same number of patches for a further 24 hours of treatment. |
None of the subjects in either arm received any other tocolytic at any time. |
| Smith,57 2007 | Canada | Inclusion: women with singleton pregnancy in preterm labor (at least 4 painful uterine contractions per 20 min and evidence of cervical change [change in the Bishop score or Bishop score >6]) and intact membranes. Exclusion: any maternal/fetal condition necessitating delivery, multiple gestation, rupture of membranes, intrauterine fetal death, treatment with tocolytics within 24 hours, lethal fetal anomaly, cervical dilatation >5 cm, previous enrollment in the trial, sensitivity to nitroglycerin, or failure to give consent. |
24–32; no data on cervical dilatation and effacement, and frequency of uterine contractions at trial entry. | Nitroglycerin (n = 74): one 10 mg/24 h (0.4 mg/h) transdermal patch was applied to the skin of the abdomen. If there was ongoing uterine activity (4 contractions per 20 minutes) or evidence of further cervical change 1 hour after placement of the first study patch, one additional study patch was placed. Placebo (n = 79): similar placebo patch. Twenty-four hours after initiation of treatment, the patches were replaced with the same number of patches for a further 24 hours of treatment. |
None of the subjects in either arm received any other tocolytic at any time. |
| Nitroglycerin compared with β2-adrenergic-receptor agonists | |||||
| Bisits,58 1998 | Australia | Inclusion: women with singleton pregnancy in preterm labor (painful regular uterine contractions at <5-minute intervals) and intact membranes. Exclusion: multiple pregnancy, intrauterine infection, severe fetal distress, rapidly progressing labor, advanced cervical dilatation (>5 cm), active vaginal bleeding, and the presence of contraindications to nitrate therapy or albuterol. |
24–34; no data on cervical dilatation and effacement, and frequency of uterine contractions at trial entry. | Nitroglycerin (n = 13): one 10 mg/24 h (0.4 mg/h) transdermal patch was applied to the skin of the upper abdomen and replaced, if necessary, every 24 hours. If no decrease in uterine contractility was noted after 1 hour, a second patch was applied. If there was still no decrease in uterine activity after another hour, the patient received intravenous albuterol and the patch removed. If uterine activity ceased, the patch was removed 12 hours later. If after this period further tocolytic treatment was indicated, one patch could be placed on the abdomen every 24 hours. Albuterol (n = 13): 25 μg/min intravenously. The infusion rate was subsequently titrated against uterine activity and maternal heart rate. If uterine contractions ceased, the infusion rate was halved every 30 minutes until the patient was completely weaned. |
Albuterol in nitroglycerin group (15.4%) |
| Lees,59 1999 | United Kingdom, Italy, Belgium, Germany, Thailand, and Indonesia | Inclusion: women with singleton or multiple pregnancy in preterm labor (at least three painful, uterine contractions every 10 min for more than 1 hour with or without cervical change) and intact membranes. Exclusion: hypotension, major fetal congenital abnormality, non reassuring fetal cardiotocography, antepartum hemorrhage, placenta previa, rupture of membranes, chorioamnionitis, cervical suture in situ, unexplained pyrexia, urinary tract infection, contraindication to nitrates or β-agonists, and previous treatment with tocolytics in current pregnancy. |
24–36; no data on cervical dilatation and effacement, and frequency of uterine contractions at trial entry. | Nitroglycerin (n = 113): one 10 mg/24 h (0.4 mg/h) transdermal patch was applied to the skin of the abdomen. If, after one hour, there was no reduction in contraction frequency or strength, an additional patch was placed. Patches remained in place for full 24 hours, at the end of which they were removed. Ritodrine (n = 120): 50 μg/min intravenously. The rate of administration was titrated to the woman’s contractions and increased according to local guidelines. Treatment was terminated after cessation of contractions for 24 hours, or progress of labor to delivery. In two hospitals that did not use any tocolytics routinely, a double-masked placebo controlled trial was done: Nitroglycerin (n = 7): two patches applied per 24 hours with a maximum duration of 48 hours. Placebo (n = 5): similar placebo patch. |
Cross over to other treatment (6% in each group) |
| Szulc,60 2000 | Poland | Inclusion: women with singleton pregnancy in preterm labor (at least four uterine contractions every 20 min with effacement ≥80% or cervical dilatation up to 3 cm), no contraindications to tocolysis, and intact membranes. Exclusion: Not reported |
23–34; no data on cervical dilatation and effacement, and frequency of uterine contractions at trial entry. | Nitroglycerin (n = 30): one 10 mg/24 h (0.4 mg/h) transdermal patch was applied to the skin of the lower abdomen. If contractions persisted after 1 h, a second patch of 5 mg was placed. Depending on uterine activity, one additional patch was placed at 24 hours. Fenoterol (n = 30): 1 mg plus verapamil (10 mg) in 500 ml of 5% dextrose solution at a rate of 10–30 drops per minute which was increased according to uterine contractions and tolerance to drug. After contractions ceased, patients received fenoterol (5 mg) + verapamil (40 mg) orally every 4–6 hours. |
Not reported |
| Schleussner,61 2001 | Germany | Inclusion: women with singleton pregnancy in preterm labor (at least three uterine contractions every 30 min with Bishop score ≥3), maternal age ≥18 years, and intact membranes. Exclusion: multiple pregnancy, preterm rupture of membranes, chorioamnionitis, placenta previa, abruption placentae, antepartum hemorrhage, contraindication to nitroglycerin or β-agonists, and inclusion in other study. |
27–35; mean Bishop score, sonographic cervical length, and cervical funneling at trial entry was 4.5 +1.2, 2.3+ 0.8, and 51%, and 4.7 +1.4, 2.4+0.9, and 62% for nitroglycerin and control groups, respectively. Mean uterine activity was 6 contractions per 30 min in both groups. | Nitroglycerin (n = 57): two 10 mg/24 h (0.8 mg/h) transdermal patches were applied to the skin of the periumbilical area. Fenoterol (n = 61): 60–120 μg/h plus magnesium sulfate (1.2 g/h) and verapamil (0.6–1.4 mg/h) intravenously or oral metoprolol (47.5 mg/d). The doses of the study medications were halved after 24 hours without contractions and stopped after 48 hours without contractions. Then, all women from both arms received magnesium aspartame oral 300 mg/d until 35 weeks’ gestation. |
Not reported |
| Bisits,62 2004 | Australia, Singapore, and Hong Kong | Inclusion: women with singleton pregnancy in preterm labor (at least two uterine contractions every 10 min) with positive cervicovaginal fetal fibronectin or ruptured membranes. Exclusion: multiple pregnancies, chorioamnionitis, cervical dilatation ≥5 cm, a history of hypotension, and a negative cervicovaginal fetal fibronectin in the presence of intact membranes. |
24–35; mean cervical dilatation at trial entry was 0.71 and 0.63 cm for nitroglycerin and β2-adrenergic-receptors antagonists groups, respectively. | Nitroglycerin (n = 120): one 10 mg/24 h (0.4 mg/h) transdermal patch was placed on the skin of the anterior chest wall. If contractions did not settle in 1 hour, then one additional patch was placed. If the contractions settled, the patch was left on for 12 hours and then removed. If the contractions did not settle after 2 hours of nitroglycerin treatment, they were then removed and β2 sympathomimetic treatment was commenced. Salbutamol or ritodrine (n = 116) according to local practice. No data on doses used. |
β2-adrenergic-receptors agonists in nitroglycerin group (33.1%) |
| Lee,63 2004 | South Korea | Inclusion: women with singleton pregnancy in preterm labor (labor (≥4 uterine contractions in 20 min or at least 8 in 60 min and cervical dilatation >1 cm) and intact membranes. Exclusion: premature rupture of membranes, fetal malformation, preeclampsia, hypotension, non-reassuring fetal cardiotocography, chorioamnionitis, placenta previa, urinary tract infection, history of maternal cardiovascular disease, and hypersensitivity or contraindication to study drugs, |
24–34; no data on cervical dilatation and effacement, and frequency of uterine contractions at trial entry. | Nitroglycerin (n = 24): one 5 mg/24 h (0.2 mg/h) transdermal patch was applied to the skin of the abdomen, followed by an additional patch after 1 hour if contractions continued. Patches were left on for 24 hours and then removed. Ritodrine (n = 35): 25 μg/min intravenously increasing every 15 minutes until contractions were inhibited or side effects became intolerable (maximum dose, 200 μg/min). |
Magnesium sulfate |
| Wani,64 2004 | United Arab Emirates | Inclusion: women in preterm labor (no definition provided), intact membranes, no vaginal bleeding, and no cardiovascular disease. Exclusion: abnormal fetal cardiotocography, intrauterine infection, rupture of membranes, fetal death, severe intrauterine growth retardation, antepartum hemorrhage with hemodynamic instability, and cervical dilatation >4 cm. |
23–34; no data on cervical dilatation and effacement, and frequency of uterine contractions at trial entry. | Nitroglycerin (n = 67): one 10 mg/24 h (0.4 mg/h) transdermal patch was applied to the skin of the abdomen, followed by an additional patch after 1 hour if contractions continued. Patches were replaced after 24 hours. Ritodrine (n = 65): 150 μg/min intravenously increasing by 50 μg/min every 10 minutes until contractions ceased, a maximum dose of 350 μg/min was reached or the occurrence of side effects. After cessation of contractions, a minimal dose of ritodrine was continued to maintain suppression of uterine activity. Treatment was continued for at least 24 hours after cessation of contractions, nitroglycerin being used for up to 5 days and ritodrine for a maximum of 3 days. Recurrence of preterm labor was treated as randomized. |
Not reported |
| Latif,65 2010 | India | Inclusion: women with singleton pregnancy in preterm labor (>20 uterine contractions per hour documented by external tocography, and/or cervical dilatation ≥2 cm) and intact membranes. Exclusion: multiple pregnancy, premature rupture of membranes, known contraindications to tocolytics, and treatment with tocolytics in current pregnancy. |
28–36; among women allocated to receive nitroglycerin, 70% had a cervical dilatation >1 cm and 80% had 2–4 uterine contractions per minute at trial entry. The corresponding values among women allocated to receive salbutamol were 77% and 77%, respectively. | Nitroglycerin (n = 30): one 10 mg/24 h (0.4 mg/h) transdermal patch was applied to the skin of the abdomen, followed by an additional patch after 1 hour if there was no reduction in contraction frequency or intensity. Patches were left in place for 24 hours. Salbutamol (n = 30): 5 mg in 500 ml of 5% dextrose solution. The infusion was started at a rate of 10 drops per minute and then increased by 10 drops every 5–10 min until contractions ceased. Then tapered off slowly in next 12 hours. Both treatments were discontinued if contractions ceased for 24 hours or delivery occurred. |
Not reported |
| Rekha,66 2012 | India | Inclusion: women with singleton pregnancy in preterm labor (≥4 uterine contractions in 20 min or at least 8 in 60 min, cervical dilatation >1 cm, and effacement ≥80%). Exclusion: active labor, preterm premature rupture of membranes, chorioamnionitis, severe hypertension, eclampsia, antepartum hemorrhage, fetal distress, severe intrauterine growth restriction, lethal congenital anomaly, intrauterine death, and sensitivity or contraindication to tocolysis |
24–36; no data on cervical dilatation and effacement, and frequency of uterine contractions at trial entry. | Nitroglycerin (n = 50): one 10 mg/24 h (0.4 mg/h) transdermal patch was applied to the skin of the anterior abdominal wall. If, after one hour, there was no reduction in uterine activity, an additional 10 mg/24 h patch was placed and both patches were continued for 24 hours. Twenty-four hours after initiation of treatment, the patches were replaced with the same number of patches for a further 24 hours of treatment Isoxsuprine (n = 50): 10 mg intramuscularly every 8 hours until 24 hours of uterine relaxation. Thereafter 10 mg orally every 8 hours for 7 days. |
Not reported |
| Nitroglycerin compared with nifedipine | |||||
| Amorim,67 2009 | Brazil | Inclusion: women with singleton pregnancy in preterm labor (≥4 uterine contractions in 30 min with a duration ≥30 seconds and cervical changes) and intact membranes. Exclusion: premature rupture of membranes, preeclampsia, diabetes, placental abruption, fetal malformation, and previous treatment with tocolytics. |
24–34; median (range) cervical dilatation and number of uterine contractions per 10 min at trial entry was 2 (2–4) cm and 3 (2–4), respectively. | Nitroglycerin (n = 26): one 10 mg/24 h (0.4 mg/h) transdermal patch was applied to the skin of arm or forearm. If contractions persisted after 6 h, a second patch of 10 mg was placed (maximum dose of 20 mg/24 h). Nifedipine (n = 24): 10 mg sublingually repeated after 30 min. Then 20 mg orally every 6 hours for at least 24 hours. |
Terbutaline |
| Nitroglycerin compared with magnesium sulfate | |||||
| Mirteimoori,68 2009 | Iran | Inclusion: women with singleton pregnancy in preterm labor (≥4 uterine contractions in 20 min with cervical dilatation <4 cm and effacement ≥80%), and intact membranes. Exclusion: placenta previa, abruptio placentae, hypertension, fetal growth restriction, fetal abnormality, chorioamnionitis, and a history of recurrent vaginal bleeding, urinary tract infection, sensitivity or contraindication to nitrates or magnesium sulfate, rupture of the membranes, and renal insufficiency. |
27–36; no data on cervical dilatation and effacement, and frequency of uterine contractions at trial entry. | Nitroglycerin (n = 21): one 5 mg/24 h (0.2 mg/h) transdermal patch was applied to the skin of the upper abdomen. No additional patches were placed. Magnesium sulfate (n = 21): 4-g bolus then 2 g/h until suppression of uterine contractions. |
Not reported |
Overall, nitroglycerin dosing regimens were similar across the trials. Ten studies used one 10 mg/24 h (0.4 mg/h) transdermal patch which was applied to the skin of the abdomen, anterior chest wall, arm or forearm.56–60,62,64–67 One study used two 10 mg/24 h (0.8 mg/h) transdermal patches61 and two used one 5 mg/24 (0.2 mg/h) transdermal patch.63,68 In 10 studies, one additional patch was placed if contractions persisted after 1 hour of application of the first patch.56–60,62–66 In one study, a second patch was applied if contractions persisted after 6 hours.67 No additional patches were placed in the remaining two studies.61,68 In six studies, one additional patch was placed at 24 hours after initiation of treatment.56,57,60,61,64,66 One study used transdermal nitroglycerine for up to 5 days after cessation of contractions.64 Verapamil or metoprolol was used for suppressing the tachycardia caused by fenoterol in the 2 studies that compared transdermal nitroglycerin with this β2-adrenergic-receptor agonist.60,61 Moreover, magnesium sulfate was added to the fenoterol infusion in one of these studies.61 Use of alternative tocolytic therapy was explicitly mentioned in 5 studies.58,59,62,63,67 In two studies, none of the subjects in either arm received any other tocolytic at any time.56,57 The use of alternative tocolytic therapy was not reported in the remaining 6 studies.60,61,64–66,68 Six trials reported administration of antenatal corticosteroids for most women enrolled.56,57,61,64,66,67 The use of antenatal corticosteroids was not reported in the remaining 7 trials.58–60,62,63,65,68 The main primary outcome measures were interval between trial entry and delivery (7 studies), delivery within 48 hours (5 studies) and 7 days of treatment (5 studies), and delivery before 37 weeks of gestation(4 studies).
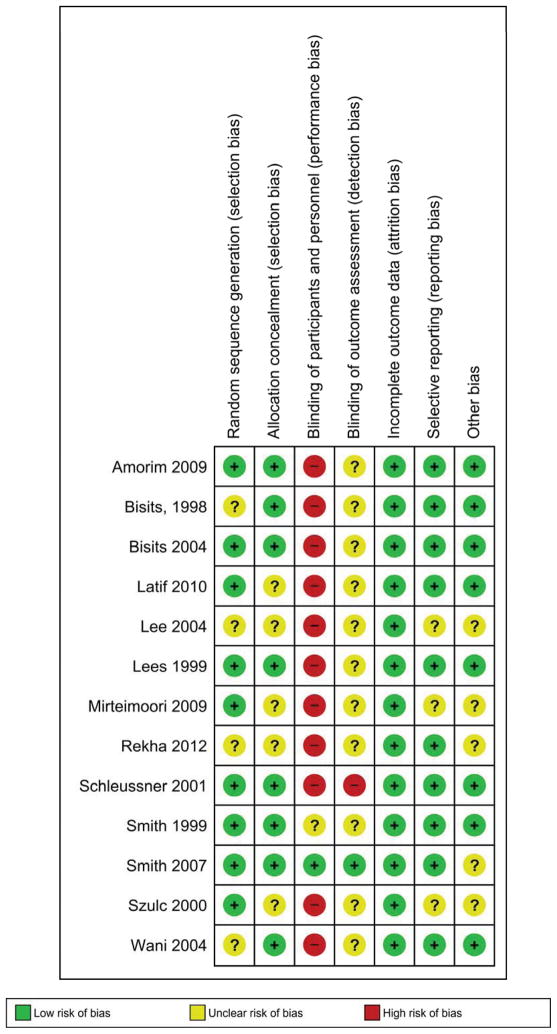
Figure 2 shows the risk of bias in each included study. Nine studies had adequate generation of allocation sequence56,57,59–62,65,67,68 and 8 reported adequate concealment of allocation.56–59,61,62,64,67 Blinding of the intervention was not performed in any of the 11 studies that evaluated transdermal nitroglycerin versus any other tocolytic, and masking assessment of outcomes was not reported in 10 of them. Of the 2 trials that evaluated transdermal nitroglycerin versus placebo, only one was actually double-blinded placebo-controlled.57 In the remaining study,56 true placebo patches were not available and the nursing staff who applied the patches were not blinded to the treatment. However, we judged that assessment and measurement of most outcomes that were included in our review are considered objective in nature and were not likely to be influenced by a lack of blinding. All studies had an adequate handling of incomplete outcome data and 10 were free of suggestion of selective outcome reporting. Eight studies appeared to be free of other sources of bias. The study by Smith et al57 was stopped early because of feasibility issues after 158 women were recruited (26% of the original sample size calculated). Six trials met ≥5 criteria, 3 met four criteria and the remaining 4 met <3 criteria.
Figure 2.
Methodologic quality summary: risk of bias for each included study
Transdermal nitroglycerin versus placebo
Two trials, with a total of 186 women, compared transdermal nitroglycerin with placebo.56,57 There were no significant differences between transdermal nitroglycerin and placebo for delivery within 48 hours of initiation of treatment or before 28, 34 or 37 weeks’ gestation, or gestational age at birth (Table 2). Maternal side effects secondary to study medication were significantly more common among women allocated transdermal nitroglycerin rather than placebo (67% vs 45%; RR 1.49, 95% CI 1.14–1.94; NNT for harm 5, 95% CI 2–16). In addition, 1 trial reported that headache and local irritation were significantly more frequent among women in the transdermal nitroglycerin group than among women in the placebo group.57 No statistically significant differences were seen for flushing, dizziness, hypotension and fetal bradycardia.
TABLE 2.
Transdermal nitroglycerin compared with placebo
| Outcome | Number of trials | Number of events/total number or total number | Relative risk or mean difference (95% CI) | I2 (%) | |
|---|---|---|---|---|---|
| Nitroglycerin | Placebo | ||||
| Pregnancy outcomes | |||||
| Delivery within 48 h of treatment | 256,57 | 24/91 | 31/95 | 0.80 (0.51–1.24) | 6 |
| Preterm birth <28 weeks’ gestation | 157 | 8/74 | 17/79 | 0.50 (0.23–1.09) | NA |
| Preterm birth <34 weeks’ gestation | 157 | 26/74 | 30/79 | 0.93 (0.61–1.41) | NA |
| Preterm birth <37 weeks’ gestation | 257,59 | 37/81 | 41/84 | 0.68 (0.19–2.43) | 52 |
| Gestational age at birth, wks | 256,57 | 91 | 95 | 1.1 (−0.5 to 2.7) | 0 |
| Any maternal side effect | 256,57 | 61/91 | 43/95 | 1.49 (1.14–1.94) | 0 |
| Headache | 157 | 42/74 | 23/79 | 1.95 (1.31–2.90) | NA |
| Flushing | 157 | 11/74 | 13/79 | 0.90 (0.43–1.89) | NA |
| Local irritation | 157 | 10/74 | 3/79 | 3.56 (1.02–12.43) | NA |
| Dizziness | 157 | 9/74 | 6/79 | 1.60 (0.60–4.28) | NA |
| Hypotension | 157 | 9/74 | 8/79 | 1.20 (0.49–2.95) | NA |
| Fetal bradycardia | 157 | 7/74 | 2/79 | 3.74 (0.80–17.41) | NA |
| Perinatal and neonatal outcomes | |||||
| Birthweight, g | 156 | 17 | 16 | 327 (−272 to 926) | NA |
| Respiratory distress syndrome | 156 | 3/17 | 6/16 | 0.47 (0.14–1.57) | NA |
| Necrotizing enterocolitis | 256,57 | 1/91 | 2/95 | 0.67 (0.11–4.07) | 27 |
| Grade II/IV intraventricular hemorrhage | 157 | 2/74 | 1/79 | 2.14 (0.20–23.06) | NA |
| Periventricular leukomalacia | 157 | 0/74 | 2/79 | 0.21 (0.01–4.37) | NA |
| Chronic lung disease | 157 | 1/74 | 7/79 | 0.15 (0.02–1.21) | NA |
| Perinatal mortality | 256,57 | 1/91 | 4/95 | 0.34 (0.05–2.13) | 0 |
| Composite of neonatal morbidity/perinatal mortality | 157 | 3/74 | 11/79 | 0.29 (0.08–1.00) | NA |
| Children’s developmental performance total score at 1 year follow-up | 157 | 55 | 56 | 3.3 (−15.1 to 21.7) | NA |
| Children’s developmental performance total score at 2 years follow-up | 157 | 42 | 41 | 16.6 (−7.5 to 40.6) | NA |
CI, confidence interval; NA, not applicable
There were no significant differences between the groups in the risk of major adverse perinatal/neonatal outcomes and neurodevelopmental status at 12 and 24 months of age. Nevertheless, the study by Smith et al.57 found a marginally significant reduction in the risk of a composite outcome (occurrence of 1 or more of chronic lung disease, necrotizing enterocolitis, grade 3 or 4 intraventricular hemorrhage, periventricular leukomalacia, and perinatal mortality) in the transdermal nitroglycerin group compared with the placebo group (4% vs 14%; RR 0.29, 95% CI 0.08–1.00).
Transdermal nitroglycerin versus β2-adrenergic-receptor agonists
This subgroup analysis included data from 9 trials with a total of 1024 women.58–66 Compared with women receiving β2-adrenergic-receptor agonists, those using transdermal nitroglycerin had a statistically significant reduction in the risk of preterm birth <34 weeks’ gestation (20% vs 28%; RR 0.71, 95% CI 0.51–0.99; I2=47%; NNT for benefit 12, 95% CI 7–362) and preterm birth <37 weeks’ gestation (44% vs 57%; RR 0.76, 95% CI 0.60–0.96; I2=67%; NNT for benefit 7, 95% CI 4–44) (Table 3). A significant increase in the interval between trial entry and delivery (MD 1.4 days, 95% CI 0.7–2.0; I2=49%) and birthweight (MD 331 g, 95% CI 67–595; I2=53%) was also shown. No differences were seen in the risk of delivery within 48 hours and 7 days of initiation of treatment.
TABLE 3.
Transdermal nitroglycerin compared with β2-adrenergic-receptor agonists
| Outcome | Number of trials | Number of events/total number or total number | Relative risk or mean difference (95% CI) | I2 (%) | |
|---|---|---|---|---|---|
| Nitroglycerin | β2-Agonists | ||||
| Pregnancy outcomes | |||||
| Delivery within 48 hours of treatment | 759,61–66 | 90/461 | 77/477 | 1.18 (0.91–1.53) | 28 |
| Delivery within 7 days of treatment | 758,59,61,62,64–66 | 140/450 | 140/455 | 1.00 (0.83–1.20) | 47 |
| Preterm birth <34 weeks’ gestation | 359,64,66 | 45/230 | 65/235 | 0.71 (0.51–0.99) | 44 |
| Preterm birth <37 weeks’ gestation | 758,59,61,62,64–66 | 199/450 | 258/455 | 0.76 (0.60–0.96) | 67 |
| Pregnancy prolongation, days | 361,63,64 | 148 | 161 | 1.4 (0.7 to 2.0) | 49 |
| Maternal tachycardia | 559,60,63,64,66 | 3/265 | 166/277 | 0.03 (0.01–0.07) | 0 |
| Headache | 758–61,63,64,66 | 169/335 | 33/351 | 5.92 (2.04–17.22) | 82 |
| Hypotension | 263,66 | 10/74 | 0/85 | 30.24 (1.86–492.7) | NA |
| Flushing | 360,61,66 | 16/137 | 48/141 | 0.35 (0.22–0.57) | 34 |
| Palpitations | 459,61,64,66 | 7/268 | 125/273 | 0.07 (0.03–0.13) | 39 |
| Nausea/vomiting | 658–61,63,64 | 21/285 | 39/301 | 0.57 (0.35–0.94) | 0 |
| Chest pain | 559–61,63,64 | 1/272 | 30/288 | 0.10 (0.03–0.33) | 0 |
| Dyspnea | 258,59 | 1/107 | 16/110 | 0.09 (0.02–0.46) | 0 |
| Pulmonary edema | 259,66 | 0/144 | 1/147 | 0.34 (0.01–8.34) | NA |
| Dizziness | 259,61 | 7/151 | 16/158 | 0.59 (0.12–2.78) | 58 |
| Discontinuation of treatment because of adverse effects | 359,61,64 | 19/237 | 39/246 | 0.52 (0.31–0.86) | 45 |
| Fetal tachycardia | 363–65 | 0/121 | 11/130 | 0.08 (0.01–0.61) | 0 |
| Perinatal and neonatal outcomes | |||||
| Birthweight (grams) | 261,64 | 121 | 123 | 331 (67–595) | 53 |
| Respiratory distress syndrome | 261,66 | 4/104 | 12/108 | 0.35 (0.12–1.04) | 0 |
| Necrotizing enterocolitis | 162 | 10/120 | 10/116 | 0.97 (0.42–2.24) | NA |
| Intracerebral/intraventricular hemorrhage | 261,62 | 4/174 | 11/174 | 0.37 (0.12–1.12) | 0 |
| Neonatal sepsis | 161 | 0/54 | 4/58 | 0.12 (0.01–2.16) | NA |
| Retinopathy of prematurity | 161 | 1/54 | 0/58 | 3.22 (0.13–77.34) | NA |
| Chronic lung disease | 162 | 9/120 | 9/116 | 0.97 (0.40–2.35) | NA |
| Perinatal mortality | 459,61,62,64 | 5/335 | 12/336 | 0.44 (0.16–1.18) | 0 |
| Admission to NICU | 361,64,66 | 32/171 | 58/173 | 0.57 (0.40–0.81) | 0 |
| Use of mechanical ventilation | 361,64,66 | 6/171 | 16/173 | 0.38 (0.15–0.95) | 0 |
| Patent ductus arteriosus | 162 | 3/120 | 10/116 | 0.29 (0.08–1.03) | NA |
| Griffiths mental development total score at 18 months of age below 2 SD of the mean | 162 | 5/58 | 4/54 | 1.16 (0.33–4.11) | NA |
CI, confidence interval; NA, not applicable; NICU, neonatal intensive care unit
Treatment with transdermal nitroglycerin was associated with a significant decrease in the rates of maternal tachycardia, flushing, palpitations, nausea/vomiting, chest pain, dyspnea, discontinuation of treatment because of adverse events and fetal tachycardia, and a significant increase in the rates of headache and hypotension. There were no significant differences between the groups in the risk of neonatal morbidity, although a significant reduction was seen in the risk of admission to NICU (19% vs 34%; RR 0.57, 95% CI 0.40–0.81; I2=0%; NNT for benefit 7, 95% CI 5–16), and use of mechanical ventilation (4% vs 9%; RR 0.38, 95% CI 0.15–0.95; I2=0%; NNT for benefit 17, 95% CI 13–216) in the transdermal nitroglycerin group compared with the β2-adrenergic-receptor agonists group. Moreover, transdermal nitroglycerin was associated with a nonsignificant 56% reduction in the risk of perinatal mortality (RR 0.44, 95% CI 0.16–1.18). One study reported that there was no significant difference in the risk of neurodevelopmental delay at 18 months of age, assessed by means of the Revised Griffiths Mental Development Scales, between infants in the transdermal nitroglycerin group and infants in the β2-adrenergic-receptor agonist group.62,70 The same study found that treatment with transdermal nitroglycerin was associated with a nonsignificant 71% reduction in the risk of patent ductus arteriosus (RR 0.29, 95% CI 0.08–1.03).
After the sensitivity analysis limited to trials with adequate allocation concealment, the effect of transdermal nitroglycerin on reduction in the risk of preterm birth <37 weeks’ gestation and admission to NICU did not change (RR 0.71, 95% CI 0.51–0.99 and RR 0.58, 95% CI 0.41–0.83, respectively) whereas the reduction in the risk of preterm birth <34 weeks’ gestation and mechanical ventilation turned non-significant (RR 0.75, 95% CI 0.52–1.10 and RR 0.39, 95% CI 0.14–1.06, respectively). However, it should be noted that the sensitivity analyses yielded effect sizes similar in magnitude and direction to those obtained in the overall analyses. The funnel plots of trials that compared transdermal nitroglycerin and β2-adrenergic-receptor agonists showed no asymmetry, either visually or in terms of statistical significance (P>.10 for all, by Egger test).
Transdermal nitroglycerin versus nifedipine and magnesium sulfate
The comparison of transdermal nitroglycerin versus nifedipine included only 1 trial involving 50 women.67 There were no significant differences between transdermal nitroglycerin and nifedipine in the risk of delivery within 48 hours of treatment, recurrent preterm labor, and maternal side effects (Table 4). There was only 1 study that compared transdermal nitroglycerin and magnesium sulfate (42 women).68 No statistically significant differences were found between these 2 agents in pregnancy prolongation. No other outcomes were reported in this study.
TABLE 4.
Transdermal nitroglycerin compared with nifedipine and magnesium sulfate
| Outcome | Number of trials | Number of events/total number or total number | Relative risk or mean difference (95% CI) | I2 (%) | |
|---|---|---|---|---|---|
| Nitroglycerin | Other tocolytic | ||||
| Transdermal nitroglycerin compared with nifedipine | |||||
| Delivery within 48 hours of treatment | 167 | 4/26 | 3/24 | 1.23 (0.31–4.94) | NA |
| Recurrent preterm labor | 167 | 7/26 | 4/24 | 1.62(0.54–4.83) | NA |
| Any maternal side effect | 167 | 9/26 | 5/24 | 1.66 (0.65–4.26) | NA |
| Headache | 167 | 8/26 | 2/24 | 3.69 (0.87–15.69) | NA |
| Flushing | 167 | 2/26 | 1/24 | 1.85 (0.18–19.08) | NA |
| Maternal tachycardia | 167 | 0/26 | 1/24 | 0.31 (0.01–7.23) | NA |
| Hypotension | 167 | 1/26 | 3/24 | 0.31 (0.03–2.76) | NA |
| Nausea/vomiting | 167 | 2/26 | 3/24 | 0.62 (0.11–3.37) | NA |
| Transdermal nitroglycerin compared with magnesium sulfate | |||||
| Pregnancy prolongation, days | 168 | 21 | 21 | 1.4 (−7.4 to 10.2) | NA |
CI, confidence interval; NA, not applicable; NICU, neonatal intensive care unit
COMMENT
Principal findings
The main findings of our study included the following: (1) there were no statistically significant differences between transdermal nitroglycerin and placebo patches in delivery within 48 hours of initiation of treatment, preterm birth <28, <34 and <37 weeks’ gestation, gestational age at birth, adverse perinatal and neonatal outcomes, and neurodevelopmental status at 12 and 24 months of age. However, treatment with transdermal nitroglycerin was associated with a marginally significant reduction in the risk of a composite of significant neonatal morbidity and perinatal mortality; 2) maternal side effects, mainly headache and local irritation, were significantly more common in women that used transdermal nitroglycerin patches than in women that used placebo patches; (3) overall, transdermal nitroglycerin appears to be more effective than β2-adrenergic-receptor agonists because its use was associated with a significant reduction in preterm birth <34 and <37 weeks’ gestation (approximately 25%), admission to NICU, and use of mechanical ventilation despite there were no significant differences between the 2 tocolytic agents in the risk of perinatal morbidity and mortality; (4) in general, transdermal nitroglycerin was less likely than β2-adrenergic-receptor agonists to cause maternal side effects. Nevertheless, its use was associated with a significant increase in headache and hypotension; (5) there were no significant differences between children exposed in utero to either transdermal nitroglycerin or β2-adrenergic-receptor agonists in neurodevelopmental status at 18 months of age; and (6) the paucity of trials that evaluated transdermal nitroglycerin versus nifedipine or magnesium sulfate precludes conclusions about the comparative effectiveness of these agents.
Strengths and limitations of the study
Strengths of the study include: (1) the use of the best available methods to perform a systematic review and meta-analysis of randomized controlled trials; (2) the extensive literature search using different databases, including sources of grey literature, and without language restrictions, allowed us to find and include studies published in English, German, Korean, Polish, and Portuguese in the systematic review; (3) the inclusion of a largest number of studies in the review (n = 13) in comparison with those included in the Cochrane review (n = 4)33; (4) the study quality assessment which was based on strict predetermined criteria; (5) the performance of individual comparisons between transdermal nitroglycerin and placebo, β2-adrenergic-receptor agonists, nifedipine, and magnesium sulfate; (6) the quantitative summary of the evidence; (7) the meta-analyses of the studies at low risk of bias were consistent with and thus supportive of our overall findings in the comparison of transdermal nitroglycerin versus β2-adrenergic-receptor agonists; and (8) the symmetrical funnel plots of trials that compared transdermal nitroglycerin and β2-adrenergic-receptor agonists suggesting absence of publication and related biases in the meta-analyses performed.
Several limitations must be noted when interpreting the results of this review. First, only a few trials, involving a small number of patients, evaluated transdermal nitroglycerin versus placebo, nifedipine and magnesium sulfate. As a result, our analysis was limited in its power to estimate effects within these subgroups and the CIs around estimates of differences were often wide which may have resulted in a failure to detect clinically important differences. In addition, we did not identify studies that compared transdermal nitroglycerin versus cyclooxygenase inhibitors or oxytocin-receptor antagonists. Therefore, most of the evidence generated in this systematic review is based on the comparison of transdermal nitroglycerin versus β2-adrenergic-receptor agonists. Second, about half of the trials included in the review were considered to be at moderate to high risk of bias and just one was truly double-blind. However, sensitivity analyses restricted to trials with adequate concealment of allocation showed no significant differences in the results obtained with overall meta-analyses. In addition, assessment and measurement of most outcomes included in our review are considered objective in nature, and thereby, not likely to be influenced by lack of blinding. Third, we were unable to explore reasons for the statistical heterogeneity found in several of the meta-analyses performed which might be due to differences in study population, definition of preterm labor used, cervical dilatation/effacement at trial entry, dose of transdermal nitroglycerin, and use of alternative tocolytic agents and antenatal corticosteroid therapy, among others. We used random effects models to pool data across studies in an attempt to minimize the effects of statistical heterogeneity. Fourth, 8 studies did not report data on cervical dilatation and effacement, and frequency of uterine contractions at trial entry which did not allow us to judge if participating women in such trials were in true preterm labor. The inclusion of women who were not in true preterm labor or at risk of preterm birth could have greatly hampered the assessment of transdermal nitroglycerin as tocolytic agent. Fifth, we could not assess the potential effect of the use of concomitant co-interventions on the tocolytic efficacy of transdermal nitroglycerin. The difference in frequency of use and/or type of alternative tocolytic therapy between groups could have increased the apparent benefit of transdermal nitroglycerin compared with β2-adrenergic-receptor agonists. Finally, several trials did not report results for some outcome measures assessed in our systematic review. It is possible that if these results were reported more consistently, effect sizes might be different.
Currently, there is evidence that the use of a tocolytic agent, rather than placebo, is associated with a successful delaying of delivery for at least 48 hours with no significant effect on neonatal morbidity or mortality.71 In contrast, in the present study we found that there was no overall difference between transdermal nitroglycerin and placebo for delivery within 48 hours of treatment. However, the study by Smith et al.57 reported a reduction in a neonatal morbidity/perinatal mortality composite outcome, which was borderline for statistical significance, despite no difference in overall gestational age at delivery or overall corticosteroid use between the treatment groups. The authors of the study attributed this reduction to a 23 day prolongation of pregnancy (P= .019) and a trend (P= .04) toward completing a course of corticosteroids in the subgroup of women allocated to receive transdermal nitroglycerin prior to 28 weeks’ gestation.35 It is also possible that the decrease in the composite outcome was due to a gestational age-specific nontocolytic effect of transdermal nitroglycerin on uterine blood flow, the placenta, or the fetus. Nevertheless, the beneficial effect of transdermal nitroglycerin reported in this study could have been due to a type I statistical error (incorrect rejection of a true null hypothesis). In addition, although this study was not stopped early for benefit, there is evidence that randomized controlled trials that are stopped early for benefit (whether or not as a result of a formal stopping rule) are associated with greater effect sizes than randomized controlled trials that continue to the end.72,73 Moreover, differences in treatment effect size between truncated and non-truncated randomized controlled trials are greatest in small trials that are stopped early.72 Finally, it should be stressed that the difference in the neonatal morbidity/perinatal mortality composite outcome between the two groups (3/74 in the transdermal nitroglycerin group vs 11/79 in the placebo group) was driven almost entirely by a reduction in chronic lung disease (1/74 in the transdermal nitroglycerin group vs 7/79 in the placebo group) with minimal differences in the other components of the composite outcome. Although the use of composite end points is appropriate in some instances, they can potentially provide misleading impressions about the nature and magnitude of treatment benefits. This occurs when the composite outcome includes components of varying importance, but the less important components are primarily responsible for the observed effect.74–77
The results from our meta-analysis also suggest that transdermal nitroglycerin has a greater beneficial effect than β2-adrenergic-receptor agonists. In fact, when compared with this class of tocolytic agents, the use of transdermal nitroglycerin resulted in a statistically significant decrease in preterm birth <34 and <37 weeks’ gestation, admission to NICU, use of mechanical ventilation and maternal side effects. Nevertheless, there were no significant differences between the groups in the risk of neonatal morbidity. Recently, we have published a systematic review and meta-analysis that included 16 trials, with a total of 1278 women, comparing nifedipine versus β2-adrenergic-receptor agonists.7 We found that nifedipine was clearly superior to β2-adrenergic-receptor agonists because its use was associated with a significant reduction in preterm birth within 7 days of initiation of treatment and <34 weeks’ gestation, respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage, neonatal jaundice, admission to NICU, length of stay in NICU, and maternal side effects. Although the only trial that evaluated transdermal nitroglycerin versus nifedipine (N=50)67 showed no significant differences in delivery within 48 hours of treatment or maternal adverse effects, the overall evidence from our two meta-analyses suggests that nifedipine is superior to transdermal nitroglycerin as tocolytic agent.
The role of tocolysis in preventing preterm delivery
The inhibition of myometrial contractions has been the focus of therapeutic approaches for managing preterm labor. Nonetheless, the term “preterm labor” simply describes clinical manifestations (signs and symptoms) without consideration of the specific etiology. Preterm labor is a syndrome in which multiple pathological processes may lead to myometrial contractions, membrane/decidual activation and cervical ripening.78–83 Therefore it is unlikely that one treatment will prevent all cases of preterm birth. In addition, the conceptual framework that has justified the use of tocolysis needs to be revisited. Tocolysis is aimed at achieving myometrial relaxation, which is only one of the components of the common pathway of parturition. If activation of this pathway is the consequence of a pathologic process such as infection or maternal anti-fetal rejection, it is easy to envision how tocolysis would be only of temporary benefit. On the other hand, if a specific mechanism of disease was to be identified in myometrium (e.g. an increase in oxytocin receptors or a primary disorder such as apoptosis of myometrium) targeting this organ may have more success than it has been reported thus far. It is also possible that tocolysis may assist in gaining time while other pathologic processes responsible for the activation of the common pathway are being treated. Preterm labor has a reversible and irreversible phase. In the reversible phase, tocolytic agents may be effective, but not when administered to patients who have entered the irreversible phase. It is possible that the discouraging results of tocolysis thus far, may represent the inclusion of patients who are in the irreversible phase of preterm labor. Therefore, the role for tocolysis in the management of preterm labor may be strengthened by identifying biophysical and biochemical markers of preterm labor prior to the irreversible phase of parturition. Some pathologic insults may be of such nature (e.g. intrauterine infection) in that the onset of labor has survival value for the mother (to maintain reproductive fitness) and/or fetus (e.g. to exit an intrauterine environment that is hostile).84, 85 In these cases tocolysis is likely to be ineffective.
Implications for practice and research
Based on the findings of this systematic review, currently, there is insufficient evidence to recommend the use of transdermal nitroglycerin for the treatment of preterm labor. If tocolysis is considered for women in preterm labor, the current available evidence suggests indirectly (i.e. without direct comparison) that nifedipine is preferable to transdermal nitroglycerin. Notwithstanding, the marginally significant reduction in the frequency of neonatal morbidity/perinatal mortality reported in one trial57 deserves further adequately powered double-blind placebo-controlled trials focusing in women with preterm labor before 28 weeks’ gestation. Information about the long-term growth and development of the child exposed in utero to transdermal nitroglycerin is also needed.
Acknowledgments
Financial support: This research was supported, in part, by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
We are very grateful to Dr Ekkehard Schleussner for assistance in providing unpublished data from his study and for clarification of other queries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010–2011. Pediatrics. 2013;131:548–58. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 4.Jongbloed-Pereboom M, Janssen AJ, Steenbergen B, Nijhuis-van der Sanden MW. Motor learning and working memory in children born preterm: a systematic review. Neurosci Biobehav Rev. 2012;36:1314–30. doi: 10.1016/j.neubiorev.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–52. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conde-Agudelo A, Romero R, Kusanovic JP. Nifedipine in the management of preterm labor: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;204:134. e1–20. doi: 10.1016/j.ajog.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis R, Mercer BM, Salama M, Walsh MA, Sibai BM. Oral terbutaline after parenteral tocolysis: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 1996;175:834–7. doi: 10.1016/s0002-9378(96)80008-1. [DOI] [PubMed] [Google Scholar]
- 9.Guinn DA, Goepfert AR, Owen J, Brumfield C, Hauth JC. Management options in women with preterm uterine contractions: a randomized clinical trial. Am J Obstet Gynecol. 1997;177:814–8. doi: 10.1016/s0002-9378(97)70274-6. [DOI] [PubMed] [Google Scholar]
- 10.Moutquin JM, Sherman D, Cohen H, Mohide PT, Hochner-Celnikier D, Fejgin M, et al. Double-blind, randomized, controlled trial of atosiban and ritodrine in the treatment of preterm labor: a multicenter effectiveness and safety study. Am J Obstet Gynecol. 2000;182:1191–9. doi: 10.1067/mob.2000.104950. [DOI] [PubMed] [Google Scholar]
- 11.Anotayanonth S, Subhedar NV, Garner P, Neilson JP, Harigopal S. Betamimetics for inhibiting preterm labour. Cochrane Database Syst Rev. 2004;4:CD004352. doi: 10.1002/14651858.CD004352.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Dodd JM, Crowther CA, Middleton P. Oral betamimetics for maintenance therapy after threatened preterm labour. Cochrane Database Syst Rev. 2012;12:CD003927. doi: 10.1002/14651858.CD003927.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis DF, Bergstedt S, Edwards MS, Burlison S, Gallaspy JW, Brooks GG, et al. Successful magnesium sulfate tocolysis: is “weaning” the drug necessary? Am J Obstet Gynecol. 1997;177:742–5. doi: 10.1016/s0002-9378(97)70261-8. [DOI] [PubMed] [Google Scholar]
- 14.Terrone DA, Rinehart BK, Kimmel ES, May WL, Larmon JE, Morrison JC. A prospective, randomized, controlled trial of high and low maintenance doses of magnesium sulfate for acute tocolysis. Am J Obstet Gynecol. 2000;182:1477–82. doi: 10.1067/mob.2000.107334. [DOI] [PubMed] [Google Scholar]
- 15.How HY, Zafaranchi L, Stella CL, Recht K, Maxwell RA, Sibai BM, et al. Tocolysis in women with preterm labor between 32 0/7 and 34 6/7 weeks of gestation: a randomized controlled pilot study. Am J Obstet Gynecol. 2006;194:976–81. doi: 10.1016/j.ajog.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev. 2002;4:CD001060. doi: 10.1002/14651858.CD001060. [DOI] [PubMed] [Google Scholar]
- 17.Morales WJ, Madhav H. Efficacy and safety of indomethacin compared with magnesium sulfate in the management of preterm labor: a randomized study. Am J Obstet Gynecol. 1993;169:97–102. doi: 10.1016/0002-9378(93)90138-9. [DOI] [PubMed] [Google Scholar]
- 18.Bivins HA, Jr, Newman RB, Fyfe DA, Campbell BA, Stramm SL. Randomized trial of oral indomethacin and terbutaline sulfate for the long-term suppression of preterm labor. Am J Obstet Gynecol. 1993;169:1065–70. doi: 10.1016/0002-9378(93)90055-n. [DOI] [PubMed] [Google Scholar]
- 19.Macones GA, Robinson CA. Is there justification for using indomethacin in preterm labor? An analysis of neonatal risks and benefits. Am J Obstet Gynecol. 1997;177:819–24. doi: 10.1016/s0002-9378(97)70275-8. [DOI] [PubMed] [Google Scholar]
- 20.Stika CS, Gross GA, Leguizamon G, Gerber S, Levy R, Mathur A, et al. A prospective randomized safety trial of celecoxib for treatment of preterm labor. Am J Obstet Gynecol. 2002;187:653–60. doi: 10.1067/mob.2002.125281. [DOI] [PubMed] [Google Scholar]
- 21.Sawdy RJ, Lye S, Fisk NM, Bennett PR. A double-blind randomized study of fetal side effects during and after the short-term maternal administration of indomethacin, sulindac, and nimesulide for the treatment of preterm labor. Am J Obstet Gynecol. 2003;188:1046–51. doi: 10.1067/mob.2003.255. [DOI] [PubMed] [Google Scholar]
- 22.Khanprakob T, Laopaiboon M, Lumbiganon P, Sangkomkamhang US. Cyclo-oxygenase (COX) inhibitors for preventing preterm labour. Cochrane Database Syst Rev. 2012;10:CD007748. doi: 10.1002/14651858.CD007748.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glock JL, Morales WJ. Efficacy and safety of nifedipine versus magnesium sulfate in the management of preterm labor: a randomized study. Am J Obstet Gynecol. 1993;169:960–4. doi: 10.1016/0002-9378(93)90035-h. [DOI] [PubMed] [Google Scholar]
- 24.Carr DB, Clark AL, Kernek K, Spinnato JA. Maintenance oral nifedipine for preterm labor: a randomized clinical trial. Am J Obstet Gynecol. 1999;181:822–7. doi: 10.1016/s0002-9378(99)70308-x. [DOI] [PubMed] [Google Scholar]
- 25.Larmon JE, Ross BS, May WL, Dickerson GA, Fischer RG, Morrison JC. Oral nicardipine versus intravenous magnesium sulfate for the treatment of preterm labor. Am J Obstet Gynecol. 1999;181:1432–7. doi: 10.1016/s0002-9378(99)70388-1. [DOI] [PubMed] [Google Scholar]
- 26.Papatsonis DN, Van Geijn HP, Dekker GA. Nifedipine as a safe and effective tocolytic agent in the treatment of preterm labor. Am J Obstet Gynecol. 2000;183:513–4. doi: 10.1067/mob.2000.105047. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Sibai BM, Sanchez-Ramos L, Valenzuela GJ, Veille JC, Tabor B, et al. An oxytocin receptor antagonist (atosiban) in the treatment of preterm labor: a randomized, double-blind, placebo-controlled trial with tocolytic rescue. Am J Obstet Gynecol. 2000;182:1173–83. doi: 10.1067/mob.2000.95834. [DOI] [PubMed] [Google Scholar]
- 28.Valenzuela GJ, Sanchez-Ramos L, Romero R, Silver HM, Koltun WD, Millar L, et al. Maintenance treatment of preterm labor with the oxytocin antagonist atosiban. The Atosiban PTL-098 Study Group. Am J Obstet Gynecol. 2000;182:1184–90. doi: 10.1067/mob.2000.105816. [DOI] [PubMed] [Google Scholar]
- 29.Moutquin JM, Sherman D, Cohen H, Mohide PT, Hochner-Celnikier D, Fejgin M, et al. Double-blind, randomized, controlled trial of atosiban and ritodrine in the treatment of preterm labor: a multicenter effectiveness and safety study. Am J Obstet Gynecol. 2000;182:1191–9. doi: 10.1067/mob.2000.104950. [DOI] [PubMed] [Google Scholar]
- 30.Papatsonis D, Flenady V, Cole S, Liley H. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst Rev. 2005;3:CD004452. doi: 10.1002/14651858.CD004452.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Thornton S, Goodwin TM, Greisen G, Hedegaard M, Arce JC. The effect of barusiban, a selective oxytocin antagonist, in threatened preterm labor at late gestational age: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2009;200:627. e1–10. doi: 10.1016/j.ajog.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 32.O’Grady JP, Parker RK, Patel SS. Nitroglycerin for rapid tocolysis: development of a protocol and a literature review. J Perinatol. 2000;20:27–33. doi: 10.1038/sj.jp.7200303. [DOI] [PubMed] [Google Scholar]
- 33.Duckitt K, Thornton S. Nitric oxide donors for the treatment of preterm labour. Cochrane Database Syst Rev. 2002;3:CD002860. doi: 10.1002/14651858.CD002860. [DOI] [PubMed] [Google Scholar]
- 34.Morgan PJ, Kung R, Tarshis J. Nitroglycerin as a uterine relaxant: a systematic review. J Obstet Gynaecol Can. 2002;24:403–9. doi: 10.1016/s1701-2163(16)30403-0. [DOI] [PubMed] [Google Scholar]
- 35.Smith GN, Guo Y, Wen SW, Walker MC. Secondary analysis of the use of transdermal nitroglycerin for preterm labor. Am J Obstet Gynecol. 2010;203:565. e1–6. doi: 10.1016/j.ajog.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Buxton IL, Crow W, Mathew SO. Regulation of uterine contraction: mechanisms in preterm labor. AACN Clin Issues. 2000;11:271–82. doi: 10.1097/00044067-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Maul H, Longo M, Saade GR, Garfield RE. Nitric oxide and its role during pregnancy: from ovulation to delivery. Curr Pharm Des. 2003;9:359–80. doi: 10.2174/1381612033391784. [DOI] [PubMed] [Google Scholar]
- 38.Ito E, Obayashi S, Nagai A, Imamura M, Azuma H. Regulation of myometrial contractivity during pregnancy in the rat: potential role for DDAH. Mol Hum Reprod. 2009;15:507–12. doi: 10.1093/molehr/gap041. [DOI] [PubMed] [Google Scholar]
- 39.Buhimschi I, Yallampalli C, Dong YL, Garfield RE. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am J Obstet Gynecol. 1995;172:1577–84. doi: 10.1016/0002-9378(95)90500-6. [DOI] [PubMed] [Google Scholar]
- 40.Norman JE, Ward LM, Martin W, Cameron AD, McGrath JC, Greer IA, Cameron IT. Effects of cGMP and the nitric oxide donors glyceryl trinitrate and sodium nitroprusside on contractions in vitro of isolated myometrial tissue from pregnant women. J Reprod Fertil. 1997;110:249–54. doi: 10.1530/jrf.0.1100249. [DOI] [PubMed] [Google Scholar]
- 41.David M, Hamann C, Chen FC, Bruch L, Lichtenegger W. Comparison of the relaxation effect in vitro of nitroglycerin vs. fenoterol on human myometrial strips. J Perinat Med. 2000;28:232–42. doi: 10.1515/JPM.2000.032. [DOI] [PubMed] [Google Scholar]
- 42.Wetzka B, Schäfer WR, Stehmans A, Zahradnik HP. Effects of nitric oxide donors on the contractility and prostaglandin synthesis of myometrial strips from pregnant and non-pregnant women. Gynecol Endocrinol. 2001;15:34–42. [PubMed] [Google Scholar]
- 43.Lees C, Campbell S, Jauniaux E, Brown R, Ramsay B, Gibb D, Moncada S, Martin JF. Arrest of preterm labour and prolongation of gestation with glyceryl trinitrate, a nitric oxide donor. Lancet. 1994;343:1325–6. doi: 10.1016/s0140-6736(94)92468-6. [DOI] [PubMed] [Google Scholar]
- 44.Duley L, Elbourne D. Glyceryl trinitrate in management of preterm labour. Lancet. 1994;344:553. [PubMed] [Google Scholar]
- 45.Hendrickx B. Glyceryl trinitrate in management of preterm labour. Lancet. 1994;344:553. [PubMed] [Google Scholar]
- 46.Groom KM, Bennett PR, Shennan AH. Randomised, double-blind, placebo controlled pilot study assessing nitroglycerin as a tocolytic. BJOG. 2000;107:1182–3. doi: 10.1111/j.1471-0528.2000.tb11132.x. [DOI] [PubMed] [Google Scholar]
- 47.Iams JD. Transdermal nitroglycerin for preterm labor. Am J Obstet Gynecol. 2007;196:4. doi: 10.1016/j.ajog.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Nassar AH, Usta IM. Randomized, double-blind, placebo-controlled trial of transdermal nitroglycerin for preterm labor. Am J Obstet Gynecol. 2007;197:325–6. doi: 10.1016/j.ajog.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Di Renzo GC, Facchinetti F. Transdermal nitroglycerin for preterm labor. Expert Rev Obstet Gynecol. 2007;2:417–21. [Google Scholar]
- 50.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 51.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Google Scholar]
- 52.Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Google Scholar]
- 53.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–12. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analyses detected by a simple graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith GN, Walker MC, McGrath MJ. Randomised, double-blind, placebo controlled pilot study assessing nitroglycerin as a tocolytic. BJOG. 1999;106:736–9. doi: 10.1111/j.1471-0528.1999.tb08376.x. [DOI] [PubMed] [Google Scholar]
- 57.Smith GN, Walker MC, Ohlsson A, O’Brien K, Windrim R. Randomized double-blind placebo-controlled trial of transdermal nitroglycerin for preterm labor. Am J Obstet Gynecol. 2007;196:37. e1–8. doi: 10.1016/j.ajog.2006.10.868. [DOI] [PubMed] [Google Scholar]
- 58.Bisits A, Madsen G, McLean M, O’Callaghan S, Smith R, Giles W. Corticotropin-releasing hormone: a biochemical predictor of preterm delivery in a pilot randomized trial of the treatment of preterm labor. Am J Obstet Gynecol. 1998;178:862–6. doi: 10.1016/s0002-9378(98)60503-2. [DOI] [PubMed] [Google Scholar]
- 59.Lees CC, Lojacono A, Thompson C, Danti L, Black RS, Tanzi P, White IR, Campbell S. Glyceryl trinitrate and ritodrine in tocolysis: an international multicenter randomized study. Obstet Gynecol. 1999;94:403–8. doi: 10.1016/s0029-7844(99)00296-3. [DOI] [PubMed] [Google Scholar]
- 60.Szulc E, Leibschang J. Comparative evaluation of efficiency and tolerance of two alternative methods for premature uterine contractions suppression using fenoterol and nitroglycerin [in Polish] Med Wieku Rozwoj. 2000;4:307–16. [PubMed] [Google Scholar]
- 61.Schleussner E, Richter S, Gross W, Kähler C, Möller A, Möller U, Seewald HJ. Nitroglycerin patch for tocolysis--a prospective randomized comparison with fenoterol by infusion [in German] Z Geburtshilfe Neonatol. 2001;205:189–94. doi: 10.1055/s-2001-18504. [DOI] [PubMed] [Google Scholar]
- 62.Bisits A, Madsen G, Knox M, Gill A, Smith R, Yeo G, et al. The Randomized Nitric Oxide Tocolysis Trial (RNOTT) for the treatment of preterm labor. Am J Obstet Gynecol. 2004;191:683–90. doi: 10.1016/j.ajog.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 63.Lee BK, Park IW. Doppler findings and tocolytic effect of transdermal glyceryl trinitrate and intravenous ritodrine as tocolysis of preterm labor [in Korean] Korean J Obstet Gynecol. 2004;47:2447–52. [Google Scholar]
- 64.Wani MP, Barakzai N, Graham I. Glyceryl trinitrate vs. ritodrine for the treatment of preterm labor. Int J Gynaecol Obstet. 2004;85:165–7. doi: 10.1016/j.ijgo.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Latif F, Hussain U, Bano B. Preterm labour; comparison of efficacy of glyceryl trinitrate patch with salbutamol for prolonging gestation for more than 48 hours, 7 days and up to 37 weeks of gestation. Professional Med J. 2010;17:84–90. [Google Scholar]
- 66.Rekha S, Pooja G, Patel ML, Chaudhary S, Agarwal R. Clinical evaluation of transdermal nitroglycerine in preterm labor in tertiary care teaching hospital in North India. Int J Sci Res Pub. 2012;2:1–7. [Google Scholar]
- 67.Amorim MM, Lippo LA, Costa AA, Coutinho IC, Souza AS. Transdermal nitroglycerin versus oral nifedipine administration for tocolysis: a randomized clinical trial [in Portuguese] Rev Bras Ginecol Obstet. 2009;31:552–8. [PubMed] [Google Scholar]
- 68.Mirteimoori M, Sakhavar N, Teimoori B. Glyceryl trinitrate versus magnesium sulfate in the suppression of preterm labor. Shiraz E Med J. 2009;10:73–8. [Google Scholar]
- 69.Guo Y, Xie R, Wen SW, Walker MC, Smith GN. Maternal transdermal nitroglycerin use and early childhood development. J Obstet Gynaecol Can. 2010;32:1147–52. doi: 10.1016/S1701-2163(16)34738-7. [DOI] [PubMed] [Google Scholar]
- 70.Gill A, Madsen G, Knox M, Bisits A, Giles W, Tudehope D, Rogers Y, Smith R. Neonatal neurodevelopmental outcomes following tocolysis with glycerol trinitrate patches. Am J Obstet Gynecol. 2006;195:484–7. doi: 10.1016/j.ajog.2006.01.103. [DOI] [PubMed] [Google Scholar]
- 71.Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. BMJ. 2012;345:e6226. doi: 10.1136/bmj.e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303:1180–7. doi: 10.1001/jama.2010.310. [DOI] [PubMed] [Google Scholar]
- 73.Briel M, Bassler D, Wang AT, Guyatt GH, Montori VM. The dangers of stopping a trial too early. J Bone Joint Surg Am. 2012;94(Suppl 1):56–60. doi: 10.2106/JBJS.K.01412. [DOI] [PubMed] [Google Scholar]
- 74.Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA. 2003;289:2554–9. doi: 10.1001/jama.289.19.2554. [DOI] [PubMed] [Google Scholar]
- 75.Ross S. Composite outcomes in randomized clinical trials: arguments for and against. Am J Obstet Gynecol. 2007;196:119. e1–6. doi: 10.1016/j.ajog.2006.10.903. [DOI] [PubMed] [Google Scholar]
- 76.Ferreira-González I, Busse JW, Heels-Ansdell D, Montori VM, Akl EA, Bryant DM, et al. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials. BMJ. 2007;334:786. doi: 10.1136/bmj.39136.682083.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cordoba G, Schwartz L, Woloshin S, Bae H, Gøtzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ. 2010;341:c3920. doi: 10.1136/bmj.c3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 79.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kramer MS, Papageorghiou A, Culhane J, Bhutta Z, Goldenberg RL, Gravett M, et al. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol. 2012;206:108–12. doi: 10.1016/j.ajog.2011.10.864. [DOI] [PubMed] [Google Scholar]
- 82.Goldenberg RL, Gravett MG, Iams J, Papageorghiou AT, Waller SA, Kramer M, et al. The preterm birth syndrome: issues to consider in creating a classification system. Am J Obstet Gynecol. 2012;206:113–8. doi: 10.1016/j.ajog.2011.10.865. [DOI] [PubMed] [Google Scholar]
- 83.Villar J, Papageorghiou AT, Knight HE, Gravett MG, Iams J, Waller SA, et al. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol. 2012;206:119–23. doi: 10.1016/j.ajog.2011.10.866. [DOI] [PubMed] [Google Scholar]
- 84.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989 May;160(5 Pt 1):1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 85.Alfirevic Z. Tocolytics: do they actually work? BMJ. 2012;345:e6531. doi: 10.1136/bmj.e6531. [DOI] [PubMed] [Google Scholar]