Abstract
Background
Although ambient concentrations of particulate matter ≤10μm (PM10) are often used as proxies for total personal exposure, correlation (r) between ambient and personal PM10 concentrations varies. Factors underlying this variation and its effect on health outcome-PM exposure relationships remain poorly understood.
Methods
We conducted a random-effects meta-analysis to estimate effects of study, participant and environmental factors on r; used the estimates to impute personal exposure from ambient PM10 concentrations among 4,012 non-smoking, diabetic participants in the Women’s Health Initiative clinical trial; and then estimated the associations of ambient and imputed personal PM10 concentrations with electrocardiographic measures such as heart rate variability.
Results
We identified fifteen studies (in years 1990-2009) of 342 participants in five countries. The median r was 0.46 (range = 0.13 to 0.72). There was little evidence of funnel-plot asymmetry but substantial heterogeneity of r, which increased 0.05 (95% confidence interval [CI]= 0.01 to 0.09) per 10 μg/m3 increase in mean ambient PM10 concentration. Substituting imputed personal exposure for ambient PM10 concentrations shifted mean percent changes in electrocardiographic measures per 10μg/m3 increase in exposure away from the null and decreased their precision, e.g. −2.0% (95% CI= −4.6% to 0.7%) versus −7.9% (−15.9% to 0.9%) for the standard deviation of normal-to-normal RR interval duration.
Conclusions
Analogous distributions and heterogeneity of r in extant meta-analyses of ambient and personal PM2.5 concentrations suggest that observed shifts in mean percent change and decreases in precision may be generalizable across particle size.
Particulate matter (PM) exposure is associated with numerous adverse health outcomes, particularly those involving the cardiovascular and respiratory systems.1 Although these health effects may be strongest for small particles,2 many studies have found that large particles have independent, adverse effects on health.3 This fact, combined with global interest in PM10, suggests that focus on larger-size fractions is still merited when examining PM-disease associations. In studies of these associations, researchers have quantified PM exposure using ambient, micro-environmental, or personal sampling. Although personal concentrations may represent the most accurate assessment of total exposure, it is ambient concentrations that are federally regulated by the Environmental Protection Agency under the Clean Air Act.4 Moreover, using ambient data is often less costly for sponsors, less burdensome for participants, and sometimes the only feasible method of retrospectively characterizing PM exposure in longitudinal cohort studies. As a result, many epidemiologic studies rely on ambient concentrations of PM, which are associated with varying degrees of measurement error.
The characteristics and determinants of such exposure measurement error have been largely unknown or ignored in analyses of PM-health outcome associations, although the body of literature on this topic is growing.5-11 Further, researchers can examine the potential effects of measurement error if the relationship between ambient and personal exposures can be quantified. Fortunately, many studies have uniformly reported the correlation (r) of ambient and personal PM10 concentrations in a variety of geographic locations, often with an emphasis on vulnerable populations.12-27 However, these studies have not been systematically assessed. We therefore reviewed the literature examining the longitudinal, within-person, ambient-personal PM10 concentration correlation to identify and characterize factors influencing the observed distribution and heterogeneity of r. We illustrate how results from such a review can be used to impute personal PM concentrations from ambient concentrations, and we clarify effects of exposure measurement error on the relation of PM exposure to health outcomes in epidemiologic studies.
METHODS
Search and data abstraction strategy
We searched seven electronic databases using the strategy described in eAppendix 1. We downloaded articles to Endnote (EndNote X1; Thomson Reuters, New York, NY), de-duplicated, and examined the list for potential omissions. We reviewed each article, excluding those without PM10 concentrations measured in both ambient (central site or outside participant home) and personal environments, and those lacking an ambient-personal Pearson or Spearman correlation coefficient (r) or at least four paired ambient-personal concentrations. We then abstracted the following data: individual participant r (study mean or median if individual participant unavailable) or paired ambient-personal concentrations; number of paired concentrations; and selected characteristics of the study, participants, and environment (eTables 1-3). Article review, exclusion, and abstraction were conducted in duplicate by two authors who resolved discrepancies by consensus. We requested additional data from primary authors as needed. Coordinates were assigned to cities in which studies were conducted using the United States Geological Survey Geographic Names Information System.28 We then linked additional weather variables (eTable 3) from the National Climatic Data Center29 to the coordinates by downloading data from the three nearest monitors and calculating inverse distance-weighted means across study dates.
Meta-analysis statistical procedures
When possible, we calculated a study-level mean r (rj) that weighted each participant’s contribution by the number of that participant’s paired ambient-personal PM10 concentrations. To do this, we “r-to-z transformed” participant-level measures of r30 and then calculated a study-level mean z using the Hedges-Olkin and Rosenthal-Rubin method under a random-effects model.10,11,31 In this method, , where k denotes the number of participants in the study i identifies the participant zri the participant’s r-to-z transformed correlation coefficient, and wi the corresponding weight.31 The weight is composed of within- and between-participant variances: 1 / (ni – 3) and τ2. It is calculated as [(1 / (ni – 3)) + τ2] −1, where ni is the participant-level number of paired ambient-personal PM10 concentrations, τ2 = [Q – (k – 1)] / c , , and .31 Negative values of τ2 were set to zero.31 When participant-level data were unavailable, was calculated under a fixed-effects model as follows: , where zr is the study-level median r-to-z transformed correlation coefficient, wi is (n - 3), and n is the study-level mean number of paired ambient-personal PM10 concentrations per participant.31 The standard errors of the study-level random-effects and fixed-effects were calculated as .31 Funnel-plot asymmetry was examined by plotting the study-level versus its weight , computing Begg and Egger test statistics32,33 and completing a trim-and-fill analysis.34 We evaluated homogeneity of r using Cochran’s Q35 and explored potential sources of heterogeneity by first assembling study, participant, and environmental characteristics with putative effects on r, then dichotomizing interval-scale characteristics at their medians, and computing summary random-effects correlation coefficients within strata defined by the characteristics. We also conducted univariate, random-effects meta-regressions to examine differences in r among strata, estimated changes in r per one-unit increase in interval-scale measures,36 and examined their sensitivity to exclusion of outlying observations identified using an extreme studentized deviate multiple-outlier procedure.37 Potential sources of heterogeneity identified in univariate random-effects meta-regressions were dichotomized at their median values (for continuous variables) and included in bivariable random-effects meta-regressions when cross-classification cell size was ≥ 2 to examine the possibility that one variable might explain all or part of the relationship observed between r and the other variables.
Imputation of Personal PM10 Concentration
We used the results of the meta-analysis to impute personal PM10 concentrations from ambient concentrations. Imputation was performed among 4,012 non-smoking, diabetic women who participated in the Women’s Health Initiative clinical trial. Women had to be residing in the contiguous U.S. at the time of their first resting, standard, twelve-lead electrocardiogram (ECG), for which measures of RR, PR, QRS, and QT interval durations, as well as the root mean square of successive differences in and the standard deviation of normal-to-normal RR interval duration, were available.38-40 Collectively, the ECG measures reflect the rate of atrioventricular conduction, rate of ventricular depolarization / repolarization, and variation in heart rate.41 Each has been recommended as a candidate outcome in studies of air-pollution health effects under a mechanistic hypothesis postulating that the cardiovascular effects of air pollution depend in part on autonomic and myocardial pathophysiology.42
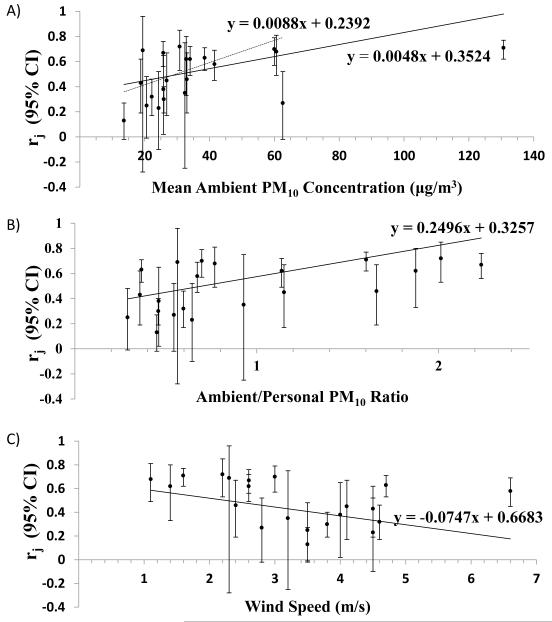
Imputation was completed in two steps. In step 1, we estimated participant-specific correlations between ambient and personal PM concentrations using the random-effects meta-regression equation, r = β0 + β1x (Figure 3A, solid line), where β0 is the intercept and x is the participant-specific ambient PM10 concentration, a plausible, consistently identified, and important source of between-study heterogeneity in r.10,11 In this setting, ambient PM10 concentrations were the geocoded address-specific daily means43,44 averaged over the day of and two days before (lag0-2) the ECG recording.
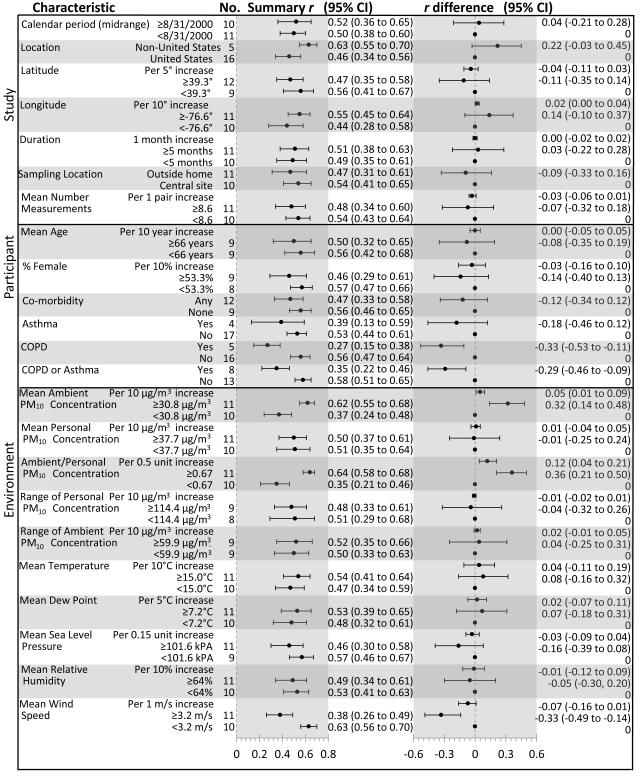
Figure 3.
Summary Random-Effects Correlation Coefficients and Meta-Regression Differences by Study, Participant, and Environment Characteristics. Summary r computed within strata of each characteristic. Difference in r from meta-regression analyses predicting r from the characteristics.
In step 2, we assumed that the distributions of the ambient and personal PM concentrations are bivariate normal and estimated the participant-specific mean personal PM10 concentration (p) at a given ambient PM10 concentration (x) using the equation, , where for each participant i, r is estimated as in Step 1; is the mean (standard deviatio ambient PM10 concentration among the Women’s Health Initiative participants; and is the mean (standard deviation) personal PM10 concentration estimated from the distributions of the personal concentrations observed in the studies contributing to the meta-analysis. The variance of μp∣x was calculated as .
Bias Analysis
The authors assessed effects of exposure measurement error by (i) iterating participant-specific estimation of μp∣x as in Step 2 using y and sy from each of the d studies contributing both pieces of information to the meta-analysis, (ii) computing the random-effects weighted mean and variance of the d estimates of μp∣x for each participant, (iii) regressing each of the ECG measures on the weighted mean μp∣x, and then (iv) comparing the estimated associations with conventional estimates obtained by regressing the same ECG measures on xi. In (iii), error-in-variables regression models were implemented in SAS® Proc Calis (SAS; Cary, NC) to accommodate the random-effects weighted variance of the weighted mean μp∣x, averaged across all participants. A covariable adjustment strategy similar to that in Whitsel et al.45 was adopted in both (iii-iv). This strategy involved adjusting for the previously described sociodemographic, geographic, temporal, clinical, behavioral, and environmental variables footnoted in the Table.
Table.
Percent Change in Electrocardiographic Measures per 10μg/m3 Increase in PM10 Concentration Among 4,012 Non-smoking, Diabetic Women’s Health Initiative Clinical Trial Participants, United States, 1993-2004.
| ECG Measure | Ambient PM10 | Imputed Personal PM10 | ||
|---|---|---|---|---|
| % (95% CI)a | Posterior probability of %>0 |
% (95% CI)a | Posterior probability of %>0 |
|
| Root mean square of successive differences in normal-to-normal RR interval duration |
−1.5 (−4.3 to 1.3) | 0.14 | −6.7 (−15.3 to 2.8) | 0.08 |
| Standard deviation of normal-to- normal RR interval duration |
−2.0 (−4.6 to 0.7) | 0.07 | −7.9 (−15.9 to 0.9) | 0.04 |
| RR interval duration | −0.2 (−0.8 to 0.4) | 0.25 | −1.0 (−2.9 to 0.9) | 0.15 |
| PR interval duration | −0.2 (−0.7 to 0.3) | 0.21 | −0.5 (−2.2 to 1.3) | 0.30 |
| QRS interval duration | 0.1 (−0.4 to 0.6) | 0.66 | 0.2 (−1.5 to 1.9) | 0.59 |
| QT interval duration | 0.0 (−0.3 to 0.3) | 0.50 | −0.2 (−1.2 to 0.8) | 0.34 |
Fully Adjusted (age, race/ethnicity, education, region, time of day (minutes), day of week, season, body mass index (kg/m2), hypertension, systolic blood pressure (mm Hg), anti-arrhythmia medication use, total energy expenditure (kcal/kg*week), chronic lung disease, hypercholesterolemia, coronary heart disease, revascularization, congestive heart failure, lag0-1 temperature (°C), dew point (°C), and barometric pressure (kPa))
RESULTS
The electronic search strategy identified 698 articles, of which 14 (2.0%) met inclusion criteria. We identified an additional unpublished thesis, yielding a total of 15 studies. In addition, three studies provided results for sub-studies, totaling 21 for analysis. The studies were conducted over 20 years (1988-2007) and encompassed a large geographic area including 19 cities, 8 U.S. states, and 5 countries. The studies included 342 participants (median: 14 per sub-study) who were assessed over widely varying durations (0.3 to 21.0 months); however, samples were collected for 24-hour periods in 19 (90%) sub-studies (eTable 1).
The mean participant age ranged from 9 to 85 years and several sub-studies focused on populations with conditions commonly associated with increased susceptibility to PM health effects: chronic obstructive pulmonary disease (COPD, 24%), asthma (19%), and coronary artery disease (19%) (eTable 2).
As several studies spanned multiple seasons, mean weather variables should be viewed cautiously; however, the ranges of mean temperature (−4° to 30°C) and wind speed (1 to 7 m/s) were large. The ranges of mean personal and ambient PM10 concentrations also were large among studies: 12 to 115 μg/m3 and 14 to 131 μg/m3, respectively. Despite these ranges, personal concentrations were typically greater than ambient concentrations (eTable 3), and only one value was identified as an outlier: the mean ambient PM10 concentration in the paper by Watchalayann et al.23
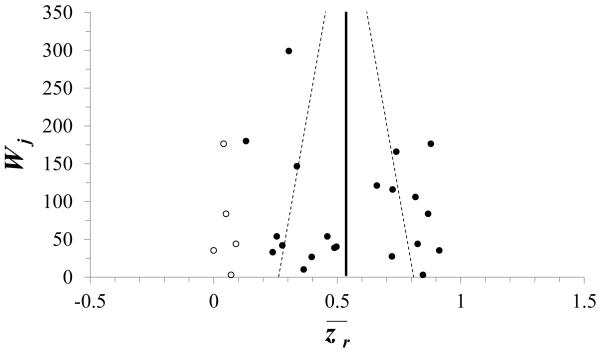
The median of rj was 0.46 (range = 0.13 to 0.72) (Figure 1; eTable 3), with no outlying r values. Although the funnel plot symmetry test P-values were high (PEgger=0.6, PBegg=0.9), the visual impression of the plot suggested asymmetry and the trim-and-fill analysis imputed five hypothetically missing results, all with rj near zero (Figure 2). In addition, there was substantial evidence of heterogeneity (PCochran’s Q <0.001). Consequently, an overall summary r was not estimated.
Figure 1.
Forest plot of 21 estimates of rj (95% confidence interval) from twenty-one sub-studies of the within-participant correlation between ambient and personal PM10 concentration. (See eTable 3 for details).
Figure 2.
Funnel plot of 21 reported (•) and five imputed (○) estimates of the z-transformed rj from twenty-one sub-studies of the within-participant correlation coefficient between ambient and personal PM10 concentrations, where wj is the inverse variance of the z-transformed rj.
The magnitude and precision of stratum-specific, random-effects correlation coefficients suggested that participants without COPD or asthma, and those exposed to higher ambient PM10 concentrations, higher ambient-to-personal concentration ratios, and lower wind speeds, had more strongly correlated ambient and personal PM10 concentrations (Figure 3). Random-effects meta-regression results were consistent with these suggestions (Figures 3-4), as was the strengthened association between the ambient PM10 concentration and r after excluding an outlying ambient PM10 concentration (Figure 4). Although study location appeared to influence r, 76% of studies were located in the U.S., and r was similar among north-south and east-west dichotomization of coordinates (Figure 3). In addition, r was comparable among studies relying on PM10 measured at a central site versus outside home (0.54 [95% CI= 0.41 to 0.65] versus 0.47 [0.31 to 0.61]) and over the range of ambient and personal concentrations (Figure 3). Between-group differences were slightly attenuated in bivariable meta-regressions including combinations of mean ambient PM10 concentration, ambient-to-personal PM10 concentration ratio, and wind speed; however overall conclusions did not change (eTable 4).
Figure 4.
Plot of 21 estimates of rj (95% confidence interval) from twenty-one sub-studies of the within-participant correlation between ambient and personal PM10 concentrations versus (A) mean ambient PM10 concentration (μg/m3), (B) mean ambient to personal concentration ratio, and (C) mean wind speed (m/s). Univariate random-effects regression lines (solid line). Excluding the outlying 130.7 μg/m3 ambient PM10 concentration from Watchalayann et al. (dotted lines).
The median ambient PM10 concentration measured among Women’s Health Initiative participants was 25.7 (range = 7.3 to 109.6) μg/m3. Before and after excluding the outlying results,23 the median imputed r was 0.46 (range = 0.38 to 0.71) and 0.44 (0.30 to 0.84), respectively, while the corresponding median imputed personal PM10 concentration was 34.1 (23.8 to 135.7) μg/m3 and 28.6 (20.6 to 152.8) μg/m3.
The relationships between PM10 concentration, the root mean square of successive differences in normal-to-normal RR interval duration, and the standard deviation of normal-to-normal RR interval duration were notable in the bias analysis (Table). When the ambient PM10 concentration was used as the exposure, percent changes in the root mean square of successive differences in normal-to-normal RR interval duration and the standard deviation of normal-to-normal RR interval duration per 10 μg/m3 increase were −1.5% (95% CI= −4.3 to 1.3) and −2.0% (−4.6 to 0.7), but when the imputed personal concentration was substituted for the ambient PM10 concentration, corresponding estimates shifted away from the null and their precision decreased: −6.7% (95% CI= −15.3 to 2.8) and −7.9% (−15.9 to 0.9). The posterior probabilities of a positive percent change also decreased, from 0.14 to 0.08 and 0.07 to 0.04, respectively. Similar changes were observed across ECG measures in sensitivity analyses excluding (1) Watchalayann et al,23 (2) child studies14, 19, 22, and (3) both 1 and 2. For example, percent changes in the root mean square of successive differences in normal-to-normal RR interval duration and the standard deviation of normal-to-normal RR interval duration per 10 μg/m3 increase were −5.5% (95% CI= −12.6 to 2.2) and −6.5% (−13.1 to 0.7) when Watchalayann et al.23 and child studies14,19, 22 were excluded from the bias analysis.
DISCUSSION
The use of ambient PM10 concentrations in health association studies remains commonplace. Although potentially important sources of measurement error in this surrogate of true personal exposure have been suggested by many investigators, 5-11 no systematic review or application of results from studies examining the correlation between ambient and personal PM10 concentrations has been carried out to date. We therefore summarized these studies, characterized factors influencing the among-study heterogeneity of r, and then described an accessible framework for using quantitative information about the sources of heterogeneity to impute personal from ambient PM concentrations and clarify the effects of exposure measurement error on health outcome-PM exposure relationships.
The summary included a funnel plot suggesting that the historically high costs and burdens associated with personal PM monitoring may have resulted in more studies of r enrolling few participants (scattered near the bottom of the plot) and few studies enrolling many participants (near the top). The results imputed by the accompanying trim-and-fill analysis could represent those that remain unpublished for a variety of reasons, such as implausibility (correlations near or below zero) or discordance with the extant literature. Had such low correlations actually been withheld from publication, the observed among-study heterogeneity of r would have been even greater. Despite this possibility, the tests of funnel-plot asymmetry support the ability of the included studies to represent the literature and their suitability for meta-analysis.
Because the meta-analysis provided substantial evidence of among-study heterogeneity of r, presentation of an overall fixed- or random-effects summary correlation coefficient was not warranted. Instead, we characterized the potential sources of heterogeneity. As r changed little with the range of the study-specific ambient or personal PM10 concentration, its association with other variables was anticipated. That expectation was substantiated by the observed increase in the ambient-personal PM10 concentration correlation with increasing ambient PM10 concentration, increasing ambient-to-personal PM10 concentration ratio, and decreasing wind speed. Additionally, we observed higher correlations in participants without versus with COPD or asthma.
The observed patterns appear plausible. In areas where ambient concentrations or ambient-to-personal concentration ratios are high, ambient PM may contribute more to total personal exposure than in areas where ambient concentrations are low. Direct increases in exposure to ambient concentrations, changes in ventilation, or altered activity patterns may account for this. Wind speed also may influence the ambient-personal PM10 concentration correlation, as it affects the distribution of PM in the environment. Lower wind speed impedes dispersion of PM10 from its sources, thus allowing central site monitors to better predict an individual participant’s exposure to ambient PM.46 Persons with and without COPD (or asthma) may also have different activity patterns, such as time spent outdoors,47 which could influence the relationship between their personal and ambient concentrations of PM.
We used bivariable meta-regression models to address the possibility that one of the aforementioned factors could explain part or all of the association of another with r (eTable 4). However, too few studies included participants with COPD, thereby preventing examination of this characteristic in bivariable meta-regression. Estimates of r did not differ substantially among the uni- and bi-variable meta-regression models, suggesting that meta-confounding of the univariable association of r with ambient PM10 concentration, ambient-to-personal PM10 concentration ratio, and wind speed may be less of a concern in this context. Nevertheless, all bivariable meta-regressions should be interpreted cautiously, given sample size constraints.
The observed pattern of ambient-personal PM10 correlation coefficients (r > 0; low median; high range) is similar to those previously reported in meta-analyses of PM2.5.10,11 Further, the meta-analyses of both PM10 and PM2.5 suggest that ambient PM concentrations are an important source of heterogeneity in r.10,11 Although PM2.5 concentrations comprise a large portion of PM10 concentrations, the extent of the similarity was unexpected. The differing distributive properties of the two size fractions48 suggest that r would be somewhat higher for PM2.5 than PM10. While the ambient-personal PM10 correlation may have been driven by PM2.5, data availability and methodological constraints limit ability of the present study to determine the extent to which this is true. Nonetheless, the similarity suggests that a variable and non-negligible degree of measurement error is incurred when using ambient PM concentrations as proxies for personal exposures in studies of PM-health associations, regardless of particle size.
The direction of PM effects on heart-rate variability in this setting is consistent with that described by a recent review of the topic. Ambient PM was inversely associated with the root mean square of successive differences in (and the standard deviation of) normal-to-normal RR interval duration, overall and among a variety of sub-groups.49 However, the review did not address the error inherent in substituting ambient for personal exposures, which has several components.8 In the present study, we addressed the component most likely to produce bias (the difference between average personal and true ambient exposure), because the remaining components are largely Berksonian and therefore less likely to produce bias. The results suggest that this non-Berksonian component behaves like classical exposure measurement error to the extent that it biases PM10 health-effect estimates toward the null when r depends on the ambient PM10 concentration, as in the current meta-analysis. This observation may well generalize across particle size, given the analogous dependence of r on centrally and proximally measured ambient PM2.5 concentrations in prior meta-analyses.10,11 As such, the true magnitude of PM effects on heart-rate variability may be larger than previously anticipated by Pieters and colleagues.49
Controlling for the effects of PM measurement error as described herein has some general disadvantages when compared with error correction methods such as regression calibration and hierarchical Bayesian analyses. One is its dependence on relatively small, technically complex and, in some cases, incompletely documented studies of potentially low-level exposures measured with behavior-altering personal monitors. Another is that bias and precision may vary among populations with ambient or personal PM concentrations that are unlike those observed in the Women’s Health Initiative or the meta-analyses, and perhaps unpredictably so among populations that smoke. Simultaneously evaluating multiple sources of heterogeneity in the ambient-personal PM10 correlation within a meta-analysis of 21 studies is an additional challenge. Our frequentist methods also assume bivariate normality of ambient and personal PM concentrations, which may be unrealistic. Nevertheless, the range of ambient PM concentrations is wide in both the Women’s Health Initiative 44,45, 50-52 and these meta-analyses10,11; four of five U.S. adults aged ≥ 18 years do not smoke53; and robustness to modest departure from normality is well-known. Moreover, error-in-variables regression and quantitative bias assessments are familiar to epidemiologists54 and readily accessible to a wide variety of users. In this case, they are illustrative of the meta-analytic foundation on which more comprehensive and rigorous (e.g. hierarchical Bayesian) approaches to improving estimation of air-pollution effects could be built and applied in settings where only ambient PM concentration data are available.55
Such application may well benefit from the fact that we relied on a systematic review encompassing a wide variety of settings and allowing for broad examination of study, participant, and environmental effects on the ambient-personal PM10 correlation. By focusing on total personal PM10 exposure instead of personal exposure to PM10 of ambient origin, the data collection effort also avoided complications associated with the potentially unrealistic assumption that personal exposure is best assessed by relying on a distinctly smaller and less accessible group of microenvironmentally homogenous, single-marker (e.g. sulfate) studies. In contrast, the data that were collected, quality controlled, and tabulated in eTables 1-3, readily facilitate sensitivity analyses at the discretion of future users, an option infrequently available with regression calibration factors published in isolation.
Other powerful methods for improving estimation of ambient exposures at geocoded participant addresses have been proposed.44, 56-61 The dual benefit of improving estimation of total personal exposure to PM—a particular interest in etiologic studies—and clarifying the downstream effects of the measurement error with which it is associated, helps distinguish the meta-analytically informed interpolation method illustrated here from those alternatives. Although total personal exposure to PM is not regulated under the Clean Air Act, the effect of aggregate PM exposure on health is of no less scientific interest. The current and previously published meta-analyses10,11 provide the necessary data and an accessible statistical framework for estimating such exposure and conducting participant-level analysis of bias in ambient PM concentration-health association studies. In combination, the data and framework detailed here can be leveraged to increase understanding of the true, but often masked, relationships underlying such associations.
Supplementary Material
Acknowledgements
We thank Jingjing Li and P. Miguel Quibrera for their assistance in compiling the dataset, and we acknowledge the contributions of WHI Investigators in the NHLBI Program Office, Clinical Coordinating Center and affiliated Academic Centers. A complete list of academic centers and investigators who have contributed to WHI science is available at https://cleo.whi.org/researchers/SitePages/Write%20a%20Paper.aspx.
Conflicts of Interest and Sources of Funding This work was supported by the National Institute of Environmental Health Sciences (grant R01-ES012238) and a National Research Service Award from the National Heart, Lung and Blood Institute, U.S. Department of Health and Human Services (grant T32-HL007055 to K.M.H.). The NHLBI/DHHS also funded the Women’s Health Initiative program (contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, and HHSN268201100004C). Smith RL received funding from a SAMSI grant, NSF-DMS 0635449.
Although this work was reviewed by EPA and approved for publication, it may not necessarily reflect official Agency policy.
Footnotes
We have no conflicts of interest to declare.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com). This content is not peer-reviewed or copy-edited; it is the sole responsibility of the author.
REFERENCES
- 1.United States Environmental Protection Agency . Integrated Science Assessment for Particulate Matter (Final Report) United States Environmental Protection Agency; Washington, DC: 2009. EPA/600/R-08/139F. [PubMed] [Google Scholar]
- 2.Peng RD, Chang HH, Bell ML, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. JAMA. 2008;299(18):2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunekreef B, Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur Respir J. 2005;26(2):309–318. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- 4.Clean Air Act 1990. Public Law 101-549. 42 U.S.C. §7401-7671q, 104 Stat. 2399.
- 5.Shy CM, Kleinbaum DG, Morgenstern H. The effect of misclassification of exposure status in epidemiological studies of air pollution health effects. Bull N Y Acad Med. 1978;54(11):1155–1165. [PMC free article] [PubMed] [Google Scholar]
- 6.United States Environmental Protection Agency . United States Environmental Protection Agency. Air quality criteria for particulate matter. United States Environmental Protection Agency; Washington DC: 1996. Human exposure to particulate matter: relations to ambient and indoor concentrations. EPA/600/P-95/001aF-cF. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=2832#Download. [Google Scholar]
- 7.Wallace L. Correlations of personal exposure to particles with outdoor air measurements: a review of recent studies. Aerosol Science and Technology. 2000;32(1):15–25. [Google Scholar]
- 8.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominici F, Zeger SL. A measurement error model for time-series studies of air pollution and mortality. Biostatistics. 2000;1(2):157–175. doi: 10.1093/biostatistics/1.2.157. [DOI] [PubMed] [Google Scholar]
- 10.Avery CL, Mills KT, Williams R, et al. Estimating error in using ambient PM2.5 concentrations as proxies for personal exposures: a review. Epidemiology. 2010;21(2):215–223. doi: 10.1097/EDE.0b013e3181cb41f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avery CL, Mills KT, Williams R, et al. Estimating error in using residential outdoor PM2.5 concentrations as proxies for personal exposures: a meta-analysis. Environ Health Perspect. 2010;118(5):673–678. doi: 10.1289/ehp.0901158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lioy PJ, Waldman JM, Buckley T, et al. The personal, indoor and outdoor concentrations of PM-10 measured in an industrial community during the winter. Atmospheric Environment. 1990;24(1):57–66. [Google Scholar]
- 13.Wallace L. Indoor particles: a review. J Air Waste Manag Assoc. 1996;46(2):98–126. doi: 10.1080/10473289.1996.10467451. [DOI] [PubMed] [Google Scholar]
- 14.Janssen NAH, Hoek G, Harssema H, et al. Childhood exposure to PM10: relation between personal, classroom, and outdoor concentrations. Occup Environ Med. 1997;54(12):888–894. doi: 10.1136/oem.54.12.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen NAH, Hoek G, Brunekreef B, et al. Personal sampling of particles in adults: relation among personal, indoor, and outdoor air concentrations. Am J Epidemiol. 1998;147(6):537–547. doi: 10.1093/oxfordjournals.aje.a009485. [DOI] [PubMed] [Google Scholar]
- 16.Linn WS, Gong H, Jr, Clark KW, et al. Day-to-day particulate exposures and health changes in Los Angeles area residents with severe lung disease. J Air Waste Manag Assoc. 1999;49(9 Spec No):108–115. doi: 10.1080/10473289.1999.10463890. [DOI] [PubMed] [Google Scholar]
- 17.Rojas-Bracho L, Suh HH, Koutrakis P. Relationships among personal, indoor, and outdoor fine and coarse particle concentrations for individuals with COPD. J Expo Anal Environ Epidemiol. 2000;10(3):294–306. doi: 10.1038/sj.jea.7500092. [DOI] [PubMed] [Google Scholar]
- 18.Sarnat JA, Koutrakis P, Suh HH. Assessing the relationship between personal particulate and gaseous exposures of senior citizens living in Baltimore, MD. J Air Waste Manag Assoc. 2000;50(7):1184–1198. doi: 10.1080/10473289.2000.10464165. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler AJ, Williams I, Beaumont RA, et al. Characterisation of particulate matter sampled during a study of children’s personal exposure to airborne particulate matter in a UK urban environment. Environ Monit Assess. 2000;65(1-2):69–77. [Google Scholar]
- 20.Rodes CE, Lawless PA, Evans GF, et al. The relationships between personal PM exposures for elderly populations and indoor and outdoor concentrations for three retirement center scenarios. J Expo Anal Environ Epidemiol. 2001;11(2):103–15. doi: 10.1038/sj.jea.7500155. [DOI] [PubMed] [Google Scholar]
- 21.Evans GF, Highsmith RV, Sheldon LS, et al. The 1999 Fresno particulate matter exposure studies: comparison of community, outdoor, and residential PM mass measurements. J Air Waste Manag Assoc. 2000;50(11):1887–1896. doi: 10.1080/10473289.2000.10464224. [DOI] [PubMed] [Google Scholar]
- 22.Yip FY, Keeler GJ, Dvonch JT, et al. Personal exposures to particulate matter among children with asthma in Detroit, Michigan. Atmospheric Environment. 2004;38(31):5227–5236. doi: 10.1289/ehp.02110s2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watchalayann P, Srisatit T, Watts D, et al. Exposure to PM-10 of shop house dwellers in Bangkok, Thailand. Science Asia. 2005;31(4):359–367. [Google Scholar]
- 24.Williams R, Case M, Yeatts K, et al. Personal coarse particulate matter exposures in an adult cohort. Atmospheric Environment. 2008;42(28):6743–6748. [Google Scholar]
- 25.Arhami M, Polidori A, Delfino RJ, et al. Associations between personal, indoor, and residential outdoor pollutant concentrations: implications for exposure assessment to size-fractionated particulate matter. J Air Waste Manag Assoc. 2009;59(4):392–404. doi: 10.3155/1047-3289.59.4.392. [DOI] [PubMed] [Google Scholar]
- 26.Scapellato ML, Canova C, de Simone A, et al. Personal PM10 exposure in asthmatic adults in Padova, Italy: seasonal variability and factors affecting individual concentrations of particulate matter. Int J Hyg Environ Health. 2009;212(6):626–636. doi: 10.1016/j.ijheh.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Hsu S. Characterization of Exposures to Urban Particulate Matter (PM) and its Components in COPD Patients in New York City and Seattle [dissertation] New York University; New York, NY: 2009. [Google Scholar]
- 28.United States National Geological Survey [Accessed February 2010];Geographic Names Information System. Available: http://geonames.usgs.gov/pls/gnispublic/f?p=154:1:559715803173776.
- 29.National Climatic Data Center [Accessed February 2010];Weather/Climate Events. Available: http://www.ncdc.noaa.gov/oa/climateresearch.html.
- 30.Fisher RA. Statistical Methods for Research Workers. Oliver and Boyd; Edinburgh: 1925. [Google Scholar]
- 31.Field AP. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed- and random-effects methods. Psychol Methods. 2001;6(2):161–180. doi: 10.1037/1082-989x.6.2.161. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 34.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 35.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 36.Berkey CS, Hoaglin DC, Mosteller F, et al. A random-effects regression model for meta-analysis. Stat Med. 1995;14(4):395–411. doi: 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- 37.Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25:165–172. [Google Scholar]
- 38.The Women’s Health Initiative Study Group Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 39.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 40.Schroeder EB, Whitsel EA, Evans GW, et al. Repeatability of heart rate variability measures. J Electrocardiol. 2004;37(3):163–172. doi: 10.1016/j.jelectrocard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Prineas RJ, Crow RS, Zhang Z. The Minnesota Code Manual of Electrocardiographic Findings. 2nd ed Springer-Verlag London Limited; London: 2010. [Google Scholar]
- 42.Zareba W, Nomura A, Couderc JP. Cardiovascular effects of air pollution: what to measure in ECG? Environ Health Perspect. 2001;109(Suppl 4):533–538. doi: 10.1289/ehp.01109s4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitsel EA, Quibrera PM, Smith RL, et al. Accuracy of commercial geocoding: assessment and implications. Epidemiol Perspect Innov. 2006;3(1):8. doi: 10.1186/1742-5573-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao D, Peuquet DJ, Duan Y, et al. GIS approaches for the estimation of residential-level ambient PM concentrations. Environ Health Perspect. 2006;114(9):1374–1380. doi: 10.1289/ehp.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitsel EA, Quibrera PM, Christ SL, et al. Heart rate variability, ambient particulate matter air pollution, and glucose homeostasis: the environmental epidemiology of arrhythmogenesis in the Women’s Health Initiative. Am J Epidemiol. 2009;169(6):693–703. doi: 10.1093/aje/kwn400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcazzan GM, Vaccaro S, Valli G, et al. Characterization of PM10 and PM2.5 particulate matter in the ambient air of Milan (Italy) Atmospheric Environment. 2001;35(27):4639–4650. [Google Scholar]
- 47.Liu LJS, Box M, Kalman D, et al. Exposure assessment of particulate matter for susceptible populations in Seattle. Environ Health Perspect. 2003;111(7):909–918. doi: 10.1289/ehp.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godoy SM, Mores PL, Santa Cruz ASM, et al. Assessment of impact distances for particulate matter dispersion: a stochastic approach. Reliability Engineering and System Safety. 2009;94(10):1658–1665. [Google Scholar]
- 49.Pieters N, Plusquin M, Cox B, et al. An epidemiological appraisal of the association between heart rate variability and particulate air pollution: a meta-analysis. Heart. 2012;98(15):1127–1135. doi: 10.1136/heartjnl-2011-301505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–58. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 51.Zhang ZM, Whitsel EA, Quibrera PM, et al. Ambient fine particulate matter exposure and myocardial ischemia in The Environmental Epidemiology of Arrhythmogenesis in the Women’s Health Initiative (EEAWHI) Environ Health Perspect. 2009;117(5):751–756. doi: 10.1289/ehp.0800046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao D, Whitsel EA, Duan Y, et al. Ambient particulate air pollution and ectopy – The Environmental Epidemiology of Arrhythmogenesis in WHI (EEAWHI), 1999-2004. J Toxicol Environ Health A. 2009;72(1):30–38. doi: 10.1080/15287390802445483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged 18 years—United States, 2005–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1207–1212. [PubMed] [Google Scholar]
- 54.Lash T, Fox P, Fink A. Applying Quantitative Bias Analysis to Epidemiologic Data. Springer Science+Business Media; New York, NY: 2009. [Google Scholar]
- 55.McBride SJ, Williams RW, Creason J. Bayesian hierarchical modeling of personal exposure to particulate matter. Atmospheric Envionrment. 2007;41:6143–6155. [Google Scholar]
- 56.Raghunathan TE, Diez-Roux A, Chen W. Predicting cumulative particulate matter exposure using space-time models and historical monitor data. Epidemiol. 2006;17(6S):S250. [Google Scholar]
- 57.Yanosky JD, Paciorek CP, Schwartz J, et al. Spatio-temporal modeling of chronic PM10 exposure for the Nurses’ Health Study. Atmos Environ. 2008;42:4047–4062. doi: 10.1016/j.atmosenv.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paciorek CJ, Yanosky JD, Suh HH. Practical large-scale modeling of particulate matter. Ann Appl Stat. 2009;3:370–397. [Google Scholar]
- 59.Szpiro AA, Sheppard L, Lumley T. Efficient measurement error correction with spatially misaligned data. Biostatistics. 2011;12(4):610–623. doi: 10.1093/biostatistics/kxq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yanosky JD, Paciorek CJ, Suh HH. Predicting chronic fine and coarse particulate exposures using spatiotemporal models for the Northeastern and Midwestern United States. Environ Health Perspect. 2009;117(4):522–529. doi: 10.1289/ehp.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christakos G, Serre ML. BME analysis of spatiotemporal particulate matter distributions in North Carolina. Atmospheric Environment. 2000;34:3393–3406. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.