Abstract
Context is an essential component of learning and memory processes, and the hippocampus is critical for encoding contextual information. However, connecting hippocampal physiology with its role in context and memory has only recently become possible. It is now clear that contexts are represented by coherent ensembles of hippocampal neurons and new optogenetic stimulation studies indicate that activity in these ensembles can trigger the retrieval of context appropriate memories. We interpret these findings in light of recent evidence that the hippocampus is critically involved in using contextual information to prevent interference, and propose a theoretical framework for understanding contextual influence of memory retrieval. When a new context is encountered, a unique hippocampal ensemble is recruited to represent it. Memories for events that occur in the context become associated with the hippocampal representation. Revisiting the context causes the hippocampal context code to be re-expressed and the relevant memories are primed. As a result, retrieval of appropriate memories is enhanced and interference from memories belonging to other contexts is minimized.
Keywords: Context, Memory, Hippocampus, Interference
1. Introduction
The context plays an undeniably profound role in memory. Learned information is bound to the learning context, and the context can be a remarkably potent retrieval cue (Smith, 1988). Anyone who has returned to their childhood neighborhood after decades away can attest to the striking experience of long lost memories that come flooding back in vivid detail. Empirical studies of contextual cueing of memory have a long history in psychology. Items learned in one context are better recalled when testing takes place in the same context (Godden and Baddely, 1975). The context can also serve as a disambiguating cue that allows subjects to retrieve information associated with one context without interference from items learned in other contexts. For example, subjects who learn two lists of items in distinct contexts exhibit better recall than those who learn both lists in the same context (for review, see Smith, 1988). In fact, the association between context and memory is so strong that simply asking subjects to think about the learning environment is sufficient to improve recall (Smith, 1979).
The hippocampus has been known to be involved in processing contextual information since the 1970s (Hirsh, 1974). In the decades since, several theories of hippocampal context coding have been proposed. Several authors have noted the similarity between spatial mapping functions of the hippocampus and representations of the environmental context (Mizumori, 2007; Nadel et al., 1985). Another theory holds that the hippocampus binds the various components of the context into a complex multimodal configural cue (Sutherland and Rudy, 1989). Yet another theory suggests that context representations are a natural consequence of the relational memory encoding functions of the hippocampus (Cohen and Eichenbaum, 1994). Despite these theoretical accounts, detailed knowledge about the form of these hippocampal context representations has only recently become available, with the advent of large scale neuronal population recording and ensemble stimulation techniques. In this article, we review new findings about the nature of hippocampal context representations and present evidence that each context a subject encounters is encoded by a unique ensemble of hippocampal neurons. With experience, these hippocampal ensemble context codes become associated with the memories and behaviors that are appropriate for that context. When subjects revisit a familiar context, the hippocampal context code is automatically re-expressed, thereby priming the relevant memories and reducing the interference from memories associated with other contexts.
2. The Hippocampus and Context
In this article, we focus our discussion on the nature of hippocampal context representations and their functional significance for preventing interference. More general discussion of the hippocampal role in contextual memory can be found in several comprehensive reviews (Eichenbaum et al., 2012; Holland and Bouton, 1999; Lee and Lee, 2013; Maren et al., 2013; Mizumori, 2013; Mizumori et al., 1999; Rudy, 2009). Current ideas about the hippocampal role in context coding have come primarily from two parallel streams of research on conditioning and spatial navigation. Conditioning research has shown that learned behaviors are linked to the learning environment (i.e. the context) and that hippocampal lesions reliably disrupt contextual associations (for reviews see Anagnostaras et al., 2001; Maren, 2001; Myers and Gluck, 1994). The most well studied of these behaviors is contextual fear conditioning, in which rats quickly learn to fear an environment where foot shock occurs. Hippocampal lesions selectively impair conditioned fear responses to the context but do not impair fear responses to phasic cues, such as a tone or light (Kim and Fanselow, 1992; Phillips and LeDoux, 1992). Other studies have shown that subjects with hippocampal lesions are insensitive to changes in the context. For example, intact control subjects trained in one context showed reduced responding when tested in another context, but subjects with hippocampal or entorhinal cortical damage continued to respond as if they did not notice the context had changed (Freeman et al., 1997; Honey and Good, 1993; Penick and Solomon, 1991). Finally, the hippocampus is needed for the ability to match a learned behavior with the appropriate context (Good and Honey, 1991; Kim et al., 2012; Smith et al., 2004). In one study (Smith et al., 2004), intact controls were readily able to learn one auditory discrimination problem in one context and a different discrimination in another context. In contrast, subjects with fornix lesions were severely impaired and were only able to learn one discrimination problem at a time. These findings suggest that the context can directly elicit conditioned responses or prime the relevant behaviors so that when an appropriate cue is encountered retrieval is facilitated.
As a number of authors have noted, the well-known spatial firing properties of hippocampal neurons (i.e. place fields, O’Keefe and Nadel, 1978) are consistent with the idea of a hippocampal role in representing contexts (Mizumori et al., 2007; Nadel et al., 1985; Smith, 2008). Hippocampal neurons reliably change their activity patterns in response to changes in the spatial/environmental context (Anderson and Jeffery, 2003; Muller and Kubie, 1987). However, it is now apparent that hippocampal neurons are also highly sensitive to a variety of non-environmental aspects of the experimental situation. For example, small changes in the task demands, such as switching from a random foraging strategy to following an experimenter-defined path for rewards, cause large changes in hippocampal place fields (i.e. remapping, Markus et al., 1995). This kind of hippocampal sensitivity to task demands has been seen in a variety of experimental conditions (Eichenbaum and Cohen, 1988; Ferbinteanu and Shapiro, 2003; Smith and Mizumori, 2006b; Wood et al., 2000). Hippocampal firing is also influenced by other non-environmental aspects of the situation, including whether the subject plays an active or passive role in the task (Terrazas et al., 2005), the strategy needed to solve the task (Eschenko and Mizumori, 2007), expectations (Skaggs and McNaughton, 1998) and even the subject’s motivational state (Kennedy and Shapiro, 2009).
These observations complicate how we should think about hippocampal context coding. The term ‘context’ can be problematic due to the difficulty in clearly defining a concept that has been used in many different ways. By convention, most conditioning studies have operationally defined the context as the continuously present background cues. However, this convention should not limit the way we think about the neural systems that encode contexts. The notion of context is necessarily broad because it refers to any situation defined by a coherent set of conditions, and meaningful contextual distinctions frequently occur within a single environment. For example, a staff meeting and the department holiday party are very different contexts even though they may occur in the same conference room. Animals also differentiate these kinds of abstractly defined contexts, as do their hippocampal neurons. We will use the term context to refer to any experimental situation that has a coherent set of expectations and appropriate behaviors. More importantly, when we refer to a hippocampal context code, we specifically mean a representation that can uniquely identify a given experimental situation, regardless of whether that situation is characterized by a particular environment or by more abstract features such as the task demands.
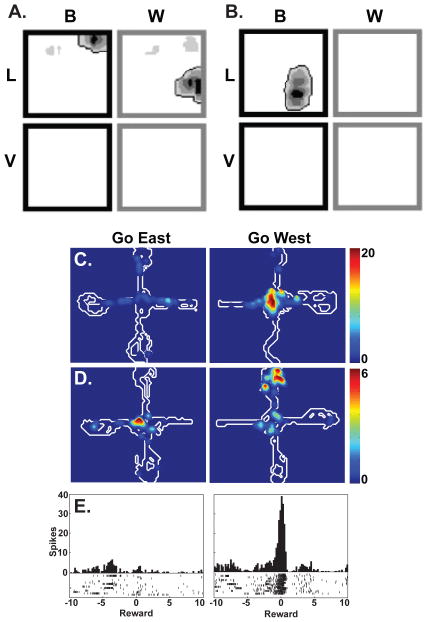
We examined the hippocampal role in encoding contexts in a series of neuronal recording studies in which rats learned to distinguish two different behavioral contexts (Fig. 1, Smith and Mizumori, 2006b). In this task, rats were trained to approach the east arm of a plus maze during the first block of fifteen trials of each session and to approach the west arm during the second block of trials, creating two distinct behaviorally-defined contexts. After learning, hippocampal neuronal firing was markedly different in the “go east” and “go west” contexts. Differential responses included changes in spatial firing as well as firing that occurred during the intertrial interval and firing associated with retrieving the reward. In short, hippocampal neurons responded to a variety of task events and stimuli and these responses were highly specific to each of the behavioral contexts. The context specific firing patterns developed as the rats learned and they did not develop in a control condition that did not involve a context manipulation. Moreover, muscimol inactivation of the dorsal hippocampus impaired learning, suggesting that differential firing patterns were necessary for the ability to distinguish the two contexts. These studies and other similar experiments (Eschenko and Mizumori, 2007; Ferbinteanu and Shapiro, 2003) suggest that hippocampal neurons respond to changes in behaviorally defined contexts in much the same way that they respond to changes in the spatial and environmental context, by generating a new representation.
Figure 1. Single Unit Studies of Hippocampal Context Coding.
Hippocampal neurons are sensitive to various aspects of the context, including the color and ambient odor. Rats were exposed black or white (B or W) boxes that could be scented with lemon or vanilla (L or V). The spatial firing of two different neurons are shown in A and B. Both were highly sensitive to the context (adapted from Anderson and Jeffery, 2003). In C-E, rats were trained to approach the east and west arms of a plus maze for reward in different blocks of trials (adapted from Smith and Mizumori, 2006a). In this task, the ‘go east’ and ‘go west’ contexts were defined by the behavioral demands rather than by the environment, which remained constant. As with environmental manipulations, hippocampal neurons were highly sensitive to the behavioral context. Place fields of the neurons in C and D were selective for the east and west conditions, as was the firing in response to the reward on the west arm in E.
On the basis of these results, we proposed the hypothesis that hippocampal firing patterns, when considered at the population level, could serve as a neural representation of the context (Smith and Mizumori, 2006a). However, although these studies recorded from dozens or hundreds of neurons, the basic unit of analysis was the individual neuron and population dynamics cannot readily be inferred from the activity of individual neurons. For example, it has been difficult to ascertain whether neurons behave as part of a coherent hippocampal representation or whether they reorganize their firing in a piecemeal fashion. However, with exponential growth in the number of simultaneously recordable neurons and new population analysis techniques that capture the interactions among neurons (Stevenson and Kording, 2011), questions about the characteristics and functions of hippocampal ensembles have become tractable. In the next section, we discuss recent studies that treat neural populations as the unit of analysis.
3. Hippocampal Ensembles Represent the Context
Recent findings have suggested that each context is encoded by a distinct ensemble of hippocampal neurons. Keleman and Fenton (2010) found that the hippocampus can hold two distinct representations of the same apparatus and alternate between them when they are placed in conflict. Rats were trained in a rotating arena that allowed the experimenters to establish two distinct reference frames, or contexts, defined by unmarked danger zones where shocks were delivered (Fig. 2A). One danger zone was stationary within the room framework, which was defined by distal visual cues. However, the rat had to move periodically in order to avoid being passively brought into the danger zone by the rotation of the arena. A second unmarked danger zone rotated along with the floor and transparent walls of the arena. This danger zone could be avoided by attending to local cues placed on the transparent walls of the rotating arena. The rat had to keep track of both danger zones simultaneously in order to avoid the shock.
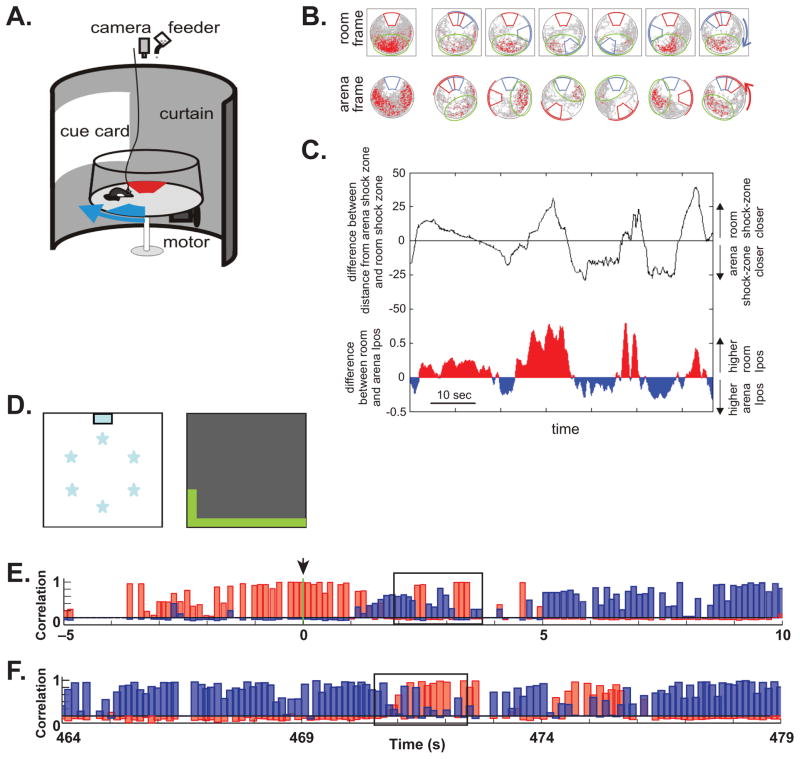
Figure 2. Ensemble Coding of Context in the Hippocampus.
Panels A-C illustrate an experiment in which rats had to avoid two danger zones associated with shock (Kelemen and Fenton, 2010). One danger zone (shown in red, panel A) was stationary within the room framework while the other (shown in blue) rotated along with the local environment of the arena. The danger zones were not marked and the rat kept track of them using local arena cues (not shown) and distal room cues. Some hippocampal place fields were stationary within the room frame, while others rotated along with the local arena (panel B). This created two distinct representational frames, or contexts (room frame and arena frame), each encoded by its own ensemble of neurons. The current state of the hippocampal representation was measured in terms of the positional information content of a population vector including all of the recorded neurons measured with respect to the room and the arena (Ipos, panel C). The relative proximity of the rat to the two shock zones is indicated by the black trace in plot C. When the rat approached one danger zone or the other the hippocampus switched to the relevant representation (room or area Ipos was greater). In another experiment (panels D-F, Jezek et al., 2011), rats foraged in a box that could have two different visual configurations (contexts) controlled by LED lighting, either white lights under the floor or green lights along part of the upper walls (panel D). Population analyses were used to assess the current representation on a moment by moment basis. The context was instantaneously switched from the white-floor box to the green-wall box at time zero (arrow, panel E), and the representation quickly shifted (red bars show correlation of the current firing with the “white-floor” representation and blue bars show correlation with the “green-wall” representation). However, even after the representation had switched to context B, the hippocampus frequently ‘flickered’ back to the context A representation (box). Flickers were most frequent after a context change but they also occurred spontaneously after several minutes in a new context (box, panel F). Panels A-C were adapted from (Kelemen and Fenton, 2010), and panels D-F were adapted from (Jezek et al., 2011).
Some hippocampal neurons exhibited place fields that were stationary within the room framework, while other place fields rotated along with the local cues of the rotating arena (Fig. 2B). Thus, the hippocampus generated two distinct representations, one defined by the stable danger zone (aligned to the room reference frame) and one defined by the rotating danger zone (aligned to the arena reference frame), and each was represented by its own neural ensemble. By using the population of neurons as the unit of analysis rather than individual neurons, the authors were able to measure the representational state of the hippocampus on a moment by moment basis. They did this by computing the spatial information content of the population, a measure that reflects how well the firing of the neurons predicts the rat’s current location with respect to the stable and rotating danger zones. Tracking the preferred reference frame of the population at very brief intervals (~100 milliseconds) revealed that the hippocampus switched between ensembles: at any given moment one representation or the other tended to be active. Remarkably, each representation was most active when the rat was close to the relevant danger zone (Fig. 2C). This result suggests that the hippocampus treated the environment as having two distinct behavioral contexts, one defined by the need to avoid the stable shock zone and the other defined by the need to avoid the rotating shock zone, and the hippocampus switched between these two representations as needed. Reactivation of the relevant representation whenever a danger zone is nearby provides a mechanism for retrieving the appropriate avoidance response without interference from other potential responses. We return to the role of hippocampal context representations in preventing interference in section 5.
Another recent study showed that after a sudden shift from one context representation to another, the hippocampus spontaneously ‘flickers’ back to the original representation (Jezek et al., 2011, Fig. 2D–F). These authors used lighting behind the translucent walls and floor to design an apparatus in which two visually distinct contexts could be instantaneously interchanged. The rats were initially trained in each of the two contexts separately until they developed distinct hippocampal representations of each context. On the test day, the experimenters instantaneously switched from one context to the other and examined the effects of this switch on hippocampal representations. Here again the authors used the neural population as the unit of analysis, correlating the activity of all recorded neurons at a given moment with the average activity pattern in each of the two contexts at the current location. In this way, the authors estimated the extent to which either representation was active on a moment by moment basis. Not surprisingly, the context switches were associated with a sudden change in the hippocampal representation. However, in the seconds following the switch, hippocampal activity frequently returned to the previous representation for brief periods of time. Alternations between representations were locked to individual cycles of the theta rhythm, with activity on any given theta cycle exclusively indicating one context or the other, suggesting that the theta rhythm may be an important organizing factor in the switch between context representations.
The tendency for the hippocampus to ‘flicker’ between context representations has also been observed in relation to learning (Dupret et al., 2013). In this study, rats learned a new set of reward locations each day and hippocampal representations gradually shifted from the previous representation to a new representation as the rats learned. However, detailed population analysis showed that the new and old representations were both expressed, but during different theta cycles. The apparently gradual change in the hippocampal representation was actually the result of a new ensemble representation occurring with increasing strength and frequency as the rats learned.
These results suggest that the hippocampus can rapidly transition between representational states, and that contexts are represented by ensembles of hippocampal neurons that become engaged as a coherent unit rather than by piecemeal responses of individual neurons. Thus, the ensemble code carries critical information about the context that may be obscured at the level of individual neuronal responses. Moment by moment shifts in representations, as seen in the above studies, are impossible to observe in individual principal neurons because they are silent most of the time. These ensemble representations are not driven solely by sensory input about the environment but can be reactivated internally, either spontaneously after a sudden context switch or when they are needed to solve the problem at hand, such as the need to retrieve the memory of a danger zone (Fig. 2A–C) or learn a new set of reward locations (Fig. 1C, D). The tendency to switch between coherent context representations explains why small changes in the input to the hippocampus, including changes in non-sensory input such as the subject’s expectations and motivational state, can produce wholesale reorganization of the hippocampal code (e.g. Kennedy and Shapiro, 2009; Skaggs and McNaughton, 1998).
In order for hippocampal ensembles to represent contexts, they have to be stable and reproducible over the long time scales of contextual memory. Neuronal recordings and studies of immediate early gene expression from the 1990s showed that repeated visits to a context elicit activity within the same ensemble over a period of minutes or hours (Guzowski et al., 1999; Wilson and McNaughton, 1993). More recent techniques have allowed for the observation of neural ensembles over extended time frames. Tayler and colleagues (2013) used genetically engineered mice that express a long lasting, activity dependent form of green florescent protein to compare the active neuronal population at the time of encoding a new contextual fear memory with the active population during retrieval of the memory up to two weeks later. About 40% of the CA1 neurons were active at both time points, suggesting that this ensemble encoded a stable representation of the context. Consistent with this idea, the ensembles that represented the fear conditioning context were not active in a different context. Another recent study used calcium imaging to simultaneously monitor the activity of hundreds of CA1 neurons in freely moving mice over a 45 day period (Ziv et al., 2013). Interestingly, although many neurons exhibited place fields on any given day, most of them were inactive during subsequent sessions. Indeed, of the place fields that were present on a given day, only 25% were still present five days later, suggesting that neurons continuously join and then bow out of the population representation. This is consistent with recent observations of continual rate remapping over the course of several days, suggesting an ongoing process in which hippocampal neurons become active and then bow out (Mankin et al., 2012). The functional role of these neurons is not clear, although an interesting possibility is that they are part of the slowly changing activity patterns that have been proposed to represent a kind of temporal context (Manns et al., 2007). Despite this day to day variability, a core subset of about 15% of the place cells continued to fire reliably across as many as 30 days of training, and these neurons were sufficient to unambiguously represent the environment. Thus, this stable subset could serve as the long term ensemble that represented the context.
Overall, these findings support the idea that a new ensemble of hippocampal neurons is recruited to represent any new context a subject encounters. After these ensembles have stabilized, the hippocampus can readily switch between them, even on a moment by moment basis, as needed to distinguish among contexts. Moreover, coherent ensembles act as the critical unit of information in representing contexts.
4. Direct Manipulation of Hippocampal Ensembles
Although the kinds of ensemble firing patterns described above are correlated with the context and with context appropriate behaviors, the causal links have been uncertain. Do hippocampal ensemble codes drive the retrieval of context appropriate memories as we have suggested? Or does sensory input from the current context drive both the hippocampal representation and context appropriate behavior? Although it is difficult to conclusively establish a direct causal link between neural firing and memory, recent studies of Pavlovian fear conditioning have advanced this goal substantially. These studies have adopted a strategy in which mice were given training and the active neurons were tagged using molecular neuroscience techniques that allowed them to be reactivated with a drug or with light. They were therefore able to determine whether reactivation of the ensemble causes the retrieval of the trained memory.
An initial step in this direction was taken in a recent experiment in which subjects learned to fear a context representation that included an artificially activated ensemble of neurons (Garner et al., 2012). The experiment used a DREADD (designer receptor exclusively activated by a designer drug) procedure in which transgenic mice express a designer (hM3Dq) receptor in an activity (c-fos) dependent manner. Mice were initially allowed to explore one context (context A) while the active neurons were allowed to express the hM3Dq receptor, which responds specifically to clozapine-N-oxide (CNO). The expression of the hM3Dq receptors was suppressed with doxycycline at all other times. The result was that the ensemble of neurons that was active in context A expressed the hM3Dq receptor and could be artificially reactivated by CNO injection. The mice were then given foot shocks in a second context (context B) while the context A neurons were artificially reactivated. The goal was to determine whether the rats could learn to fear an artificial context representation. In fact, the rats only exhibited a fear response when they were tested in the same context conditions as training, with the artificial ensemble reactivated in context B. One caveat is that the expression of the hM3Dq receptor was not limited to the hippocampus and the artificial reactivation involved neurons in many brain areas. Nevertheless, this study indicates that reactivation of a population of context sensitive neurons evokes an appropriate memory and that an artificially controlled ensemble can serve as part of a context representation capable of evoking a fear memory.
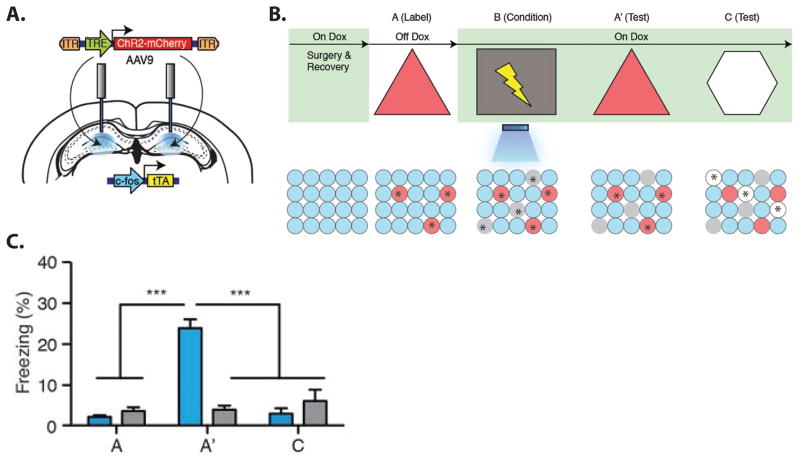
An even more striking demonstration of the causal link between ensemble activity and a conditioned fear memory came from two recent studies that used optogenetics to specifically stimulate hippocampal neural ensembles (Liu et al., 2012; Ramirez et al., 2013). These studies used transgenic mice in which channel rhodopsin was selectively expressed in active (c-fos expressing) neurons in the dentate gyrus. Using this procedure, the neurons that were active in a given context could be tagged and subsequently reactivated with light (Fig. 3A). In one study (Liu et al., 2012), mice were given tone shock pairings in a distinctive context and the active dentate gyrus neurons were labeled with channel rhodopsin. Later, optical reactivation of the neurons in a different context produced a freezing response. Since the hippocampus is not needed for tone-cued fear conditioning (Kim and Fanselow, 1992; Phillips and LeDoux, 1992), this ensemble presumably represented the context rather than the tone. Thus, reactivation of the same hippocampal ensemble that was active in the training context was sufficient to trigger retrieval of the fear memory. In a second study (Ramirez et al., 2013), mice were exposed to one context (context A) and the active neurons were tagged with channel rhodopsin. The next day the mice were given foot shocks in a different context (context B) while the labeled neurons (from context A) were optically reactivated. When the mice were subsequently tested in context A with no light stimulation, they exhibited a fear response. No such fear response was elicited by testing in a novel context. Thus, the mice learned to fear an artificially reactivated representation of context A, even though they had never been shocked there. The fact that the hippocampal ensemble activity was a serviceable substitute for the physical context provides compelling evidence that the ensemble truly represented the context.
Figure 3. Artificial Reactivation of Hippocampal Ensembles.
Channel rhodopsin was selectively expressed in active dentate gyrus neurons using a c-fos promoter which could be suppressed with doxycycline (panel A, for details see Ramirez et al., 2013). Later, the same neurons could be stimulated using light delivered via an optic fiber implanted in the brain. The training procedure is illustrated in panel B. The rat was exposed to context A without doxycycline (Off Dox), allowing the active hippocampal ensemble (stars) to be labeled with channel rhodopsin (red). Selective labeling was achieved by keeping the mice on Dox at all other times (green shading). On the day after labeling, the mice were given foot shocks in a different context (lightning bolt in context B) while the context A neurons were artificially reactivated with light. Grey circles represent neurons that were activated by exposure to context B, but which were not activated by light because they were not labeled with channel rhodopsin. The rats were subsequently tested in context A where they had never been shocked (A′), and in a novel context (C), where a new population of neurons was active (white circles). Test results are shown in panel C. Experimental rats exhibited fear responses (elevated freezing, blue bars) during the test in A′ but not during the initial exposure to context A or in the novel context C. Control rats given similar treatment, but without the channel rhodopsin, did not exhibit fear responses in either test context (grey bars). Illustrations were adapted from (Ramirez et al., 2013).
These are only the first of what will surely be many studies that directly manipulate neural memory representations. Advances in molecular neuroscience allow increasing precision in the stimulation of neural populations and this approach will likely yield important and unexpected insight into memory functions. For example, the work described above indicates that some kinds of memories (i.e. conditioned fear) can be retrieved without the normal temporal dynamics of neural firing that occur in natural situations. The study by Garner and colleagues (2012) used systemic drug injections that increased neural activity over a period of many minutes and the optogenetics experiments (Liu et al., 2012; Ramirez et al., 2013) used pulses of light stimulation to activate all of the ensemble neurons simultaneously. Despite the highly abnormal temporal patterns of activity in these studies, the fear memory was retrieved. It is not currently known whether simultaneous activation of whole ensembles can trigger the retrieval of more complex memories.
Evidence from the artificial reactivation studies and the neuronal population recording studies reviewed above suggests that hippocampal ensembles encode contexts and that they provide a mechanism for contextual cueing of memory. How new ensembles are formed in response to a new context is less clear. The large scale optical recording study discussed above (Ziv et al., 2013) suggests that new ensembles are continually and spontaneously being formed and dissolved. Recent findings suggest that these spontaneously formed ensembles can then be assigned to new contexts, thereby allowing for very rapid formation of highly distinct representations (Dragoi and Tonegawa, 2011). Indeed, the hippocampus appears to continually have many ensembles ready for assignment to any context the subject might encounter (Dragoi and Tonegawa, 2013). Interestingly, medial prefrontal cortical input to the hippocampus via the nucleus reuniens may play a role in establishing or modifying these ensemble representations (Xu and Sudhof, 2013). This is a primary route of information flow from the medial prefrontal cortex to the hippocampus, and optogenetic stimulation of the nucleus reuniens causes either an increase or decrease in the generalization of contextual fear memories depending on the pattern of stimulation.
5. The Adaptive Value of Hippocampal Context Representations: Preventing Interference
Converging evidence from large scale neuronal recordings, immediate early gene expression and direct manipulation of neural firing suggests that hippocampal neural ensembles represent contextual information. But what is the adaptive value of these context representations? The ability to encode new contexts and recognize familiar ones is important in itself. However, we suggest that the primary utility of context coding is that contextual information provides an important mechanism for preventing mnemonic interference. The fact that the context is a potent retrieval cue means that returning to a familiar context automatically results in the priming of relevant memories, making them easier to retrieve than potentially interfering memories that belong to other contexts. Interference is a critical problem for any high capacity memory system and classic studies from cognitive psychology have repeatedly demonstrated the value of contextual information for preventing interference (for review see Smith, 1988). In one commonly used procedure, subjects are trained on two or more lists of items in either the same context or in different contexts (e.g. Bilodeau and Schlosberg, 1951). Invariably, retrieval is better when each list is learned in a different context, suggesting that associating memories with the training context protects them from interference by items learned in other contexts.
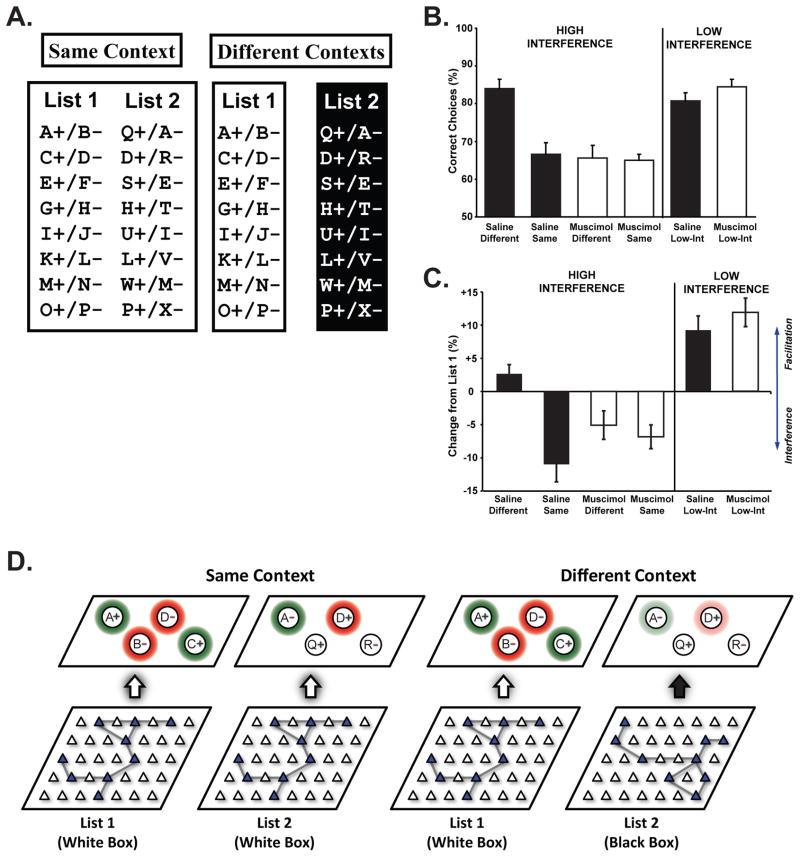
Recently, we adapted this training procedure for use in rodents to ask whether the hippocampus mediates the ability to use contextual information to overcome interference (Fig 4). Rats were trained on two lists of eight odor discrimination problems either in the same context or in different contexts. In order to induce interference, some of the odors appeared on both lists with their predictive value reversed. Just as with human subjects, rats that learned the two lists in different contexts experienced less interference and, consequently, they performed better than rats that learned both lists in the same context. However, rats given muscimol infusions into the dorsal hippocampus gained no advantage from learning in different contexts. Instead, their performance was similar to rats that learned the two lists in the same context. Interestingly, the muscimol rats were not impaired in the same context condition, relative to controls, when contextual information was not available to help prevent interference. Inactivation also had no effect on performance in a low interference version of the task involving non-overlapping odor lists. These findings indicate that the hippocampus was specifically involved in the ability to use contextual information to overcome interference.
Figure 4. Hippocampal Context Representations and Interference.
Rats were trained on two lists of odor discrimination problems (Panel A, for details see Butterly et al., 2012). On each trial, one of the discrimination problems was presented in the form of cups containing odorants mixed into a digging medium with a buried reward in the designated (+) cup. All of the rats first learned List 1 in a white box, followed by training on List 2, in either the same white box (Same Context) or in a different black box (Different Contexts). Note that the two lists contained overlapping items with their valence reversed, so that the second list was a mixture of previously rewarded odors, previously non-rewarded odors and novel odors. Muscimol or saline infusions into the dorsal hippocampus were given during training on list two. The percentage of trials with a correct choice for rats in each condition is shown in B. Control rats that learned the second list in a different context (Saline Different) performed significantly better than rats that learned the two lists in the same context (Saline Same), but muscimol rats showed no contextual learning advantage (Muscimol Different compared to Muscimol Same). Rats that were trained on another version of the task in which there were no overlapping items on the two lists (Low-Int) performed at a high level regardless of whether they were given muscimol or saline infusions, suggesting that the hippocampus was not needed when there was little interference. We also computed the change in performance from list one to list two (panel C). Negative values indicate proactive interference from List 1 while positive values indicate facilitation. Control rats that learned the two lists in different contexts did not experience interference. In contrast, control rats in the same context condition and muscimol rats experienced significant interference. Rats in the low interference condition showed no proactive interference but instead showed significant facilitation. Panel D illustrates our theoretical account of how hippocampal ensemble representations of the context influence memory retrieval and interference. During learning of List 1, the hippocampal ensemble representation of the context (represented by the filled triangles) becomes associated with the relevant odor memories and their association with reward (shading indicates primed odor memories, while green and red colors indicate rewarded and non-rewarded odor associations). When List 2 is encountered in the same context, the old ensemble representation continues to prime the old odor memories (e.g. Odor A as a rewarded odor and D as a non-rewarded odor although the valences are now different), resulting in interference. In the different context condition, a new ensemble representation of the black box is generated and the old odor memories are not primed by the context (reduced priming is indicated by weaker colors). Because each memory representation is primed by the hippocampal context representation, context-appropriate retrieval is facilitated and interference from memories that belong to other contexts is minimized.
When combined with results indicating that hippocampal neural ensembles represent the context, these findings suggest a working model for how hippocampal context coding promotes the interference free retrieval of memories (Fig 4D). When a subject encounters a new context, a unique hippocampal ensemble is recruited to represent it. With experience, this context code becomes associated with the stimuli, events and behaviors that occur in that context. For example, the memory for a particular odor is presumably established and stored in extra-hippocampal circuitry since memory for individual odors and their association with reward does not depend on the hippocampus (Eichenbaum et al., 1986). With experience, the active hippocampal ensemble becomes associated with the memory representation of the odor and the appropriate response (dig or do not dig). When the rat is later returned to the context, the hippocampal ensemble is automatically reactivated and this primes the retrieval of the odor memory. If the rat is then asked to learn a new list of odors in a different context, a new hippocampal ensemble is activated and it quickly becomes associated with the new odor memories. Because the two ensembles are very different and each ensemble primes a different set of odor memories, retrieval is not hampered by interference. However, if the rat needs to retrieve a new set of odor memories within the same context, the old hippocampal ensemble continues to prime the old odor memories, resulting in persistent intrusions of inappropriate memories and severe interference. As a result, learning is delayed until the rat forms new associations over the course of many repetitions. Since rats with lesions do not have hippocampal context representations, they are left with only the intrinsic (hippocampal independent) strength of the odor memories, they perseverate on the previously rewarded odors and they cannot prime the new odor memories, resulting in very slow learning.
Thus, hippocampal context representations provide a crucial means of priming relevant memories so that other memories are less likely to interfere. This role in resolving interference is supported by a number of other findings. Interference is a prominent characteristic of many hippocampal dependent tasks that require subjects to respond appropriately to a cue that has been rewarded some times and not others (Agster et al., 2002; Fortin et al., 2002; Rajji et al., 2006; Smith et al., 2004) or that require memory for the events of the current trial without interference from previous trials (Olton and Papas, 1979). Disruption of adult hippocampal neurogenesis has also been shown to increase susceptibility to interference (Luu et al., 2012; Winocur et al., 2012). Pattern separation is another hippocampal mechanism for preventing interference by generating highly distinctive representations even when sensory input is similar (Colgin et al., 2008; Hunsaker and Kesner, 2013; Yassa and Stark, 2011). Our account of how hippocampal context representations prevent interference depends on the capacity to efficiently form highly unique representations for each context, and pattern separation plays a critical role in this process (Leutgeb et al., 2007; Leutgeb and Leutgeb, 2007).
Previous accounts have suggested that the hippocampus acts as an index that connects the various neocortical elements that are active during a behavioral experience, thereby binding them into a distributed episodic or contextual memory (Squire and Zola-Morgan, 1991; Teyler and DiScenna, 1986; Teyler and Rudy, 2007). Subsequently, partial reactivation of the hippocampal index leads to pattern completion and automatic retrieval of the full blown episodic memory. Our account also posits that reactivation of hippocampal ensembles automatically reactivates extra-hippocampal representations. However, we suggest that the role of this hippocampus is not to link the individual components of an episodic memory, but rather to link memories with contexts. This is consistent with the observation that animals with hippocampal lesions can form and retrieve many kinds of memories, but they are unable to associate them with the context. In addition to episodic memory, Pavlovian and instrumental learning, emotional responses, motor skills and perceptual priming are all sensitive to manipulations of the context (for reviews see Maren et al., 2013; Thomson and Davies, 1988). Interestingly, many of these kinds of memory do not depend on the hippocampus in their basic (i.e. non-contextual) form, but they become highly sensitive to hippocampal damage when a contextual component is added. A key benefit of this mechanism for encoding contexts is that hippocampal output to the various memory systems of the brain can be used to modulate processing in each system according to the context, thereby supporting a remarkable degree of behavioral and cognitive flexibility across contexts.
6. Conclusions and Remaining Questions
Although many studies have shown a hippocampal role in contextual memory, there has not been a detailed account of the form and function of hippocampal context representations. The evidence reviewed above suggests that the hippocampus encodes contexts that are defined by a variety of environmental, behavioral and motivational factors. These representations take the form of a coherent ensemble code that changes as a whole rather than in a piecemeal fashion. The generation of a new context representation can be triggered when subjects encounter a new environment or by significantly changing the behavioral and motivational demands of the situation. Thus, each context is encoded by a distinct neural ensemble within the hippocampus. These ensemble context codes play a key role in resolving interference by priming extra-hippocampal memory representations that are associated with the context. This provides a highly adaptive mechanism for contextual cueing of relevant memories and, because only the appropriate memories are primed for retrieval, they are less susceptible to interference from irrelevant memories.
The idea of ensemble context representations raises some interesting questions about the functional significance of the individual neurons that make up the ensembles. For an ensemble code to be useful as a context representation, it needs only to provide a distinct and reproducible pattern of hippocampal output for each context. From this perspective, the details of what drives the individual neurons to respond are irrelevant. Information flow through the hippocampal subregions results in highly patterned output by unique neural ensembles regardless of the specific details of the sensory input (Colgin et al., 2008; Leutgeb et al., 2007; McNaughton et al., 2006) and hippocampal ensembles appear to form spontaneously and only later are they assigned to represent experiences (Dragoi and Tonegawa, 2011). Are individual responses such as place fields or other responses to task stimuli (e.g. odors) only useful to the extent that they participate in these ensembles? Alternatively, do hippocampal neurons carry information at two levels, with contextual information at the level of activity in large ensembles and information about spatial location, events and stimuli at the level of specific responses? Indeed, it is possible that individual responses are the critical encoding unit (e.g. for episodic memory functions) and that the utility of the ensemble activity as a context code is simply a fortuitous byproduct. Definitive answers to these questions await further study.
Another important question relates to the roles of different kinds of representational change in encoding contexts. Hippocampal firing patterns can change in different ways. A neuron can exhibit entirely different responses in different contexts (commonly referred to as complete remapping). For example, a neuron with a place field in one context can become silent, exhibit a place field in a different location or even exhibit a different kind of response (e.g. an odor response) in another context (Eichenbaum et al., 1987; Smith and Mizumori, 2006a). Neurons can also change their response properties by maintaining the same preferred location but changing their firing rate (i.e. rate remapping). Complete remapping is the predominant response to large scale changes in the context, including changes in behavioral demands such as those discussed above. However, rate remapping can occur in response to smaller changes in the environment (Hetherington and Shapiro, 1997; Leutgeb et al., 2005) or simply with the passage of time (Mankin et al., 2012). At present, the factors that determine whether the hippocampus generates an entirely new ensemble representation or engages in rate remapping are not fully understood, nor are the functional differences between these two kinds of representational change (for a review of this topic, see Colgin et al., 2008).
The observation of time dependent rate remapping raises the question of how the temporal dynamics of hippocampal firing patterns interact with context coding. As described above, simultaneously reactivating the neurons of an ensemble can be sufficient to trigger the retrieval of a context appropriate memory, indicating that some contextual information can be conveyed without the temporal firing patterns normally present in behaving subjects. However, other findings suggest that the temporal characteristics of neural firing are very important to context coding. Recent reports suggest that rate remapping causes hippocampal representations to continuously change over time (Mankin et al., 2012; Ziv et al., 2013). These slow systematic changes in firing may be useful to represent a kind of temporal context that is important for episodic memory (Manns et al., 2007). Yet another kind of temporal dynamics is involved in newly discovered ‘time cells,’ which fire during discrete periods of time during experimental delay periods (Pastalkova et al., 2008). Like place cells, these time cells are sensitive to behavioral and mnemonic demands of the task, suggesting that they may play a role in distinguishing contexts (Eichenbaum, 2013; Gill et al., 2011; Macdonald et al., 2011). However, additional research will be needed for a detailed account of the significance of these temporal firing characteristics for context representations.
Highlights.
Contextual information plays a critical role in memory.
Contexts are represented by coherent ensembles of hippocampal neurons.
Activity in these ensembles primes the retrieval of context appropriate memories.
Priming the relevant memories prevents interference by other, irrelevant memories.
Acknowledgments
This work was supported by NIH grant MH083809 to D. Smith.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. Journal of Neuroscience. 2002;22:5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. Journal of Neuroscience. 2003;23:8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau IM, Schlosberg H. Similarity in stimulating conditions as a variable in retroactive inhibition. Journal of experimental psychology. 1951;41:199–204. doi: 10.1037/h0056809. [DOI] [PubMed] [Google Scholar]
- Butterly DA, Petroccione MA, Smith DM. Hippocampal context processing is critical for interference free recall of odor memories in rats. Hippocampus. 2012;22:906–913. doi: 10.1002/hipo.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Mit Press; Cambridge: 1994. [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31:469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature. 2011;469:397–401. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. Distinct preplay of multiple novel spatial experiences in the rat. Proc Natl Acad Sci U S A. 2013;110:9100–9105. doi: 10.1073/pnas.1306031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O’Neill J, Csicsvari J. Dynamic reconfiguration of hippocampal interneuron circuits during spatial learning. Neuron. 2013;78:166–180. doi: 10.1016/j.neuron.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Memory on time. Trends Cogn Sci. 2013;17:81–88. doi: 10.1016/j.tics.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Representation in the hippocampus: what do hippocampal neurons code? Trends in Neurosciences. 1988;11:244–248. doi: 10.1016/0166-2236(88)90100-2. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Fagan A, Cohen NJ. Normal olfactory discrimination learning set and facilitation of reversal learning after medial-temporal damage in rats: implications for an account of preserved learning abilities in amnesia. J Neurosci. 1986;6:1876–1884. doi: 10.1523/JNEUROSCI.06-07-01876.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Kuperstein M, Fagan A, Nagode J. Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. Journal of Neuroscience. 1987;7:716–732. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Mizumori SJ. Memory influences on hippocampal and striatal neural codes: effects of a shift between task rules. Neurobiol Learn Mem. 2007;87:495–509. doi: 10.1016/j.nlm.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Weible A, Rossi J, Gabriel M. Lesions of the entorhinal cortex disrupt behavioral and neuronal responses to context change during extinction of discriminative avoidance behavior. Experimental Brain Research. 1997;115:445–457. doi: 10.1007/pl00005714. [DOI] [PubMed] [Google Scholar]
- Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. Generation of a synthetic memory trace. Science. 2012;335:1513–1516. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill PR, Mizumori SJ, Smith DM. Hippocampal episode fields develop with learning. Hippocampus. 2011;21:1240–1249. doi: 10.1002/hipo.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godden D, Baddely A. Context-dependent memory in two natural environments: On land and underwater. Brittish Journal of Psychology. 1975;66:325–331. [Google Scholar]
- Good M, Honey RC. Conditioning and contextual retrieval in hippocampal rats. Behav Neurosci. 1991;105:499–509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hetherington PA, Shapiro ML. Hippocampal place fields are altered by the removal of single visual cues in a distance-dependent manner. Behav Neurosci. 1997;111:20–34. doi: 10.1037//0735-7044.111.1.20. [DOI] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behavioral Biology. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Honey RC, Good M. Selective hippocampal lesions abolish the contextual specificity of latent inhibition and conditioning. Behav Neurosci. 1993;107:23–33. doi: 10.1037//0735-7044.107.1.23. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci Biobehav Rev. 2013;37:36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Jezek K, Henriksen EJ, Treves A, Moser EI, Moser MB. Theta-paced flickering between place-cell maps in the hippocampus. Nature. 2011;478:246–249. doi: 10.1038/nature10439. [DOI] [PubMed] [Google Scholar]
- Kelemen E, Fenton AA. Dynamic grouping of hippocampal neural activity during cognitive control of two spatial frames. PLoS biology. 2010;8:e1000403. doi: 10.1371/journal.pbio.1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proc Natl Acad Sci U S A. 2009;106:10805–10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee J, Lee I. The hippocampus is required for visually cued contextual response selection, but not for visual discrimination of contexts. Frontiers in behavioral neuroscience. 2012;6:66. doi: 10.3389/fnbeh.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Lee SH. Putting an object in context and acting on it: neural mechanisms of goal-directed response to contextual object. Reviews in the neurosciences. 2013;24:27–49. doi: 10.1515/revneuro-2012-0073. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14:745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Sill OC, Gao L, Becker S, Wojtowicz JM, Smith DM. The role of adult hippocampal neurogenesis in reducing interference. Behav Neurosci. 2012;126:381–391. doi: 10.1037/a0028252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, Leutgeb JK. Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci U S A. 2012;109:19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature reviews. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. Journal of Neuroscience. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nature reviews. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ. Context Prediction Analysis and Episodic Memory. Frontiers in behavioral neuroscience. 2013;7:132. doi: 10.3389/fnbeh.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, Ragozzino KE, Cooper BG, Leutgeb S. Hippocampal representational organization and spatial context. Hippocampus. 1999;9:444–451. doi: 10.1002/(SICI)1098-1063(1999)9:4<444::AID-HIPO10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Smith DM, Puryear CB. Hippocampal and neocortical interactions during context discrimination: Electrophysiological evidence from the rat. Hippocampus. 2007;17:851–862. doi: 10.1002/hipo.20317. [DOI] [PubMed] [Google Scholar]
- Mizumori SJY. Mnemonic contributions of hippocampal place cells. In: Martinez JL, Kesner RP, editors. Neurobiology of Learning and Memory. Academic Press; Burlington, MA: 2007. pp. 155–189. [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CE, Gluck M. Context, conditioning, and hippocampal rerepresentation in animal learning. Behavioral Neuroscience. 1994;108:835–847. doi: 10.1037//0735-7044.108.5.835. [DOI] [PubMed] [Google Scholar]
- Nadel L, Willner J, Kurz EM. Cognitive maps and environmental context. In: Balsam P, Tomie A, editors. Context and Learning. Erlbaum; Hillsdale, NJ: 1985. pp. 385–406. [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford, UK: 1978. [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penick S, Solomon PR. Hippocampus, context, and conditioning. Behavioral Neuroscience. 1991;105:611–617. doi: 10.1037//0735-7044.105.5.611. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Rajji T, Chapman D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. J Neurosci. 2006;26:908–915. doi: 10.1523/JNEUROSCI.4194-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Spatial firing properties of hippocampal CA1 populations in an environment containing two visually identical regions. Journal of Neuroscience. 1998;18:8455–8466. doi: 10.1523/JNEUROSCI.18-20-08455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM. The hippocampus, context processing and episodic memory. In: Huston JP, Dere Ekrem, Easton Alexander, Nadel Lynn, Huston Joseph P, editors. Handbook of Behavioral Neuroscience, Vol 18, Handbook of Episodic Memory. Elsevier; The Netherlands: 2008. pp. 465–481. [Google Scholar]
- Smith DM, Mizumori SJY. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006a;16:716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJY. Learning-Related Development of Context-Specific Neuronal Responses to Places and Events: The Hippocampal Role in Context Processing. Journal of Neuroscience. 2006b;26:3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Wakeman D, Patel J, Gabriel M. Fornix Lesions Impair Context-Related Cingulothalamic Neuronal Patterns and Concurrent Discrimination Learning. Behavioral Neuroscience. 2004;118:1225–1239. doi: 10.1037/0735-7044.118.6.1225. [DOI] [PubMed] [Google Scholar]
- Smith SM. Remembering in and out of Context. Journal of Experimental Psychology: Human Learning and Memory. 1979;5:460–471. [Google Scholar]
- Smith SM. Environmental context-dependent memory. In: Davies G, Thomson DM, editors. Memory in context: context in memory. John Wiley and Sons, Ltd; New York: 1988. pp. 13–34. [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stevenson IH, Kording KP. How advances in neural recording affect data analysis. Nat Neurosci. 2011;14:139–142. doi: 10.1038/nn.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Rudy JW. Configural Association Theory - the Role of the Hippocampal-Formation in Learning, Memory, and Amnesia. Psychobiology. 1989;17:129–144. [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol. 2013;23:99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Terrazas A, Krause M, Lipa P, Gothard KM, Barnes CA, McNaughton BL. Self-motion and the hippocampal spatial metric. J Neurosci. 2005;25:8085–8096. doi: 10.1523/JNEUROSCI.0693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. The hippocampal memory indexing theory. Behav Neurosci. 1986;100:147–154. doi: 10.1037//0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Rudy JW. The hippocampal indexing theory and episodic memory: updating the index. Hippocampus. 2007;17:1158–1169. doi: 10.1002/hipo.20350. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Davies GM. Introduction. In: Davies GM, Thomson DM, editors. Memory in context: Context in memory. John Wiley and Sons; New York: 1988. pp. 1–10. [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space [see comments] [published erratum appears in Science 1994 Apr 1;264(5155):16] Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Winocur G, Becker S, Luu P, Rosenzweig S, Wojtowicz JM. Adult hippocampal neurogenesis and memory interference. Behavioral Brain Research. 2012;227:464–469. doi: 10.1016/j.bbr.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Xu W, Sudhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]