Abstract
Cardiolipins (CLs) are ancient and unusual dimeric phospholipids localized in the plasma membrane of bacteria and in the inner mitochondrial membrane of eukaryotes. In mitochondria, two types of asymmetries – trans-membrane and molecular - are essential determinants of CL functions. In this review, we describe CL-based signaling mitochondrial pathways realized via modulation of trans-membrane asymmetry and leading to externalization and peroxidation of CLs in mitophagy and apoptosis, respectively. We discuss possible mechanisms of CL translocations from the inner leaflet of the inner to the outer leaflet of the outer mitochondrial membranes. We present redox reaction mechanisms of cytochrome c-catalyzed CL peroxidation as a required stage in the execution of apoptosis as well as a possible source of lipid mediators. We also emphasize the significance of CL-related metabolic pathways as new targets for drug discovery. Finally, a remarkable diversity of polyunsaturated CL species and their oxidation products have evolved in eukaryotes vs. prokaryotes. This diversity - associated with CL molecular asymmetry - is presented as the basis for mitochondrial communications language.
“The universe is an asymmetrical entity. I am inclined to believe that life as it is manifested to us must be a function of the asymmetry of the universe or of the consequence of this fact…”
Louis Pasteur
Cardiolipin asymmetries
Cardiolipins (CLs) belong to a class of ancient phospholipids found in membranes of both prokaryotes and eukaryotes albeit at different locations: they comprise an abundant component of the bacterial plasma membrane, but are confined almost exclusively to mitochondria in eukaryotes (Daum and Vance, 1997; Hoch, 1992; Schlame, 2008). CL’s biosynthetic pathways are also different: they are biosynthesized from two molecules of phosphatidylglycerol (PG) in bacteria, but from a PG and a cytidine diphosphate diacylglycerol (CDP-DAG) in eukaryotes (Tian et al., 2012). Molecular speciations of bacterial and mitochondrial CLs are also significantly different: while shorter carbon chain and saturated or mono-unsaturated CL species are typical of the former, longer chain polyunsaturated CLs are predominant in mitochondria (Schlame, 2008). In spite of this, the simultaneous appearance of CLs in both bacteria and eukaryotic mitochondria as well as their exclusive localization to inner mitochondrial membrane (IMM) in eukaryotes - have been viewed as a strong evidence for the endosymbiotic origin of mitochondria from bacteria (Tian et al., 2012).
Two types of asymmetries – trans-membrane and molecular – seem to be essential determinants of CL’s functions in mitochondria. The first one is based on preferential localization of CLs in mitochondrial membranes and includes not only highly selective distribution of CLs into IMM compared to outer mitochondrial membrane (OMM) but also possible enrichment of the inner vs. outer leaflets of IMM with CLs (Gallet et al., 1997; Harb et al., 1981). The second type - molecular asymmetry of CLs - is associated with the presence of chiral carbons in a dimeric structure with two PGs connected via a glycerol backbone thus including four acyl (fatty acid, FA) chains and two negative charges of phosphate groups. If all four FA-residues are identical – CL molecules are symmetric; however, integration of at least one different FA-residue in the CL disturbs the symmetric organization of the molecule. In several tissues – heart, muscles, liver –symmetric CL molecules with all four FA represented by C18:2 are most common (Schlame et al., 2005). Interestingly, symmetrical CL species with more unsaturated FA-residues - C22:6n-3 and C20:5n-3 - were found in marine mollusk bivalves with taxon specificity that paralleled the bivalve phylogeny (Kraffe et al., 2008). Notably, these CL species are prone to peroxidation (see Tyurina et al., this issue) resulting in the oxygenation of one or more of C18:2 residues, hence to the emergence of asymmetric structure of CLs. In this mini-review, we describe biological significance as well as major mechanisms and pathways through which the two above types of CL asymmetry participate in cells signaling.
Reduced trans-membrane asymmetry and externalization of CLs leads to mitophagy
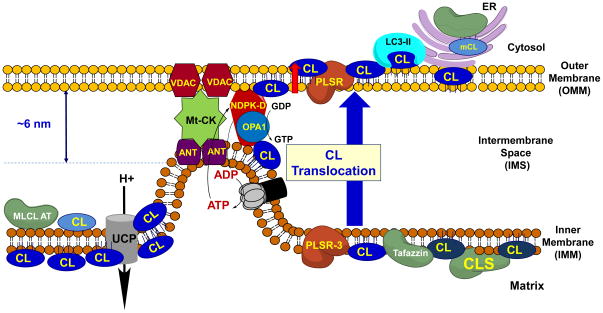
The topography of cardiolipin synthase (CLS) – the catalyst of the final stage in CL biosynthesis – is one of the major defining factors for CL asymmetry in the IMM. The enzyme is an integral IMM protein with hydrophilic domains exposed to the matrix side of mitoplasts (Schlame and Haldar, 1993). This suggests that CL’s biosynthesis places it predominantly in the inner leaflet of IMM. While some of the reactions of CL remodeling (Cao et al., 2004) may be occurring in endoplasmic reticulum (ER), thus physiologically necessitating CL‘s trans-membrane translocations (Esposti et al., 2001), it is believed that in normally functioning mitochondria CLs are found only in IMM whereby the inner leaflet may contain higher amounts of CLs than the outer leaflet (Hovius et al., 1990; Krebs et al., 1979). This highly asymmetric distribution of CLs across mitochondrial membranes changes dramatically upon mitochondrial injury and depolarization: a significant portion of CLs are translocated to the OMM (Baile et al., 2013; Garcia Fernandez et al., 2002; Gonzalvez and Gottlieb, 2007). Moreover, a fraction of CLs becomes exposed on the mitochondrial surface resulting in its accessibility to CL- metabolizing enzymes such as PLA2 (Buckland et al., 1998; Malhotra et al., 2009; Marinetti, 1964). Recent studies revealed the physiological significance of the appearance of externalized CL as an “eat-me-signal” for the autophageal machinery resulting in targeted removal of damaged mitochondria (Chu et al., 2013). The mitophagy mechanisms include specific recognition of externalized CL by microtubule-associated protein 1 light chain 3 (LC3), a component of the autophageal machinery which mediates both autophagosome formation and cargo recognition. The cytosolic form of the protein (LC3-I) is processed and recruited to autophagosomes, where the membrane bound form, LC3-II, is generated by covalent lipidation (with phosphatidylethanolamine) (Eskelinen, 2008; Kabeya et al., 2000). While detailed studies demonstrated the effectiveness of this CL-based recognition mechanism of depolarized dysfunctional mitochondria in several different types of cells – primary neurons, SH-SY5Y cells, HeLa cells, and mouse lung epithelial MLE-15 cells (Chu et al., 2013) – the pathways involved in CL transmembrane redistribution remain elusive. Externalization of CL from the inner leaflet of IMM to the surface of OMM requires at least three translocations: 1) from the inner to the outer leaflet of IMM, 2) from the outer leaflet of IMM to the inner leaflet of OMM and, finally 3) from the inner to the outer leaflet of OMM (Figure 1).
Figure 1.
Mitophagic Translocation of Cardiolipin: Cardiolipin (CL) is synthesized by Cardiolipin Synthase (CLS) in the inner leaflet of IMM, where it can be remodeled by Tafazzin and MLCLAT, but can also translocate to OMM and ER for remodeling during its maturation. In healthy mitochondria, the large majority of CL remains at the site of its synthesis in the IMM. Externalization of cardiolipin to the mitochondrial surface through a mechanism involving phospholipid scramblase-3 (PLS3) is a prerequisite for its recognition by LC3 as a mitophagic “eat-me signal” that targets dysfunctional mitochondria to the autophagosomal machinery. While the precise mechanism of PLS3 regulation remains to be delineated, nucleoside diphosphate kinase (NDPK-D) may physically facilitate such a transfer. NDPK-D regulates the balance between di- and tri-phospho-nucleotides, providing GTP for the GTPase activity of OPA1. By forming a hexamer in the intermembrane space, NDPK-D can physically bridge the IMM and OMM. The switch from a phosphotransfer to a cardiolipin-translocating mode would thus faciliate the redistribution of cardiolipins between IMM and OMM.
Both protein-dependent and independent mechanisms may operate in realization of the transverse distribution of CL and its inter-leaflet IMM transfer. The former may include CL interactions with several integral proteins of the IMM such as adenylate translocase (ANT) and uncoupling proteins (UCPs) (Hoang et al., 2012). Alternatively or concomitantly, massive accumulation of CLs at the site of its synthesis may result in its non-bilayer arrangements with the formation of hexagonal-phase-like defects capable of disturbing the bilayer structure (Cullis et al., 1978; Van Venetie and Verkleij, 1982) and facilitating translocation of CLs to the IMM surface.
The second stage of CL redistribution from the IMM to the OMM may be mostly occurring within the areas of contact sites (Ardail et al., 1990; Hovius et al., 1990), whereby one or more “rotary” translocators bind and transfer CL molecules through an ~6nm gap between the membranes (Rassow et al., 1989; Rassow and Pfanner, 1991). One of possible candidates for this role is mitochondrial nucleoside diphosphate kinase (NDPK-D), which displays high affinity for CL binding and the ability to transfer it between liposomes in vitro (Tokarska-Schlattner et al., 2008). Lately, realization of this possible translocator role of NDPK-D’s hexamer has been also confirmed in cells whereby yet to be fully understood interactions of the protein with one of mitochondrial GTPases, optic atrophy 1 (OPA1), participate in switching the kinase function of NDPK-D to its CL-translocase activity (Schlattner et al, 2013; see Schlattner et al., in this issue). Importantly, the essentiality of this CL-translocase activity of NDPK-D and CL externalization on the surface of mitochondria has been demonstrated for mitophagy (Huang et al., unpublished) and apoptosis (Schlattner et al., 2013). The results of in vitro biochemical experiments are also compatible with the involvement of yet another mitochondrial kinase, mitochondrial creatine phosphokinase (mCPK) in the transfer of CL between IMM and OMM whereby the octameric mCPK may act as a “rotary” mechanism of CL binding/unbinding and trans-membrane delivery (Karo et al., 2012; Schlattner and Wallimann, 2000).
Finally, transgression of CL from the inner to the outer leaflet of the OMM is required for its externalization. It is possible that one of phospholipid scramblases is involved in this move of CL to the mitochondrial surface (Liu et al., 2003; Van et al., 2007). Indeed, RNAi knockdown of phospholipid scramblase 3 (PLS3) profoundly decreased the delivery of injured mitochondria to autophagosomes (Chu et al., 2013). While the (trans)-mitochondrial localization of PLS3 has not yet been firmly established (Korytowski et al., 2011), it is also possible that other intermembrane space proteins (including cytochrome c) (Xu et al., 2013) and integral OMM proteins - capable of binding CL - including members of Bcl-2 family (Asciolla et al., 2012; Epand et al., 2003; Ott et al., 2009) and voltage-dependent anion channel (VDAC) (Rostovtseva and Bezrukov, 2008) - may facilitate its redistribution to the mitochondrial surface (Garcia Fernandez et al., 2002). Importantly, autophageal machinery recognizes CLs more effectively than its metabolites, including mono- and di-lyso-CLs as well as CL oxidation products (Chu et al., 2013). This implies that CL oxidation is not a requirement for mitophageal elimination of injured mitochondria. This seems to be contradictory to the accepted opinion that oxidative stress and lipid peroxidation are inherent to mitochondrial injury and mitophagy (Kirkland et al., 2002). However, association is not the same as causation, and oxidative reactions may indirectly promote mitophagy by causing mitochondrial damage with accumulation of oxidized lipids as side effect. It is also important to consider that triggers for both mitophagy and apoptosis may occur simultaneously in a population of injured cells, some of which are dying. However, under sublethal treatments with rotenone that were sufficient to trigger autophagy, CL peroxidation products were not detected (Chu et al., 2013).
Translocation and Peroxidation of CL in apoptosis
Redistribution of CLs from IMM to OMM is essential for the execution and completion of apoptotic program (Garcia Fernandez et al., 2002; Gonzalvez and Gottlieb, 2007). This suggests that the same mechanisms involved in CL externalization and signaling in mitophagy are also required for the apoptotic death pathway. One may wonder how intracellular regulatory mechanisms switch from mitophageal to apoptotic pathways? The major specific feature of CL’s engagement in pro-apoptotic mechanisms requires not only the appearance of CLs in the OMM but also its oxidative modification (Kagan et al., 2005). Detailed studies have established the major corner-stone events in pro-apoptotic CL oxidation in mitochondria that is enzymatically catalyzed by the peroxidase function of an intermembrane space hemoprotein, cytochrome c (cyt c) (Atkinson et al., 2011; Kagan et al., 2009). In the absence of CL, cyt c has a very weak peroxidase activity (Basova et al., 2007). Met80 – one of its distal ligands – is located only 2.5Å away from Fe, thus precluding access of the heme to H2O2 in the native protein (Pinheiro, 1994). Upon binding of CL, Met80 moves away from the heme and weakens the sixth coordination Met80-Fe bond thus conferring peroxidase competence on cyt c that is specific towards CL peroxidation. While the structural details and hierarchies of foldons in the re-arrangements of cyt c’s molecule induced by its interactions with CL-containing membrane surfaces are currently being deciphered (Kapralov et al., 2007; Muenzner et al., 2013) (see Pletneva et al., in this issue) it is likely that both electrostatic interactions of one or more positively charged aminoacid (lysine) residues with negatively charged phosphate groups of CL as well as hydrophobic interactions of one or more of CL’s fatty acid residues with apolar sites of the protein are involved in the formation of its “molten” globule. Understanding of the catalytic mechanisms of the reaction also started to emerge. It is believed that tyrosine residues - via the generation of tyrosyl radicals (Tyr) -are likely reactive intermediates of the peroxidase cycle leading to CL peroxidation whereby the highly conserved Tyr67 is a likely electron-donor (radical acceptor) in the oxygenase half-reaction of the cyt c/CL peroxidase complex (Kapralov et al., 2011).
A recent discovery of tyrosine phosphorylation sites in cyt c implies that its peroxidase function may be also regulated through this type of signaling (Pecina et al., 2010). Indeed, it has been established that Tyr48 phosphorylation might serve as an anti-apoptotic switch, possibly via its effects on CL peroxidation during apoptosis. Another interesting opportunity for regulation of CL peroxidation by cyt c is offered by the involvement of lipid hydroperoxides – in place of H2O2 – as a source of oxidizing equivalents feeding the peroxidase cycle. Indeed, it turns out that fatty acid hydroperoxides (and CL hydroperoxides) can accelerate the CL peroxidation reaction more than two orders of magnitude (Belikova et al., 2009). This suggests that the initial dependence of CL peroxidation on the sufficient production of H2O2 (likely via dismutation of superoxide radicals) by dysfunctional electron carriers of injured mitochondria (Petrosillo et al., 2003), loses its regulatory role with the accumulation of CL hydroperoxides. In other words, the CL peroxidation process becomes independent on the supply of oxidative equivalents by damaged mitochondria.
While the essentiality of CL peroxidation for the release of pro-apoptotic factors from mitochondria into the cytosol has been established (Belikova et al., 2007; Petrosillo et al., 2009), specific mechanisms through which peroxidized CL species fulfill their action are much less clear. This relates to i) identification of one or more individual molecular species of peroxidized CLs (CLox) from their highly diversified multiple forms accumulating during apoptosis (Domingues et al., 2008; Ji et al., 2012; Tyurin et al., 2010; see Tyurina et al., in this issue), and ii) the necessity and identity of potential protein partners engaged in the formation of a “pore” in the OMM through which pro-apoptotic factors are liberated into the cytosol (Bender and Martinou, 2013). While complexes of CLs and CLox with several OMM and intermembrane space proteins –Bcl-2 family proteins, VDAC, cyt c – have been suggested to play a role in apoptotic membrane permeabilization and pore formation (Korytowski et al., 2011; Rostovtseva and Bezrukov, 2008; Veenman et al., 2008; Xu et al., 2013), neither specific mechanisms nor physiological role of these interactions have been firmly established. Moreover, there are indications that sequestration of CLox molecules occurring without participation of any proteins may lead to the formation of pores large enough to allow for the release of pro-apoptotic factors (Jurkiewicz et al., 2012).
Molecular Asymmetry of Cardiolipins and Their Oxidation products
The dimeric architecture of CLs with the two phosphatidyl residues containing different fatty acid residues suggests that a huge diversity of stereochemically non-equivalent species of CL may be realized (Schlame, 2008). This non-equivalency does not exist for selected CL molecular species with all four identical fatty acid residues. Notably, one of these species – tetra-linoleyl-CL (TLCL) – is the most abundant type of CL in several tissues (heart, muscles, liver) where its content may be accountable for ~80–85% of all CLs (Cheng et al., 2008; Han et al., 2006). It is believed that this specific enrichment with symmetric TLCL is achieved through maturation of diversified nascent species (Cheng et al., 2008; Gebert et al., 2009). It has been also hypothesized that the CL species of this type are particularly useful for structural purposes whereby their symmetric arrangement simplifies adjustments and arrangements within lipid-protein mitochondrial complexes (Kiebish et al., 2010). On the other hand, highly diversified molecular speciation of CLs is associated with their role as precursors of signaling molecules. In this regard, of particular interest may be the involvement of polyunsaturated CLs in (per)oxidative reactions resulting in the formation of numerous long-chain oxygenated CL species (containing hydroperoxy-, hydroxy-, epoxy-, and oxo-groups) as well as oxidatively truncated CL species (Tyurina et al., 2011). Notably, the appearance of one or more oxygenated fatty acid residues in symmetric CL molecules produces stereochemical non-equivalency, i.e. converts symmetric CL species into non-symmetric ones. It is possible that myriads of highly diversified asymmetric species of CLs and CLox are utilized as a yet to be identified signaling language used for the intracellular communications of mitochondria with other intra- and extracellular compartments. Interestingly, several phospholipases A2 specific towards oxidatively modified anionic phospholipids and capable of releasing oxygenated fatty acids have been recently described (Hsu et al., 2013). While the meaning of individual species of CLs and their metabolites as words in the signaling language has not been deciphered the significance of CL/CLox commands in mitophagy and apoptosis is firmly established (Kagan et al., 2005, Chu et al., 2013). It is noteworthy that acute tissue injury – is accompanied by the generation of multiple CLox species. One of the most prominent examples of this is massive apoptotic response after acute brain injury (Chan and Di Paolo, 2012; Ji et al., 2012). In this case, prevention of CL oxidation resulted in significant protection not only on biochemical but also on physiological and behavioral levels suggesting the essentiality of the accumulated CLox in the apoptotic signaling in injured brain. The important and still unanswered issue is the decoding of individual CLox signals generated by injured cells.
CL asymmetry as a new therapeutic target
It is tempting to speculate that regulation of CL asymmetry – transmembrane and molecular – and understanding of the CL/CLox signaling language may be important as a new target for drug discovery. Given a well-known pro-survival role of mitophagy in cells one can imagine that stimulation of mechanisms of CL externalization in damaged mitochondria will lead to their enhanced and selective elimination thus preserving cells from a risk of uncontrolled oxidative injury. Similarly, controlled regulation of excessive CL peroxidation may be essential for the protection against apoptosis. Based on the established catalytic role of cyt c/CL peroxidase complexes, at least two approaches can be envisioned to regulate CL peroxidation: (i) “locking” of the heme-iron coordination bond with a strong ligand delivered through the hydrophobic channel into immediate proximity of the heme; (ii) removing the source of oxidizing equivalents (H2O2) feeding the peroxidase activity of cyt c/CL complexes. Optimistically, both of these approaches have been found effective in anti-apoptotic protection of cells in vitro and, most importantly, in vivo (Atkinson et al., 2011; Ji et al., 2012).
Outlook and perspectives
Transition from anaerobic to aerobic life and appearance of eukaryotic cells required many adaptive changes among which one of the most prominent was the emergence of mitochondria as a product of endosymbiotic relationships. In eukaryotic mitochondria, a class of ancient phospholipids, CLs, were insulated from the cytosol by the OMM and cloaked within the IMM, thus creating its asymmetric trans-membrane distribution. This asymmetry has been used as an important recognition mechanism for distinguishing healthy mitochondria capable of maintaining the CL trans-membrane gradient from injured organelles where this asymmetry is collapsing. This results in CL externalization on the surface of mitochondria, recognition of CLs by components of the autophageal system (LC3) and elimination of the damaged mitochondria, in analogy to the recognition of externalized phosphatidylserine on the cell surface for elimination by professional phagocytes. Diversification of CLs in eukaryotic cells yielded multiplicity of its polyunsaturated species whose oxidation may serve additional signaling purposes. Indeed, peroxidation of CLs and accumulation of CLox is essential for the completion of the cell death program, thus offering new opportunities for drug discovery. Our understanding of mitochondrial functions – in addition to being a powerhouse of cells - is currently extended to a concept of their role as a major regulatory platform involved in numerous intra- and extracellular functions, from coordination of metabolism and cell death to immune responses in which CLs/CLox are considered as important signaling molecules. There is an intuitive perception that the remarkably diversified CLs/CLox species in eukaryotes relative to prokaryotes may represent a mitochondrial communications language. The biochemical and signaling principles of this language, its vocabulary and meaning of its words are yet to be deciphered.
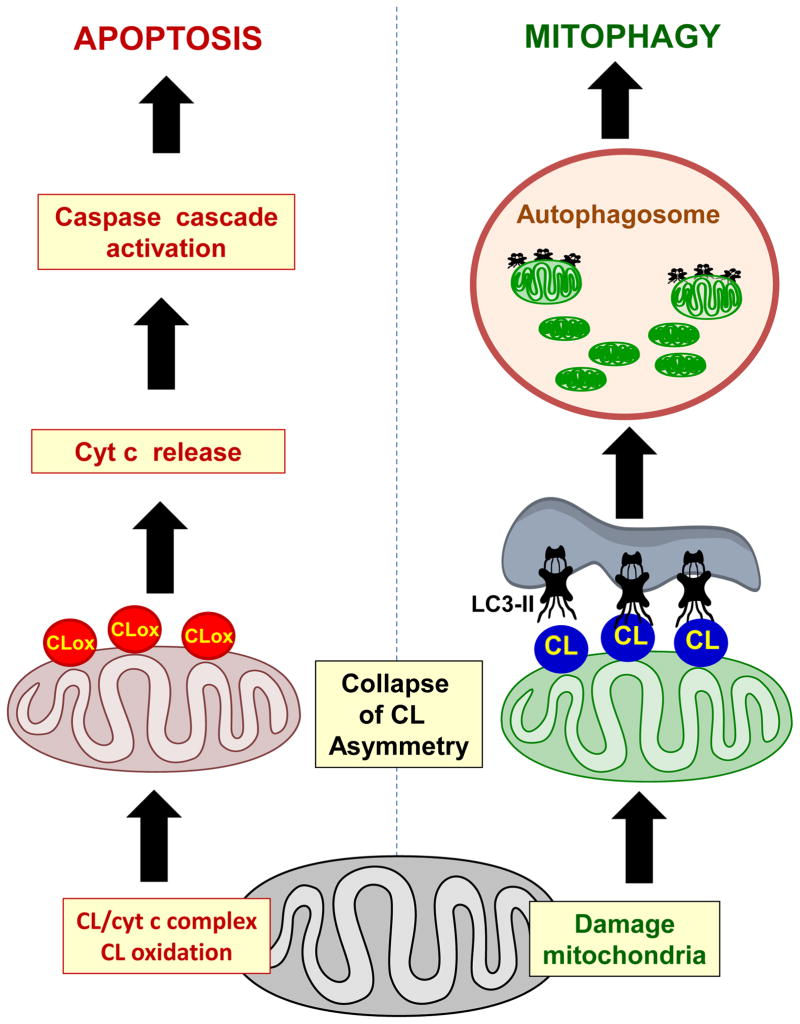
Figure 2.
Collapse of cardiolipin asymmetry and its redistribuition from IMM top OMM is required for the execution of both mitophagy and apoptosis. However, cardiolipin peroxidation is not necessary for mitophagy but it is a pre-requisite for the release of pro-apoptotic factors from mitochondria into the cytosol.
Highlights.
Cardiolipin asymmetries.
Reduced trans-membrane asymmetry and externalization of CLs leads to mitophagy.
Translocation and peroxidation of CL in apoptosis.
Molecular asymmetry of cardiolipins and their oxidation products
CL asymmetry as a new therapeutic target.
Acknowledgments
Supported by NIH: ES020693, ES021068, U19AIO68021, NS076511, NS061817, NIOSH OH008282, AG026389 and NS065789, Fulbright U.S/Canada Scholar Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardail D, Privat JP, Egret-Charlier M, Levrat C, Lerme F, Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. The Journal of biological chemistry. 1990;265:18797–18802. [PubMed] [Google Scholar]
- Asciolla JJ, Renault TT, Chipuk JE. Examining BCL-2 family function with large unilamellar vesicles. Journal of visualized experiments. 2012 Oct 5;(68):4291. doi: 10.3791/4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Kapralov AA, Yanamala N, Tyurina YY, Amoscato AA, Pearce L, Peterson J, Huang Z, Jiang J, Samhan-Arias AK, Maeda A, Feng W, Wasserloos K, Belikova NA, Tyurin VA, Wang H, Fletcher J, Wang Y, Vlasova II, Klein-Seetharaman J, Stoyanovsky DA, Bayir H, Pitt BR, Epperly MW, Greenberger JS, Kagan VE. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nature communications. 2011;2:497. doi: 10.1038/ncomms1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baile MG, Whited K, Claypool SM. Deacylation on the matrix side of the mitochondrial inner membrane regulates cardiolipin remodeling. Molecular biology of the cell. 2013;24:2008–2020. doi: 10.1091/mbc.E13-03-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basova LV, Kurnikov IV, Wang L, Ritov VB, Belikova NA, Vlasova II, Pacheco AA, Winnica DE, Peterson J, Bayir H, Waldeck DH, Kagan VE. Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity. Biochemistry. 2007;46:3423–3434. doi: 10.1021/bi061854k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belikova NA, Jiang J, Tyurina YY, Zhao Q, Epperly MW, Greenberger J, Kagan VE. Cardiolipin-specific peroxidase reactions of cytochrome C in mitochondria during irradiation-induced apoptosis. International journal of radiation oncology, biology, physics. 2007;69:176–186. doi: 10.1016/j.ijrobp.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Belikova NA, Tyurina YY, Borisenko G, Tyurin V, Samhan Arias AK, Yanamala N, Furtmuller PG, Klein-Seetharaman J, Obinger C, Kagan VE. Heterolytic reduction of fatty acid hydroperoxides by cytochrome c/cardiolipin complexes: antioxidant function in mitochondria. Journal of the American Chemical Society. 2009;131:11288–11289. doi: 10.1021/ja904343c. [DOI] [PubMed] [Google Scholar]
- Bender T, Martinou JC. Where killers meet--permeabilization of the outer mitochondrial membrane during apoptosis. Cold Spring Harbor perspectives in biology. 2013;5:a011106. doi: 10.1101/cshperspect.a011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland AG, Kinkaid AR, Wilton DC. Cardiolipin hydrolysis by human phospholipases A2. The multiple enzymatic activities of human cytosolic phospholipase A2. Biochimica et biophysica acta. 1998;1390:65–72. doi: 10.1016/s0005-2760(97)00170-7. [DOI] [PubMed] [Google Scholar]
- Cao J, Liu Y, Lockwood J, Burn P, Shi Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. The Journal of biological chemistry. 2004;279:31727–31734. doi: 10.1074/jbc.M402930200. [DOI] [PubMed] [Google Scholar]
- Chan RB, Di Paolo G. Knockout punch: cardiolipin oxidation in trauma. Nature neuroscience. 2012;15:1325–1327. doi: 10.1038/nn.3222. [DOI] [PubMed] [Google Scholar]
- Cheng H, Mancuso DJ, Jiang X, Guan S, Yang J, Yang K, Sun G, Gross RW, Han X. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry. 2008;47:5869–5880. doi: 10.1021/bi7023282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CH, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Wang KZQ, Zhu J, Klein-Seetharaman JJ, Balasubramanian K, Amoscato AA, Borisenko GG, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Catherine Baty C, Watkins S, Bahar I, Bayir H, Kagan VE. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in mammalian cells. Nature Cell Biology. 2013 Sep 15; doi: 10.1038/ncb2837. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis PR, Verkleij AJ, Ververgaert PH. Polymorphic phase behaviour of cardiolipin as detected by 31P NMR and freeze-fracture techniques. Effects of calcium, dibucaine and chlorpromazine. Biochimica et biophysica acta. 1978;513:11–20. doi: 10.1016/0005-2736(78)90107-4. [DOI] [PubMed] [Google Scholar]
- Daum G, Vance JE. Import of lipids into mitochondria. Progress in lipid research. 1997;36:103–130. doi: 10.1016/s0163-7827(97)00006-4. [DOI] [PubMed] [Google Scholar]
- Domingues MR, Reis A, Domingues P. Mass spectrometry analysis of oxidized phospholipids. Chemistry and physics of lipids. 2008;156:1–12. doi: 10.1016/j.chemphyslip.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Epand RF, Martinou JC, Montessuit S, Epand RM. Transbilayer lipid diffusion promoted by Bax: implications for apoptosis. Biochemistry. 2003;42:14576–14582. doi: 10.1021/bi035348w. [DOI] [PubMed] [Google Scholar]
- Eskelinen EL. New insights into the mechanisms of macroautophagy in mammalian cells. International review of cell and molecular biology. 2008;266:207–247. doi: 10.1016/S1937-6448(07)66005-5. [DOI] [PubMed] [Google Scholar]
- Esposti MD, Erler JT, Hickman JA, Dive C. Bid, a widely expressed proapoptotic protein of the Bcl-2 family, displays lipid transfer activity. Molecular and cellular biology. 2001;21:7268–7276. doi: 10.1128/MCB.21.21.7268-7276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet PF, Petit JM, Maftah A, Zachowski A, Julien R. Asymmetrical distribution of cardiolipin in yeast inner mitochondrial membrane triggered by carbon catabolite repression. The Biochemical journal. 1997;324 ( Pt 2):627–634. doi: 10.1042/bj3240627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Fernandez M, Troiano L, Moretti L, Nasi M, Pinti M, Salvioli S, Dobrucki J, Cossarizza A. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 2002;13:449–455. [PubMed] [Google Scholar]
- Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, Rehling P, Meisinger C, Ryan MT, Wiedemann N, Greenberg ML, Pfanner N. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Current biology. 2009;19:2133–2139. doi: 10.1016/j.cub.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez F, Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis: an international journal on programmed cell death. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- Han X, Yang K, Yang J, Cheng H, Gross RW. Shotgun lipidomics of cardiolipin molecular species in lipid extracts of biological samples. Journal of lipid research. 2006;47:864–879. doi: 10.1194/jlr.D500044-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb JS, Comte J, Gautheron DC. Asymmetrical orientation of phospholipids and their interactions with marker enzymes in pig heart mitochondrial inner membrane. Archives of biochemistry and biophysics. 1981;208:305–318. doi: 10.1016/0003-9861(81)90153-3. [DOI] [PubMed] [Google Scholar]
- Hoang T, Smith MD, Jelokhani-Niaraki M. Toward understanding the mechanism of ion transport activity of neuronal uncoupling proteins UCP2, UCP4, and UCP5. Biochemistry. 2012;51:4004–4014. doi: 10.1021/bi3003378. [DOI] [PubMed] [Google Scholar]
- Hoch FL. Cardiolipins and biomembrane function. Biochimica et biophysica acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- Hovius R, Lambrechts H, Nicolay K, de Kruijff B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochimica et biophysica acta. 1990;1021:217–226. doi: 10.1016/0005-2736(90)90036-n. [DOI] [PubMed] [Google Scholar]
- Hsu YH, Dumlao DS, Cao J, Dennis EA. Assessing phospholipase A2 activity toward cardiolipin by mass spectrometry. PloS one. 2013;8:e59267. doi: 10.1371/journal.pone.0059267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Kline AE, Amoscato A, Samhan-Arias AK, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio AM, Okonkwo DO, Cheng JP, Alexander H, Clark RS, Kochanek PM, Wipf P, Kagan VE, Bayir H. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nature neuroscience. 2012;15:1407–1413. doi: 10.1038/nn.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz P, Olzynska A, Cwiklik L, Conte E, Jungwirth P, Megli FM, Hof M. Biophysics of lipid bilayers containing oxidatively modified phospholipids: insights from fluorescence and EPR experiments and from MD simulations. Biochimica et biophysica acta. 2012;1818:2388–2402. doi: 10.1016/j.bbamem.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. The EMBO journal. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free radical biology & medicine. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature chemical biology. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Kapralov AA, Kurnikov IV, Vlasova II, Belikova NA, Tyurin VA, Basova LV, Zhao Q, Tyurina YY, Jiang J, Bayir H, Vladimirov YA, Kagan VE. The hierarchy of structural transitions induced in cytochrome c by anionic phospholipids determines its peroxidase activation and selective peroxidation during apoptosis in cells. Biochemistry. 2007;46:14232–14244. doi: 10.1021/bi701237b. [DOI] [PubMed] [Google Scholar]
- Kapralov AA, Yanamala N, Tyurina YY, Castro L, Samhan-Arias A, Vladimirov YA, Maeda A, Weitz AA, Peterson J, Mylnikov D, Demicheli V, Tortora V, Klein-Seetharaman J, Radi R, Kagan VE. Topography of tyrosine residues and their involvement in peroxidation of polyunsaturated cardiolipin in cytochrome c/cardiolipin peroxidase complexes. Biochimica et biophysica acta. 2011;1808:2147–2155. doi: 10.1016/j.bbamem.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karo J, Peterson P, Vendelin M. Molecular dynamics simulations of creatine kinase and adenine nucleotide translocase in mitochondrial membrane patch. The Journal of biological chemistry. 2012;287:7467–7476. doi: 10.1074/jbc.M111.332320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebish MA, Bell R, Yang K, Phan T, Zhao Z, Ames W, Seyfried TN, Gross RW, Chuang JH, Han X. Dynamic simulation of cardiolipin remodeling: greasing the wheels for an interpretative approach to lipidomics. Journal of lipid research. 2010;51:2153–2170. doi: 10.1194/jlr.M004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Adibhatla RM, Hatcher JF, Franklin JL. Loss of cardiolipin and mitochondria during programmed neuronal death: evidence of a role for lipid peroxidation and autophagy. Neuroscience. 2002;115:587–602. doi: 10.1016/s0306-4522(02)00512-2. [DOI] [PubMed] [Google Scholar]
- Korytowski W, Basova LV, Pilat A, Kernstock RM, Girotti AW. Permeabilization of the mitochondrial outer membrane by Bax/truncated Bid (tBid) proteins as sensitized by cardiolipin hydroperoxide translocation: mechanistic implications for the intrinsic pathway of oxidative apoptosis. The Journal of biological chemistry. 2011;286:26334–26343. doi: 10.1074/jbc.M110.188516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraffe E, Grall J, Le Duff M, Soudant P, Marty Y. A striking parallel between cardiolipin fatty acid composition and phylogenetic belonging in marine bivalves: a possible adaptative evolution? Lipids. 2008;43:961–970. doi: 10.1007/s11745-008-3219-9. [DOI] [PubMed] [Google Scholar]
- Krebs JJ, Hauser H, Carafoli E. Asymmetric distribution of phospholipids in the inner membrane of beef heart mitochondria. The Journal of biological chemistry. 1979;254:5308–5316. [PubMed] [Google Scholar]
- Liu J, Dai Q, Chen J, Durrant D, Freeman A, Liu T, Grossman D, Lee RM. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Molecular cancer research. 2003;1:892–902. [PubMed] [Google Scholar]
- Malhotra A, Edelman-Novemsky I, Xu Y, Plesken H, Ma J, Schlame M, Ren M. Role of calcium-independent phospholipase A2 in the pathogenesis of Barth syndrome. Proceedings of the National Academy of Sciences of USA. 2009;106:2337–2341. doi: 10.1073/pnas.0811224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti GV. Hydrolysis of Cardiolipin by Snake Venom Phospholipase A. Biochimica et biophysica acta. 1964;84:55–59. doi: 10.1016/0926-6542(64)90100-3. [DOI] [PubMed] [Google Scholar]
- Muenzner J, Toffey JR, Hong Y, Pletneva EV. Becoming a Peroxidase: Cardiolipin-Induced Unfolding of Cytochrome c. The journal of physical chemistry. B. 2013 May 28; doi: 10.1021/jp402104r. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M, Norberg E, Zhivotovsky B, Orrenius S. Mitochondrial targeting of tBid/Bax: a role for the TOM complex? Cell death and differentiation. 2009;16:1075–1082. doi: 10.1038/cdd.2009.61. [DOI] [PubMed] [Google Scholar]
- Pecina P, Borisenko GG, Belikova NA, Tyurina YY, Pecinova A, Lee I, Samhan-Arias AK, Przyklenk K, Kagan VE, Huttemann M. Phosphomimetic substitution of cytochrome C tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry. 2010;49:6705–6714. doi: 10.1021/bi100486s. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Moro N, Ruggiero FM, Paradies G. Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome c release. Free radical biology & medicine. 2009;47:969–974. doi: 10.1016/j.freeradbiomed.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Ruggiero FM, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB journal. 2003;17:2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- Pinheiro TJ. The interaction of horse heart cytochrome c with phospholipid bilayers. Structural and dynamic effects. Biochimie. 1994;76:489–500. doi: 10.1016/0300-9084(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Rassow J, Guiard B, Wienhues U, Herzog V, Hartl FU, Neupert W. Translocation arrest by reversible folding of a precursor protein imported into mitochondria. A means to quantitate translocation contact sites. The Journal of cell biology. 1989;109:1421–1428. doi: 10.1083/jcb.109.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J, Pfanner N. Mitochondrial preproteins en route from the outer membrane to the inner membrane are exposed to the intermembrane space. FEBS letters. 1991;293:85–88. doi: 10.1016/0014-5793(91)81157-4. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Bezrukov SM. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. Journal of bioenergetics and biomembranes. 2008;40:163–170. doi: 10.1007/s10863-008-9145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. Journal of lipid research. 2008;49:1607–1620. doi: 10.1194/jlr.R700018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Haldar D. Cardiolipin is synthesized on the matrix side of the inner membrane in rat liver mitochondria. The Journal of biological chemistry. 1993;268:74–79. [PubMed] [Google Scholar]
- Schlame M, Ren M, Xu Y, Greenberg ML, Haller I. Molecular symmetry in mitochondrial cardiolipins. Chemistry and physics of lipids. 2005;138:38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Schlattner U, Tokarska-Schlattner M, Ramirez S, Tyurina YY, Amoscato AA, Mohammadyani D, Huang Z, Jiang J, Yanamala N, Seffouh A, Boissan M, Epand RF, Epand RM, Klein-Seetharaman J, Lacombe ML, Kagan VE. Dual function of mitochondrial Nm23-H4 protein in phosphotransfer and intermembrane lipid transfer: a cardiolipin-dependent switch. The Journal of biological chemistry. 2013;288:111–121. doi: 10.1074/jbc.M112.408633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlattner U, Wallimann T. Octamers of mitochondrial creatine kinase isoenzymes differ in stability and membrane binding. The Journal of biological chemistry. 2000;275:17314–17320. doi: 10.1074/jbc.M001919200. [DOI] [PubMed] [Google Scholar]
- Tian HF, Feng JM, Wen JF. The evolution of cardiolipin biosynthesis and maturation pathways and its implications for the evolution of eukaryotes. BMC evolutionary biology. 2012;12:32. doi: 10.1186/1471-2148-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarska-Schlattner M, Boissan M, Munier A, Borot C, Mailleau C, Speer O, Schlattner U, Lacombe ML. The nucleoside diphosphate kinase D (NM23-H4) binds the inner mitochondrial membrane with high affinity to cardiolipin and couples nucleotide transfer with respiration. The Journal of biological chemistry. 2008;283:26198–26207. doi: 10.1074/jbc.M803132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Ritov VB, Lysytsya A, Amoscato AA, Kochanek PM, Hamilton R, Dekosky ST, Greenberger JS, Bayir H, Kagan VE. Oxidative lipidomics of apoptosis: quantitative assessment of phospholipid hydroperoxides in cells and tissues. Methods in molecular biology. 2010;610:353–374. doi: 10.1007/978-1-60327-029-8_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina YY, Tyurin VA, Kapralova VI, Wasserloos K, Mosher M, Epperly MW, Greenberger JS, Pitt BR, Kagan VE. Oxidative lipidomics of gamma-radiation-induced lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Radiation research. 2011;175:610–621. doi: 10.1667/RR2297.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Q, Liu J, Lu B, Feingold KR, Shi Y, Lee RM, Hatch GM. Phospholipid scramblase-3 regulates cardiolipin de novo biosynthesis and its resynthesis in growing HeLa cells. The Biochemical journal. 2007;401:103–109. doi: 10.1042/BJ20060373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Venetie R, Verkleij AJ. Possible role of non-bilayer lipids in the structure of mitochondria. A freeze-fracture electron microscopy study. Biochimica et biophysica acta. 1982;692:397–405. doi: 10.1016/0005-2736(82)90390-x. [DOI] [PubMed] [Google Scholar]
- Veenman L, Shandalov Y, Gavish M. VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. Journal of bioenergetics and biomembranes. 2008;40:199–205. doi: 10.1007/s10863-008-9142-1. [DOI] [PubMed] [Google Scholar]
- Xu J, Vanderlick TK, Beales PA. Lytic and non-lytic permeabilization of cardiolipin-containing lipid bilayers induced by cytochrome C. PloS one. 2013;8:e69492. doi: 10.1371/journal.pone.0069492. [DOI] [PMC free article] [PubMed] [Google Scholar]