Abstract
Purpose
Basal-like breast tumors are typically (ER/PR/HER2) triple-negative and are associated with a high incidence of brain metastases and poor clinical outcomes. The molecular chaperone αB-crystallin is predominantly expressed in triple-negative breast cancer (TNBC) and contributes to an aggressive tumor phenotype in preclinical models. We investigated the potential role of αB-crystallin in brain metastasis in TNBC.
Experimental Design
αB-crystallin expression in primary breast carcinomas and brain metastases was analyzed by immunohistochemistry among breast cancer patients with brain metastases. αB-crystallin was overexpressed or silenced in two different TNBC cell lines. The effects on cell adhesion to human brain microvascular endothelial cells (HBMECs) or extracellular matrix proteins, transendothelial migration, and transmigration across a HBMEC/astrocyte co-culture blood-brain barrier (BBB) model were examined. Additionally, the effects of overexpressing or silencing αB-crystallin on brain metastasis in vivo were investigated using orthotopic TNBC models.
Results
In a cohort of women with breast cancer brain metastasis, αB-crystallin expression in primary breast carcinomas was associated with poor overall survival and poor survival after brain metastasis, even among TNBC patients. Stable overexpression of αB-crystallin in TNBC cells enhanced adhesion to HBMECs, transendothelial migration, and BBB transmigration in vitro, while silencing αB-crystallin inhibited these events. αB-crystallin promoted adhesion of TNBC cells to HBMECs at least in part through an α3β1 integrin-dependent mechanism. αB-crystallin overexpression promoted brain metastasis, while silencing αB-crystallin inhibited brain metastasis in orthotopic TNBC models.
Conclusion
αB-crystallin is a novel regulator of brain metastasis in TNBC and represents a potential biomarker and drug target for this aggressive disease.
Keywords: Brain metastasis, Breast Cancer, Blood-brain barrier, αB-crystallin, Cell Adhesion
Introduction
Brain metastases are a devastating complication in 10–16% of women with advanced breast cancer that result in neurologic deficits, including headaches, cognitive problems, seizures, and/or motor deficits (1). Brain metastases are often a late occurrence in women with pre-existing metastatic disease in other organs and typically follow a rapidly progressive course with one-year survival less than 20%. Unfortunately, the incidence of brain metastases appears to be increasing with the advent of improved systemic therapies, which are frequently ineffective against brain metastases because of their limited ability to cross the blood-brain barrier (BBB), a distinctive permeability barrier composed of human brain microvascular endothelial cells (HBMECs) interconnected by tight junctions and reinforced by astrocytes, pericytes and a basement membrane (2, 3). The BBB is also a formidable obstacle for circulating tumor cells to extravasate into the brain, a critical step in brain metastasis.
Recent studies in animal models have provided new insights into the mechanisms by which tumor cells cross the BBB and colonize the brain. Circulating tumor cells arrest in the brain microvasculature, often at capillary branches, where they adhere to HBMECs and/or the subendothelial basement membrane, distending the capillary and extravasating through gaps in the microvessel wall (2–5). Tumor cells proliferate in the brain in close proximity to the exterior surface of capillaries from which they extravasate, a process termed “vascular cooption”, although some metastatic tumor cells rely on angiogenesis for perivascular growth (2–6). The metastatic tumor cells initiate a localized inflammatory response through reciprocal interactions with reactive astrocytes and microglia that promote tumor growth (2, 3, 5). Hence, dynamic interactions between tumor cells and diverse cell types in the brain contribute to brain metastasis.
Molecular profiling of breast tumors has provided additional insights into brain metastasis. Brain metastases are most prevalent in HER2/ErbB2-positive and basal-like breast tumors; the latter express basal epithelial genes and are often “triple (ER/PR/HER2)-negative” (7–9). Brain metastases in triple-negative breast cancer (TNBC) are particularly challenging given the lack of targeted agents and poor clinical outcomes. In one cohort, brain metastases were present in 46% of patients with metastatic TNBC and were associated with median survival of 4.9 months after diagnosis (10). Moreover, patients with early stage TNBC have a high incidence (4.7%) of brain metastasis as a first site of relapse (11). Although a brain metastasis gene signature was recently reported (12), the specific genes that regulate brain metastasis in TNBC are poorly understood. Identification of these genes is a critical first step in the development of biomarkers and targeted therapies for brain metastases.
The molecular chaperone αB-crystallin is predominantly expressed in basal-like breast cancer/TNBC and is associated with poor outcomes (13–15). αB-crystallin has been linked to many biological characteristics of these aggressive tumors. αB-crystallin promotes apoptosis-resistance at least in part by inhibiting caspase-3 activation, thereby enhancing cell survival in the setting of oncogenic stress, growth factor depletion, chemotherapy and other cellular stressors (16–19). αB-crystallin promotes cell migration and invasion and localizes to the infiltrative edge of malignant glioblastomas (13, 20–22). These effects are likely mediated by direct interactions of αB-crystallin with actin and intermediate filaments, which regulate cytoskeletal stability and dynamics (23, 24). Additionally, αB-crystallin expression is dramatically increased in two highly metastatic TNBC cell lines identified by in vivo selection (including GILM2 cells used in our experiments) compared to the less metastatic parental cells (25). However, the functional role of αB-crystallin in metastasis has not been studied. We postulated that αB-crystallin might contribute to the observed proclivity of TNBCs to metastasize to the brain.
Here we report that αB-crystallin is commonly expressed in breast cancer brain metastases and demonstrate that its expression in primary breast carcinomas predicts poor survival in patients with brain metastasis. Stable overexpression of αB-crystallin in TNBC cells enhanced adhesion to HBMECs, transendothelial migration, and transmigration through a BBB model in vitro, while silencing αB-crystallin inhibited these events. αB-crystallin promoted adhesion of TNBC cells to HBMECs at least in part by an α3β1 integrin-dependent mechanism. Moreover, αB-crystallin promoted brain metastasis in vivo in orthotopic TNBC models. Our findings indicate that αB-crystallin is a novel regulator of brain metastasis in TNBC and point to αB-crystallin and α3β1 integrin as potential drug targets for this devastating disease.
Materials and Methods
Breast cancer brain metastasis cohort and immunohistochemistry (IHC) analyses
Patients with a diagnosis of breast cancer and brain metastases who were treated at the University of North Carolina at Chapel Hill (1989–2006) and Duke University Medical Center (1985–2005) with available tumor tissue (breast, brain or both) and survival data were included. Additional data included age, gender, race, tumor estrogen receptor (ER)/progesterone receptor (PR)/HER2 status, and therapies. For cases with sufficient tissue, ER, PR and HER2 status was determined by IHC. Eighty-seven formalin-fixed, paraffin-embedded (FFPE) tissues (49 brain metastases and 38 breast tumors, including 11 paired tumors) were available from 76 patients. The study was approved by the respective Institutional Review Boards.
FFPE tissue sections were incubated in 3% hydrogen peroxide/methanol for 10 min, followed by antigen retrieval in steaming citrate buffer for 30 min. Sections were preincubated in horse serum (Vector Laboratories) and then incubated for 60 min with Abs against ER (1D5, 1:50, Dako), PR (16, 1:70, Vision BioSystems), HER2 (CB11, 1:100, BioGenex) or αB-crystallin (1B6.1-3G4, 1:200, Enzo Life Sciences/Stressgen) using a DakoCytomation autostainer. An avidin-biotin complex (Vectastain Elite) was applied for 30 min followed by diaminobenzidine (Innovex) and hematoxylin (DakoCytomation). ER and PR staining were scored using the Allred system (26). HER2 was scored using ASCO/CAP guidelines (27). Breast cancer subtype was assigned by primary tumor IHC as ER/PR or hormone receptor (HR)-positive/HER2-negative (HR+/HER2−), triple-negative (HR−/HER2−), or HR-positive/negative and HER2-positive (HER2+). αB-crystallin was scored as negative (0%) or positive (> 0%) based on tumor cell expression.
Statistical analysis for associations between subtypes and αB-crystallin expression was performed using Fisher’s Exact Test. Time-to-event analyses were done for overall survival (time from breast tumor diagnosis to death or last contact) and overall survival from brain metastasis (time from the date of brain metastasis to the date of death or last contact). The Kaplan-Meier method and Log rank statistics were used to estimate survival and to evaluate associations with αB-crystallin expression. Breast cancer subtype was available for 71 of 76 patients. Date of brain metastasis was available for 75 patients. Statistical analyses were performed with SAS 9.2 software.
Cell lines and culture
Human GILM2 and MDA-MB-231 TNBC cells expressing mCherry fluorescent protein (231-mCherry) were described (28). 231-mCherry cells were grown in DMEM/F12 media with 5% FBS, 100 units/mL penicillin/streptomycin, nonessential amino acids, and 1 mM sodium pyruvate (Invitrogen). GILM2 cells were cultured in DMEM/F12 media with 10% FBS, 100 units/mL penicillin/streptomycin and Insulin/Transferrin/Sodium Selenite mix (Invitrogen). Primary HBMECs and human astrocytes (ScienCell) were cultured according to the manufacturer’s protocol.
Lentiviral and retroviral transduction
pLL3.7RSV-mCherry lentivirus was generated in 293T cells using the ViraPower Lentiviral Expression system (Invitrogen) and used to stably transduce GILM2 cells. Retroviruses were produced in Phoenix cells and used to infect breast cancer cells as described (13, 29). 231-mCherry cells were infected with pLXSN or pLXSN-αB-crystallin retrovirus (13), while GILM2-mCherry cells were infected with pSM2, pSM2-CRYABsh1 or pSM2-CRYABsh2 (Open Biosystems, RHS1764-9393967 and RHS1764-9691062, respectively). Cells were selected for growth in the appropriate antibiotics. Silent point mutations in αB-crystallin, which rendered it resistant to RNAi but did not alter its coding sequence, were made using the QuikChange Site-Directed Mutagenesis kit (Stratagene) with the primer 5′-ccgcctcttctttgaccagttctt-3′.
Immunocytochemistry of HBMECs
HBMECs (passage #4–5) were grown to confluence on tissue-culture plastic, fixed with 4% paraformaldehyde (or 100% ice-cold methanol), and permeabilized with 0.1% Triton-X100. Cells were blocked with 10% goat serum and then incubated with primary Abs against β-catenin (1:100, BD Biosciences), claudin-5 (1:100, Life Technologies), GLUT-1 (1:50, SPM498 clone, Thermo-Fisher), Occludin (1:100, Life Technologies), PECAM-1 (1:50, Thermo-Fisher), VE-cadherin (1:50, F8 clone, Santa Cruz) and ZO-1 (1:100, Life Technologies) overnight at 4°C. Anti-mouse and anti-rabbit conjugated Alexa Fluor® secondary Abs (1:200, Life Technologies) were incubated with the monolayers for 1 h. Nuclei were counterstained with 30 nM DAPI (Sigma). Immunolabeled HBMECs were visualized with an inverted epifluorescence microscope (Olympus) and images acquired using a 16-bit SPOT camera (Diagnostic Instruments) with Metavue software (Molecular Devices). Images were visualized and processed using NIH ImageJ software.
Adhesion to brain endothelium
HBMECs were grown on fibronectin-coated 24-well plates until confluent. Fifty thousand cancer cells were added to each well. After 2 h (231-mCherry) or 4 h (GILM2-mCherry), cells were washed, and the attached cells were fixed in 10% formalin and scored per 10x magnification field (Leica MZ10F stereomicroscope). In some experiments, cells were preincubated for 1 h with integrin blocking antibodies (Millipore).
Adhesion to extracellular matrix proteins
Twenty-four-well plates were coated with fibronectin, collagen I or laminin (20 μg/ml, Invitrogen), washed, and blocked with 2% BSA for 1 h at 37°C. Fifty thousand cells were added to each well, incubated for 20–30 min at 37°C, washed, and the attached cells were fixed, stained with crystal violet, and scored per 10x field.
Transendothelial migration and BBB transmigration in vitro
Transendothelial migration was assayed as described (30). Transwell inserts with 8 μm pores (BD Bioscience) were coated with fibronectin (20 μg/ml) overnight. Fifty thousand HBMECs (passage #4–5) were plated on the upper chamber of the inserts, and cells were grown to confluence. One day post-confluence, fifty thousand cancer cells were added to each well. After 24 h (231-mCherry) or 48 h (GILM2-mCherry), the non-migrating cancer cells and HBMECs were removed, and the mCherry-flourescent migrating cells were fixed in 10% formalin and scored per 10x field (Leica MZ10F stereomicroscope). For the BBB assay, primary HBMECs were co-cultured with human primary astrocytes on opposite sides of transwell inserts as described (12, 31). Transwell inserts with 8 μm pores were coated with fibronectin as described above and placed upside-down in a 15 cm plate. Twenty thousand primary human astrocytes were plated on the membrane surface and incubated for 30 min at 37°C. The inserts were then inverted, HBMECs added as in the transendothelial migration assay, and grown to confluence. One day post-confluence, fifty thousand cancer cells were plated on the upper chamber of the inserts, and mCherry-fluorescent cancer cells migrating through the endothelial and astrocyte layers were scored per 10x field at 48 h (231-mCherry) or 72 h (GILM2-mCherry).
Immunoblotting
Immunoblotting was performed as described (13) with antibodies for αB-crystallin (Enzo Life Sciences/Stressgen) or β-actin (Sigma).
Cell Surface expression of integrins
The cell surface expression of integrins was determined using an α/β Integrin-mediated Cell Adhesion Array kit (CHEMICON). Absorbance at 570 nm was measured using a SpectraMax Plus384 Absorbance MicroPlate Reader (Molecular Devices).
Orthotopic models of breast cancer brain metastasis
231-mCherry cells stably expressing vector or αB-crystallin (2.5 × 105) or GILM2-mCherry cells stably expressing a non-silencing construct or shRNA1 targeting αB-crystallin (1 × 106) were resuspended in 100% Matrigel (BD Bioscience) and injected bilaterally into the ducts of the 4th mammary glands of 4- to 5-week old female NOD scid IL2 receptor γ chain knockout (NSG) mice (Jackson Laboratory). Tumor volume was measured weekly as described (32). For GILM2 xenografts, mammary tumors were resected 9 weeks after tumor inoculation to allow sufficient time for metastasis. Mice were euthanized at 7 weeks (231-mCherry) or 12 weeks (GILM2-mCherry). Images of isolated whole brains were obtained (Leica MZ10F fluorescent stereomicroscope) and analyzed with ImageJ software. All animal experiments were approved by the institutional Animal Care and Use Committee.
Statistical Methods
Statistical significance was assessed as described in “Breast cancer brain metastasis cohort and immunohistochemistry analyses” or by ANOVA with posttests using GraphPad Prism software.
Results
αB-crystallin predicts poor survival in breast cancer patients with brain metastases
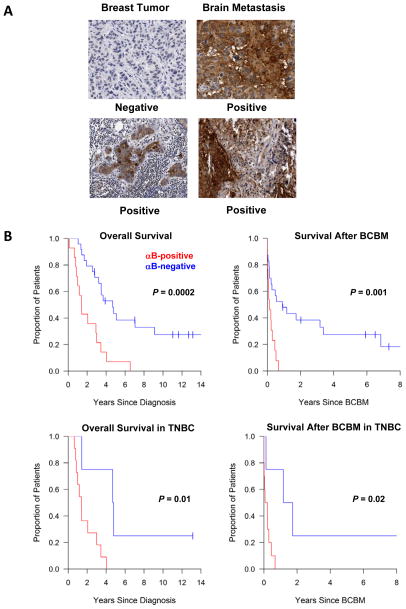
We examined αB-crystallin expression by IHC in primary breast carcinomas and brain metastases in 76 breast cancer patients who developed brain metastases (Table S1). Thirty-seven percent (14 of 38) of primary breast cancers and 47% (23 of 49) of brain metastases expressed αB-crystallin. Representative IHC staining of paired breast tumor and brain metastases from two patients is shown (Fig. 1A). Concordance between paired breast tumors and brain metastases was 55% (6 of 11, Table S2). It was more common for brain metastases to gain αB-crystallin expression (36%, 4 of 11) than to lose expression of this protein (9%, 1 of 11). αB-crystallin expression in primary breast tumors was associated with breast cancer subtype defined by IHC (P <0.0001, Table S3). Triple-negative (HR−/HER2−) breast cancers were more likely to express αB-crystallin (73%, 11 of 15) compared to HR+/HER2− (10%, 1 of 10) or HER2+ tumors (0%, 0 of 11). These findings indicate that αB-crystallin is commonly expressed in breast cancer brain metastases and confirm the association between αB-crystallin expression and TNBC.
Figure 1. αB-crystallin expression in primary breast tumors is associated with poor survival in patients with breast cancer brain metastases.
(A) Representative IHC staining of paired breast tumors and brain metastases from two patients. (B) Kaplan Meier survival curves by αB-crystallin expression (positive in red and negative in blue) for all breast cancer patients (upper panels) and for patients with TNBC (lowers panel). Overall survival (OS) is plotted in the left-hand panels and survival after breast cancer brain metastasis (BCBM) is shown in the right-hand panels.
We next examined the relationship between αB-crystallin expression and survival. The median follow-up for survivors was 6.5 years from diagnosis and 1.7 years from time of brain metastases; 79% (60 of 76) of patients had died when these analyses were completed. Overall survival was inferior among patients with αB-crystallin-positive breast tumors compared to αB-crystallin-negative breast tumors (1.4 [95% CI: 0.79 – 3.01] versus 4.7 [95% CI: 2.79 – 9.11] years; P = 0.0002) (Fig. 1B). Similarly, overall survival from the time of brain metastases was inferior among patients with αB-crystallin-positive breast tumors compared to αB-crystallin-negative tumors (0.13 [95% CI: 0.01 – 0.3] versus 0.91 [95% CI: 0.13 – 3.37] years, P = 0.001). Among patients with TNBC, αB-crystallin expression in the primary breast tumors was associated with lower overall survival rates (1.4 [95% CI: 0.79 – 3.01] versus 4.7 [95% CI: 1.43 – not estimable] years, P = 0.01) and survival following brain metastases (0.14 [95% CI: 0 – 0.3] versus 1.5 [95% CI: 0.13 – not estimable] years, P = 0.02) compared to those with αB-crystallin-negative breast tumors. αB-crystallin expression in brain metastases did not predict survival in this cohort (data not shown). These results indicate that αB-crystallin expression in primary breast carcinomas is associated with poor overall survival and poor survival after brain metastasis, even among TNBC patients.
αB-crystallin promotes adhesion of TNBC cells to brain endothelium, transendothelial migration, and BBB transmigration in vitro
The extravasation of breast cancer cells into the brain is a critical step-wise process in brain metastasis that begins with their adhesion to brain microvascular endothelium, followed by migration through the BBB (2, 3). We modeled these metastatic steps in vitro by examining the ability of TNBC cells to adhere to HBMECs, migrate through a layer of HBMECs (transendothelial migration), and pass through a model of the BBB composed of HBMECs and human astrocytes grown on opposite sides of transwell inserts (12, 31). HBMECs formed a modest restrictive barrier that was enhanced by co-culture with astrocytes (Fig. S1). In addition, HBMEC monolayers expressed the vascular markers PECAM-1 and VE-cadherin, the BBB marker GLUT-1, and the BBB cell junction-associated proteins claudin-5, ZO-1, occludin, and β-catenin (Fig. 2A).
Figure 2. αB-crystallin overexpression in TNBC cells enhances adhesion to brain endothelium, transendothelial migration, and BBB transmigration in vitro.
(A) Immunocytochemistry analysis of confluent primary HBMEC (passage #4–5) monolayers. The tight junction proteins claudin-5, occludin and ZO-1 were expressed at varied levels throughout the monolayer. HBMEC clusters with elevated expression and appropriate localization of these proteins are shown. Negative controls (mouse or rabbit IgG) are also included. Scale bar = 5 μm. (B) Immunoblot of 231-mCherry breast cancer cells stably expressing vector or αB-crystallin. (C) Adhesion of 231-mCherry cells stably expressing vector or αB-crystallin (αB) to HBMECs. Cells were seeded onto confluent HBMECs for 2 h, washed and attached mCherry-positive cells were scored (mean ± SEM, n = 3, **P < 0.01). (D) Transendothelial migration (TEM) of 231-mCherry-Vector or 231-mCherry-αB cells at 24 h (mean ± SEM, n = 3, **P < 0.01). (E) BBB transmigration in vitro. 231-mCherry-Vector or 231-mCherry-αB cells that transmigrated through two cell layers (HBMECs and astrocytes) were scored at 48 h (mean ± SEM, n = 3, *P < 0.05).
To determine the role of αB-crystallin in these steps, we stably overexpressed vector or αB-crystallin in MDA-MB-231-mCherry TNBC cells (abbreviated 231-mCherry-Vector and 231-mCherry-αB cells, respectively). Immunoblot analysis confirmed robust expression of αB-crystallin in 231-mCherry-αB cells, while 231-mCherry-Vector cells did not express detectable αB-crystallin protein (Fig. 2B). αB-crystallin overexpression increased adhesion to HBMECs (Fig. 2C), transendothelial migration through HBMECs (Fig. 2D) and BBB transmigration in vitro (Fig. 2E). Additionally, we stably silenced αB-crystallin in GILM2-mCherry TNBC cells with two different shRNAs (sh-αB1 and sh-αB2), which reduced αB-crystallin levels compared to GILM2-mCherry cells stably expressing a non-silencing (NS) construct (Fig. 3A). To control for potential off-target effects, we coexpressed sh-αB1 with an RNAi-resistant mutant αB-crystallin that restored expression of the wild-type protein (sh1-αBM). Silencing αB-crystallin in GILM2-mCherry cells inhibited adhesion to HBMECs (Fig. 3B), transendothelial migration (Fig. 3C), and BBB transmigration in vitro (Fig. 3D) compared to GILM2-mCherry-NS and GILM2-mCherry-sh1-αBM cells. Notably, altering αB-crystallin levels by overexpression and/or gene silencing did not affect cell viability under standard cell culture conditions (Fig. S2A and S2B). Collectively, these findings indicate that αB-crystallin promotes adhesion of TNBC cells to HBMECs, transendothelial migration and BBB transmigration in vitro.
Figure 3. Silencing αB-crystallin in TNBC cells inhibits adhesion to brain endothelium, transendothelial migration, and BBB transmigration in vitro.
(A) Immunoblot of GILM2-mCherry breast cancer cells stably expressing a non-silencing (NS) construct, shRNAs targeting different αB-crystallin sequences (sh1-αB and sh2-αB), or sh1-αB and an αB-crystallin mutant that disrupts gene silencing but does not alter its coding sequence (sh1-αBM). (B) Adhesion of GILM2-mCherry cells stably expressing NS, sh1-αB, sh2-αB or sh1-αBM to HBMECs. Cells were seeded onto confluent HBMECs for 4 h, washed and attached mCherry-positive cells were scored (mean ± SEM, n = 3). (C) Transendothelial migration (TEM) of GILM2-mCherry cells stably expressing NS, sh1-αB, sh2-αB or sh1-αBM at 48 h (mean ± SEM, n = 3). (D) BBB transmigration in vitro of GILM2-mCherry cells stably expressing NS, sh1-αB, sh2-αB or sh1-αBM at 72 h (mean ± SEM, n = 3). For (B) – (D), *P < 0.05, **P < 0.01, ***P < 0.001 versus NS.
αB-crystallin increases adhesion of TNBC cells to HBMECs at least in part through an α3β1 integrin-dependent mechanism and promotes adhesion to ECM proteins
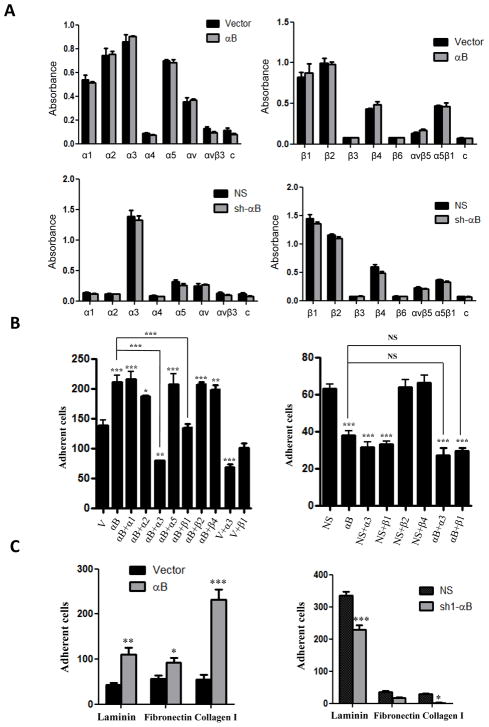
Because integrins play an important role in the adhesion of cancer cells to endothelial cells in the microvasculature (33–35), we postulated that the observed effects of αB-crystallin in promoting adhesion to HBMECs might be mediated by one or more integrins. Neither overexpression of αB-crystallin in 231-mCherry cells nor silencing αB-crystallin in GILM2-mCherry cells affected the cell surface expression of several α or β-integrin subunits (Fig. 4A). Nevertheless, integrin α3 and β1 blocking Abs (but not several other integrin blocking Abs) inhibited the enhanced adhesion of 231-mCherry-αB cells to HBMECs (Fig. 4B, left panel). Similarly, integrin α3 and β1 blocking Abs attenuated the adhesion of GILM2-mCherry-NS cells to HBMECs to a level comparable to that observed in GILM2-mCherry-sh1-αB cells (Fig. 4B, right panel). In contrast, the integrin β1 blocking Ab had no effect on the adhesion of 231-mCherry-Vector or GILM2-sh-αB cells to HBMECs. The integrin α3 blocking Ab inhibited adhesion of 231-mCherry-Vector cells (albeit to a lesser extent than observed in 231-mCherry-αB cells) but not GILM2-mCherry-sh-αB cells to HBMECs. These results indicate that αB-crystallin promotes adhesion of TNBC cells to HBMECs at least in part by an α3β1 integrin-dependent mechanism.
Figure 4. αB-crystallin promotes adhesion to HBMECs at least in part by an α3β1 integrin-dependent mechanism and enhances adhesion to extracellular matrix proteins.
(A) The cell surface expression of various integrins in 231-mCherry breast cancer cells stably expressing vector or αB-crystallin (upper panels) and GILM2-mCherry cells stably expressing NS or sh-αB (lower panels) was determined using an α/β Integrin-mediated Cell Adhesion Array. Data is expressed as absorbance at 570 nm (mean ± SEM, n = 3). A negative control (c) was included. (B) 231-mCherry-Vector, 231-mCherry-αB, GILM2-mCherry-NS, and GILM2-sh-αB cells were preincubated with integrin blocking Abs for 1 h, seeded onto confluent HBMECs for 2h (231-mCherry cells) or 4h (GILM2-mCherry cells), washed, and attached mCherry-positive cells were scored (mean ± SEM, n = 3). (C) Adhesion of breast cancer cells to different ECM proteins. Cells were plated on fibronectin, collagen or laminin coated 24-well plates for 20 min (231-mCherry cells) or 30 min (GILM2-mCherry cells) at 37°C, washed, and attached cells were counted (mean ± SEM, n = 3). For (A) – (C), *P < 0.05, **P < 0.01, ***P < 0.001.
We next examined whether αB-crystallin regulated adhesion of TNBC cells to ECM proteins, which are present in subendothelial microvasculature and on the exterior of microvessels in organs (6, 36). 231-mCherry-αB cells adhered more robustly to laminin, fibronectin and collagen than 231-mCherry-vector cells, while GILM2-sh1-αB cells exhibited reduced adhesion to laminin and collagen compared to control GILM2-mCherry-NS cells (Fig. 4C). These results indicate that αB-crystallin promotes adhesion of TNBC cells to multiple ECM proteins including laminin, a key step in both extravasation and perivascular growth of micrometastases.
αB-crystallin overexpression increases brain metastases in an orthotopic TNBC model
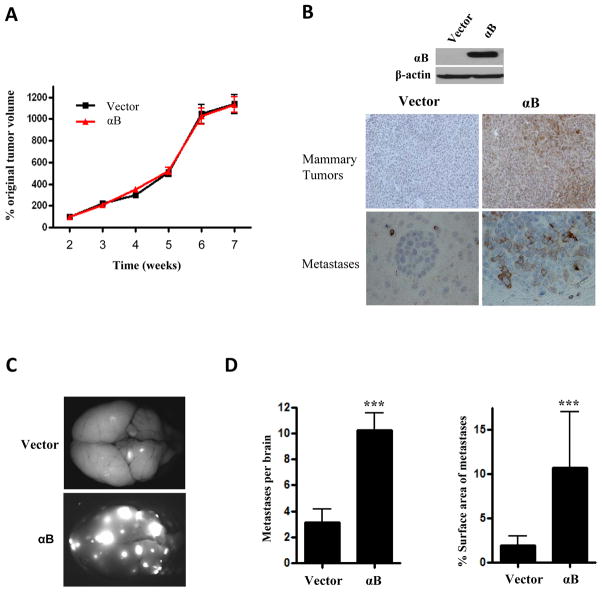
To explore the potential role of αB-crystallin in brain metastasis in vivo, we injected 231-mCherry-αB or 231-mCherry-Vector cells intraductally into the 4th mammary glands of NSG mice. αB-crystallin overexpression did not affect mammary tumor growth in this model (Fig. 5A). αB-crystallin overexpression in mammary tumors was confirmed by immunoblotting and IHC (Fig. 5B). Mice were euthanized 7 weeks after tumor inoculation and mCherry-fluorescent metastatic lesions were identified at autopsy. Both groups of mice had widespread metastases to many organs, including the brain, lungs, liver, lymph nodes and other tissues (Table S4). NSG mice with 231-mCherry-αB tumors had a greater number of mCherry-positive brain metastatic lesions and more extensive tumor burden as determined by the percentage of the surface area of the brain occupied by metastases compared to mice with 231-mCherry-Vector tumors (Fig. 5C and 5D). In addition, αB-crystallin overexpression resulted in increased metastatic tumor burden in the liver and bone (Table S4 and Fig. S3). αB-crystallin expression in brain metastases was observed by IHC in mice with 231-mCherry-αB tumors but not vector controls (Fig. 5B, lower panels). Intriguingly, αB-crystallin overexpression did not affect proliferation as determined by Ki67 IHC or apoptosis as determined by active caspase-3 IHC of mammary tumors or brain metastases analyzed at 7 weeks (Fig. S5A). These observations indicate that αB-crystallin promotes brain metastases in an orthotopic TNBC model with widespread metastatic dissemination.
Figure 5. αB-crystallin overexpression increases brain metastases in an orthotopic TNBC model.
231-mCherry cells stably expressing vector or αB-crystallin (αB) were injected bilaterally into the ducts of the 4th mammary gland of NSG mice. (A) Mammary tumor volume expressed as the percentage original tumor volume at 2 weeks in mice with 231-mCherry-Vector and 231-mCherry-αB xenografts (n=10 mice per group). (B) Immunoblot of 231-mCherry-Vector and 231-mCherry-αB mammary tumors and αB-crystallin IHC staining of mammary tumors and brain metastases in both groups. (C) Representative fluorescent whole brain images from mice with 231-mCherry xenografts overexpressing vector or αB. (D) Number of mCherry-fluorescent metastases per brain in vector and αB groups, and percentage surface area of brain metastases in vector and αB groups (mean ± SEM, n = 10 mice per group, ***P < 0.001).
Silencing αB-crystallin inhibits brain metastases in an orthotopic TNBC model
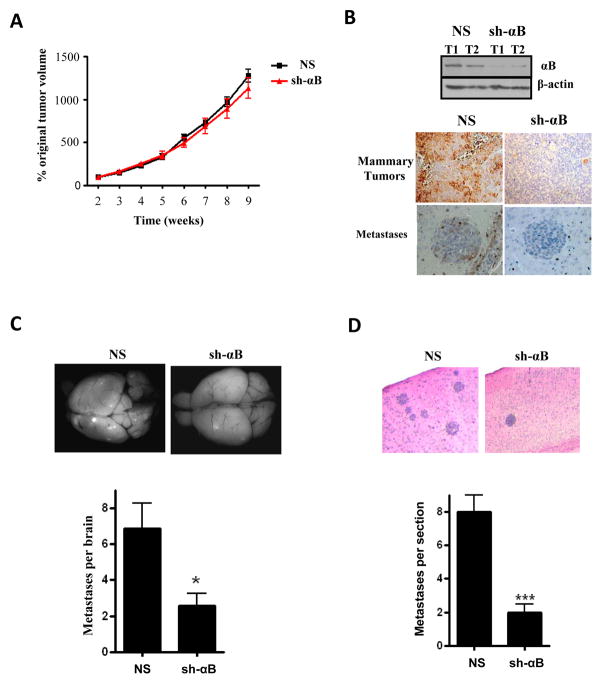
We also examined the effect of silencing αB-crystallin in TNBC cells on brain metastases in vivo. GILM2-mCherry-sh1-αB and GILM2-mCherry-NS cells were injected intraductally into the 4th mammary glands of NSG mice. Silencing αB-crystallin did not alter mammary tumor growth (Fig. 6A) despite robust reduction of αB-crystallin levels (Fig. 6B). Mammary tumors were resected at 9 weeks to allow additional time for metastasis; mCherry-positive metastases were identified at autopsy three weeks later. Both groups of mice had metastases to many organs, including the brain, lungs, liver, lymph nodes and other organs (Table S4). Mice with GILM2-mCherry-sh-αB tumors had fewer brain metastases as determined by mCherry-fluorescence (Fig. 6C) and H&E staining (Fig. 6D). GILM2-mCherry-sh-αB brain metastases had reduced expression of αB-crystallin compared to NS controls (Fig. 6B). Moreover, silencing αB-crystallin reduced metastates in other organs including the liver (Table S4 and Fig. S4). Silencing αB-crystallin did not affect proliferation or apoptosis as determined by IHC of mammary tumors or brain metastases analyzed at 12 weeks (Fig. S5B). These findings provide additional evidence that αB-crystallin promotes brain metastasis in vivo in a second orthotopic TNBC model.
Figure 6. Silencing αB-crystallin inhibits brain metastases in an orthotopic TNBC model.
GILM2-mCherry cells stably expressing a non-silencing (NS) construct or αB-crystallin shRNA (sh1-αB) were injected bilaterally into the ducts of the 4th mammary gland of immunodeficient NSG mice. (A) Mammary tumor volume expressed as the percentage original tumor volume at 2 weeks in mice with GILM2-mCherry-sh-αB and GILM2-mCherry-NS xenografts (n=10 mice per group). (B) Immunoblot of GILM2-mCherry-NS and GILM2-sh-αB mammary tumors and αB-crystallin IHC staining of mammary tumors and brain metastases in both groups. (C) Representative fluorescent whole brain images and number of fluorescent metastases per brain for NS and sh-αB groups (mean ± SEM, n = 10 mice per group, *P < 0.05). (D) Representative H&E staining of brain sections and number of metastatic lesions per section for NS and sh-αB groups (mean ± SEM, ***P < 0.001).
Discussion
The molecular pathogenesis of breast cancer brain metastasis remains poorly understood due to limited access to brain metastases from patients, a dearth of clinical trials, and the lack of animal models that recapitulate the entire metastatic cascade (37). The vast majority of animals models reported rely on intracardiac or carotid artery injection of breast tumor cells (38). We have demonstrated that αB-crystallin, a molecular chaperone previously linked to an aggressive tumor phenotype in TNBC, glioblastoma multiforme (GBM) and other neoplasms (13, 19, 20, 22, 39), is a novel regulator of breast cancer brain metastasis. Specifically, we have shown that αB-crystallin is commonly expressed in clinical breast cancer brain metastases, including some brain metastases that developed from breast tumors that did not express αB-crystallin. αB-crystallin expression in breast carcinomas was associated with triple-negative IHC status and with poor overall survival and poor survival after brain metastasis in a cohort of breast cancer cases across all subtypes and also within the subset of TNBC cases. We have also demonstrated a direct causal role for αB-crystallin in promoting breast cancer brain metastasis in vivo in two orthotopic models in which fluorescently labeled TNBC cells metastasize from the mammary gland to the brain in NSG mice. These models recapitulate the entire metastatic cascade and several clinical aspects of breast cancer brain metastasis, including triple-negative status and αB-crystallin expression by tumors, widespread metastatic disease, and late-onset brain metastases. Using overexpression and gene silencing to alter αB-crystallin levels in mammary tumors, we showed that αB-crystallin promotes brain metastases in vivo without accelerating mammary tumor growth. Collectively, our results point to a previously unrecognized role for αB-crystallin in breast cancer brain metastasis and suggest that αB-crystallin may be a useful biomarker to identify poor-prognosis breast cancer patients who might be enrolled in clinical trials for early detection or prevention/treatment of brain metastases. Moreover, our observation that silencing αB-crystallin inhibits brain metastases suggests that αB-crystallin may be promising drug target.
We have shown that αB-crystallin promotes several of the earliest steps in the extravasation of circulating tumor cells across the BBB, including cell adhesion to HBMECs, transendothelial migration and transmigration across a model of the BBB in vitro. Of note, our in vitro BBB model mimics some features of the BBB, but like most in vitro BBB models, the barrier is not as tight as that observed in vivo (31). Nevertheless, our observation that αB-crystallin enhances transmigration through our in vitro BBB model and breast cancer brain metastasis in vivo strongly suggests that αB-crystallin promotes BBB penetration. Moreover, αB-crystallin promotes adhesion to several ECM proteins including laminin, which are present in the subendothelial vessel wall and mediate adhesion to intraluminal circulating tumor cells and support perivascular growth of newly extravasated tumor cells (6, 36). Notably, the initial adhesion of TNBC cells to HBMECs is dependent at least in part on α3β1 integrin, which has been broadly implicated in metastasis, including brain metastasis. Specifically, α3β1 integrin has been reported to mediate arrest of circulating tumor cells in the pulmonary vasculature by engaging its ligand laminin-5 in exposed regions of the vessel wall; pulmonary arrest in vivo was inhibited by an integrin β1 blocking Ab (36). α3β1 integrin is also robustly expressed in a non-small cell lung cancer cell line highly metastatic to the brain and mediates adhesion to brain slices and invasion in vitro, while an α3 integrin blocking Ab dramatically suppressed brain metastases when these cells were injected into the left ventricle of mice (40, 41). In addition, β1 integrin plays a key role in the perivascular growth of early brain micrometastases upon intracardiac injection of breast and other tumor cells by mediating “vascular cooption”, the adhesion of metastatic tumor cells to the exterior surface of the vascular basement membrane of pre-existing vessels and subsequent expansion of micrometastses in the perivascular niche (6). Intriguingly, deletion of β1 integrin in the MMTV-activated ErbB2 model did not affect mammary tumor induction but suppressed lung metastases in this model (42). Although we have yet to determine the nature of the interaction between αB-crystallin and α3β1 integrin, these studies suggest that inhibition of α3β1 integrin might be a promising therapeutic strategy against brain metastases arising from αB-crystallin-positive TNBCs.
In our orthotopic TNBC models, αB-crystallin increased brain metastases without affecting mammary tumor growth, suggesting that the enhanced adhesion to HBMECs and BBB transmigration may be the principal mechanisms by which αB-crystallin promotes brain metastases. However, we cannot exclude other potential mechanisms, including increased intravasation and survival of circulating tumor cells, increased perivascular growth of micrometastases and/or diminished tumor dormancy. Although we did not observe differences in apoptosis or proliferation of mammary tumors or brain metastases at the conclusion of the experiments, αB-crystallin might affect proliferation or apoptosis during the initial perivascular expansion of micrometastases. Furthermore, we have previously reported that ectopic expression of high levels of αB-crystallin in a single clone of MDA-MB-231 cells promoted mammary tumor growth (43). No such mammary tumor growth advantage was observed in these experiments using a polyclonal population of MDA-MB-231 cells with more modest levels of αB-crystallin, suggesting that these effects may be dose-dependent. Additionally, the prometastatic activity of αB-crystallin is not limited to the brain. In both orthotopic models, αB-crystallin promoted liver metastases, indicating that αB-crystallin may also regulate cell adhesion and/or extravasation in the liver microvasculature. Consistent with this idea, integrin β1 has been implicated in tumor cell extravasation and hepatic colonization (44). Although we did not observe a difference in lung metastatic tumor burden at autopsy by altering αB-crystallin levels, the lungs in all animals had extensive tumor burden by the time brain metastases became apparent, suggesting the need for more detailed analyses at earlier time points or different tumor models to determine whether αB-crystallin may affect lung metastasis as well.
In summary, our results underscore a previously unrecognized role for αB-crystallin in brain metastasis in TNBC and point to αB-crystallin and α3β1 integrin as potential drug targets for this devastating disease. We are currently utilizing our orthotopic TNBC models to further delineate the prometastatic mechanisms of αB-crystallin and to evaluate novel therapies including neutralizing α3β1 integrin antibodies. Moreover, our findings point to αB-crystallin as a potential biomarker to help identify breast cancer patients who might benefit from additional diagnostic or therapeutic interventions.
Supplementary Material
Translational Relevance.
The prognosis for patients with breast cancer brain metastases remains dismal with survival typically measured in months. Unfortunately, the limited availability of brain metastases from patients and dearth of animal models that recapitulate the entire metastatic cascade have been major impediments to understanding the molecular pathogenesis of brain metastasis and developing effective therapies. Here we report that the molecular chaperone αB-crystallin, previously linked to the pathogenesis of triple-negative breast cancer (TNBC) and malignant glioblastomas, is commonly expressed in primary breast carcinomas and brain metastases from patients and predicts poor clinical outcomes. αB-crystallin promotes adhesion to brain microvascular endothelial cells at least in part by an α3β1 integrin-dependent mechanism, transmigration through a model of the blood-brain barrier (BBB) in vitro, and brain metastasis in two orthotopic TNBC models that capture the entire metastatic cascade. Our findings point to αB-crystallin and α3β1 integrin as potential drug targets for this deadly disease.
Acknowledgments
Grant support: Susan G. Komen for the Cure Postdoctoral Fellowship Award (DM), CALGB Young Investigator Award (CKA), UNC Hematology-Oncology NCI K12 (CKA), and the Breast Cancer Research Foundation (VLC).
We are indebted to Drs. Jennifer Koblinski and Janet Price for providing cell lines.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–17. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 2.Arshad F, Wang L, Sy C, Avraham S, Avraham HK. Blood-brain barrier integrity and breast cancer metastasis to the brain. Patholog Res Int. 2011:920509. doi: 10.4061/2011/920509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11:352–63. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–22. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 5.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176:2958–71. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbonell WS, Ansorge O, Sibson N, Muschel R. The vascular basement membrane as “soil” in brain metastasis. PLoS One. 2009;4:e5857. doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaedcke J, Traub F, Milde S, Wilkens L, Stan A, Ostertag H, et al. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol. 2007;20:864–70. doi: 10.1038/modpathol.3800830. [DOI] [PubMed] [Google Scholar]
- 8.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–14. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 9.Toft DJ, Cryns VL. Minireview: Basal-like breast cancer: from molecular profiles to targeted therapies. Mol Endocrinol. 2011;25:199–211. doi: 10.1210/me.2010-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638–45. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer. Cancer. 2012;118:4652–9. doi: 10.1002/cncr.27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, et al. αB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–70. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sitterding SM, Wiseman WR, Schiller CL, Luan C, Chen F, Moyano JV, et al. αB-crystallin: a novel marker of invasive basal-like and metaplastic breast carcinomas. Ann Diagn Pathol. 2008;12:33–40. doi: 10.1016/j.anndiagpath.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Lee Y, Lim YA, Kang HJ, Kim LS. αB-crystallin is a novel oncoprotein associated with poor prognosis in breast cancer. J Breast Cancer. 2011;14:14–19. doi: 10.4048/jbc.2011.14.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamradt MC, Chen F, Cryns VL. The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–63. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 17.Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein αB-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277:38731–6. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- 18.Petrovic V, Malin D, Cryns VL. αB-Crystallin promotes oncogenic transformation and inhibits caspase activation in cells primed for apoptosis by Rb inactivation. Breast Cancer Res Treat. 2013;138:415–425. doi: 10.1007/s10549-013-2465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, et al. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci U S A. 2008;105:10703–10708. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho PY, Chueh SC, Chiou SH, Wang SM, Lin WC, Lee IL, et al. αB-crystallin in clear cell renal cell carcinoma: tumor progression and prognostic significance. Urol Oncol. 2012 Mar 12; doi: 10.1016/j.urolonc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 21.van de Schootbrugge C, Bussink J, Span P, Sweep F, Grénman R, Stegeman H, et al. αB-crystallin stimulates VEGF secretion and tumor cell migration and correlates with enhanced distant metastasis in head and neck squamous cell carcinoma. BMC Cancer. 2012;13:128. doi: 10.1186/1471-2407-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goplen D, Bougnaud S, Rajcevic U, Boe SO, Skaftnesmo KO, Voges J, et al. αB-crystallin is elevated in highly infiltrative apoptosis-resistant glioblastoma cells. Am J Pathol. 2010;177:1618–28. doi: 10.2353/ajpath.2010.090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and αB-crystallin. J Cell Sci. 1999;112:2099–112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- 24.Singh BN, Rao KS, Ramakrishna T, Rangaraj N, Rao ChM. Association of αB-crystallin, a small heat shock protein, with actin: role in modulating actin filament dynamics in vivo. J Mol Biol. 2007;366:756–67. doi: 10.1016/j.jmb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Chelouche-Lev D, Kluger HM, Berger AJ, Rimm DL, Price JE. αB-crystallin as a marker of lymph node involvement in breast carcinoma. Cancer. 2004;100:2543–8. doi: 10.1002/cncr.20304. [DOI] [PubMed] [Google Scholar]
- 26.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68. [PubMed] [Google Scholar]
- 27.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 28.Malin D, Chen F, Schiller C, Koblinski J, Cryns VL. Enhanced metastasis suppression by targeting TRAIL receptor 2 in a murine model of triple-negative breast cancer. Clin Cancer Res. 2011;17:5005–15. doi: 10.1158/1078-0432.CCR-11-0099. [DOI] [PubMed] [Google Scholar]
- 29.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–6. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee S, Wei H. Roles of glycosphingolipids in cell signaling: adhesion, migration, and proliferation. Methods Enzymol. 2003;363:300–312. doi: 10.1016/S0076-6879(03)01059-0. [DOI] [PubMed] [Google Scholar]
- 31.Eugenin EA, Berman JW. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods. 2003;29:351–61. doi: 10.1016/s1046-2023(02)00359-6. [DOI] [PubMed] [Google Scholar]
- 32.Lu M, Strohecker A, Chen F, Kwan T, Bosman J, Jordan VC, et al. Aspirin sensitizes cancer cells to TRAIL-induced apoptosis by reducing survivin levels. Clin Cancer Res. 2008;14:3168–76. doi: 10.1158/1078-0432.CCR-07-4362. [DOI] [PubMed] [Google Scholar]
- 33.Price EA, Coombe DR, Murray JC. Beta-1 integrins mediate tumour cell adhesion to quiescent endothelial cells in vitro. Br J Cancer. 1996;74:1762–6. doi: 10.1038/bjc.1996.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujisaki T, Tanaka Y, Fujii K, Mine S, Saito K, Yamada S, et al. CD44 stimulation induces integrin-mediated adhesion of colon cancer cell lines to endothelial cells by up-regulation of integrins and c-Met and activation of integrins. Cancer Res. 1999;59:4427–4434. [PubMed] [Google Scholar]
- 35.Reymond N, Im JH, Garg R, Vega FM, Borda d’Agua B, Riou P, et al. Cdc42 promotes transendothelial migration of cancer cells through β1 integrin. J Cell Biol. 2012;199:653–68. doi: 10.1083/jcb.201205169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Fu W, Im JH, Zhou Z, Santoro SA, Iyer V, et al. Tumor cell α3β1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J Cell Biol. 2004;164:935–41. doi: 10.1083/jcb.200309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gril B, Evans L, Palmieri D, Steeg PS. Translational research in brain metastasis is identifying molecular pathways that may lead to the development of new therapeutic strategies. Eur J Cancer. 2010;46:1204–10. doi: 10.1016/j.ejca.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruz-Munoz W, Kerbel RS. Preclinical approaches to study the biology and treatment of brain metastases. Semin Cancer Biol. 2011;21:123–30. doi: 10.1016/j.semcancer.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang XY, Ke AW, Shi GM, Zhang X, Zhang C, Shi YH, et al. αB-Crystallin complexes with 14-3-3ζ to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology. 2013;57:2235–47. doi: 10.1002/hep.26255. [DOI] [PubMed] [Google Scholar]
- 40.Shintani Y, Higashiyama S, Ohta M, Hirabayashi H, Yamamoto S, Yoshimasu T, et al. Overexpression of ADAM9 in non-small cell lung cancer correlates with brain metastasis. Cancer Res. 2004;64:4190–6. doi: 10.1158/0008-5472.CAN-03-3235. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimasu T, Sakurai T, Oura S, Hirai I, Tanino H, Kokawa Y, et al. Increased expression of integrin α3β1 in highly brain metastatic subclone of a human non-small cell lung cancer cell line. Cancer Sci. 2004;95:142–8. doi: 10.1111/j.1349-7006.2004.tb03195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huck L, Pontier SM, Zuo DM, Muller WJ. β1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc Natl Acad Sci U S A. 2010;107:15559–64. doi: 10.1073/pnas.1003034107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, et al. The small heat shock protein αB-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280:11059–66. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- 44.Kato H, Liao Z, Mitsios JV, Wang HY, Deryugina EI, Varner JA, et al. The primacy of β1 integrin activation in the metastatic cascade. PLoS One. 2012;7:e46576. doi: 10.1371/journal.pone.0046576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.