Abstract
The anterior hippocampus is associated with emotional functioning and hippocampal volume is reduced in depression. We reported reduced neuropil volume and number of glia in the dentate gyrus (DG) and cornu ammonis (CA)1 of the anterior hippocampus in behaviorally depressed adult female cynomolgus macaques. To determine the biochemical correlates of morphometric and behavioral differences between behaviorally depressed and nondepressed adult female monkeys, glial and synaptic transcripts and protein levels were assessed in the DG, CA3 and CA1 of the anterior hippocampus. Glial fibrillary acidic protein (GFAP) was increased whereas spinophilin and postsynaptic density (PSD)-95 protein were decreased in the CA1 of depressed monkeys. GFAP was reciprocally related to spinophilin and PSD-95 protein in the CA1. Gene expression of GFAP paralleled the protein changes observed in the CA1 and was inversely related to serum estradiol levels in depressed monkeys. These results suggest that behavioral depression in female primates is accompanied by astrocytic and synaptic protein alterations in the CA1. Moreover, these findings indicate a potential role for estrogen in modulating astrocyte-mediated impairments in synaptic plasticity.
Keywords: GFAP, PSD-95, Spinophilin, CA1, Macaca fascicularis
INTRODUCTION
Numerous studies support an association between structural and functional alterations of the hippocampus and the pathophysiology of depression. Hippocampal volume is consistently reduced in depressed patients [15]. Studies in the postmortem hippocampus of depressed patients reported limited evidence of neuron loss [5,17,20], while others found decreased density of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes [20], increased neuron packing densities, decreased neuron size [32], and decreased cell layer volume [5], suggesting that neuropil alterations may contribute to volume reductions in depression. Studies in male rodents also indicate neuropil alterations, with dendritic retraction and fewer synapses in the cornu ammonis (CA)3 of the hippocampus in response to stress or glucocorticoid exposure [18,35]. Similarly, stress decreased levels of the presynaptic marker synaptophysin (SYN) and the postsynaptic marker postsynaptic density (PSD)-95 in the CA3 of male rodents [6,23], suggesting that synaptic integrity may be compromised in the neuropil of these animals. However, no differences were reported between major depressive disorder (MDD) patients and controls in SYN immunoreactivity [20] or PSD-95 expression in the postmortem hippocampus [37]. Importantly, MDD patients are frequently medicated with antidepressants that reversed or prevented deficits in SYN, PSD-95 and GFAP-immunoreactive astrocytes in stressed animal models [7,23], which may account for a lack of observed differences in patients.
Despite the increased prevalence of depression in women, little is known about the depressed female hippocampus. Sex differences in behavioral and neurobiological responses to stress have been reported [11,36]. Glial deficits associated with depression-like behavior in prenatally stressed mice are limited to female offspring [4], and estrogen protects stressed female rodents from the dendritic retraction observed in the CA3 of males [10]. In the CA1 of the hippocampus, estrogen increases spine density in rodents [12], and immunoreactivity for spinophilin, a marker of dendritic spines, in monkeys [14]. As such, estrogen-dependent alterations in hippocampal neuropil, particularly in response to stress, may be central to the neurobiology of depression in females.
To increase understanding of the depressed female primate brain, we have studied social stress-associated depressive behavior and the accompanying physiology and neurobiology in adult female cynomolgus macaques (Macaca fascicularis) for over two decades [28,40]. Cynomolgus macaques have menstrual cycles like women in length and hormone fluctuations, and the macaque hippocampus closely resembles the organization and connectivity of the human hippocampus. Behaviorally depressed monkeys have poor ovarian function relative to their nondepressed counterparts [28,40]. Recently, we reported morphological deficits in the CA1 and dentate gyrus (DG) of the anterior hippocampus in antidepressant-naïve, behaviorally depressed females, including reductions in region and cell layer volumes, neuropil size, and glia number [39]. The present study was designed to elucidate the molecular correlates of anterior hippocampal volume reduction in depressed female primates, while eliminating the confound of treatment. Protein and gene expression of glial and synaptic markers were measured in subregions of the anterior hippocampus from the same matched set of adult, female monkeys characterized for behavioral depression used in the previous morphologic study [39]. We hypothesized that markers of glia and synaptic integrity would be dysregulated in the anterior CA1 and DG of behaviorally depressed female monkeys.
MATERIALS AND METHODS
Subjects
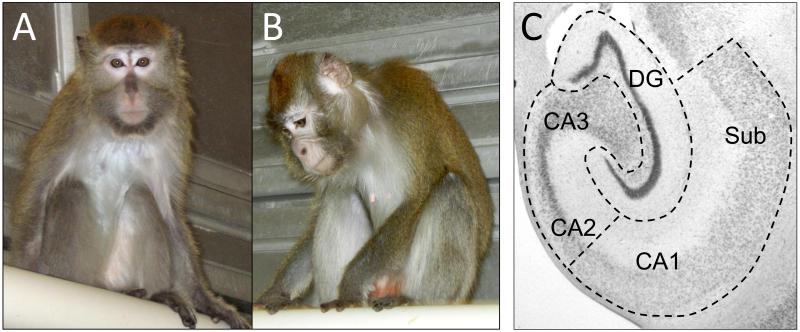
As previously described, 28 reproductive-aged female cynomolgus macaques (Macaca fascicularis) were housed in stable social groups (N=4/group) under a 12/12 light/dark cycle for 24 months [25-27,39]. All procedures involving primates were conducted in compliance with institutional, state, and federal laws for the usage of primates in laboratory settings. As part of a larger study investigating the comorbidity of depression and cardiovascular disease risk, the animals consumed a Western diet, containing 0.28 mg cholesterol/Cal and 42% of calories as fat [27]. Characteristics of the behavior and physiology of this specific cohort of animals have been described in detail elsewhere [25-27]. Briefly, social status was determined monthly by recording the outcomes of agonistic interactions [24,27,29], and the resulting social status hierarchy within each social group was stable over time, as in previous experiments. Behavioral depression was operationally defined as a slumped or collapsed body posture (head lower than shoulders), in which an animal’s eyes are open, yet the animal lacks interest or responsivity to environmental stimuli [24,27,29] (Figure 1A-B). A dexamethasone suppression test (DST) was administered one month before necropsy to assess the sensitivity of the hypothalamic-pituitary-adrenal axis to glucocorticoid negative feedback [27]. Estradiol and progesterone were assayed in serum collected at necropsy [27].
Figure 1.
A nondepressed monkey (A) compared to a behaviorally depressed (B) monkey. The nondepressed monkey (A) is alert and attentive to the photographer, whereas the behaviorally depressed monkey (B) remains in a slumped, collapsed body posture with eyes pointed downward, and is unresponsive to environmental events. Behavioral depression in captive cynomolgus macaques occurs spontaneously and is not induced by a specific experimental manipulation. (C) Cresyl violet stained section of the hippocampus in the coronal plane indicating subregional dissection boundaries.
Tissue Preparation
At necropsy, brains were rapidly removed, hemisected, and frozen at −80°C. From the initial population of 28 animals, eight animals from the upper tertile of the distribution of time spent in the depressed posture were matched with eight animals from the lower tertile as reported in Willard et al. [39]. Briefly, to reduce variance from characteristics known to affect hippocampal structure and function, animals were matched for body weight, age, social status, basal cortisol levels, cortisol response in the DST, % suppression of cortisol, and estradiol and progesterone levels at the time of necropsy. The anterior hippocampus was delineated from the posterior hippocampus by the presence of the uncus [38,39], and the anterior DG, CA1 and CA3 regions were dissected from one hemisphere and pulverized (Figure 1C). To control for effects of laterality, the dissected hemisphere was counterbalanced within groups, as reported previously [38,39]. Dissections were pulverized in liquid nitrogen and stored at −80°C for protein and gene expression analyses.
Western Blotting
Dissected hippocampal regions were fractionated into membrane and cytosolic subcellular compartments using a modified procedure [34]. Briefly, tissue samples were homogenized in 10 mM HEPES, 10 mM NaCl, 1 mM KH2PO4, 5 mM NaHCO3, 1 mM CaCl2, 0.5 mM MgCl2, 0.5 mM EDTA, Halt™ Protease Inhibitor Cocktail (PI cocktail; Pierce, Rockford, IL, USA), and centrifuged in an Eppendorf 5414 D at 7500 rpm for 5 min at 4°C. Total supernatant was centrifuged at 60,000 × g for 40 min at 4°C, and the cytosolic supernatant was stored at −80°C. The plasma membrane pellet was resuspended in phosphate-buffered saline (pH 7.4) with PI cocktail, and stored at −20°C.
Standard Western blot analyses [21] were used to assess the relative expression of anti-PSD-95 (1:5000; 6G6-1C9, Millipore, Billerica, MA), anti-spinophilin (1:500; 06-852, Millipore), anti-synaptophysin (1:5000; MAB368, Millipore), and anti-Iba1 (1:1000; ab5076, Abcam, Cambridge, MA) in the membrane fraction, and anti-GFAP (1:1000; 173002, Synaptic Systems, Gottingen, Germany) and anti-MAP2 (1:500; M4403, Sigma-Aldrich, St. Louis, MO) in the cytosolic fraction, to that of mouse monoclonal anti-neuronal tubulin (1:20,000; 05-559; Millipore). For each hippocampal region, 20μg protein was analyzed. Blots were scanned with the LI-COR Odyssey infrared scanner (LI-COR Biosciences, Lincoln, NE) and signal intensities calculated using Odyssey version 3.0 software. Data are reported as signal intensities for proteins of interests relative to those of neuronal tubulin. Preliminary analyses of synaptohphysin, MAP2 and Iba1 revealed no effects of depression, therefore these markers were eliminated from further analyses and data are not reported.
Quantitative real-time RT-PCR
Total RNA was isolated from 30 mg pulverized tissue and reverse transcribed to cDNA as previously described [1]. RNA quality was verified for each sample using the Agilent 2100 BioAnalyzer (Agilent Technologies, Inc., Santa Clara, CA), and the resulting RNA integrity values ranged from 7.7 to 8.9, indicating high quality. Taqman assays (Life Technologies, Carlsbad, CA) were used to assess gene expression of GFAP (Rh02840886_m1), Spinophilin (Hs00261636_m1), and PSD-95 (Hs00176354_m1) using an ABI Prism 7900HTS real-time detector (Life Technologies) [1]. From a panel of nine candidate reference transcripts, geNorm software was used to select the three most stable endogenous control gene transcripts [1]: TATA box binding protein (TBP: Hs99999910_m1), hypoxanthine phosphoribosyltransferase 1 (HPRT1: Hs99999909_m1), and phosphoglycerate kinase 1 (PGK1: Hs99999906_m1). Gene expression was calculated using SDS 2.1 software (Applied Biosystems, Foster City, CA, USA) to interpolate the Ct values for each well onto a standard curve generated from the Ct values of a dilution series of standards. Quantity values were then averaged across triplicates and expressed relative to the quantity value for the mean of the endogenous control genes. Data are reported as relative expression (gene of interest quantity mean/geometric mean of endogenous control quantity means).
Statistical Analyses
T-tests were used determine group differences for GFAP, spinophilin and PSD-95 protein expression. In the CA1, follow-up gene expression analyses were conducted and group differences determined using t-tests. Pearson r was used to determine correlations between variables. All analyses were two-tailed with the α-level set at 0.05, and data are depicted as means ± SEMs.
RESULTS
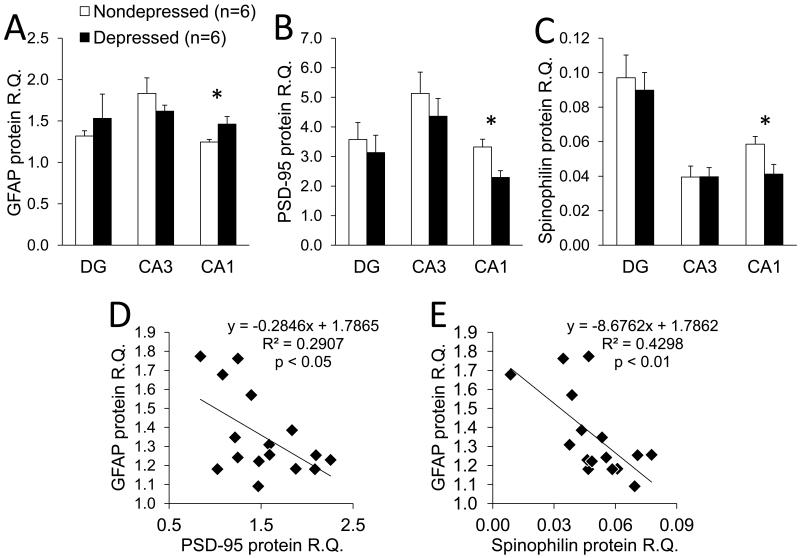
Western blotting revealed that alterations in glial and synaptic proteins were limited to the CA1, with no group differences observed for any protein examined in the DG or the CA3 (Figure 2). Relative quantification of GFAP revealed that depressed animals had on average 15% more GFAP protein in the CA1 compared to nondepressed animals (t(1,14) = 2.2, p < 0.05; Figure 2A). By contrast, significant reductions were observed in PSD-95 (t(1,14) = 2.4, p < 0.05; Figure 2B) and spinophilin (t(1,14) = 3.0, p < 0.01; Figure 2C); both were nearly 30% lower in the CA1 of depressed compared to nondepressed animals. Also only in the CA1, GFAP protein expression was inversely correlated with that of PSD-95 (r = −0.54, p < 0.05; Figure 2D) and spinophilin (r = −0.66, p < 0.01; Figure 2E), suggesting a reciprocal relationship between glial and synaptic proteins.
Figure 2.
Effects of behavioral depression on GFAP, spinophilin and PSD-95 in the anterior hippocampus. (A-C) Protein levels were quantified by densitometry, and the resulting signal intensities are reported as relative quantification (R.Q.) to n-tubulin. GFAP was increased whereas spinophilin and PSD-95 were decreased in the CA1 of depressed compared to nondepressed monkeys. Data are depicted as means ± SEMS; *p < 0.05 in two-tailed t-tests. (D-E) In the CA1, GFAP protein was inversely correlated with spinophilin and PSD-95 protein, as determined in Pearson r correlations.
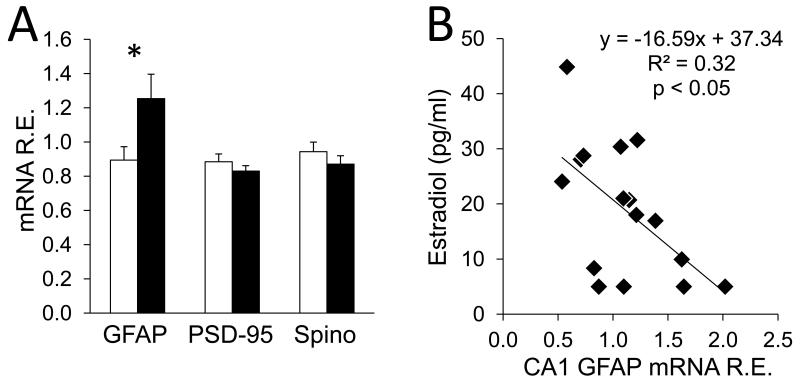
Given that differences in protein expression were observed only in the CA1, secondary analyses of GFAP, spinophilin and PSD-95 gene expression were conducted in this region to determine if protein levels were paralleled by the expression of the genes encoding those proteins. Relative gene expression of GFAP was ~28% greater in the CA1 of depressed compared to nondepressed monkeys (t(1,14) = 2.2, p < 0.05), but there were no differences in gene expression of spinophilin (t(1,14) = 1.1, p = 0.3) or PSD-95 (t(1,14) = 0.71, p = 0.5; Figure 3A). Although the groups were carefully balanced on characteristics known to affect hippocampal structure and function, correlational analyses were used to determine whether any matching variable was associated with proteins or genes observed to vary between groups. There was an inverse correlation between estradiol levels at the time of necropsy and GFAP gene expression (r = −0.57, p < 0.05; Figure 3B).
Figure 3.
Gene expression of glial and synaptic markers in the CA1. (A) GFAP gene expression was increased in the CA1 of depressed female monkeys compared to their nondepressed counterparts. Data are reported as relative expression (gene of interest quantity mean/geometric mean of endogenous control quantity means), and depicted as means ± SEMs; *p < 0.05 in two-tailed t-tests. (B) Gene expression of GFAP in the CA1 was reciprocally related to estradiol at the time of necropsy in a Pearson r correlation.
DISCUSSION
This study represents the first examination of the relationship between glial and synaptic markers in the anterior hippocampus of behaviorally depressed female primates. These observations are compelling because many of the variables that differentially affect hippocampal structure in human studies are controlled in this primate model, including housing condition, diet, and drug and alcohol exposure. We observed increased GFAP protein expression in the CA1 in the present study, whereas previously we found decreased number of glia in the CA1 of the opposite hemisphere from the same cohorts used in this study [39]. Although we did not distinguish glial subtypes in the previous study, astrocytes are the most abundant glial cell in the brain and are implicated in depression, and thus may contribute to the lower glia count observed in the previous study. In healthy tissue, many astrocytes express little to no GFAP, while expression of GFAP is upregulated during astrocyte activation in response to injury or insult [19,31]. If there are less astrocytes, yet more GFAP, then there may be fewer healthy astrocytes, and the astrocytes remaining may be reactive and thus expressing higher levels of GFAP. As such, increased GFAP protein expression in the present study may reflect fewer, yet more activated astrocytes. Increased GFAP also reflects perturbed glutamatergic neurotransmission and impaired synaptic plasticity [19]. GFAP and synaptic protein expression in the CA1 were inversely correlated in the present study, suggesting the possibility that GFAP may be regulating the observed changes in synaptic proteins, and may reflect a mechanism of altered synaptic plasticity in female depression.
Chronic psychosocial stress reduced the number of GFAP-immunoreactive astrocytes in male tree shrews [7], and GFAP protein expression in male rats [3]. However, sex differences exist in astrocyte function such that astrocytes contain estrogen receptors and can synthesize estrogen [8]. In hippocampal astrocytes, estrogen inhibits both gene and protein expression of GFAP, and increases glial glutamate transporters and glutamine synthetase [22,33]. Although the groups in the present study were carefully balanced for ovarian steroids, GFAP gene expression in the CA1 was inversely related to estradiol in depressed female monkeys. The ovarian suppression that is characteristic of depressed female monkeys [27,29] might influence the effects of estrogen in astrocytes, and may be central to understanding hippocampal alterations, particularly within the CA1 of depressed female primates. Future studies are warranted to determine whether the present findings represent a sex difference, and whether increased protein expression of GFAP is related to GFAP-immunoreactive astrocyte number.
Decreased spinophilin and PSD-95 in the CA1 of behaviorally depressed monkeys suggest alterations in postsynaptic integrity. This is supported by studies in which stress decreased spine density and synapses in the CA1 of female rodents [13,30], and our observation of reduced neuropil in the CA1 of depressed female monkeys [39]. Stress causes dendritic retraction and synapse loss in the CA3 of male and not female rodents [18]. Interestingly, we observed reduced PSD-95 and spinophilin protein levels in the CA1 and not the CA3, suggesting that subregional alterations in hippocampal plasticity may be sex-dependent. Since N-methyl-D-aspartate (NMDA) receptor activation is necessary for estrogen-mediated effects on CA1 spines [42], and astrocytes regulate NMDA receptor activation [41], the reduction in glia observed in the CA1 of our previous study might result in reduced astrocyte-mediated NMDA receptor activation, and thus decreased expression of synaptic proteins as observed in the present study.
Decreased spinophilin and PSD-95 protein in the CA1 were not accompanied by parallel changes in expression of the genes that encode these proteins, likely due to post-transcriptional mechanisms. Protein levels of PSD-95 are regulated by activity [9], and translation of PSD-95 is under local control at the level of the synapse [16]. In addition, estrogen stimulates protein synthesis of PSD-95 [2]. Whereas stress results in deficits in synaptic plasticity [18], estrogen has the opposite effect, and the interaction between these processes may be related to reduced synthesis of PSD-95 protein in depressed female monkeys. Similar mechanisms may affect the translation of spinophilin, since it also takes place within the dendrite [43].
A major strength of this study is the careful documentation of depressive behavior over several years, which limits spurious short term observations. Despite the relatively small sample size, differences between groups were large enough to detect, thus indicating variables that represent major molecular perturbations in the hippocampus of depressed female primates. This model also reflects spontaneously occurring depression, not the experimental manipulation of stress as in rodent models, or the pharmacologically treated brains of depressed patients. As such, this model provides the unique opportunity to study the effects of depressive behavior objectively documented over a long period of time without the confounds of pharmacotherapy or induced stress.
Additional research is warranted to further characterize the mechanisms contributing to altered anterior hippocampal structure and function in female depression. The present study supports a relationship between depressive behavior and compromised postsynaptic integrity in depressed female monkeys. Furthermore, increased GFAP expression in the CA1 may be detrimental to the structure of the anterior hippocampus, as evidenced by association with decreased synaptic proteins, thus suggesting astrocyte-mediated synaptic plasticity impairments. On the other hand, increased CA1 GFAP in depressed monkeys could be a response to atrophy in additional brain regions initiated through other mechanisms. Additional studies examining the complex relationship between estrogen and these mechanisms are necessary to better understand the neurobiology of female depression. Likewise, the hippocampus is one node in a network of neural areas implicated in depression; these relationships should be studied in other nodes as well.
Highlights.
Behaviorally depressed female primates have increased GFAP protein in the CA1
Depressed female primates have decreased spinophilin and PSD-95 protein in the CA1
Estrogen may modulate astrocyte-mediated impairments in synaptic plasticity
Behavioral depressed female cynomolgus monkeys are a useful model of depression
ACKNOWLEDGEMENTS
The authors would like to thank M. Elizabeth Forbes, Elizabeth J. Burnett, and Lindsey P. Pattison for their technical assistance, and Dr. Bruce McEwen for his advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Acosta G, Hasenkamp W, Daunais JB, Friedman DP, Grant KA, Hemby SE. Ethanol self-administration modulation of NMDA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields in cynomolgus monkeys. Brain Res. 2010;1318:144–54. doi: 10.1016/j.brainres.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Araya-Callis C, Hiemke C, Abumaria N, Flugge G. Chronic psychosocial stress and citalopram modulate the expression of the glial proteins GFAP and NDRG2 in the hippocampus. Psychopharmacology (Berl) 2012;224:209–222. doi: 10.1007/s00213-012-2741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Behan AT, van den Hove DL, Mueller L, Jetten MJ, Steinbusch HW, Cotter DR, Prickaerts J. Evidence of female-specific glial deficits in the hippocampus in a mouse model of prenatal stress. Eur Neuropsychopharmacol. 2011;21:71–79. doi: 10.1016/j.euroneuro.2010.07.004. [DOI] [PubMed] [Google Scholar]
- [5].Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cohen JW, Louneva N, Han LY, Hodes GE, Wilson RS, Bennett DA, Lucki I, Arnold SE. Chronic corticosterone exposure alters postsynaptic protein levels of PSD-95, NR1, and synaptopodin in the mouse brain. Synapse. 2011;65:763–770. doi: 10.1002/syn.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- [8].Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol. 2007;42:70–75. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- [9].Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- [10].Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- [11].Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hajszan T, MacLusky NJ, Leranth C. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm Behav. 2008;53:638–646. doi: 10.1016/j.yhbeh.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- [15].Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu-Yesucevitz L, Bassell GJ, Gitler AD, Hart AC, Klann E, Richter JD, Warren ST, Wolozin B. Local RNA translation at the synapse and in disease. J Neurosci. 2011;31:16086–16093. doi: 10.1523/JNEUROSCI.4105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lucassen PJ, Muller MB, Holsboer F, Bauer J, Holtrop A, Wouda J, Hoogendijk WJ, De Kloet ER, Swaab DF. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- [19].Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- [20].Muller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14:1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- [21].O’Connor JA, Hasenkamp W, Horman BM, Muly EC, Hemby SE. Region specific regulation of NR1 in rhesus monkeys following chronic antipsychotic drug administration. Biol Psychiatry. 2006;60:659–662. doi: 10.1016/j.biopsych.2006.03.044. [DOI] [PubMed] [Google Scholar]
- [22].Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- [23].Reines A, Cereseto M, Ferrero A, Sifonios L, Podesta MF, Wikinski S. Maintenance treatment with fluoxetine is necessary to sustain normal levels of synaptic markers in an experimental model of depression: correlation with behavioral response. Neuropsychopharmacology. 2008;33:1896–1908. doi: 10.1038/sj.npp.1301596. [DOI] [PubMed] [Google Scholar]
- [24].Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- [25].Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci Biobehav Rev. 2009;33:133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shively CA, Register TC, Adams MR, Golden DL, Willard SL, Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosom Med. 2008;70:637–645. doi: 10.1097/PSY.0b013e31817eaf0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- [28].Shively CA, Willard SL. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp Neurol. 2012;233:87–94. doi: 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- [30].Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stone DJ, Song Y, Anderson CP, Krohn KK, Finch CE, Rozovsky I. Bidirectional transcription regulation of glial fibrillary acidic protein by estradiol in vivo and in vitro. Endocrinology. 1998;139:3202–3209. doi: 10.1210/endo.139.7.6084. [DOI] [PubMed] [Google Scholar]
- [34].Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–924. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498:363–374. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- [36].Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiol Behav. 2009;97:239–249. doi: 10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- [37].Toro C, Deakin JF. NMDA receptor subunit NRI and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophr Res. 2005;80:323–330. doi: 10.1016/j.schres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- [38].Willard SL, Friedman DP, Henkel CK, Shively CA. Anterior hippocampal volume is reduced in behaviorally depressed female cynomolgus macaques. Psychoneuroendocrinology. 2009;34:1469–1475. doi: 10.1016/j.psyneuen.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Willard SL, Riddle DR, Forbes ME, Shively CA. Cell Number and Neuropil Alterations in Subregions of the Anterior Hippocampus in a Female Monkey Model of Depression. Biol Psychiatry. 2013;19:00266–00267. doi: 10.1016/j.biopsych.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Willard SL, Shively CA. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis) Am J Primatol. 2012;74:528–542. doi: 10.1002/ajp.21013. [DOI] [PubMed] [Google Scholar]
- [41].Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci U S A. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhong J, Zhang T, Bloch LM. Dendritic mRNAs encode diversified functionalities in hippocampal pyramidal neurons. BMC Neurosci. 2006;7:17. doi: 10.1186/1471-2202-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]