Abstract
Background
Evidence indicates that cardiac hypothyroidism may contribute to heart failure (HF) progression. It is also known that HF is associated with an increased risk of atrial fibrillation (AF). While it is established that hyperthyroidism increases AF incidence, the effect of hypothyroidism on AF is unclear. This study investigated the effects of different thyroid hormone levels, ranging from hypothyroidism to hyperthyroidism on AF inducibility in thyroidectomized rats.
Methods and Results
Thyroidectomized rats with serum confirmed hypothyroidism 1 month after surgery were randomized into hypothyroid (n=9), euthyroid (n=9) and hyperthyroid (n=9) groups. Rats received placebo, 3.3mg L-thyroxine (T4), or 20 mg T4 pellets (60 day release form) for 2 months, respectively. At the end of treatment, hypothyroid, euthyroid and hyperthyroid status was confirmed. Hypothyroid animals showed cardiac atrophy and reduced cardiac systolic and diastolic function, while hyperthyroid rats exhibited cardiac hypertrophy and increased cardiac function. Hypothyroidism and hyperthyroidism produced opposite electrophysiological changes in heart rates and atrial effective refractory period, but both significantly increased AF susceptibility. AF incidence was 78% in hypothyroid, 67% in hyperthyroid, and the duration of induced AF was also longer, compared with 11% in the euthyroid group (all p<0.05). Hypothyroidism increased atrial interstitial fibrosis, but connexin 43 was not affected.
Conclusions
Both hypothyroidism and hyperthyroidism lead to increased AF vulnerability in a rat thyroidectomy model. Our results stress that normal thyroid hormone levels are required to maintain normal cardiac electrophysiology and prevent cardiac arrhythmias and AF.
Keywords: thyroid hormones, atrial fibrillation, arrhythmia, electrophysiology
Introduction
Atrial fibrillation (AF) is the most common clinically significant cardiac arrhythmia. The estimated prevalence of AF is 0.4% to 1% in the general population and its prevalence increases with age, up to 8% in those older than 80 years. It was estimated that about 2.2 million Americans have AF.1 AF has a very complex pathophysiology that depends strongly on underlying cardiovascular diseases, in particular heart failure (HF).2 As a result, AF and HF frequently coexist, with up to 50% of severe HF patients developing AF.2 HF is a leading cause of morbidity and mortality, affecting more than 5 million patients in the U.S.2, 3 At 40 years of age, the lifetime risk of developing HF for both men and women is 20%.3
Normal thyroid hormone (TH) levels are required in adult life to maintain normal cardiovascular structure and function.4–6 Thyroid dysfunction, in both hyperthyroidism and hypothyroidism, is associated with an increased risk of HF.6, 7 While it is well known that thyrotoxicity is associated with an increased AF risk in patients,1 the role of hypothyroidism on AF is unclear. While several clinical case reports suggest hypothyroidism might be associated with AF,8–10 a recent population cohort study found low risk of AF in overt hypothyroidism.11
Previous reports have demonstrated that long-term hypothyroidism can lead to cardiac chamber dilatation and HF in rats.5 Epidemiological data indicate that TH deficiency may be responsible for an increased risk of HF in patients.6 Due to the fact that there is a strong coexistence of HF and AF, 2 we hypothesized that hypothyroidism may lead to increased AF as well. Currently there is no direct experimental data examining whether hypothyroidism affects AF, although the effects of hyperthyroidism on AF have been more extensively studied in animals.12–14 This study was designed to investigate cardiac electrophysiology and arrhythmia inducibility with low, normal, and high thyroid levels.
Method
This study was approved by the Institutional Animal Care and Use Committee at the New York Institute of Technology College of Osteopathic Medicine and is in compliance with the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 85–23, Revised 1996).
Animal model and study design
Thyroidectomized adult female Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Thyroid status was confirmed using blood samples taken 4 weeks after surgery. Animals were then randomized into the following groups: hypothyroid (n=9), euthyroid (n=9), and hyperthyroid (n=9). In hypothyroid rats, placebo pellets were implanted subcutaneously in the posterior neck, while 3.3 mg L-thyroxine (T4) pellets and 20 mg T4 pellets were implanted in euthyroid and hyperthyroid rats, respectively. The dosages of T4 pellets were chosen based on our previous study showing 3.3 mg T4 pellets normalize cardiac TH levels in thyroidectomized rats.15 Placebo and T4 pellets (prepared in 60-day release form) were purchased from Innovative Research of America (IRA, Sarasota, FL). Terminal experiments were performed 2 months after placebo or T4 treatment. A group of normal rats with same age and sex (n=8) served as normal control for TH levels.
Animals were housed in our institutional animal facility and kept on a 12:12-h light-dark cycle and given standard rat chow and water ad libitum.
Serum TH measurements
Total triiodothyronine (T3), T4, and thyroid-stimulating hormone (TSH) levels were measured using ELISA kits according to the manufacturers' specification. T3 and T4 kits were obtained from Monobind Inc. (Lake Forest, CA). The kits are human but have been shown to produce excellent results in rats in our laboratory.16 TSH kit was purchased from ALPCO Diagnostics (Salem, NH) and is specific for rat TSH.
Echocardiographic measurements
A GE Vivid 7 Dimension System (GE VINGMED ULTRASOUND A/S, Horten, Norway) coupled with a M12L linear (Matrix) array ultrasound transducer probe (5–13 MHz) was used to acquire echocardiographic data in a blinded manner. Briefly, rats were anesthetized with 1.5% isoflurane. After the chest was shaved, the animals were placed on an isothermal pad maintained at ~40°C. Two-dimensional echocardiograms were obtained from short-axis and long axis views of the left ventricle (LV). Two-dimensionally targeted M-mode echocardiograms were used to measure the LV dimensions in systole and diastole. Left atrial diameter at diastole was measured to estimate atrial size.
Cardiac hemodynamic measurements
LV hemodynamics were obtained under anesthesia by catheterization of the right carotid artery using a 1.9F SciSense pressure-volume catheter (Transonic Scisense Inc., London, Ontario, Canada). The tip of the catheter was advanced through the aorta into the left ventricle. The following parameters were measured: heart rate, LV peak systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), and positive/negative change in pressure over time (±dP/dt). The data were acquired and analyzed by the LabScribe software. (iWorx Systems, Inc., Dover, NH)
Electrophysiology study and AF inducibility test
Under anesthesia, a 1.6F octopolar Millar electrophysiology catheter (EPR-802, Millar Instruments, Inc., Houston, Texas) was inserted through right jugular vein and advanced into the right atrium with 8 poles recording atrial electrograms. Standard surface ECG lead II and 3 right atrial electrocardiograms from 3 pairs of electrodes were displayed and recorded using a PowerLab data acquisition system (ADInstruments, Colorado Springs, CO). The purpose of recording 3 atrial electrograms from distal, middle, and proximal pairs was to facilitate determination of atrial capturing and AF pattern. Poles 5 and 6 (the third pairs counted from catheter tip) were used for pacing.
Regular pacing and standard S1S2 pacing protocols were used to determine sinus node recovery time and atrial effective refractory period (ERP). The atria were paced at 3x threshold at cycle length of 150 ms or 20 ms shorter than the spontaneous sinus cycle length (in hyperthyroid rats due to fast heart rate). Atrial ERP was defined as the longest coupling intervals which did not capture the atria.
Burst pacing containing 200 impulses at 50 Hz was used to induce AF. The duration of the subsequent spontaneous AF after burst pacing was documented. The median AF duration based on 5 such tests was used to reflect the AF substrate in each animal. If induced spontaneous AF lasted more than 10 minutes, AF was considered permanent.
AF was defined as irregular, rapid atrial activations with varying electrogram morphology lasting ≥0.5second. The atrial rates were typically >1500bpm in rats.
Atrial histology and connexin (Cx) 43 immunohistochemical staining
Left atrial appendages were taken and immersion-fixed with 4% paraformaldehyde, processed, and paraffin embedded. Serial histological sections (6.0-µm-thick) were cut and stained with hematoxylin and eosin (H&E) and Masson’s trichrome stain.
A separate group of sections were immunostained with a monoclonal antibody against rat connexin-43 (1:500, MAB3068; Millipore, Temecula, CA). Connexin-43 (Cx43) antibody was visualized using goat-anti-mouse Rhodamine Red-X-conjugated antibody (1:200, 115-295-146; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and cell nuclei were counterstained with DAPI (4', 6-Diamidino-2-phenylindole dihydrochloride).
Stained sections were examined using an Olympus BX53 microscope and high-resolution digital images captured at x40 magnification with an Olympus DP72 digital camera. Morphometric and stereological analyses of digitized images were performed in a blinded manner using Image-Pro Analyzer 7.0 software (Media Cybernetics, Inc., Bethesda, MD).
The mean cross-sectional area (CSA) and diameter of cardiac myocytes were estimated using optical fields in which cardiac myocytes showed a relatively circular outline and presence of a round-shaped nucleus. On average, about 60 to 80 nucleated myocyte profiles were examined in each appendage.
Interstitial fibrosis was estimated using Masson-stained sections and expressed as percentage of the total area occupied by interstitial tissue and cardiac myocytes.
Density of Cx43 was estimated using the sections immunostained with anti-Cx43 antibody. Cx43 is best viewed and was examined in areas of longitudinally-oriented cardiac myocytes and expressed as percentage of area examined.
Statistical analysis
Data are described with mean and the 95% confidence interval for continuous variables. Fisher’s exact test was used to compare the AF incidence. Because AF duration data are not normally distributed, they are expressed as median, first and third quartile (Q1-Q3) values delimiting bottom 25% and top 25% of the distribution, and the extremes. A nonparametric Kruskal-Wallis test followed by Mann-Whitney U tests were used to compare the AF duration data. Analyses of variance were performed to compare all other continuous variables among groups followed by Tukey’s HSD tests. A P<0.05 was required for statistical significance.
Results
Effects of different T4 dosages on serum TH levels, heart weight and body weight
The efficacy of thyroidectomy was confirmed by the presence of serum hypothyroidism (low T3, T4 and high TSH) in all animals prior to pellet implantation (Figure 1, left panels). After 2-month treatment, serum T3 and T4 remained significantly decreased, and TSH was increased in the placebo group (confirming hypothyroidism) compared with normal control (Figure 1, right panels). Serum T3, T4 and TSH were normalized in the euthyroid group. Both T3 and T4 were significantly increased, and TSH was significantly decreased in the high-dose T4 treated hyperthyroid group (confirming hyperthyroidism).
Figure 1.
Serum thyroid hormone levels before pellet implantation (left panels) and after 2 month treatment (right panels) from the studied groups. T3, total triiodothyronine level; T4, L-thyroxine level; TSH, thyroid-stimulating hormone level. Values are mean and the 95% confidence interval. For each parameter overall ANOVA P value and significant P values versus normal control are indicated.
Placebo treated hypothyroid rats had lower body weight, compared with euthyroid and hyperthyroid rats (Table 1). Body weight in hyperthyroid rats was not statistically different from euthyroid rats. Heart weight in the hypothyroid group was significantly lower (cardiac atrophy) compared with euthyroid rats, while heart weight was higher in hyperthyroid rats (cardiac hypertrophy) compared with euthyroid rats. These changes persisted after adjustment for body weight.
Table 1.
Body weight, heart weight and ratio of heart weight/body weight
| Body weight(g) | Heart weight(mg) | HW/BW(mg/g) | |
|---|---|---|---|
| Hypothyroid | 290(259–321) | 677(590–745) | 2.33(1.98–2.67) |
| Euthyroid | 328(298–359) | 1031(886–1176) | 3.15(2.72–3.59) |
| Hyperthyroid | 345(326–364) | 1375(1224–1526) | 4.01(3.52–4.50) |
| Overall P-value (ANOVA) | 0.011 | < 0.001 | < 0.001 |
| P-value (Tukey HSD): Hypothyroid vs. Euthyroid | 0.048 | < 0.001 | 0.012 |
| P-value (Tukey HSD): Hyperthyroid vs. Euthyroid | 0.61 | 0.001 | 0.009 |
Values are mean (95% confidence interval). HW/BW indicates ratio of heart weight/body weight.
Effects of different T4 dosages on echocardiographic parameters
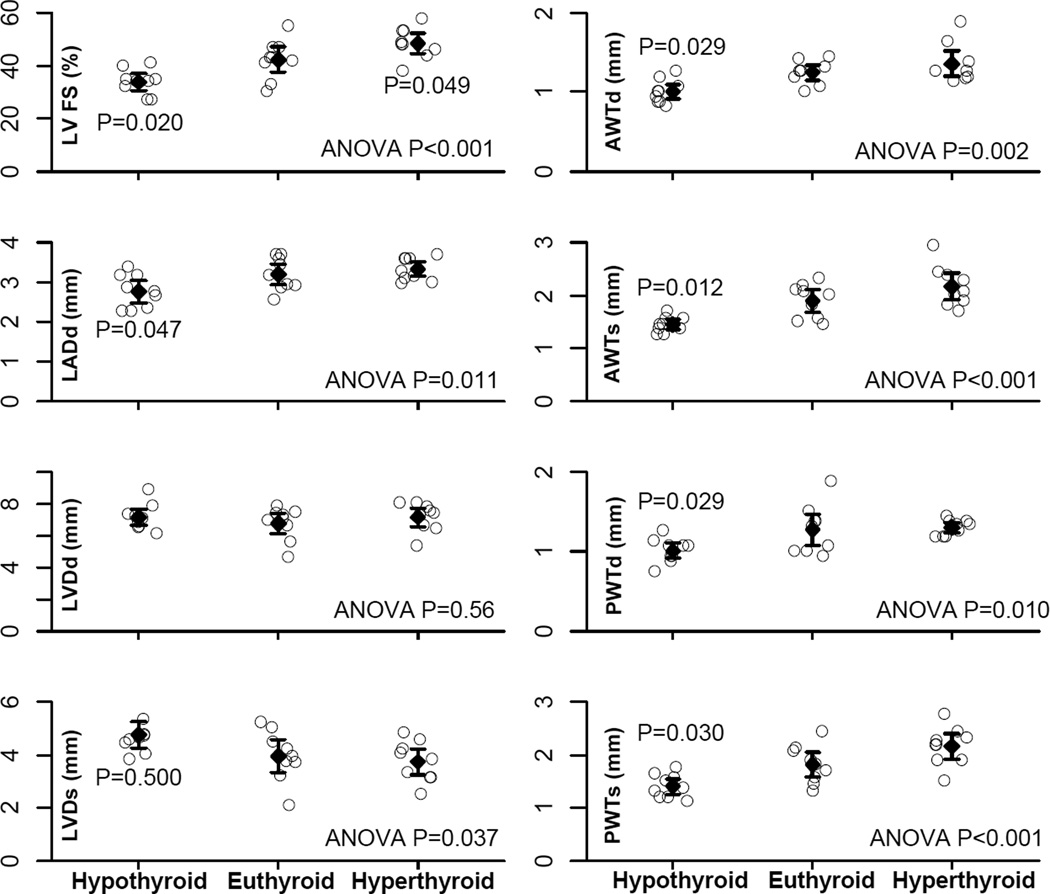
LV function and cardiac dimensions acquired by echocardiography before terminal experiments are reported in Figure 2. LV fractional shortening and wall thickness were reduced in the hypothyroid group compared with the euthyroid group and hyperthyroid group. Although hypothyroid rats had larger LV chamber dimension in systole, the LV diastolic chamber dimension was not yet increased at this relatively early stage. These data indicate cardiac atrophy with reduced ventricular contractility rather than heart failure, which can occur with long-term hypothyroidism.5 The hyperthyroid group had increased LV fractional shortening, indicating increased ventricular contractility.
Figure 2.
Echocardiographic parameters. LVFS, left ventricular fractional shortening; LADd, left atrial diameter in diastole; LVDd, left ventricular diameter in diastole; LVDs, left ventricular diameter in systole; AWTd, left ventricular anterior wall thickness in diastole; AWTs, left ventricular anterior wall thickness in systole; PWTd, left ventricular posterior wall thickness in diastole; PWTs, left ventricular posterior wall thickness in systole. Values are mean and the 95% confidence interval. For each parameter overall ANOVA P value and significant P values versus euthyroid group are indicated.
Effects of different T4 dosages on LV hemodynamics
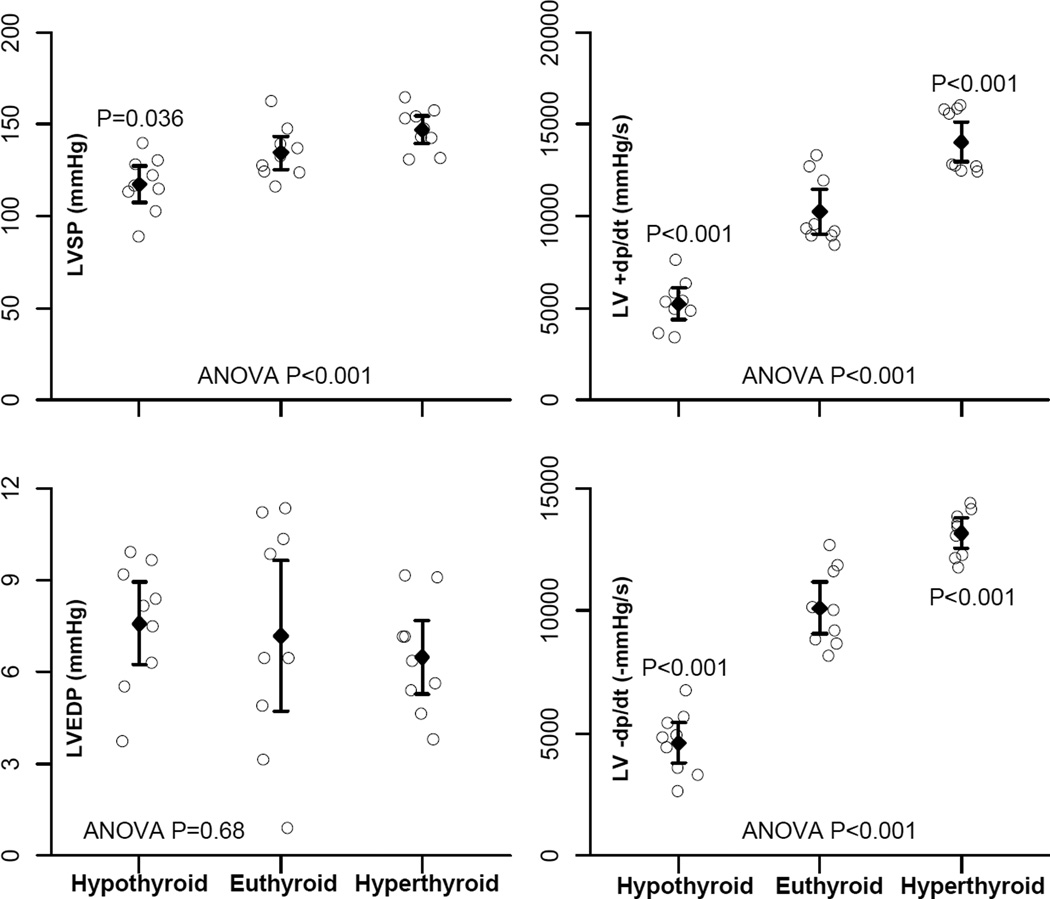
Hypothyroid rats showed a reduction in ±dp/dt, compared with euthyroid rats (Figure 3). However, LV end-diastolic pressure was not significantly changed in hypothyroid rats, suggesting the absence of heart failure. In contrast, the hyperthyroid group showed an increase in ±dp/dt compared with euthyroid rats, indicating increased LV systolic and diastolic function in hyperthyroid rats.
Figure 3.
Left ventricular hemodynamics. LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; +dp/dt, positive change in pressure over time; −dP/dt, negative change in pressure over time. Data presentation and P values are indicated as in Figure 2.
Effects of different T4 dosages on cardiac electrophysiology and AF inducibility
Basic cardiac electrophysiology data are shown in Table 2. As expected, the heart rate was lower in hypothyroid rats and higher in hyperthyroid rats, compared to euthyroid rats (Table 2). Hypothyroid rats had longer corrected sinus node recovery time compared with euthyroid rats. In addition, the atrial effective refractory period was significantly longer in hypothyroid rats compared with euthyroid rats and was shorter in hyperthyroid rats. Atrioventricular conduction time was not different among the groups.
Table 2.
Electrophysiological parameters
| Heart rate(bpm) | SNRTc(ms) | AERP(ms) | AVCT(ms) | |
|---|---|---|---|---|
| Hypothyroid | 229(199–260) | 44(33–55) | 45(37–54) | 47(43–50) |
| Euthyroid | 360(312–409) | 32(27–37) | 31(28–34) | 45(43–48) |
| Hyperthyroid | 508(449–568) | 30(23–38) | 26(22–30) | 43(40–47) |
| Overall P-value (ANOVA) | < 0.001 | 0.008 | < 0.001 | 0.23 |
| P-value (Tukey HSD): Hypothyroid vs. Euthyroid | < 0.001 | 0.048 | 0.001 | |
| P-value (Tukey HSD): Hyperthyroid vs. Euthyroid | < 0.001 | 0.94 | 0.039 |
Values are mean (95% confidence interval). SNRTc indicates corrected sinus node recovery time; AERP, atrial effective refractory period; AVCT, atrioventricular conduction time.
AF inducibility data are shown in Figure 4 and in the online supplemental Table. AF inducibility was significantly increased in the hypothyroid (7/9 rats, p=0.008) and hyperthyroid groups (6/9 rats, p=0.025) compared with the euthyroid group (1/9 rats). Induced AF duration was also increased in hypothyroid and hyperthyroid rats (Figure 4).
Figure 4.
Atrial fibrillation (AF) inducibility test. The original ECG traces show an example of no AF induction after burst pacing (upper) and AF induction after burst pacing (bottom). The AF duration data are shown with box-and-whisker plot (data were given as median, Q1-Q3 and extremes). Significant P values are versus euthyroid group.
Effects of different T4 dosages on atrial histology and Cx43 staining
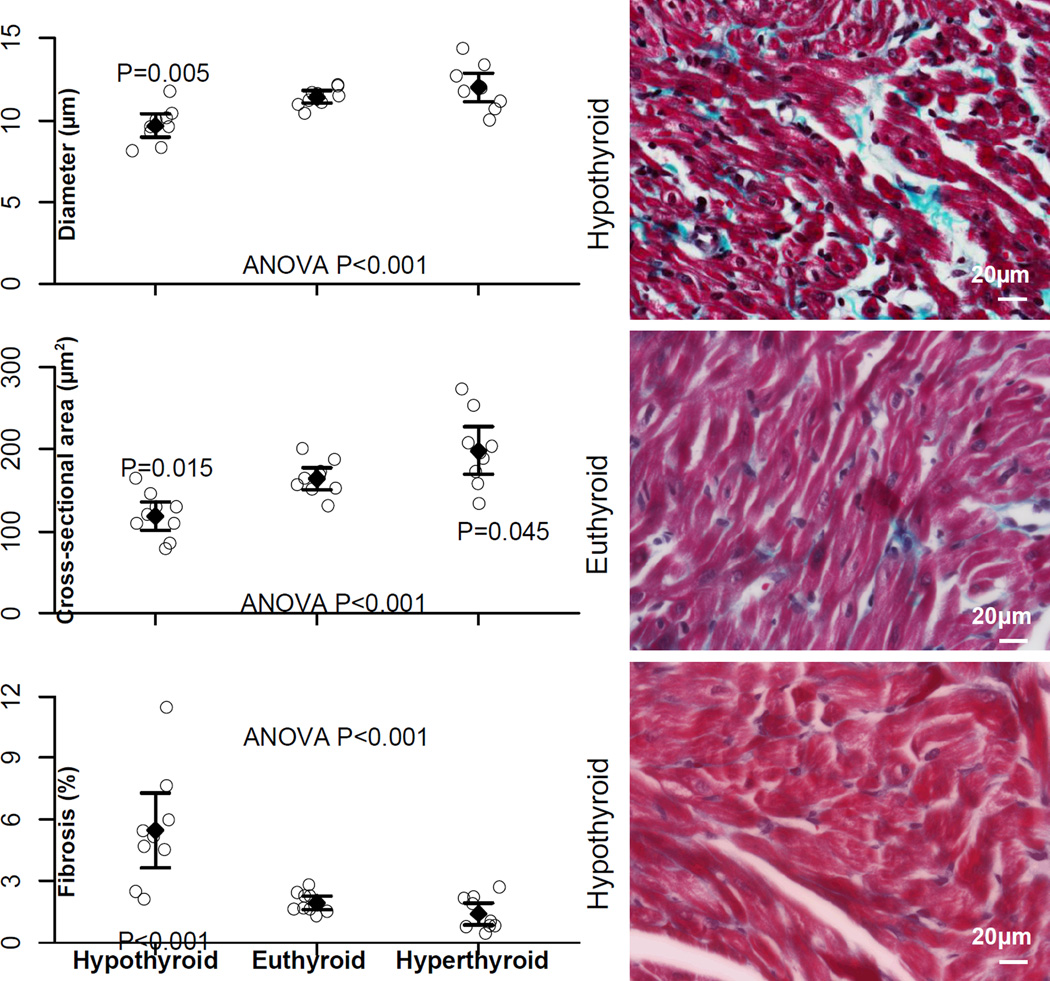
Mean cross-sectional area and diameter of left atrial myocytes were smaller in hypothyroid rats, confirming myocardial atrophy (Figure 5). The cross-sectional area was larger in hyperthyroid rats, confirming myocardial hypertrophy. Left atrial interstitial fibrosis was increased in hypothyroid rats compared with euthyroid rats. The extent of fibrosis was not statistically different in euthyroid and hyperthyroid rats.
Figure 5.
Left atrial myocyte diameter, cross-sectional area, and left atrial fibrosis content. Representative photomicrographs of left atrial histological slides (Masson Trichrome) from 1 rat in each group are shown on the right. Data presentation and P values are indicated as in Figure 2.
Left atrial myocyte Cx43 immunostaining is shown in Figure 6. Cx43 density was not statistically different among the 3 groups, although there was a tendency toward lower density in hyperthyroid rats.
Figure 6.
Left atrial myocytes connexin (Cx) 43 expression. Representative photomicrographs of left atrial myocytes Cx43 immunostaining (red) combined with green background autofluorescence outlining the myocardial tissue, from 1 rat in each group are shown on top. Data presentation and P values are indicated as in Figure 2.
Discussion
Major findings
To our knowledge, this is the first study examining the effects of different TH levels, ranging from hypothyroid to hyperthyroid, on AF arrhythmogenesis in a rat thyroidectomy model. Our results demonstrated that both hypothyroidism and hyperthyroidism were associated with enhanced AF vulnerability compared with rats having normalized TH levels. Thus, our results demonstrate that hypothyroidism, like hyperthyroidism, increases AF risk. Our data highlight the potential clinical importance of correcting thyroid dysfunction to prevent cardiac arrhythmias and AF.
Hyperthyroidism and AF
The association between hyperthyroidism and AF is well established by clinical and experimental data. AF occurs in 10–25% of patients with hyperthyroidism.1 In patients with hyperthyroidism and AF, the primary treatment goal is to restore normal thyroid function. Once a euthyroid state is achieved by effective treatment, AF usually reverts to spontaneous sinus rhythm.1
Data from this study further confirm that hyperthyroidism increases AF vulnerability. It should be noted that hyperthyroid rats demonstrated increased ventricular contractility (increased LVFS in Figure 2 and increased +dp/dt in Figure 3), which is consistent with previous reports.4–6 TH can increase contractility through many mechanisms, such as increasing ratio of α-myosin heavy chain/β-myosin heavy chain, RYR2 and decreasing phospholamban, etc. TH is known to promote physiological cardiac hypertrophy by adding sarcomeres in parallel and series, resulting in a proportional increased myocyte cross-sectional area/ventricular wall thickness and myocyte length/chamber diameter, in a manner similar to excise rather than pathologic hypertrophy.4–6 This is reflected by the increased HW/BW in Table 1. On the other hand, hyperthyroid rats had increased diastolic function (increased –dp/dt) and normal LVEDP and left atrial diameter, indicating diastolic dysfunction was not present and not expected to contribute to increased AF inducibility in these rats.
Hyperthyroidism can lead to increased automaticity, decreased atrial action potential duration, decreased atrial effective refractory period (ERP), and profoundly altered ion channel expression and function. Our results showed shortened atrial ERP in hyperthyroid rats (Table 2), which is consistent with an early report that in isolated rabbit hearts hyperthyroidism decreased atrial ERP.12 According to the wavelength theory, shortened ERP can result in a shortened wavelength (a product of ERP and conduction velocity), thus favoring re-entry.17 Moreover, hyperthyroidism can increase automaticity and enhance triggered activity in single cardiomyocytes from rabbit pulmonary veins, resulting in enhanced AF arrhythmogenesis.18 Increased ectopic activity in hyperthyroid patients has also been demonstrated.19
Hyperthyroidism can alter cardiac ion channel expression and function. In fact, the reduced atrial ERP in hyperthyroidism may be caused by increased K+ and reduced Ca++ current. In murine atria, hyperthyroidism increases delayed rectifier K+ currents, which in turn could speed up repolarization and abbreviate atrial action potential duration and ERP.13 There are reports that THs also decrease L-type calcium currents with resultant shortening of action potential duration, providing a substrate for AF. 14, 20 However, the effect of hyperthyroidism on Ca++ current is not consistent. Another study found that THs increased expression of L-type calcium channel mRNA and Ca++ current densities in both atrium and ventricle.21 Similar results were also obtained from right atrial tissue in patients.22
Hyperthyroidism also affects cardiac connexins (Cx) expression and function. Cx play an important role in cell-cell coupling and hyperthyroidism is known to up-regulate Cx 43 in rat atria.23 Up-regulation of Cx 43 should improve cell-cell coupling and electrical conduction. Improved conduction, by increasing re-entry wavelength, should reduce the chance of developing multiple wavelets in the atria.17 However, THs could decrease Cx43 phosphorylation in rat atria and may affect Cx43 channel properties and function and consequently influence cardiac arrhythmia susceptibility.24 In our experiments, by using immunohistochemical staining, we found Cx 43 densities were not increased in hyperthyroid rats. This might be due to hyperthyroidism associated myocyte hypertrophy, as reflected in the larger myocyte cross-sectional area in this group (Figure 5). Larger myocytes may reduce relative numbers of gap junctions in a given area.
Fibrosis is another important factor in AF arrhythmogenesis.25 In our study, the interstitial collagen content was not increased (Figure 5) in hyperthyroid rats compared with euthyroid animals. In fact, collagen was less pronounced. Thus, fibrosis is not a contributing factor in this animal model of hyperthyroidism. However, it should be noted that long-term hyperthyroidism may lead to increased myocardial fibrosis and reduced cardiac function.26
Hypothyroidism and AF
Currently, the relationship between hypothyroidism and AF is unclear. There are little data available on hypothyroidism and AF in patients. A few case reports suggested that hypothyroidism might be associated with AF,8–10 while a recent population cohort study found low risk of AF in overt hypothyroidism.11 It is interesting to note that a veterinary report indicates that AF and primary hypothyroidism are most often diagnosed in middle-aged and older dogs. The frequencies of primary hypothyroidism in AF dogs were significantly higher than in the control animals, suggesting hypothyroidism may contribute to AF.27 Nevertheless, there are no prior experiments specifically designed to examine the effects of hypothyroidism on AF arrhythmogenesis.
The data from this study demonstrated clearly that hypothyroidism resulted in an increased AF susceptibility (Figure 4), similar to hyperthyroidism. Although hypothyroidism and hyperthyroidism increased AF incidence, they affect other atrial electrophysiological parameters differently. Hyperthyroidism increases heart rate and shortens atrial ERP, while hypothyroidism decreases heart rate and prolongs atrial ERP. We found increased atrial interstitial collagen in hypothyroid animals, while the collagen content was not increased in hyperthyroid rats. This finding is consistent with previous reports that hypothyroidism promotes myocardial fibrosis.28 The increased fibrosis in hypothyroid rats may contribute to longer atrial ERP. Atrial fibrosis can lead to slow conduction and increased conduction heterogeneity, thus favoring re-entry formation and increasing AF vulnerability.25
Since THs profoundly affect cardiac ion channels, hypothyroidism should cause ion channel remodeling. It has been reported that hypothyroidism may reduce different K+ and Ca++ currents. 29, 30 Increased repolarization inhomogeneity and QT dispersion has also been reported in hypothyroid mice.31 All these changes may enhance arrhythmogenesis. We found that Cx43 density was not decreased in hypothyroid rats. This may be partly due to myocyte atrophy. Since hyperthyroidism and hypothyroidism produce different changes in cardiac electrophysiology, it is expected that different mechanisms may be involved for enhanced AF vulnerability in hypothyroid and hyperthyroid animals. In the current experiments, it was not possible to answer whether hypothyroidism is more arrhythmogenic than hyperthyroidism, despite the observation that induced AF duration seems longer in hypothyroid rats (Figure 4).
Clinical implication
Our results demonstrated that both hypothyroidism and hyperthyroidism can potentially lead to enhanced AF arrhythmogenesis. AF incidence was the lowest in euthyroid rats. While the relationship between hyperthyroidism and AF is well-known and its clinical importance is well- recognized,1 the role of hypothyroidism in AF arrhythmogenesis is less studied and not well-recognized. Our finding that hypothyroidism increases AF susceptibility has important clinical and pathophysiological implications.
Altered TH metabolism, characterized as low-T3 syndrome, has been described in patients with various cardiac diseases and was linked to poor prognosis of cardiac patients.32 Low-T3 syndrome was found in about 30% of admitted cardiac patients and was associated with increased cardiac and overall deaths.32 Moreover, increasing evidence suggests that blood TH levels may underestimate the extent of low cardiac tissue T3 levels in heart diseases as a result of reduced local conversion from T4 to T3 and increased myocardial T3 degradation.6, 15 Myocardial (cardiac tissue level) hypothyroidism may contribute to HF development.6 HF is known to be associated with an increased AF risk.2 Based on this evidence, it can be speculated that cardiac tissue hypothyroidism in HF, if left untreated, may contribute to AF arrhythmogenesis as well.
Accumulating evidence suggests that there are hemodynamic benefits of TH replacement therapy to normalize cardiac tissue TH level in HF.6 However, whether TH replacement therapy can decrease AF in HF remains to be investigated.
Study limitation
AF vulnerability in hypothyroidism and hyperthyroidism may be time and disease state-dependent. In this study, 3 months of hypothyroidism enhanced AF vulnerability in this animal model. Long-term hypothyroidism may lead to chamber dilation and HF.5 Thus, arrhythmia vulnerability is expected to be further increased. Certain histological changes may also be time-dependent. For example, the fibrosis content in hyperthyroid rats was not yet increased in the current study, but longstanding hyperthyroidism (10 months) has been demonstrated to impair LV function and increase interstitial fibrotic deposition in hamsters.26
In this study, cardiac tissue TH levels were not measured. However, our previous study found that the same 3.3mg T4 pellets used in the current study normalized cardiac tissue TH levels.15 While tissue TH levels may be a more precise measure of organ thyroid status, access to tissue samples in humans is, of course, problematic.
Although we have demonstrated pronounced electrophysiological changes and increased AF inducibility in hypothyroid rats, the exact mechanisms responsible for increased AF vulnerability in hypothyroidism remain to be investigated.
Conclusions
We have demonstrated that in a rat thyroidectomy model, both hypothyroidism and hyperthyroidism can increase AF vulnerability, indicating that normal TH levels are required to prevent cardiac arrhythmias and AF, especially in conditions when cardiac tissue hypothyroidism may be present.
Supplementary Material
Acknowledgments
The authors would like to acknowledge that Dr. Min-Kyung Jung, Ph.D., biostatistician in the Office of Research, has provided assistance in statistical analysis. Alice O'Connor, B.S. and Ying Li, B.S., have provided technical support during the experiments.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr, Priori SG, Estes NA, 3rd, Ezekowitz MD, Jackman WM, January CT, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Hochman JS, Kushner FG, Ohman EM, Tarkington LG, Yancy CW. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 2.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: A report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 4.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 5.Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation. 2005;112:3122–3130. doi: 10.1161/CIRCULATIONAHA.105.572883. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes AM, Iervasi G. Thyroid replacement therapy and heart failure. Circulation. 2010;122:385–393. doi: 10.1161/CIRCULATIONAHA.109.917922. [DOI] [PubMed] [Google Scholar]
- 7.Biondi B. Mechanisms in endocrinology: Heart failure and thyroid dysfunction. Eur J Endocrinol. 2012;167:609–618. doi: 10.1530/EJE-12-0627. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui AS, D'Costa DF, Moore-Smith B. Covert hypothyroidism with weight loss and atrial fibrillation. Br J Clin Pract. 1993;47:268. [PubMed] [Google Scholar]
- 9.Wong PS, Hee FL, Lip GY. Atrial fibrillation and the thyroid. Heart. 1997;78:623–624. doi: 10.1136/hrt.78.6.623a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tajiri J, Morita M, Higashi K, Sato T. Masked thyroid dysfunction in elderly patients with atrial fibrillation. Arch Intern Med. 1985;145:1140. [PubMed] [Google Scholar]
- 11.Selmer C, Olesen JB, Hansen ML, Lindhardsen J, Olsen AM, Madsen JC, Faber J, Hansen PR, Pedersen OD, Torp-Pedersen C, Gislason GH. The spectrum of thyroid disease and risk of new onset atrial fibrillation: A large population cohort study. BMJ. 2012;345:e7895. doi: 10.1136/bmj.e7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnsdorf MF, Childers RW. Atrial electrophysiology in experimental hyperthyroidism in rabbits. Circ Res. 1970;26:575–581. doi: 10.1161/01.res.26.5.575. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Jones SV, Dillmann WH. Effects of hyperthyroidism on delayed rectifier K+ currents in left and right murine atria. Am J Physiol. 2005;289:H1448–H1455. doi: 10.1152/ajpheart.00828.2004. [DOI] [PubMed] [Google Scholar]
- 14.Chen WJ, Yeh YH, Lin KH, Chang GJ, Kuo CT. Molecular characterization of thyroid hormone-inhibited atrial L-type calcium channel expression: Implication for atrial fibrillation in hyperthyroidism. Basic Res Cardiol. 2011;106:163–174. doi: 10.1007/s00395-010-0149-5. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Redetzke RA, Said S, Pottala JV, de Escobar GM, Gerdes AM. Serum thyroid hormone levels may not accurately reflect thyroid tissue levels and cardiac function in mild hypothyroidism. Am J Physiol. 2008;294:H2137–H2143. doi: 10.1152/ajpheart.01379.2007. [DOI] [PubMed] [Google Scholar]
- 16.Chen YF, Weltman NY, Li X, Youmans S, Krause D, Gerdes AM. Improvement of left ventricular remodeling after myocardial infarction with eight weeks L-thyroxine treatment in rats. J Transl Med. 2013;11:40. doi: 10.1186/1479-5876-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smeets JL, Allessie MA, Lammers WJ, Bonke FI, Hollen J. The wavelength of the cardiac impulse and reentrant arrhythmias in isolated rabbit atrium. The role of heart rate, autonomic transmitters, temperature, and potassium. Circ Res. 1986;58:96–108. doi: 10.1161/01.res.58.1.96. [DOI] [PubMed] [Google Scholar]
- 18.Chen YC, Chen SA, Chen YJ, Chang MS, Chan P, Lin CI. Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes . J Am Coll Cardiol. 2002;39:366–372. doi: 10.1016/s0735-1097(01)01731-4. [DOI] [PubMed] [Google Scholar]
- 19.Wustmann K, Kucera JP, Zanchi A, Burow A, Stuber T, Chappuis B, Diem P, Delacretaz E. Activation of electrical triggers of atrial fibrillation in hyperthyroidism. J Clin Endocrinol Metab. 2008;93:2104–2108. doi: 10.1210/jc.2008-0092. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe H, Ma M, Washizuka T, Komura S, Yoshida T, Hosaka Y, Hatada K, Chinushi M, Yamamoto T, Watanabe K, Aizawa Y. Thyroid hormone regulates mRNA expression and currents of ion channels in rat atrium. Biochem Biophys Res Commun. 2003;308:439–444. doi: 10.1016/s0006-291x(03)01420-7. [DOI] [PubMed] [Google Scholar]
- 21.Yu Z, Wang T, Xu L, Huang CX. Thyroid hormone increased L-type calcium channel mRNA expression and L-type calcium current of myocytes in rabbits. Biomed Mater Eng. 2012;22:49–55. doi: 10.3233/BME-2012-0689. [DOI] [PubMed] [Google Scholar]
- 22.Kreuzberg U, Theissen P, Schicha H, Schroder F, Mehlhorn U, de Vivie ER, Boknik P, Neumann J, Grohe C, Herzig S. Single-channel activity and expression of atrial L-type Ca(2+) channels in patients with latent hyperthyroidism. Am J Physiol. 2000;278:H723–H730. doi: 10.1152/ajpheart.2000.278.3.H723. [DOI] [PubMed] [Google Scholar]
- 23.Stock A, Sies H, Stahl W. Enhancement of gap junctional communication and connexin43 expression by thyroid hormones. Biochem Pharmacol. 1998;55:475–479. doi: 10.1016/s0006-2952(97)00473-5. [DOI] [PubMed] [Google Scholar]
- 24.Mitasikova M, Lin H, Soukup T, Imanaga I, Tribulova N. Diabetes and thyroid hormones affect connexin-43 and PKC-epsilon expression in rat heart atria. Physiol Res. 2009;58:211–217. doi: 10.33549/physiolres.931425. [DOI] [PubMed] [Google Scholar]
- 25.Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 26.Weltman NY, Wang D, Redetzke RA, Gerdes AM. Longstanding hyperthyroidism is associated with normal or enhanced intrinsic cardiomyocyte function despite decline in global cardiac function. PloS one. 2012;7:e46655. doi: 10.1371/journal.pone.0046655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerritsen RJ, van den Brom WE, Stokhof AA. Relationship between atrial fibrillation and primary hypothyroidism in the dog. Vet Q. 1996;18:49–51. doi: 10.1080/01652176.1996.9694614. [DOI] [PubMed] [Google Scholar]
- 28.Chen WJ, Lin KH, Lee YS. Molecular characterization of myocardial fibrosis during hypothyroidism: Evidence for negative regulation of the pro-alpha1(I) collagen gene expression by thyroid hormone receptor. Mol Cell Endocrinol. 2000;162:45–55. doi: 10.1016/s0303-7207(00)00203-3. [DOI] [PubMed] [Google Scholar]
- 29.Light P, Shimoni Y, Harbison S, Giles W, French RJ. Hypothyroidism decreases the ATP sensitivity of KATP channels from rat heart. J Membr Biol. 1998;162:217–223. doi: 10.1007/s002329900359. [DOI] [PubMed] [Google Scholar]
- 30.Seppet EK, Kolar F, Dixon IM, Hata T, Dhalla NS. Regulation of cardiac sarcolemmal Ca2+ channels and Ca2+ transporters by thyroid hormone. Mol Cell Biochem. 1993;129:145–159. doi: 10.1007/BF00926363. [DOI] [PubMed] [Google Scholar]
- 31.Ferrer T, Arin RM, Casis E, Torres-Jacome J, Sanchez-Chapula JA, Casis O. Mechanisms responsible for the altered cardiac repolarization dispersion in experimental hypothyroidism. Acta Physiol (Oxf) 2012;204:502–512. doi: 10.1111/j.1748-1716.2011.02364.x. [DOI] [PubMed] [Google Scholar]
- 32.Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, L'Abbate A, Donato L. Low-T3 syndrome: A strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107:708–713. doi: 10.1161/01.cir.0000048124.64204.3f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.