Abstract
Historically, the malaria vectors in western Kenya have been Anopheles funestus, Anopheles gambiae s.s., and Anopheles arabiensis. Of these species, An. funestus populations declined the most after the introduction of insecticide-treated bed nets (ITNs) in the 1990s in Asembo, and collections of An. funestus in the region remained low until at least 2008. Contrary to findings during the early years of ITN use in Asembo, the majority of the Anopheles collected here in 2010 and 2011 were An. funestus. Female An. funestus had characteristically high Plasmodium falciparum sporozoite rates and showed nearly 100% anthropophily. Female An. funestus were found more often indoors than outdoors and had relatively low mortality rates during insecticide bioassays. Together, these results are of serious concern for public health in the region, indicating that An. funestus may once again be contributing significantly to the transmission of malaria in this region despite the widespread use of ITNs/long-lasting insecticidal nets (LLINs).
Introduction
Significant regional reductions in malaria-related morbidity and mortality have occurred globally since 2001,1 but these outcomes may reverse if lessons from the past are not heeded. The Global Malaria Eradication Program (GMEP) of the 1950–60s succeeded in eliminating malaria from many countries and reducing malaria vector populations and malaria transmission substantially in others where elimination was not achieved.2,3 Unfortunately, resurgences in vector populations and transmission occurred in many of these countries, likely caused by a combination of reductions in program funding, economic and political changes, reintroductions of the malaria parasite by movement of people, development of drug resistance in malaria parasites, and evolution of insecticide resistance in vector populations.2,3 As the global health community strives once again toward the goal of malaria elimination and eradication, it is therefore vital to quantify and understand the causes of any resurgence in malaria transmission or malaria vector population size.
In western Kenya, malaria continues to cause significant morbidity and mortality despite public health efforts to reduce malaria prevalence.4,5 These efforts include the widespread distribution of long-lasting insecticidal nets (LLINs), which target indoor biting malaria vectors.6 Although high community-level coverage with LLINs reduces morbidity and mortality caused by malaria in the short term through impacts on malaria vector populations,7 the long-term effectiveness of this intervention depends, in part, on whether insecticide resistance evolves in vector populations.8,9
Historically, the primary malaria vector mosquito species in western Kenya were Anopheles gambiae sensu stricto (s.s.) and Anopheles funestus.10,11 Anopheles arabiensis was historically considered a secondary malaria vector in the region,10,11 though the role of An. arabiensis in malaria transmission has become increasingly important in the last decade as conventional insecticide-treated bed nets (ITNs) and LLINs have reduced the abundance of the other vector species.6 Following the introduction of ITNs in the Asembo region of western Kenya in 1997 during a large-scale, randomized trial, the population of An. funestus declined the most of these three species.12 The population of An. funestus remained low for several years after the trial ended and as ITNs were distributed nationally.6,13
In Kenya resistance to the pyrethroid insecticides used in ITNs has been reported largely in An. gambiae sensu lato (s.l.),14–16 a complex of at least eight closely related species including An. gambiae s.s. and An. arabiensis.17 There are few published studies on insecticide resistance in An. funestus from Kenya, although reports of resistance to pyrethroids, carbamates, and DDT in An. funestus from other regions of Africa have increased over the last decade.18 Notably, pyrethroid resistance in An. funestus was associated with an increase in malaria cases in South Africa in the 1990s.19
The purpose of this study was several-fold. First, we investigated relative abundances, human biting rates, and malaria infection rates of An. funestus and other malaria vectors in the same western Kenyan region (Asembo) where vector abundances had decreased previously as a result of ITN distribution programs,12,13 to determine if vector abundances and malaria transmission remained similarly low or had increased despite continued high coverage of ITNs and LLINs. Additionally, the sensitivity of the vector populations to pyrethroid insecticides in ITNs and LLINs was measured through standardized bioassays to investigate the possibility that pyrethroid resistance could explain changes in the malaria vector populations.
Methods
Study site.
Adult Anopheles mosquitoes were sampled in the Asembo region of Rarieda District (Figure 1). Numerous studies of malaria vectors have been conducted here since the 1970s, and the region has been described in detail.20,21 Malaria is holoendemic in Asembo and is caused chiefly by Plasmodium falciparum. Rainfall occurs year-round but is seasonally bimodal, with peaks occurring from March through May and in November and December. Mosquito sampling intervals for the indoor resting and human landing studies in 2010 and 2011 (below) were chosen to coincide with seasonal peaks of Anopheles populations, which occur after the March–May rainy season.10,11,22
Figure 1.
(A) Map of Kenya with box indicating location of the study region in Nyanza Province. (B) Map showing the location of Asembo on the shores of Lake Victoria, about 50 km west of the city of Kisumu.
Asembo was the site of a randomized, controlled trial of ITNs from 1997 to 1999.20 Following the trial, ITNs were distributed to control villages, and the ITNs were retreated every 6 to 9 months with permethrin until 2002, and then with alphacypermethrin until 2007.6,13 This program in Asembo covered a population of ∼55,000 persons as of 1997,20 which grew to 66,727 by 2011 (Odhiambo F, personal communication). Nationwide, ITNs became available at partially subsidized rates through the retail sector in 2002, and at heavily subsidized rates through health clinics in 2004.23 Furthermore, the Kenya Division of Malaria Control provided LLINs in mass distribution campaigns in 2006, targeting children < 5 years of age, and in June 2011, targeting universal coverage (one LLIN for every two people).24 The primary LLIN brands used in Asembo were PermaNet (Vestergaard Frandsen SA, Aarhus, Denmark) and Olyset (Sumitomo Chemicals, Osaka, Japan). No other vector control intervention, such as indoor residual spraying (IRS) or larval control, has been conducted at scale in Asembo over the last 20 years.
Sampling the indoor resting populations.
Indoor-resting Anopheles were sampled from 14 to 18 June 2010 and 16 May to 24 June 2011 using the pyrethrum spray catch (PSC) method as described by Gimnig and colleagues.12 Sampling was done in 416 houses across an area of 15 km2 in 2010 and in 806 houses across an area of 100 km2 in 2011, and each house was sampled once. Differences between years were examined to determine whether grouping the data across the two sampling periods was appropriate, with analyses performed using R version 2.14.2 (R Development Core Team, Vienna, Austria). Differences between years in the number collected per house within each Anopheles species within each sex were determined using non-parametric Mann-Whitney tests. Differences between years within each Anopheles species in the proportions of blood meals from humans and cattle, and in the ELISA positivity rate (see below), were determined using Fisher's exact test.
Sampling the human landing populations.
Host-seeking Anopheles were sampled indoors and outdoors in 75 villages (150 houses) covering about 200 km2 from 13 June to 22 July 2011 using the human landing collection method (HLC) as described by Gimnig and colleagues.21 Local adult men were trained as collectors and organized into 38 teams of four persons from two neighboring villages with the exception of one team that consisted of only two collectors. Each team rotated among four collection sites, sampling for 4 nights every week. Collectors working outdoors were given discretion to stop collections during rainfall, indicating any hour for which collections were stopped because of rain. Collectors working indoors were instructed to continue regardless of rainfall. All collectors were provided atovaquone proguanil as malaria prophylaxis during the sampling period and for 7 days after the last night of collection.
Historical comparison.
Anopheles females were sampled weekly in 19 villages in southern Asembo from 1993 through September 1997 using the bed net trap (BNT) sampling method. From December 1996 to September of 1997, collections were done in 10 of the villages as the remaining villages had received ITNs as part of the large-scale trial. The BNTs consisted of untreated nets hung above the sleeping spaces of volunteers so that the bottom edge of the net was ∼6 cm above the bed. Mosquitoes that fed on the sleeper and remained resting inside the net were collected the following morning using mouth aspirators. The sleepers were not at an additional risk for malaria during BNT sampling as ITNs were not the standard of care at the time.
To calibrate the BNT to the HLC, we calculated the collection rate of BNT relative to HLC using BNT and HLC samples done from 1 Nov 1992 to 1 May 1993. Anopheles females were sampled weekly from a pool of 91 houses in 15 villages covering about 70 km2 in Asembo. The HLC and BNT were done in the same houses, with HLC always done 1–3 nights after BNT for a given house. The collection rate of BNT relative to HLC was calculated using a generalized linear model with a log link function and negative binomial error distribution. To account for repeated measures within houses, an autoregressive correlation structure was used, allowing for correlation between collections to decrease with increasing time. Analyses were performed using PROC GENMOD in SAS version 9.2 (SAS Institute, Cary, NC).
From 2002 through 2008, Anopheles females were sampled monthly using Centers for Disease Control and Prevention (CDC) light traps (LT). Light traps were set inside houses next to persons sleeping under their own ITNs. Each person was instructed to turn on the trap just before they went to sleep, and mosquitoes were collected from traps in the morning. The traps were set in 30–60 houses each month and run for two consecutive nights in each house.
Collection rates of LT relative to HLC from Wong and colleagues25 were used to compare the LT data from 2002 through 2008 to the HLC data from 2011. Collection rates of BNT relative to HLC, described previously, were used to compare the BNT data from 1993 through 1997 to the HLC data from 2011. Only data from June and July of each year of BNT and LT sampling were used in the comparisons among years to avoid any potential bias caused by seasonal variation in the abundance of either An. gambiae s.l. or An. funestus.
Mosquito identification, sporozoite rates, and blood meal analysis.
All Anopheles were morphologically identified to species according to Gillies and Coetzee.26 Specimens from PSC and HLC sampling identified morphologically as part of the An. gambiae s.l. species complex were further differentiated to the species level by polymerase chain reaction (PCR) using primers specific to Anopheles gambiae s.s. and An. arabiensis.27 All female Anopheles collected during PSC and HLC were separated at the line of the thorax and abdomen to allow for separate diagnostic tests on each specimen. The heads and thoraces of those females were tested for P. falciparum sporozoite proteins by ELISA28 using the P. falciparum sporozoite ELISA reagent kit (MRA-890, MR4, ATCC, Manassas, VA). The blood meal hosts of all fed and half-gravid females collected during PSC were identified by direct sequencing of the vertebrate mitochondrial cytochrome B gene.29
Insecticide bioassays.
Insecticide susceptibility assays were carried out following the World Health Organization (WHO) protocol30 using An. gambiae s.l. and An. funestus adults collected from houses in Asembo using backpack aspirators from May through October 2012. Only unfed females not injured during collections were retained for bioassays. These mosquitoes were held for 1 h with access to 10% sugar solution, and then exposed in the field to insecticide-impregnated filter paper for 1 h. Following exposure the mosquitoes were transferred to a clean holding tube with 10% sugar solution, and mortality was determined 24 h later. The mosquitoes were exposed to the pyrethroids permethrin (at a concentration of 0.75%) and deltamethrin (0.05%), and the carbamate bendiocarb (0.01%).
Ethical approval.
This work was approved by the Institutional Review Boards of the U.S. Centers for Disease Control and Prevention and Michigan State University and by the Ethical Review Committee of the Kenya Medical Research Institute.
Results
Indoor resting samples.
Totals of 1,897 Anopheles females and 1,033 Anopheles males were collected indoors during PSC sampling, and > 99% of the Anopheles collected was identified morphologically as either An. funestus or An. gambiae s.l. The only other anopheline species found indoors during PSC sampling was Anopheles rufipes (Table 1). A total of 830 (85%) of the An. gambiae s.l. specimens were successfully differentiated into An. gambiae s.s. and An. arabiensis, whereas PCR amplification failed or was not done in 146 An. gambiae s.l. specimens. The number of Anopheles collected per house differed between the two PSC sampling periods, though An. funestus made up a large proportion of the Anopheles collected in both years (Table 1). In 2010 three-quarters (75.2%) of all female Anopheles collected were An. funestus, and in 2011 most of the female Anopheles collected were either An. funestus (37.9%) or An. arabiensis (37.0%).
Table 1.
Mean number of Anopheles collected per house during pyrethrum spray catch sampling shown by species, sex, and year*
| Species | Females | Males | ||||
|---|---|---|---|---|---|---|
| 2010 | 2011 | P value | 2010 | 2011 | P value | |
| An. funestus | 2.305 (75.2%) | 0.293 (37.9%) | < 0.001 | 1.327 (80.0%) | 0.231 (54.2%) | < 0.001 |
| An. arabiensis | 0.498 (16.2%) | 0.285 (37.0%) | 0.007 | 0.127 (7.7%) | 0.104 (24.5%) | 0.374 |
| An. gambiae s.s. | 0.099 (3.2%) | 0.122 (15.8%) | 0.230 | 0.149 (9.0%) | 0.083 (19.5%) | 0.052 |
| An. gambiae s.l.† | 0.163 (5.3%) | 0.066 (8.5%) | < 0.001 | 0.055 (3.3%) | 0.002 (0.6%) | < 0.001 |
| An. rufipes | 0.000 (0.0%) | 0.006 (0.8%) | NA‡ | 0.000 (0.0%) | 0.005 (1.2%) | NA‡ |
Percentages of the total for each column are shown in parentheses. P values are from non-parametric Mann-Whitney tests to determine differences between years for each species within each sex.
These specimens were identified as members of the Anopheles gambiae species complex according to morphological methods but could not be further differentiated by polymerase chain reaction.
NA = not applicable.
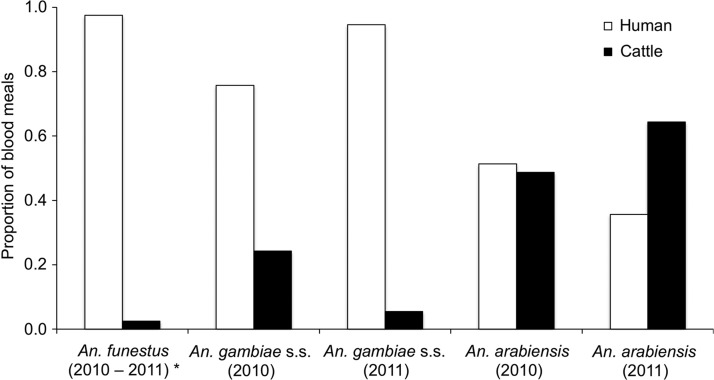
Blood meal hosts were identified in 946 of the 1,328 fed or half-gravid Anopheles females collected during PSC (Figure 2). The proportions of blood meals from humans and cattle in An. funestus did not differ between years (Fisher's exact test, P = 1.00). Nearly all of the 715 An. funestus blood meal hosts identified were from humans, though 2.5% were from cattle. The proportions of blood meals from humans and cattle in An. gambiae and An. arabiensis differed between years (Fisher's exact test, P = 0.017 and P = 0.037, respectively). In 2010, 75.7% of the 33 An. gambiae s.s. blood meals and 51.3% of the 115 An. arabiensis blood meals identified were from humans. In 2011, 94.5% of the 55 An. gambiae s.s. blood meals and 35.6% of the 73 An. arabiensis blood meals identified were from humans. The remaining blood meals in both species from both years were from cattle, except for a total of five blood meals from goats in An. arabiensis.
Figure 2.
Proportion of blood meals taken from humans and cattle by Anopheles species collected during pyrethrum spray catch in Asembo. Non-amplifiers to polymerase chain reaction for blood meal identification are not shown (29%). *Data pooled across years for Anopheles funestus because the proportions of blood meals from humans and cattle did not differ between years (Fisher's exact test, P = 1.00).
With the exception of An. rufipes, a proportion of individuals of all Anopheles species collected during PSC sampling were positive by ELISA for P. falciparum sporozoite infection, but the percentage positive varied by mosquito species (Table 2). The ELISA-positivity rate varied between years only for An. funestus (3.4% in 2010 and 8.9% in 2011, P < 0.001). The highest rate across both years was in An. gambiae s.s., and the lowest rate was in An. arabiensis.
Table 2.
Plasmodium falciparum sporozoite-negative and -positive female mosquitoes, according to enzyme-linked immunosorbent assay (ELISA), from pyrethrum spray catch (PSC) sampling in 2010 and 2011, shown by species with percent positive in parentheses
| Species | Negative | Positive (%) |
|---|---|---|
| An. funestus | 1140 | 54 (4.5)* |
| An. arabiensis | 433 | 4 (0.9) |
| An. gambiae s.s. | 127 | 12 (8.6) |
| An. gambiae s.l.† | 119 | 2 (1.7) |
ELISA positivity rates for Anopheles funestus were significantly different between PSC sampling periods in 2010 and 2011 (3.4% and 8.9%, respectively; Fisher's exact test, P < 0.001).
These specimens were identified as members of the Anopheles gambiae species complex according to morphological methods but could not be further differentiated by polymerase chain reaction.
Human landing samples.
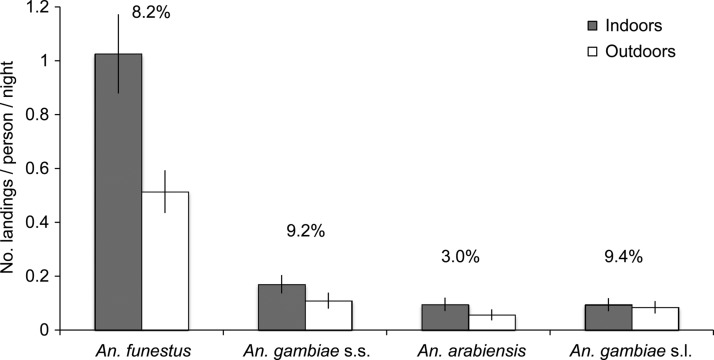
The HLC sampling was done indoors for a total of 888 collector-nights (12,432 collector-hours) and outdoors for 889.4 collector-nights (12,451 collector-hours). During HLC sampling, 1,936 female Anopheles were collected. Greater than 98% of these were morphologically identified as either An. funestus or An. gambiae s.l., whereas the remaining specimens were Anopheles coustani and An. rufipes. A total of 380 (71%) of the An. gambiae species complex specimens were differentiated into An. gambiae s.s. (43%) and An. arabiensis (28%), although PCR amplification failed in 159 (29%) of the specimens. Most of the Anopheles females collected during HLC sampling were An. funestus, and more females of each species were collected indoors than outdoors (Figure 3). Similar to specimens from PSC sampling, ELISA-positivity rates varied among the three malaria vector species during HLC sampling. Overall ELISA-positivity rates for these specimens were highest in An. gambiae s.s. and An. funestus, whereas the lowest rate was found in An. arabiensis (Figure 3).
Figure 3.
Number of Anopheles females collected per collector per night during human landing catch sampling shown by species and location (indoors/outdoors). Error bars are 95% confidence intervals. Plasmodium falciparum sporozoite rates as determined by enzyme-linked immunosorbent assays for specimens collected both indoors and outdoors are shown as percentages above each species. Anopheles gambiae s.l. specimens were identified as members of the An. gambiae species complex according to morphological methods but could not be further differentiated by polymerase chain reaction.
Historical comparison.
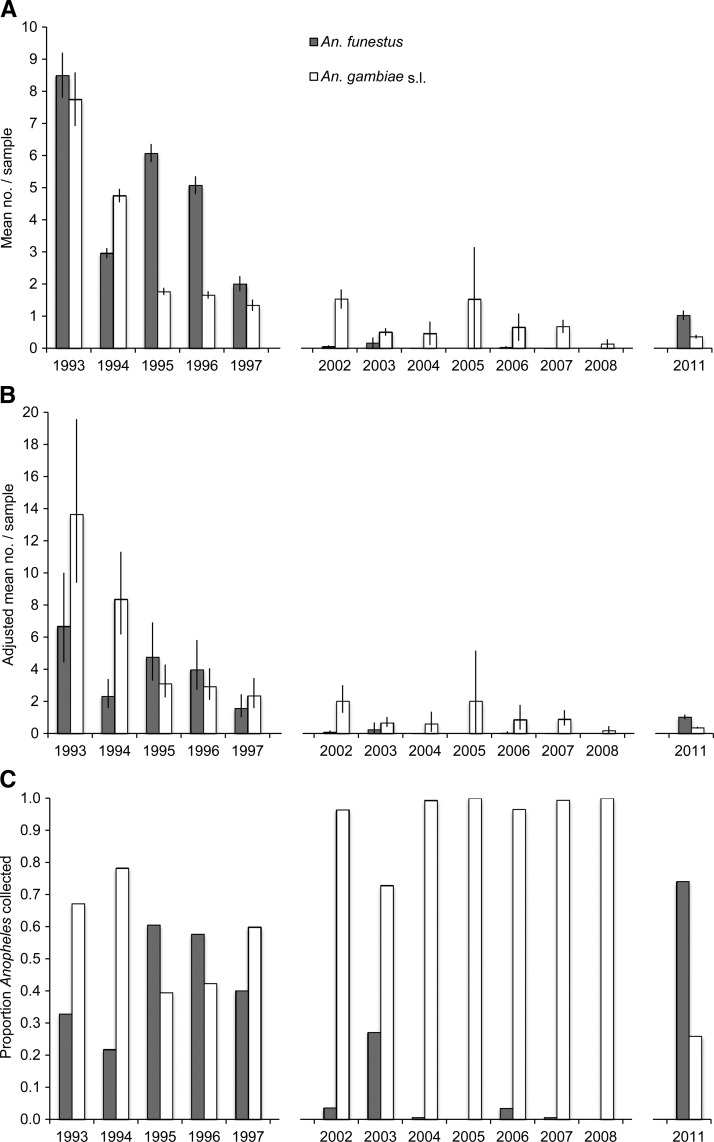
In our calibration of the collection rates of BNT relative to HLC, BNT sampling produced similar numbers of An. funestus relative to HLC sampling, but fewer An. gambiae s.l. were collected from BNT relative to HLC (Table 3). The relative collection rates shown in Table 3 were used to calibrate the 1993–1997 BNT to HLC for comparison to the 2011 HLC (Figure 4B). According to Wong and colleagues25 collections of both An. funestus and An. gambiae s.l. were lower from LT relative to HLC in Rarieda District. The collection rate of An. funestus from LT relative to HLC was 0.69 (95% CI: 0.49–0.98), whereas the relative collection rate of An. gambiae s.l. was 0.76 (95% CI: 0.61–0.96).25 These relative collection rates were used to calibrate the 2002–2008 LT to HLC for comparison to the 2011 HLC (Figure 4B ).
Table 3.
Comparison of human landing catch (HLC) and bed net trap (BNT) sampling methods for Anopheles funestus and Anopheles gambiae s.l., November 1992 to May 1993*
| Method | No. collected | Mean per night (95% CI) | Relative rate (95% CI) | P value |
|---|---|---|---|---|
| An. funestus | ||||
| HLC | 730 | 1.25 (1.05, 1.45) | 1.00† | NA‡ |
| BNT | 917 | 1.57 (1.34, 1.79) | 1.27 (0.92, 1.76) | 0.145 |
| An. gambiae | ||||
| HLC | 2408 | 4.12 (3.47, 4.78) | 1.00† | NA‡ |
| BNT | 1389 | 2.38 (2.01, 2.74) | 0.57 (0.44, 0.74) | < 0.001 |
Relative rates of collection and associated 95% confidence interval (CI) and P value were calculated using negative binomial regression. P values indicate the probability that the relative collection rate of BNT sampling was not different from that of HLC.
HLC was used as the reference sampling method.
NA = not applicable.
Figure 4.
(A) Mean number of Anopheles females collected per sample in June through July from 1993 through 2011 using bed net traps (BNT; 1993–1997), light traps (LT; 2002–2008), and human landing catch (HLC; 2011). (B) Mean number of Anopheles females collected, adjusted for comparison to HLC sampling using the relative rates of BNT to HLC (1993–1997) and LT to HLC (2002–2008). (C) Proportion of total Anopheles females collected identified as either Anopheles funestus or Anopheles gambiae s.l. Error bars indicate 95% confidence intervals. Data were not available from 1998 to 2001, 2009, and 2010.
Before the introduction of ITNs in Asembo, the populations of both An. funestus and An. gambiae s.l. were large according to BNT sampling (Figure 4). Few An. funestus were collected in Asembo using LT from 2002 through 2008. For the months of June and July, which historically were the months when An. funestus populations peaked, zero An. funestus were collected in 2 years from the period, and never were more than 0.17 An. funestus per trap collected. During the same sampling period, the monthly mean of An. gambiae s.l. collected per trap ranged from 0.13 to 1.53 with a mean of 0.75 (Figure 4).
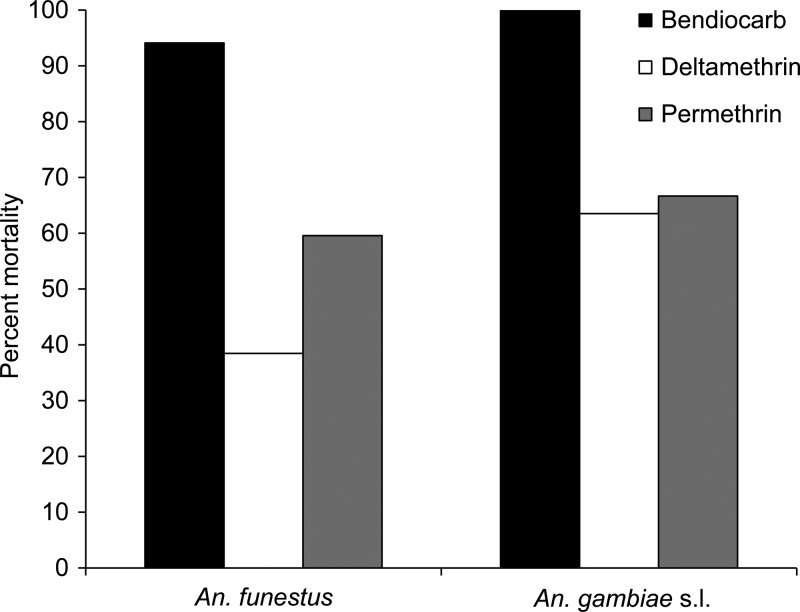
Insecticide bioassays.
Figure 5 depicts mortality to bendiocarb, deltamethrin, and permethrin in wild-caught An. gambiae s.l. and An. funestus adults from Asembo. A total of 78 An. funestus were exposed to deltamethrin, 38 to permethrin, and 34 to bendiocarb. A total of 159 An. gambiae s.l. were exposed to deltamethrin, 6 to permethrin, and 48 to bendiocarb. Although mortality rates after exposure to bendiocarb were high, much lower rates were seen for An. funestus samples exposed to deltamethrin and permethrin, indicating resistance in these specimens to the insecticides used in ITNs and LLINs.
Figure 5.
Percent mortality after 24 hours of wild-collected Anopheles funestus and Anopheles gambiae s.l. adults from Asembo when exposed to permethrin, deltamethrin, or bendiocarb in 1-hour World Health Organization tube bioassays.
Discussion
Anopheles funestus was the dominant species collected in all three of our sampling efforts in 2010 and 2011, comprising between one-third and three-quarters of the female Anopheles collected (Table 1; Figure 3). Historically, An. funestus was common in our study region,10,11,31 and it remained abundant until the introduction of ITNs in the late 1990s (Figure 4). Following ITN distribution, An. funestus became rare and likely played a minor role in malaria transmission in the period thereafter as ITN coverage increased.12,32 The population of An. funestus in Asembo remained low through 2008 according to the LT sampling reported here (Figure 4), but our sampling in 2010 and 2011 indicates a marked reversal of this trend. Although An. funestus numbers have not reached the levels observed before the introduction of ITNs, the reemergence of this species has important implications for malaria transmission, which has increased recently despite widespread distribution of ITNs and LLINs.4–6
The low mortality rate of An. funestus collected from Asembo when exposed to the pyrethroids used in ITNs and LLINs (Figure 5) supports one possible mechanism by which the An. funestus population in the region has been able to increase, though a more extensive analysis is necessary to determine the scope of insecticide resistance in An. funestus populations within western Kenya. Other potential hypotheses to explain the reemergence of An. funestus in Asembo may include changes in bed net use within LLIN-owning households, socio-economic changes, or land use–land cover changes. However, the potential for insecticide resistance to reduce the effectiveness of ITNs is widely recognized,8 and the appearance of pyrethroid resistance in populations of An. funestus after IRS in South Africa19 underscores that potential for this malaria vector. A recent review by Coetzee and Koekemoer18 covers the extent to which insecticide resistance in An. funestus is currently understood. Varying degrees of resistance have been reported across many regions of Africa, notably including Tororo District in eastern Uganda near the Kenyan border.33 In addition to the reemergence of An. funestus in the 1990s after evolving pyrethroid resistance in South Africa,19 the development of insecticide resistance in other malaria vectors during the Global Malaria Eradication Program provides numerous examples of the detrimental effects of insecticide resistance on malaria control.34 Thus, the reemergence of An. funestus in this region of high ITN and LLIN coverage indicates the need to further investigate the resistance characteristics of this population.
Anopheles funestus and An. gambiae s.s. were found here to have taken their blood meals almost exclusively from humans, highlighting one factor contributing to their high vectorial capacity, a measure of the rate at which a vector population transmits malaria.35 Similar to An. gambiae s.s.,6 the presence of ITNs or LLINs appears not to shift An. funestus host selection away from humans. Anopheles arabiensis took a larger proportion of blood meals from cattle than did either An. gambiae s.s. or An. funestus, but An. arabiensis also took blood meals from humans, showing broader variation in feeding behaviors typical of An. arabiensis.36 Overall, the results from this blood meal analysis emphasize the need for additional vector control methods beyond the indoor, insecticide-based strategies that target only highly anthropophilic vectors preferring late night, indoor feeding.
The high rate of sporozoite ELISA-positive females collected during both PSC and HLC sampling implies a substantial infective human reservoir of malaria in Asembo, corresponding with recent measures of P. falciparum prevalence in the region5. We found the highest ELISA-positivity rates in An. gambiae s.s. females, implying the continued importance of this vector to malaria transmission in the region. Similar high ELISA-positivity rates were found in An. funestus, further indicating its reemergence as one of the primary malaria vectors in Asembo. The relatively lower rates of ELISA-positive An. arabiensis females during this study agree with findings from before the introduction of ITNs suggesting it has a lower vectorial capacity in the region than An. gambiae s.s. and An. funestus.11
One limitation of this study is the lack of a consistent sampling method over the years, and interpretations of changing vector abundances should be viewed with this caveat. We attempted to mitigate this problem by using calibrations of each collection method to HLC that were done in 1993 (BNT versus HLC) and 2009 (LT versus HLC). Calibrating the relative rate of other methods to HLC is limited in part by the population targeted by the sampling method (e.g., host-seeking versus blood-fed females), but the three sampling methods used in this study to compare Anopheles female abundances across time (BNT, LT, and HLC) all target host-seeking females at night. Calibration of other methods to HLC is also limited by spatial variation in mosquito populations' response to different sampling methods, even among districts in western Kenya,25 and possibly by temporal changes in mosquito behavior. However, all of our collections were done in the Asembo area of western Kenya, and we limited our comparisons to the months of the year for which we had data collected by all three collection methods. Despite these precautions, the calibrations are unlikely to have completely eliminated the potential differences among our sampling methods. However, the observed changes in An. funestus numbers between 2002–2008 and 2010–2011 were large. Although LT undersampled An. funestus relative to HLC,25 the different sensitivities of these collection methods are unlikely to account for the differences observed between these years. Thus, the data strongly suggest that An. funestus has reemerged over this time period.
The high proportion of An. funestus relative to An. arabiensis and An. gambiae in our indoor resting and human landing samples is an important indication of changes to malaria transmission in this region, and shows how our most efficient and widely implemented malaria control interventions may have important limitations. Anopheles funestus is well known as an efficient malaria vector because of near 100% anthropophily, which was confirmed in this study along with high sporozoite rates. The distribution of ITNs in Asembo in the 1990s significantly impacted An. funestus, and collections of An. funestus in the region had remained low until at least 2008. The increased abundance of An. funestus reported in this study suggests a reemergence of this species as a primary malaria vector in western Kenya, and might be explained by the development of insecticide resistance. Public health officials here and throughout regions where ITNs or IRS are deployed should be aware of changes in malaria vector populations, especially as our findings indicate reduced effectiveness of ITNs as a long-term, stand-alone method for malaria control. The GMEP of the 1950–60s revealed the potential dangers of relying on a single method of vector control, as malaria transmission resurged in many areas where significant gains had been made previously. Strategies to attenuate insecticide resistance development and complementary control methods for integrated vector management37 deserve a renewed focus as the global health community continues to fight malaria.
ACKNOWLEDGMENTS
We thank George Olang' and Maurice Ombok for logistical support; the field staff from the KEMRI/CDC field station in Kisian and the field assistants from Asembo for assisting with mosquito sampling; lab assistants from KEMRI/CDC and MSU for assisting with laboratory analyses; the residents of Asembo for their cooperation during mosquito collections; and MR4 for providing us with P. falciparum sporozoite ELISA reagents contributed by Robert Wirtz and the MR4 Vector Activity. This work is published with the permission of the director of the Kenya Medical Research Institute.
Disclaimer: The opinions expressed by the authors of this article do not necessarily reflect the opinions of the U.S. Centers for Disease Control and Prevention.
Footnotes
Financial support: This study was supported by a National Science Foundation Ecology of Infectious Diseases grant (grant no. EF-0723770) and NIAID Grant U01AI0508542 with additional support from the Rhodes Thompson Memorial Fellowship Fund.
Authors' addresses: Robert S. McCann, Department of Entomology, Michigan State University, East Lansing, MI, E-mail: mccannr3@msu.edu. Eric Ochomo and M. Nabie Bayoh, Kenya Medical Research Institute/Centers for Disease Control and Prevention Research and Public Health Collaboration, Kisumu, Kenya, E-mails: eochomo@kemricdc.org and nbayoh@kemricdc.org. John M. Vulule, Centre for Global Health Research, Kenya Medical Research Institute, Kisumu, Kenya, E-mail: jvulule@kemricdc.org. Mary J. Hamel, John E. Gimnig, and William A. Hawley, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: mhamel@cdc.gov, jgimnig@cdc.gov, and whawley@cdc.gov. Edward D. Walker, Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI, E-mail: walker@msu.edu.
References
- 1.World Health Organization . World Malaria Report 2012. Geneva: World Health Organization; 2012. pp. 60–62. [Google Scholar]
- 2.Feachem RG, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, Sabot O, Rodríguez MH, Abeyasinghe RR, Ghebreyesus TA, Snow RW. Shrinking the malaria map: progress and prospects. Lancet. 2010;376:1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nájera JA, González-Silva M, Alonso PL. Some lessons for the future from the Global Malaria Eradication Programme (1955–1969) PLoS Med. 2011;8:e1000412. doi: 10.1371/journal.pmed.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou G, Afrane YA, Vardo-Zalik AM, Atieli H, Zhong D, Wamae P, Himeidan YE, Minakawa N, Githeko AK, Yan G. Changing patterns of malaria epidemiology between 2002 and 2010 in western Kenya: the fall and rise of malaria. PLoS ONE. 2011;6:e20318. doi: 10.1371/journal.pone.0020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamel MJ, Adazu K, Obor D, Sewe M, Vulule JM, Williamson JM, Slutsker L, Feikin DR, Laserson KF. A reversal in reductions of child mortality in western Kenya, 2003–2009. Am J Trop Med Hyg. 2011;85:597–605. doi: 10.4269/ajtmh.2011.10-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawley WA, Phillips-Howard PA, ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, Nahlen BL, Gimnig JE, Kariuki SK, Kolczak MS, Hightower AW. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg. 2003;68((Suppl 4)):121–127. [PubMed] [Google Scholar]
- 8.Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Trape J-F, Tall A, Diagne N, Ndiath O, Ly AB, Faye J, Dieye-Ba F, Roucher C, Bouganali C, Badiane A, Sarr FD, Mazenot C, Touré-Baldé A, Raoult D, Druilhe P, Mercereau-Puijalon O, Rogier C, Sokhna C. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis. 2011;11:925–932. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 10.Beier JC, Perkins PV, Onyango FK, Gargan TP, Oster CN, Whitmire RE, Koech DK, Roberts CR. Characterization of malaria transmission by Anopheles (Diptera: Culicidae) in western Kenya in preparation for malaria vaccine trials. J Med Entomol. 1990;27:570–577. doi: 10.1093/jmedent/27.4.570. [DOI] [PubMed] [Google Scholar]
- 11.Taylor KA, Koros JK, Nduati J, Copeland RS, Collins FH, Brandling-Bennett AD. Plasmodium falciparum infection rates in Anopheles gambiae, An. arabiensis, and An. funestus in western Kenya. Am J Trop Med Hyg. 1990;43:124–129. doi: 10.4269/ajtmh.1990.43.124. [DOI] [PubMed] [Google Scholar]
- 12.Gimnig JE, Vulule JM, Lo TQ, Kamau L, Kolczak MS, Phillips-Howard PA, Mathenge EM, ter Kuile FO, Nahlen BL, Hightower AW, Hawley WA. Impact of permethrin-treated bed nets on entomologic indices in an area of intense year-round malaria transmission. Am J Trop Med Hyg. 2003;68((Suppl 4)):16–22. [PubMed] [Google Scholar]
- 13.Lindblade KA, Eisele TP, Gimnig JE, Alaii JA, Odhiambo F, ter Kuile FO, Hawley WA, Wannemuehler KA, Phillips-Howard PA, Rosen DH, Nahlen BL, Terlouw DJ, Adazu K, Vulule JM, Slutsker L. Sustainability of reductions in malaria transmission and infant mortality in western Kenya with use of insecticide-treated bed nets: 4 to 6 years of follow-up. JAMA. 2004;291:2571–2580. doi: 10.1001/jama.291.21.2571. [DOI] [PubMed] [Google Scholar]
- 14.Vulule JM, Beach RF, Atieli FK, Roberts JM, Mount DL, Mwangi RW. Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Med Vet Entomol. 1994;8:71–75. doi: 10.1111/j.1365-2915.1994.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 15.Mathias DK, Ochomo E, Atieli FK, Ombok M, Bayoh MN, Olang G, Muhia D, Kamau L, Vulule JM, Hamel MJ, Hawley WA, Walker ED, Gimnig JE. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in western Kenya. Malar J. 2011;10:10. doi: 10.1186/1475-2875-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochomo E, Bayoh MN, Brogdon WG, Gimnig JE, Ouma C, Vulule JM, Walker ED. Pyrethroid resistance in Anopheles gambiae s.s. and Anopheles arabiensis in western Kenya: phenotypic, metabolic and target site characterizations of three populations. Med Vet Entomol. 2013;27:156–164. doi: 10.1111/j.1365-2915.2012.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coetzee M, Hunt RH, Wilkerson RC, della Torre A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–274. [PubMed] [Google Scholar]
- 18.Coetzee M, Koekemoer LL. Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. Annu Rev Entomol. 2013;58:393–412. doi: 10.1146/annurev-ento-120811-153628. [DOI] [PubMed] [Google Scholar]
- 19.Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 20.Phillips-Howard PA, Nahlen BL, Alaii JA, ter Kuile FO, Gimnig JE, Terlouw DJ, Kachur SP, Hightower AW, Lal AA, Schoute E, Oloo AJ, Hawley WA. The efficacy of permethrin-treated bed nets on child mortality and morbidity in western Kenya I: development of infrastructure and description of study site. Am J Trop Med Hyg. 2003;68((Suppl 4)):3–9. [PubMed] [Google Scholar]
- 21.Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Ombok M, Williamson J, Marwanga D, Abong'o D, Desai M, Kariuki S, Hamel MJ, Lobo NF, Vulule JM, Bayoh MN. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am J Trop Med Hyg. 2013;88:301–308. doi: 10.4269/ajtmh.2012.12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odiere M, Bayoh MN, Gimnig JE, Vulule JM, Irungu L, Walker ED. Sampling outdoor, resting Anopheles gambiae and other mosquitoes (Diptera: Culicidae) in western Kenya with clay pots. J Med Entomol. 2007;44:14–22. doi: 10.1603/0022-2585(2007)44[14:soraga]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noor AM, Amin AA, Akhwale WS, Snow RW. Increasing coverage and decreasing inequity in insecticide-treated bed net use among rural Kenyan children. PLoS Med. 2007;4:e255. doi: 10.1371/journal.pmed.0040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Division of Malaria Control . National Malaria Strategy 2009–2017. Nairobi, Kenya: Ministry of Public Health and Sanitation; 2009. p. 25. [Google Scholar]
- 25.Wong J, Bayoh MN, Olang G, Killeen GF, Hamel MJ, Vulule JM, Gimnig JE. Standardizing operational vector sampling techniques for measuring malaria transmission intensity: evaluation of six mosquito collection methods in western Kenya. Malar J. 2013;12:143. doi: 10.1186/1475-2875-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillies MT, Coetzee M. A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region) Johannesburg, South Africa: South African Institute for Medical Research; 1987. pp. 1–143. [Google Scholar]
- 27.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 28.Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, Esser KM, Beaudoin RL, Andre RG. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39. [PMC free article] [PubMed] [Google Scholar]
- 29.Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Walker ED. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile Virus to humans. J Med Entomol. 2008;45:125–128. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization . Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-Efficacy and Persistence of Insecticides on Treated Surfaces. Report of the WHO Informal Consultation. Geneva: World Health Organization; 1998. pp. 1–46. [Google Scholar]
- 31.Evans AM, Symes CB. Anopheles funestus and its allies in Kenya. Ann Trop Med Parasitol. 1937;31:105–112. [Google Scholar]
- 32.Lindblade KA, Gimnig JE, Kamau L, Hawley WA, Odhiambo F, Olang G, ter Kuile FO, Vulule JM, Slutsker L. Impact of sustained use of insecticide-treated bednets on malaria vector species distribution and culicine mosquitoes. J Med Entomol. 2006;43:428–432. doi: 10.1603/0022-2585(2006)043[0428:iosuoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Morgan JC, Irving H, Okedi LM, Steven A, Wondji CS. Pyrethroid resistance in an Anopheles funestus population from Uganda. PLoS ONE. 2010;5:e11872. doi: 10.1371/journal.pone.0011872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busvine JR, Pal R. The impact of insecticide-resistance on control of vectors and vector-borne diseases. Bull World Health Organ. 1969;40:731–744. [PMC free article] [PubMed] [Google Scholar]
- 35.Garrett-Jones C. The human blood index of malaria vectors in relation to epidemiological assessment. Bull World Health Organ. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- 36.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–453. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization . Global Strategic Framework for Integrated Vector Management. Geneva: World Health Organization; 2004. pp. 1–12. [Google Scholar]