Abstract
Severe malaria is frequently managed without access to laboratory testing. We report on the performance of point-of-care tests used to guide the management of a cohort of 179 children with severe malaria in a resource-limited Ugandan hospital. Correlation coefficients between paired measurements for glucose (i-STAT and One Touch Ultra), lactate (i-STAT and Lactate Scout), and hemoglobin (Hb; laboratory and i-STAT) were 0.86, 0.85, and 0.73, respectively. The OneTouch Ultra glucometer readings deviated systematically from the i-STAT values by +1.7 mmol/L. Lactate Scout values were systematically higher than i-STAT by +0.86 mmol/L. Lactate measurements from either device predicted subsequent mortality. Hb estimation by the i-STAT instrument was unbiased, with upper and lower limits of agreement of −34 and +34 g/L, and it was 91% sensitive and 89% specific for the diagnosis of severe anemia (Hb < 50 g/L). New commercially available bedside diagnostic tools, although imperfect, may expedite clinical decision-making in the management of critically ill children in resource-constrained settings.
Severe malaria claims an estimated 1.24 million lives annually, mostly among children in sub-Saharan Africa.1 Patients presenting with signs of severe malaria have a mortality of approximately 20%.2 Case management of these critically ill children requires intensive medical and nursing care, life support technologies, and laboratory support to guide clinical decision-making. However, because of resource and technological limitations in many hospitals in sub-Saharan Africa, patients are often managed with minimal laboratory guidance. Point-of-care instruments have recently become commercially available for the measurement of a number of analytes, including glucose, lactate, and hemoglobin (Hb). Markets for these products exist because of their use in the management of common diseases or other applications in industrialized countries (e.g., diabetes, exercise physiology, transport medicine, and anesthesia). The test performance characteristics in these contexts have been well-described.3–13 Hypoglycemia and anemia are common complications of malaria that require laboratory diagnosis, because clinical assessment is an unsatisfactory substitute. Furthermore, lactate level at admission is an important prognostic indicator in severe malaria.14 We used three commercially available instruments (OneTouch Ultra Mini glucometer, Lactate Scout, and i-STAT) in a resource-poor hospital in sub-Saharan Africa to guide case management of critically ill children with severe malaria. Here, we report on the agreement and diagnostic accuracy of these modalities and comment on their potential use in field settings.
Children ages 1–10 years presenting to the Regional Referral Hospital in Jinja, Uganda with severe malaria between July of 2011 and June of 2013 were enrolled in this study. These patients were prospectively identified for enrollment in a randomized controlled trial of a novel adjunctive treatment of severe malaria, which has been described in detail elsewhere.15,16 Diagnosis of malaria was confirmed by (1) a three-band malaria rapid diagnostic test (RDT), which required positivity for two distinct parasite antigens—Plasmodium lactate dehydragenase and histidine-rich protein 2 (First Response Malaria Ag. [pLDH/HRP2] Combo Rapid Diagnostic Test; Premier Medical Corporation, Nani Daman, India)—and (2) subsequent confirmation by microscopy or polymerase chain reaction (PCR) in cases where RDT was positive but microscopy was negative (10 cases). After stabilization in the emergency room, participants underwent femoral venipuncture. Venous whole blood was immediately tested using the OneTouch Ultra glucometer (LifeScan, Milpitas, CA), the Lactate Scout (EKF Diagnostics, Penarth, Cardiff, United Kingdom), and the i-STAT handheld biochemistry analyzer with CHEM8+ and CG4+ cartridges (Abbott Point of Care, Mississauga, ON, Canada) according to manufacturers' instructions. The CHEM8+ cartridge measures sodium, potassium, chloride, total carbon dioxide, ionized calcium, glucose, blood urea nitrogen, creatinine, lactate, and hematocrit; Hb concentration is automatically calculated from the hematocrit using the formula Hb (g/dL) = hematocrit (% packed cell volume [PCV]) × 0.34. The CG4+ cartridge measures lactate, pH, partial pressure of carbon dioxide (PCO2), and partial pressure of oxygen (PO2). Both cartridges require a sample volume of 95 μL.
In addition, a 0.5-mL sample of blood in (ethylenedinitrilo)tetraacetic acid was sent within 24 hours to a College of American Pathologists-certified research laboratory (the Makerere University–John's Hopkins University Core Laboratory in Kampala, Uganda), where automated complete blood count (CBC) with differential was performed using the Beckman Coulter AcT 5 Diff hematology analyzer (Beckman Coulter, Inc., Fullerton, CA). All batches of test strips and cartridges (glucose, lactate, CHEM8+, and CG4+) were routinely tested for accuracy using control solutions and were within manufacturer's specified limits of error. Instruments were compared by examining the correlation as well as Bland–Altman plots for agreement between tests. Parents or guardians of study participants provided written informed consent, and ethical oversight was provided by the Makerere University School of Medicine Research Ethics Committee and the University of Toronto Research Ethics Committee.
In total, 179 children were included. Demographic characteristics, clinical presentation (including physical examination for deep breathing and pallor), treatment, and outcome of the cohort are summarized in Table 1. Paired glucose measurements (i-STAT and OneTouch Mini) were available for 125 patients, lactate measurements (i-STAT and LactateScout) were available for 142 patients, and Hb measurements (quality-controlled laboratory CBC and i-STAT) were available for 108 patients. Reasons for missing values included lack of cartridges or test strips at the time of patient admission, repeated test failure because of elevated ambient temperature, problems with sample loading in the cartridge, coagulation of the whole-blood sample, and processing errors at the central laboratory (in the case of quality-controlled Hb measurement). The prevalence of hypoglycemia (glucose < 3 mmol/L17 based on the average of point-of-care instruments), hyperlactatemia (lactate > 5 mmol/L based on the average of point-of-care instruments), and severe anemia (Hb < 50 g/L based on reference CBC) were 4/125 (3.2%), 37/142 (26%), and 23/108 (21%), respectively.
Table 1.
Characteristics of study participants
| Characteristics of cohort | n | Percent |
|---|---|---|
| Demographics | ||
| Age (years), median (IQR) | 2.0 (1.0–3.0) | |
| Female sex | 78 | 44 |
| Physical signs at admission | ||
| Coma (BCS < 3) | 106 | 59 |
| Seizures | 144 | 80 |
| Pallor | 154 | 86 |
| Deep breathing | 86 | 48 |
| Treatment | ||
| Artesunate | 179 | 100 |
| Intravenous dextrose infusion | 63 | 35 |
| Red blood cell transfusion | 147 | 82 |
| Outcome | ||
| In-hospital mortality | 15 | 8.4 |
BCS = Blantyre Coma Scale23; IQR = interquartile range.
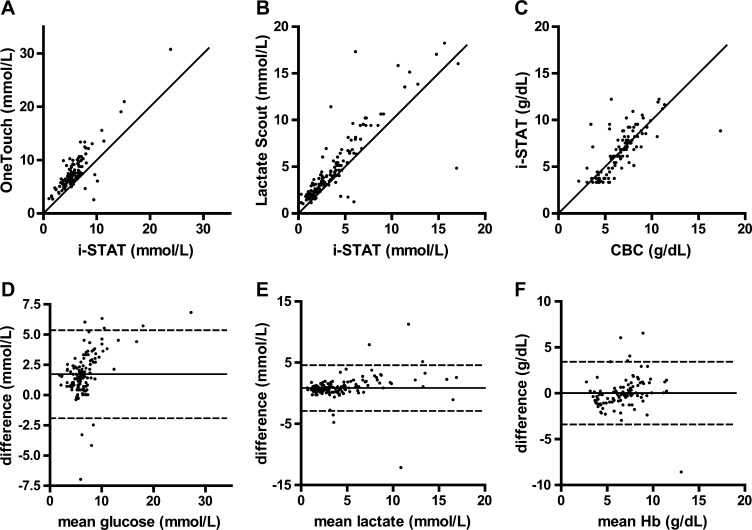
Pearson's correlation coefficients (95% confidence interval [95% CI]) between the paired measurements for glucose, lactate, and Hb were 0.86, 0.85, and 0.73, respectively (Figure 1A–C). Bland–Altman plots for agreement between the measurement methods are shown in Figure 1D–F.
Figure 1.
Correlation and Bland–Altman plots for agreement between point-of-care diagnostic tests among 179 children with severe malaria. Correlation between paired measurements of (A) glucose (i-STAT and One Touch Ultra), (B) lactate (i-STAT and Lactate Scout), and (C) Hb (laboratory CBC and i-STAT). All correlation plots are shown with the line of identity. Bland–Altman plots for (D) glucose, (E) lactate, and (F) Hb show the mean difference (solid line) as well as upper and lower limits of agreement (dashed lines).
Glucose values obtained using the One Touch Ultra glucometer were higher than the i-STAT values by +1.7 mmol/L (95% CI = +1.4 to +2.1, P < 0.001) on average. There was evidence of increasing deviation of the measurements at higher glucose concentrations (Figure 1A and D) (P = 0.029). Test variability was large, with upper and lower limits of agreement of −1.9 and +5.4 mmol/L, respectively (Figure 1D). Nine patients (5%) had hypoglycemia by one or both glucose measurements. In seven cases, the result was discordant between test methods, with six cases identified only by the i-STAT device and one case identified only by the OneTouch instrument. The OneTouch device may be a less sensitive method for detecting hypoglycemia, which may lead to underrecognition of clinically significant hypoglycemia, which was previously documented with other point-of-care glucose sensors.18 Upward adjustment of a treatment threshold (e.g., to ∼5 mmol/L) based on the instrument bias might be considered and would have captured all cases of hypoglycemia in our series. Previous studies comparing the i-STAT glucose12 and the One Touch Ultra instruments3,6,8 to reference standards have generally shown a high level of accuracy and tighter precision than suggested by our findings, perhaps related to greater variability under field conditions of either or both instruments.
Correlation and Bland–Altman plots for lactate measurement by i-STAT and Lactate Scout are shown in Figure 1B and E. Values obtained from the Lactate Scout were higher than i-STAT values by +0.86 mmol/L (95% CI = +0.54 to +1.2, P < 0.001) (Figure 1E). Increasing deviation between measurements was observed at higher lactate levels (Figure 1B and E) (P = 0.0018). Upper and lower limits of agreement were −2.9 and +4.6 mmol/L, respectively (Figure 1E). Higher lactate levels from both i-STAT (P < 0.001) and Lactate Scout (P < 0.001) were significantly associated with the clinical sign of deep breathing, supporting the convergent validity of the instruments. Lactate values from both instruments, taken at admission, predicted subsequent in-hospital mortality (area under receiver operator characteristic curve is 0.74 [95% CI = 0.59 to 0.89, P = 0.003] and 0.71 [95% CI = 0.57 to 0.85, P = 0.008] for i-STAT and Lactate Scout, respectively), suggesting that lactate measurement by either instrument has prognostic use. The prognostic value of lactate measurement has been established in previous studies,14,19 and early goal-directed therapy targeting rapid correction of hyperlactatemia has been shown to reduce mortality in patients with sepsis.20 Additional studies may be warranted to evaluate the impact of point-of-care lactate testing and goal-directed therapy in children with severe malaria.
The i-STAT Hb measurement provided an unbiased estimate of the quality-assured laboratory CBC value (Figure 1F) (mean difference = −0.18 g/L, 95% CI = −3.1 to 3.5, P = 0.92). The upper and lower limits of agreement were +34 and −34 g/L, respectively, indicating substantial variability in the measurement (Figure 1F). The sensitivity and specificity for the diagnosis of severe anemia (Hb < 50 g/L) were 91% (95% CI = 77% to 97%) and 89% (95% CI = 79% to 92%), respectively. In contrast, clinical assessment of pallor had a sensitivity of 96% (95% CI = 76% to 99%, P = 1.0) but a specificity of only 14% (95% CI =8.3% to 23%, P < 0.001) for predicting laboratory-defined severe anemia. Treatment of anemia based on clinical judgment would, therefore, result in a large number of unnecessary transfusions compared with a protocol guided by point-of-care testing. Previous studies in the context of cardiopulmonary bypass surgery have found systematic errors caused by alterations in plasma proteins and electrolytes from the conductivity-based i-STAT measurement compared with reference methods12,21; however, our patients were managed with cautious administration of fluids and electrolytes,22 such that no significant bias was observed. Variability reported in previous studies (±20 g/L) seems to be lower than in our study, perhaps related to the tightly regulated artificial conditions used for testing.13
Our study is limited by the lack of a gold standard comparator for glucose and lactate. Comparison between different point-of-care tests provided an assessment of interassay agreement, but accuracy cannot be formally evaluated. In some cases, sample size was small, which limits the strength of the conclusions (e.g., only nine cases of hypoglycemia were observed in this cohort). Multiple sources of variability, including test strip/cartridge transportation and storage conditions, practitioner technique, and testing environment, may have contributed to error in this study. However, these real life factors may provide more generalizable and realistic estimates of test variability for clinicians in the busy, uncontrolled work environments typical of resource-constrained hospitals in Africa.
In summary, we have provided a quantitative appraisal of the performance and limitations of commercially available tests for glucose, lactate, and Hb under field conditions. This information may assist clinicians in resource-limited settings to interpret the measurements provided by selected commercially available instruments. Clinicians should recognize substantial imprecision in the estimates provided by these tests under field conditions as well as possible systematic bias in the measurement of glucose and lactate. Nonetheless, specific aspects of severe malaria case management might be improved with point-of-care tests, including rational goal-directed therapy guided by lactate values and avoidance of unnecessary transfusions using point-of-care Hb measurement. Implementation of point-of-care devices in resource-limited hospitals also requires careful consideration of costs of equipment and maintenance, costs of consumables (cartridges and test strips), and continuing training and quality control measures. Although imperfect, point-of-care diagnostics may improve the case management and outcome of severe malaria in resource-limited settings by providing objective and quantitative estimates of life-threatening but correctable hematologic and metabolic abnormalities.
Footnotes
Financial support: This work was supported by a kind donation from Kim Kertland, the Tesari Foundation, the Sandra Rotman Centre for Global Health, a Post-Doctoral Research Award (to M.H. and A.L.C.), Canadian Institutes of Health Research Grants MOP-244701 (to K.C.K.) and MOP-13721 (to K.C.K.), and a Canada Research Chair (K.C.K.).
Authors' addresses: Michael Hawkes, Division of Infectious Diseases, Department of Pediatrics, University of Alberta, Edmonton, AB, Canada, E-mail: mthawkes@ualberta.ca. Andrea L. Conroy and Kevin C. Kain, Department of Medicine and Institute of Medical Sciences, University of Toronto, Toronto, ON, Canada, E-mails: andrea.conroy@gmail.com and kevin.kain@uhn.ca. Robert O. Opoka, Department of Paediatrics and Child Health, Mulago Hospital and Makerere University, Kampala, Uganda, E-mail: opokabob@yahoo.com. Sophie Namasopo, Department of Paediatrics, Jinja Regional Referral Hospital, Jinja, Uganda, E-mail: snamasopo@yahoo.com. W. Conrad Liles, Department of Medicine, University of Washington, Seattle, WA, E-mail: wcliles@medicine.washington.edu. Chandy C. John, Division of Global Pediatrics, Department of Pediatrics, University of Minnesota, Minneapolis, MN, E-mail: ccj@umn.edu.
References
- 1.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Kyu HH, Fernandez E. Artemisinin derivatives versus quinine for cerebral malaria in African children: a systematic review. Bull World Health Organ. 2009;87:896–904. doi: 10.2471/BLT.08.060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetes Research in Children Network (Direcnet) Study Group A multicenter study of the accuracy of the One Touch Ultra home glucose meter in children with type 1 diabetes. Diabetes Technol Ther. 2003;5:933–941. doi: 10.1089/152091503322640971. [DOI] [PubMed] [Google Scholar]
- 4.Arabadjief D, Nichols JH. Assessing glucose meter accuracy. Curr Med Res Opin. 2006;22:2167–2174. doi: 10.1185/030079906X148274. [DOI] [PubMed] [Google Scholar]
- 5.Chan JC, Wong RY, Cheung CK, Lam P, Chow CC, Yeung VT, Kan EC, Loo KM, Mong MY, Cockram CS. Accuracy, precision and user-acceptability of self blood glucose monitoring machines. Diabetes Res Clin Pract. 1997;36:91–104. doi: 10.1016/s0168-8227(97)00036-3. [DOI] [PubMed] [Google Scholar]
- 6.Demers J, Kane MP, Bakst G, Busch RS, Hamilton RA. Accuracy of home blood glucose monitors using forearm blood samples: FreeStyle versus One Touch Ultra. Am J Health Syst Pharm. 2003;60:1130–1135. doi: 10.1093/ajhp/60.11.1130. [DOI] [PubMed] [Google Scholar]
- 7.Kilpatrick ES, McLeod MJ, Rumley AG, Small M. A ward comparison between the One Touch II and Glucometer II blood glucose meters. Diabet Med. 1994;11:214–217. doi: 10.1111/j.1464-5491.1994.tb02023.x. [DOI] [PubMed] [Google Scholar]
- 8.Rivers SM, Kane MP, Bakst G, Busch RS, Hamilton RA. Precision and accuracy of two blood glucose meters: FreeStyle Flash versus One Touch Ultra. Am J Health Syst Pharm. 2006;63:1411–1416. doi: 10.2146/ajhp050473. [DOI] [PubMed] [Google Scholar]
- 9.Gaieski DF, Drumheller BC, Goyal M, Fuchs BD, Shofer FS, Zogby K. Accuracy of Handheld Point-of-Care Fingertip Lactate Measurement in the Emergency Department. West J Emerg Med. 2013;14:58–62. doi: 10.5811/westjem.2011.5.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopfer SM, Nadeau FL, Sundra M, Makowski GS. Effect of protein on hemoglobin and hematocrit assays with a conductivity-based point-of-care testing device: comparison with optical methods. Ann Clin Lab Sci. 2004;34:75–82. [PubMed] [Google Scholar]
- 11.Moore CC, Jacob ST, Pinkerton R, Meya DB, Mayanja-Kizza H, Reynolds SJ, Scheld WM. Point-of-care lactate testing predicts mortality of severe sepsis in a predominantly HIV type 1-infected patient population in Uganda. Clin Infect Dis. 2008;46:215–222. doi: 10.1086/524665. [DOI] [PubMed] [Google Scholar]
- 12.Steinfelder-Visscher J, Weerwind PW, Teerenstra S, Brouwer MH. Reliability of point-of-care hematocrit, blood gas, electrolyte, lactate and glucose measurement during cardiopulmonary bypass. Perfusion. 2006;21:33–37. doi: 10.1191/0267659106pf846oa. [DOI] [PubMed] [Google Scholar]
- 13.Wu P, Morey TE, Harris NS, Gravenstein N, Rice MJ. Intravenous fluids cause systemic bias in a conductivity-based point-of-care hematocrit meter. Anesth Analg. 2013;114:314–321. doi: 10.1213/ANE.0b013e31823fecbd. [DOI] [PubMed] [Google Scholar]
- 14.Newton CR, Valim C, Krishna S, Wypij D, Olola C, Agbenyega T, Taylor TE. The prognostic value of measures of acid/base balance in pediatric falciparum malaria, compared with other clinical and laboratory parameters. Clin Infect Dis. 2005;41:948–957. doi: 10.1086/432941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkes M, Opoka RO, Namasopo S, Miller C, Conroy AL, Serghides L, Kim H, Thampi N, Liles WC, John CC, Kain KC. Nitric oxide for the adjunctive treatment of severe malaria: hypothesis and rationale. Med Hypotheses. 2011;77:437–444. doi: 10.1016/j.mehy.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkes M, Opoka RO, Namasopo S, Miller C, Thorpe KE, Lavery JV, Conroy AL, Liles WC, John CC, Kain KC. Inhaled nitric oxide for the adjunctive therapy of severe malaria: protocol for a randomized controlled trial. Trials. 2011;12:176. doi: 10.1186/1745-6215-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N, Maitland K, Kivaya E, Agbenyega T, Nguah SB, Evans J, Gesase S, Kahabuka C, Mtove G, Nadjm B, Deen J, Mwanga-Amumpaire J, Nansumba M, Karema C, Umulisa N, Uwimana A, Mokuolu OA, Adedoyin OT, Johnson WB, Tshefu AK, Onyamboko MA, Sakulthaew T, Ngum WP, Silamut K, Stepniewska K, Woodrow CJ, Bethell D, Wills B, Oneko M, Peto TE, von Seidlein L, Day NP, White NJ. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–1657. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebel A, Rice MA, Fahy BG. Accuracy of point-of-care glucose measurements. J Diabetes Sci Technol. 2012;6:396–411. doi: 10.1177/193229681200600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 20.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 21.Steinfelder-Visscher J, Weerwind PW, Teerenstra S, Pop GA, Brouwer RM. Conductivity-based hematocrit measurement during cardiopulmonary bypass. J Clin Monit Comput. 2007;21:7–12. doi: 10.1007/s10877-006-9052-x. [DOI] [PubMed] [Google Scholar]
- 22.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, Evans JA, Tibenderana JK, Crawley J, Russell EC, Levin M, Babiker AG, Gibb DM. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 23.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]