Abstract
Pentoxifylline is a tumor necrosis factor-α (TNF-α) inhibitor that also attenuates the immune response and decreases tissue inflammation. The association of pentoxifylline with antimony improves the cure rate of mucosal and cutaneous leishmaniasis. In this randomized and double blind pilot trial, cure rate was higher, although not significant, in patients who received antimony plus pentoxifylline than in those patients receiving antimony plus placebo. A significant decrease in TNF-α and interferon-γ (IFN-γ) levels during therapy was more pronounced in the antimony plus pentoxifylline group, whereas CCL-3 (Chemokine [C-C motif] ligand 3) decreased similarly in both groups. The increased levels of CXCL-9 (Chemokine [C-X-C motif] ligand 9) during therapy were lower in the antimony plus pentoxifylline group. Therapy with pentoxifylline modifies cytokines and chemokines production, which may be associated with therapeutic outcome.
Introduction
Tegumentary leishmaniasis is a major public health problem in developing countries,1 and cutaneous leishmaniasis (CL) is the most common form of the disease. CL caused by Leishmania braziliensis is characterized by skin ulcerations that take months to heal, with increasing failure rates to monotherapy with pentavalent antimony (Sbv).2,3 The pathology in CL is associated with an exacerbated inflammatory response with high concentrations of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) found in supernatants of peripheral blood mononuclear cells (PBMCs) and tissue.4,5 The participation of TNF-α in the pathogenesis of inflammatory and autoimmune diseases6 as well as CL and mucosal leishmaniasis (ML) has been documented.4,5 In CL, there is an association between production of this cytokine and development of cutaneous lesions7 and an association between the frequency of cells expressing TNF-α and ulcer size.4 Although Sbv has been the drug of choice for treatment of CL for decades, therapeutic failure has been observed in up to 40% of the patients with disease caused by L. braziliensis.3,8 Pentoxifylline is a metilxantine used to treat vascular and chronic inflammatory diseases.9,10 Pentoxifylline inhibits TNF-α production,11 and the association of pentoxifylline plus antimony decreases the healing time of CL and ML,12,13 is more effective than antimony alone,10,11 and cures ML and CL patients refractory to Sbv therapy.14
This randomized, placebo-controlled pilot clinical trial was performed in the Health Post of Corte de Pedra, Bahia, Brazil, an endemic region of L. braziliensis transmission. The trial was conceived with a small sample size as a preliminary study to obtain data about changes in immunological parameters associated with the use of pentoxifylline to better understand how pentoxifylline can contribute to the healing of CL ulcers.
Methods
Thirty-six CL patients were included who presented one to three cutaneous ulcers, had a duration of illness between 1 and 3 months, and had documentation of L. braziliensis infection by parasite isolation or real-time polymerase chain reaction (PCR). Patients were assigned to receive Sbv plus pentoxifylline (study group) or Sbv plus placebo (control group) by a randomization table obtained at www.randomization.com. Sbv (Glucantime, Sanofi, São Paulo, SP, Brasil) was given at a dose of 20 mg/kg per day associated with oral pentoxifylline (400 mg) or placebo three times per day for 20 days. The Sbv dosage used is the standard therapy for the aggressive disease caused by L. braziliensis in this endemic area.15 Immunologic studies were performed on days 0 and 15 (during treatment). PBMCs were obtained by heparinized venous blood. Cells were adjusted to the concentration of 107/mL, and aliquots of 106 cells were incubated in plates with or without the addition of soluble leishmania antigen (SLA) at a concentration of 5 μg/mL. After 72 hours, supernatants were collected, and TNF-α, IFN-γ, interleukin-10 (IL-10), CCL3, CXCL9, and CXCL10 were measured by enzyme-linked immunosorbent assay (ELISA) using reagents from BD OptEIA (San Diego, CA) and R&D Systems (Minneapolis, MN). Results are expressed as picograms per milliliter. SLA was prepared from a strain isolated from an ML patient as previously described.16
Clinical outcomes.
Cure or failure was determined on day 90. Cure was defined by complete healing of the lesions with reepithelization of the skin. Failure was defined as persistence of ulceration or infiltrated borders.
Statistical analysis.
The comparison between the immunological responses of the two groups was performed by Mann–Whitney test, and Fisher's exact test was used to analyze a contingency table. The significance level was defined as P < 0.05.
Results
The demographic and clinical features of 33 patients who participated in the study are shown in Table 1. Three patients were excluded because of loss to follow-up or absence for the second immunological evaluation. There was no difference between the two groups regarding age, sex, or number and size of the lesions. The healing time was greater in the group that received Sbv plus placebo, but it was not significant (data not shown). Although 56% of patients were cured in the Sbv plus pentoxifylline group, 47% of the patients were cured in the Sbv plus placebo group. Mild and transitory side effects, like arthralgia (four patients), headache (two patients), fever (two patients), and lack of appetite (two patients), predominated in the Sbv plus pentoxifylline group (five patients) but also occurred in the Sbv plus placebo group. Nausea, vomiting, or diarrhea was not found in either group. The concentrations of TNF-α, IFN-γ, and chemokines before and during therapy in the two groups of patients are shown in Figure 1. In the Sbv plus pentoxifylline group, the median of TNF-α before therapy (Figure 1A) was 909 pg/mL, and it ranged from 384 to 1,617 pg/mL; after 15 days of therapy, it was 144 pg/mL, and it ranged from 46 to 889 pg/mL (P < 0.0001). In the Sbv plus placebo group, the median of TNF-α before therapy was 640 pg/mL, and it ranged from 402 to 1,604 pg/mL; it dropped to 333 pg/mL, ranging from 160 to 1,452 pg/mL (P = 0.04). The percentage of suppression of TNF-α production in the Sbv plus placebo group was 48%, whereas in patients who received Sbv plus pentoxifylline, the suppression was of the order of 84% (P < 0.0001). IFN-γ levels also dropped significantly in the antimony plus pentoxifylline group (from 1,703 to 605 pg/mL) compared with the antimony plus placebo group (from 1,882 to 1,275 pg/mL) (Figure 1B). IL-10 production was low in both groups, and no difference was found before and during treatment (Figure 1C). The levels of CCL3 in the two groups are shown in Figure 1D. A significant decrease in CCL3 levels during therapy was observed in both groups. Different from the observed levels of TNF-α, IFN-γ, and CCL3, the levels of CXCL9 and CXCL10 increased at 15 days of therapy. The median of CXCL9 levels before therapy in the Sbv plus pentoxifylline group was 187 pg/mL, and it ranged from 102 to 589 pg/mL (Figure 1E); after 15 days of therapy, it was 343 pg/mL, and it ranged from 164 to 777 pg/mL (P = 0.007). In the Sbv plus placebo group, it was 180 pg/mL, and it ranged from 65 to 473 pg/mL; after 15 days, it was 783 pg/mL, and it ranged from 271 to 2,683 pg/mL (P < 0.0001). Regarding CXCL10 (Figure 1F), the medians before and after 15 days of therapy in the Sbv plus pentoxifylline group were 272 and 435 pg/mL (P = 0.0005), respectively. In the Sbv plus placebo group, CXCL10 increased from 153 to 453 pg/mL (P = 0.0005).
Table 1.
Demographic, clinical and therapeutic outcome data from cutaneous leishmanisis patients
| Variables | Sbv plus placebo (N = 15) | Sbv plus pentoxifylline (N = 18) | P value |
|---|---|---|---|
| Sex (% of male) | 71 | 64 | 0.10 |
| Age (M ± SD) | 34 ± 10 | 29 ± 5 | 0.64 |
| No. of lesions (M ± SD) | 1 ± 0 | 1.7 ± 0.5 | 0.11 |
| Size of lesions | 25 × 22 | 25 × 19 | 0.12 |
| Cure rate at 90 days | 7 (47%) | 10 (56%) | 0.73* |
Fisher's exact test.
M = media; SD = standard deviation.
Figure 1.
TNF-α and chemokine levels of patients with CL before and after therapy with antimony associated with pentoxifylline or antimony plus placebo. (A) TNF-α, (B) IFN-γ, (C) IL-10, (D) CCL3, (E) CXCL9, and (F) CXCL10 levels were measured by ELISA in supernatants of PBMCs stimulated with SLA on days 0 and 15 of treatment with antimony plus placebo or antimony plus pentoxifylline.
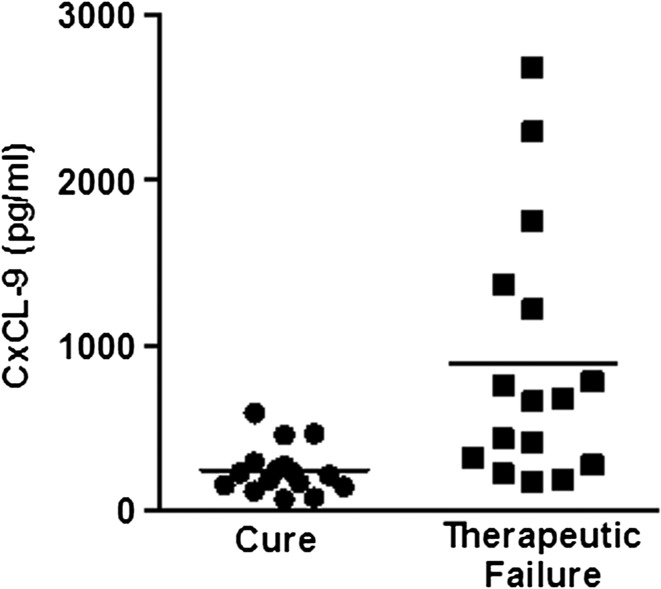
To determine if there were any associations between cytokine and chemokine levels before and after 15 days of therapy with the clinical outcomes, we compared the measurements of these cytokines and chemokines in patients who were not cured with patients who were cured (independent of the type of therapy). As shown in Figure 2, the level of CXCL9 at day 15 of therapy in patients with therapeutic failure was 669 pg/mL (range = 168–2,683 pg/mL) higher, but it was not significant in those patients who were cured in 90 days (331 pg/mL; range 164–826 pg/mL).
Figure 2.
CXCL9 levels at 15 days of therapy in CL patients who were cured or failed to antimony associated or not associated with pentoxifylline.
Discussion
This study extends previous evidence of the participation of the immunological response in the pathogenesis of CL and ML and the ability of pentoxifylline in association with antimony to improve the therapeutic response of CL patients. Herein, we show that treatment with Sbv plus pentoxifylline was associated with a more significant reduction in the TNF-α and IFN-γ levels compared with placebo, whereas a decrease in CCL3 levels and an increase in CXCL10 levels occurred similarly in both groups. Interestingly, the raised production of CXCL9 during treatment was more pronounced in the antimony plus placebo group. CXCL9 is a T-cell chemoattractant, which is induced by IFN-γ. It is closely related to CXCL10, and CXCL10 is secreted by several cell types in response to IFN-γ. CXCL10 has been attributed to several roles, such as chemoattraction for monocytes/macrophages, T cells, natural killer (NK) cells, and dendritic cells, promotion of T-cell adhesion to endothelial cells, antitumor activity, and inhibition of bone marrow colony formation and angiogenesis.16,17 Localized CL (LCL) is associated with a T helper 1 (Th1) response and characterized by opposing dermal chemokine profiles, and LCL lesions show high expression of CXCL9 and CXCL10 and only low amounts of CCL3.18 Our data indicate that pentoxifylline down-modulates preferentially not only TNF-α but also, IFN-γ and CXCL9 production. Although our major aim was to better understand how pentoxifylline acts on the immunologic response of CL patients, our data also suggest that pentoxifylline associated with Sbv is more effective than antimony alone in the treatment of CL. Regarding the changes in the immunologic response, one unexpected finding was the observation that, although TNF-α, IFN-γ, and CCL3 levels had fallen during therapy, CXCL9 and CXCL10 increased during therapy. There are two possible explanations for increasing production of these chemokines during therapy. (1) The in situ release of leishmania antigen may stimulate the production of CXCL9 and CXCL10 by clustered monocytic cells in the lesions. (2) The subpopulation of monocytes that produces TNF-α and CCL3 is different from the subpopulation that secretes CXCL9 and CXCL10. Three populations of monocytes have been described. Most of them are classic monocytes expressing low CD16 after the intermediate and non-classical monocytes that strongly express CD16.19 These non-classical monocytes produce more TNF-α than the intermediate and classical monocytes,20 but it is not known which subpopulations are responsible for the secretion of the chemokines studied here. Finally, the dropping levels of IFN-γ during therapy may be associated with not only pentoxifylline inhibition but also, leishmania killing and autocrine immune down-modulation. Therefore, the lower increased levels of CXCL9 during therapy in the antimony plus pentoxyfilline group may reflect these phenomena, which may imply that a lower inflammatory environment may lead to a cure.
Therapy with Sbv is performed for 20 days, but patients are followed for up to 60–90 days for determination of cure. Also, we observed here changes in TNF-α, IFN-γ, and chemokines levels with 15 days of therapy. Therefore, it is possible that these changes may be used to predict therapeutic response to antimony. Actually, we observed that CXCL9 levels at 15 day of therapy were higher in patients who failed than patients who were cured (independent of the groups). Therefore, the evaluation of CXCL9 levels during therapy should be performed in a higher number of patients to confirm if it may be a precocious marker of therapeutic failure. This finding is of utmost relevance, because in such a case, a new drug or a prolonged course of antimony may be incorporated to the treatment to avoid clinical failure and future complications, like mucosal or disseminated disease. A limitation to our study is the small number of subjects included, which may be associated with a low power to detect significance regarding clinical outcome. Therefore, a larger trial with enough power to address the important clinical and immunological issues raised by our data is needed.
Footnotes
Financial support: INCT-DT 573839/2008-5 and ICIDR grant AI088650.
Authors' addresses: Graça Brito, Mayra Dourado, Ludmila Polari, Daniela Celestino, Lucas P. Carvalho, Adriano Queiroz, Paulo R. L. Machado, and Sara Passos, Serviço de Imunologia, Complexo Hospitalar Universitário Professor Edgard Santos, Salvador, Bahia, Brazil, E-mails: gracaobrito@yahoo.com.br, mayradourado@hotmail.com, mila_farmacia@hotmail.com, dany_celestino@hotmail.com, carvalholp@ig.com.br, adrianoqs@gmail.com, prlmachado@uol.com.br, and saratpassos@hotmail.com. Edgar M. Carvalho, HUPES, R. Joao das Botas s/n Canela, Salvador, Bahia, Brazil, E-mail: imuno@ufba.br.
References
- 1.WHO . Leishmaniasis. Tropical Diseases Research. Geneva: WHO; 1998. [Google Scholar]
- 2.Newlove T, Guimaraes LH, Morgan DJ, Alcantara L, Glesby MJ, Carvalho EM, Machado PR. Antihelminthic therapy and antimony in cutaneous leishmaniasis: a randomized, double-blind, placebo-controlled trial in patients co-infected with helminths and Leishmania braziliensis. Am J Trop Med Hyg. 2011;84:551–555. doi: 10.4269/ajtmh.2011.10-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado PR, Ampuero J, Guimaraes LH, Villasboas L, Rocha AT, Schriefer A, Sousa RS, Talhari A, Penna G, Carvalho EM. Miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Negl Trop Dis. 2010;4:e912. doi: 10.1371/journal.pntd.0000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Oliveira A, Jr, Machado P, Bacellar O, Cheng LH, Almeida RP, Carvalho EM. Evaluation of IFN-gamma and TNF-alpha as immunological markers of clinical outcome in cutaneous leishmaniasis. Rev Soc Bras Med Trop. 2002;35:7–10. doi: 10.1590/s0037-86822002000100002. [DOI] [PubMed] [Google Scholar]
- 5.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005;101:226–230. doi: 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. Role of pro- and anti-inflammatory cytokines during inflammation: experimental and clinical findings. J Biol Regul Homeost Agents. 1997;11:91–103. [PubMed] [Google Scholar]
- 7.Melby PC, Andrade-Narvaez FJ, Darnell BJ, Valencia-Pacheco G, Tryon VV, Palomo-Cetina A. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect Immun. 1994;62:837–842. doi: 10.1128/iai.62.3.837-842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos JB, de Jesus AR, Machado PR, Magalhaes A, Salgado K, Carvalho EM, Almeida RP. Antimony plus recombinant human granulocyte-macrophage colony-stimulating factor applied topically in low doses enhances healing of cutaneous leishmaniasis ulcers: a randomized, double-blind, placebo-controlled study. J Infect Dis. 2004;190:1793–1796. doi: 10.1086/424848. [DOI] [PubMed] [Google Scholar]
- 9.Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez-Reyes G, Lopez-Ortal P, Sixtos S, Cruz S, Ramirez-Iglesias MT, Gutierrez-Ruiz MC, Sanchez-Avila F, Roldan E, Vargas-Vorackova F, Kershenobich D. Effect of pentoxifylline on levels of pro-inflammatory cytokines during chronic hepatitis C. Scand J Immunol. 2006;63:461–467. doi: 10.1111/j.1365-3083.2006.001761.x. [DOI] [PubMed] [Google Scholar]
- 11.Doherty GM, Jensen JC, Alexander HR, Buresh CM, Norton JA. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 1991;110:192–196. [PubMed] [Google Scholar]
- 12.Sadeghian G, Nilforoushzadeh MA. Effect of combination therapy with systemic glucantime and pentoxifylline in the treatment of cutaneous leishmaniasis. Int J Dermatol. 2006;45:819–821. doi: 10.1111/j.1365-4632.2006.02867.x. [DOI] [PubMed] [Google Scholar]
- 13.Machado PR, Lessa H, Lessa M, Guimaraes LH, Bang H, Ho JL, Carvalho EM. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis. 2007;44:788–793. doi: 10.1086/511643. [DOI] [PubMed] [Google Scholar]
- 14.Lessa HA, Machado P, Lima F, Cruz AA, Bacellar O, Guerreiro J, Carvalho EM. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am J Trop Med Hyg. 2001;65:87–89. doi: 10.4269/ajtmh.2001.65.87. [DOI] [PubMed] [Google Scholar]
- 15.Reed SG, Badaro R, Masur H, Carvalho EM, Lorenco R, Lisboa A, Teixeira R, Johnson WD, Jr, Jones TC. Selection of a skin test antigen for American visceral leishmaniasis. Am J Trop Med Hyg. 1986;35:79–85. doi: 10.4269/ajtmh.1986.35.79. [DOI] [PubMed] [Google Scholar]
- 16.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasama T, Muramatsu M, Kobayashi K, Yajima N, Shiozawa F, Hanaoka R, Miwa Y, Negishi M, Ide H, Adachi M. Interaction of monocytes with vascular endothelial cells synergistically induces interferon gamma-inducible protein 10 expression through activation of specific cell surface molecules and cytokines. Cell Immunol. 2002;219:131–139. doi: 10.1016/s0008-8749(02)00600-7. [DOI] [PubMed] [Google Scholar]
- 18.Ritter U, Korner H. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunol. 2002;24:295–301. doi: 10.1046/j.1365-3024.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 20.Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–e61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]