Abstract
Haitian children were monitored longitudinally in a filariasis study. Included were stool samples examined for Giardia intestinalis and Entamoeba histolytica cysts, and serum specimens analyzed for immunoglobulin G (IgG) responses to eight recombinant antigens from G. intestinalis (variant-specific surface protein [VSP1–VSP5]), E. histolytica (lectin adhesion molecule [LecA]), and Cryptosporidium parvum (17- and 27-kDa) using a multiplex bead assay. The IgG responses to VSP antigens peaked at 2 years of age and then diminished and were significantly lower (P < 0.002) in children > 4.5 years than in children < 4.5 years. The IgG responses to Cryptosporidium tended to increase with age. The IgG responses to LecA and VSP antigens and the prevalence of stools positive for cysts were significantly higher (P < 0.037 and P < 0.035, respectively) in the rainy season than in the dry season. The multiplex bead assay provides a powerful tool for analyzing serologic responses to multiple pathogens.
Introduction
Entamoeba histolytica, Cryptosporidium parvum, Cryptosporidium hominis, and Giardia intestinalis (syn. Giardia duodenalis and Giardia lamblia) are enteric protozoans that infect humans. These protozoans cause amebiasis, cryptosporidiosis, and giardiasis, respectively, and all can produce diarrhea, abdominal cramps, vomiting, malaise, and malabsorption after establishment in the intestinal tract. Pathogenic E. histolytica can invade extra- intestinal tissues such as the liver, brain, and lungs, whereas Cryptosporidium can invade the respiratory tract. All are known to infect animals except C. hominis and E. histolytica. Worldwide, it has been estimated that pathogenic E. histolytica kills 40–110 million people annually, which is second to malaria among protozoans as a cause of death.1 Ubiquitous throughout the world, C. parvum or C. hominis can cause symptoms lasting 1–14 days in immunocompetent humans. However, in immunocompromised individuals, such as those with human immunodeficiency virus (HIV), cryptosporidiosis can cause a mortality rate as high as 68%, a major burden in Africa, where HIV prevalence is as high as 30%.2 In Africa, Asia, and Latin America, it has been estimated that G. intestinalis infects 200 million people.3 In contrast to developed countries, many people in undeveloped countries with infections of G. intestinalis do not develop symptoms.4
Serological techniques, such as enzyme-linked immunosorbent assay (ELISA), enzyme-linked immunoelectrotransfer blot, and multiplex bead assay (MBA), have been used to assess the humoral immune responses in persons infected with enteric protozoans.5–13 These techniques can confirm that infection has occurred and can monitor the effectiveness of public health measures, such as chlorination or filtration of water sources, before and after implementation. Serology has been used in the assessment of treatment programs for the elimination of filariasis, comparing pre- and post-administration of anti-filarial drugs when antibody levels are high and low, respectively.14–16
We used a MBA to assess immunoglobulin G (IgG) responses to 28 antigens in longitudinal serum specimens collected from Haitian children enrolled in a filariasis study from 1990 to 1999.17 The MBA technique was selected because of its proven performance, its conservation of serum specimens (only 125 nL/well) and its relatively low labor and low cost, about $100 per plate per 10-plex assay, excluding labor and cost of antigens.16 Here, we report IgG responses from these children to the recombinant E. histolytica lectin adhesion antigen (LecA), to the recombinant C. parvum antigens 17- and 27-kDa (Cry17 and Cry27) from invasive sporozoites, to the glutathione-S-transferase (GST) from Schistosoma japonicum, a control antigen that elicits no appreciable human IgG activity even in persons infected with S. japonicum (personal observation), and to five recombinant variant-specific surface proteins (VSP1–VSP5) from G. intestinalis. For comparison, IgG responses to these antigens from adult Haitian individuals were also examined. In addition, longitudinal stool specimens were collected from most of the children and were examined by microscopy for G. intestinalis and E. histolytica cysts. Relationships of serological responses to stool examinations, to age, and to season of sample collection in Haiti were evaluated.
Materials and Methods
Study population and design.
The study was reviewed and approved by the Centers for Disease Control and Prevention Institutional Review Board and by the Ethics Committee of Hôpital Sainte Croix, Leogane, Haiti. Briefly, families with small children in the neighborhood areas of Cada, Bino, Dampus, and in urban Leogane, Haiti were recruited to participate in the filariasis study as previously described17; after parental consent, serum specimens were collected periodically from 1990 to 1999. The age range of children monitored was 3 weeks to 11.5 years, and the median age, at the time of serum specimen collection, was 4.5 years. In addition to the children, IgG responses were determined for 30 Haitian adults who also lived in urban Leogane and who submitted one serum specimen during the filariasis study. Of the 142 children enrolled, an average of 5.4 serum specimens were collected per child, and 135 of these children submitted stool specimens from 1990 to 1996. Unfortunately, not all stool specimens were collected at the time of the serum specimen collection, and at times, some children were not available for specimen collection. An average of 3.6 stool specimens per child was collected. Stool specimens were concentrated by the formalin-ethyl acetate procedure, and then examined by microscopy.18 Results were reported as positive or negative for G. intestinalis cysts (GIC) or E. histolytica cysts (EHC). The EHC prevalence may be over-estimated as cysts of E. dispar and E. moshkovskii cannot be distinguished from EHC by microscopy.19 No staining for Cryptosporidium was carried out.
Recombinant antigen preparation and purification.
The LecA recombinant antigen, containing the carbohydrate recognition domain for galactose and N-acetyl-D-galactosamine complex from E. histolytica, was expressed with six histidines at the amino terminus as previously described.20 All other antigens were GST-linked. Recombinant antigens from C.parvum, Cry17, and Cry27, have been previously described.9 The purification of GST alone and the five G. intestinalis recombinant antigens, three from assemblage A, VSP1–VSP3, and two from assemblage B, VSP4 and VSP5, has been previously described.9,13
Antigen coupling to beads.
Carboxyl groups on the surface of the polystyrene microspheres (SeroMap Beads; Luminex Corporation, Austin, TX) were chemically modified to a reactive ester, using the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide method (Calbiochem, Woburn, MA). The esters readily react with available primary amine groups on the antigen, forming a stable covalent amide bond between antigen and bead. All couplings were performed in phosphate buffered saline, pH 7.2, as previously described16; the coupling efficiency was determined by polyclonal goat anti-GST antibodies and by sera known to be highly reactive to the antigens.
Multiplex bead assay.
The procedure for the MBA was slightly modified from a previous study by extending the incubation period of the serum specimens (dilution 1:400) to the antigen-coupled beads from 45 minutes to 1.5 hours.16,17 Data were acquired using a reader (Luminex Corp) equipped with Bio-Plex Manager 6.0 software (Bio-Rad, Hercules, CA) and was reported as the average of the median fluorescence intensity from duplicate wells minus the background (MFI-bg). For each antigen, a serum blank and a negative control serum were run on each plate along with a positive control serum that was diluted to yield mid-range fluorescence intensity. The fluorescence intensity in the MBA is directly proportional to the amount of IgG, the analyte, bound to the antigen-coupled beads.
Establishment of cutoffs.
Sixty-five North American serum specimens from adults (U.S. citizens with no history of foreign travel) were used to establish cutoffs to the LecA and VSP antigen-coupled beads. The three highest responses from the 65 adults were eliminated for each antigen, and the remaining responses were used to establish the mean + 3 SD as the cutoff. The resulting cutoff for the LecA antigen (Table 1) showed 100% sensitivity on sera from ameabiasis patients compared with an in-house prepared Entamoeba Serum Test kit (TechLab, Inc., Blacksburg, VA) which also uses the LecA antigen (unpublished observation). The cutoffs for the VSP antigens (Table 1) were used in a previous study and showed 93% sensitivity for persons GIC positive.13 Methods for cutoff determination for the Cry17 and Cry27 antigens have been described and were used here.11
Table 1.
Antigen, cutoff, median, and range of IgG responses (MFI-bg) to each antigen on 142 Haitian children from 1990–1999
| Antigen | Cutoff | Median (range) of fluorescence intensities (MFI-bg)* |
|---|---|---|
| LecA | 302 | 1,040 (4–28,964) |
| Cry17 | 180 | 1,557 (1–30,317) |
| Cry27 | 500 | 2,535 (2–30,191) |
| VSP1 | 209 | 293 (0–29,148) |
| VSP2 | 270 | 395 (0–28,288) |
| VSP3 | 262 | 446 (−1–29,091) |
| VSP4 | 209 | 194 (0–27,758) |
| VSP5 | 206 | 519 (0–29,437) |
| GST | − | 13 (−2–202) |
Possible median fluorescence intensity (MFI) range is 1–32,766 without background (bg) subtracted, and possible fluorescence intensities with bg subtracted (MFI-bg) can yield a negative number −32,766-bg.
Definitions.
To be considered a serological episode, MFI-bg intensities had to be above the cutoffs. This included both Cry17 and Cry27 antigens for Cryptosporidium and at least two of the five VSP antigens for Giardia. Subsequent serological episodes required at least a 10% decrease from a previous episode followed by at least a 40% increase. Intentions were to collect one serum specimen per child per year. Because the majority of the serum specimens were collected in this manner, an arbitrary interval of at least 340 days was used as the minimum time interval between serological episodes for them to be considered distinct. This interval is related to the typical interval between sample collection dates and is a conservative approach that will result in an under-estimate of seroincidence.
Possible secondary antibody responses were serological episodes higher in MFI-bg intensities than a previous serological episode, requiring at least two of the VSP antigens for Giardia and both Cry17 and Cry27 antigens for Cryptosporidium.
Stool episodes were stool specimens found positive for GIC or EHC. Stool specimens were collected independent of serum specimens. Because stool specimens were collected twice a year for some children, we used an arbitrary interval of at least 180 days between stool episodes for them to be distinct.
Stool and serum specimens were categorized by the season they were collected. In Haiti, one dry and two rainy seasons occur annually.21 Specimens were collected in January–March (Dry), which includes part of the dry season. Specimens were also collected in April–July (Rain1), which includes the first rainy season from April–June, and in August–October (Rain2), which is the second rainy season.
Evaluation of cyst-positive stools and IgG responses.
Serum specimens collected within 6 months of positive stool specimens were evaluated for positive or negative IgG responses, using the cutoffs described previously.
Statistical analysis.
For consistency, the coefficient of variation was determined on the positive controls from all plates. The z test (SigmaPlot, version 11.0, www.systat.com) was used to determine significance of differences in prevalence of cyst-positive stools collected in the dry and rainy seasons and in prevalence of children with stool and serological episodes between children < 4.5 years of age and children ≥ 4.5 years of age. The age of 4.5 years was the median age of the children at the time serum specimens were collected. For comparison of IgG responses between dry and rainy seasons and between age groups, the generalized estimating equation (GEE) approach with negative binomial distribution was applied, accounting for correlations stemming from repeated measurements on the same child22,23; the exchangeable working correlation structure was assumed. The GEE analyses were done using the two-sided hypothesis test, and analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC). P < 0.05 was considered statistically significant.
Results
MBA controls and cutoffs.
Shown in Table 1 are the median and range of MFI-bg values (the IgG responses) to each antigen along with cutoff determinations. The coefficient of variation for positive controls on all plates, diluted to yield moderate MFI-bg intensities, was ≤ 10%, and all negative controls on all plates were consistently below the cutoffs. There were no appreciable IgG responses to GST-coupled beads as shown in Table 1. Sera known to be highly reactive to the antigens and goat anti-GST antibodies showed high MFI-bg values from the antigen-coupled beads, indicating sufficient antigen coupling (data not shown). There was an excellent range of IgG reactivity to the LecA, all VSP, and Cry17 and Cry27 antigens from the children (Table 1).
Stool episodes.
Over the 6-year period of stool collections, 65.9% (89 of 135) of the children had at least one GIC stool episode while 22.2% (30 of 135) had at least one EHC stool episode. Seventeen percent (23 of 135) had both GIC and EHC identified at one time or another in the longitudinal stool specimens from the same child. The number of stool episodes per child ranged from 1 to 6 for GIC and from 1 to 3 for EHC. Over the 6 years of stool collection, the total number of GIC and EHC stool episodes from the 135 children was 178 and 39, respectively.
No significant differences in the prevalence of children with at least one stool episode were observed between children < 4.5 years and children ≥ 4.5 years of age: for GIC, 60.0% (75 of 125) and 57.1% (40 of 70), respectively; and for EHC, 16.0% (20 of 125) and 21.3% (13 of 61), respectively.
Serological episodes.
Based on the cutoffs, 90.1% (128 of 142), 97.9% (139 of 142), and 96.5% (137 of 142) of the children had at least one serological episode to E. histolytica, Cryptosporidium, and Giardia, respectively, throughout the 10-year study. The serological episodes per child over the 10-year study, including initial and subsequent episodes, ranged from 1 to 4 for each organism and the total number of serological episodes from the 142 children to each organism was 188, 248, and 243, respectively.
Over the 10-year study, 14.1% (20 of 142), 21.1% (30 of 142), and 28.9% (41 of 142) of the children showed possible secondary IgG responses to E. histolytica, Giardia, and Cryptosporidium antigens, respectively.
There was no evidence of differential IgG responses to Giardia antigens VSP1–VSP3 from assemblage A or VSP4 and VSP5 from assemblage B.
In Table 2, the prevalence of children with at least one serological episode to the enteric organisms is shown for children < 4.5 years of age and children ≥ 4.5 years of age. The prevalence of children with at least one serologic episode decreased for all enteric pathogens for children ≥ 4.5 years of age compared with children < 4.5 years of age but was significant (P < 0.001) only for Giardia and approached significance (P = 0.079) for Cryptosporidium.
Table 2.
Comparison of the prevalence of children < 4.5 years of age with children ≥ 4.5 years of age who had at least one serological episode to Entamoeba histolytica, Cryptosporidium parvum, and Giardia intestinalis
| Organism | % Children < 4.5 years of age with serological episode | % Children ≥ 4.5 years of age with serological episode | P value* |
|---|---|---|---|
| Entamoeba histolytica | 64.9 (85/131) | 62.9 (78/124) | 0.840 |
| Cryptosporidium parvum | 78.5 (102/130) | 68.0 (85/125) | 0.079 |
| Giardia intestinalis | 91.7 (121/132) | 62.1 (77/124) | < 0.001 |
Statistical method, z test.
A comparison of antibody responses, as measured by estimated mean MFI-bg between children < 4.5 years of age and children ≥ 4.5 years of age, adjusted for seasonality, is shown in Table 3. The estimated mean MFI-bg for LecA was similar for both age groups, although for Cry17 and Cry27 antigens, the estimated mean MFI-bg was greater in the older age group than in the younger age group but was significant only for the Cry17 (P = 0.048). In contrast, the estimated mean MFI-bg for each of the VSP antigens was significantly lower (P < 0.002) in the older age group than in the younger age group.
Table 3.
Comparison of the estimated mean IgG responses to antigens from children < 4.5 years of age with children ≥ 4.5 years of age
| Antigen | Estimated mean IgG responses (MFI-bg) of children < 4.5 years of age | Estimated mean IgG responses (MFI-bg) of children ≥ 4.5 years of age | P value* |
|---|---|---|---|
| LecA | 3,876 | 4,180 | 0.542 |
| Cry17 | 5,809 | 7,196 | 0.048 |
| Cry27 | 6,933 | 7,895 | 0.169 |
| VSP1 | 2,310 | 1,166 | 0.001 |
| VSP2 | 2,647 | 1,381 | 0.001 |
| VSP3 | 3,550 | 1,491 | < 0.001 |
| VSP4 | 1,747 | 693 | < 0.001 |
| VSP5 | 4,021 | 1,756 | < 0.001 |
Generalized estimation equation (GEE) method was used with the negative binomial probability distribution; the GEE model was adjusted for seasonality.
IgG = immunoglobulin G; MFI-bg = median fluorescence intensity minus the background.
Evaluation of cyst-positive stools and IgG responses.
Shown in Table 4 are children with EHC- and GIC-positive stools and their IgG responses to the Entamoeba and Giardia antigens on serum specimens collected within 6 months of their positive stools. Of 20 children with EHC-positive stools, 18 (90%) showed positive IgG responses to the LecA antigen within 6 months of the of the EHC-positive stools. Of 103 children with GIC-positive stools, 80 (78%) showed positive IgG responses to the VSP antigens within 6 months of the GIC-positive stools. Children were more likely to be IgG positive to VSP antigens if GIC-positive stools were collected within 6 days of serum specimen than in GIC-positive stools collected within 2–8 weeks or 5–6 months. Two children with EHC-positive stools and 19 of the 23 children with GIC-positive stools who showed negative IgG responses to the LecA and VSP antigens, respectively, did show positive IgG responses to the respective antigens at least one time during the study, indicating the antigens were recognized. Four children with GIC-positive stools showed negative IgG responses in all their serum specimens.
Table 4.
Serological responses in serum specimens collected within 6 months of positive stool specimens
| Positive and negative IgG responses in serum specimens collected within 6 months of GIC-positive stool | Positive and negative IgG responses in serum specimens collected within 6 months of GIC-positive stool* | ||||||
|---|---|---|---|---|---|---|---|
| No. of GIC-positive stools | Time between stool and serum | No. children IgG+ (%) | No. children IgG– (%) | No. of EHC-positive stools | Time between stool and serum | No. children IgG+ (%) | No. children IgG– (%) |
| 20 | 5–6 months | 14 (74) | 5 (26) | 3 | 6 months | 3 (100) | 0 (0) |
| 31 | 2–8 weeks | 21 (70) | 9 (30) | 4 | 2 months | 4 (100) | 0 (0) |
| 81 | < 6 days | 45 (83) | 9 (17) | 13 | < 6 days | 11 (85) | 2 (15) |
| Total 132 | 80 | 23 | 20 | 18 | 2 | ||
Entamoeba histolytica cysts (EHC)-positive stools can include not only E. histolytica cysts but also E. histolytica-like cysts, such as Entamoeba dispar and Entamoeba moshkovskii cysts.
GIC = Giardia intestinalis cysts; IgG = immunoglobulin G.
Complexities of the IgG responses to the antigens.
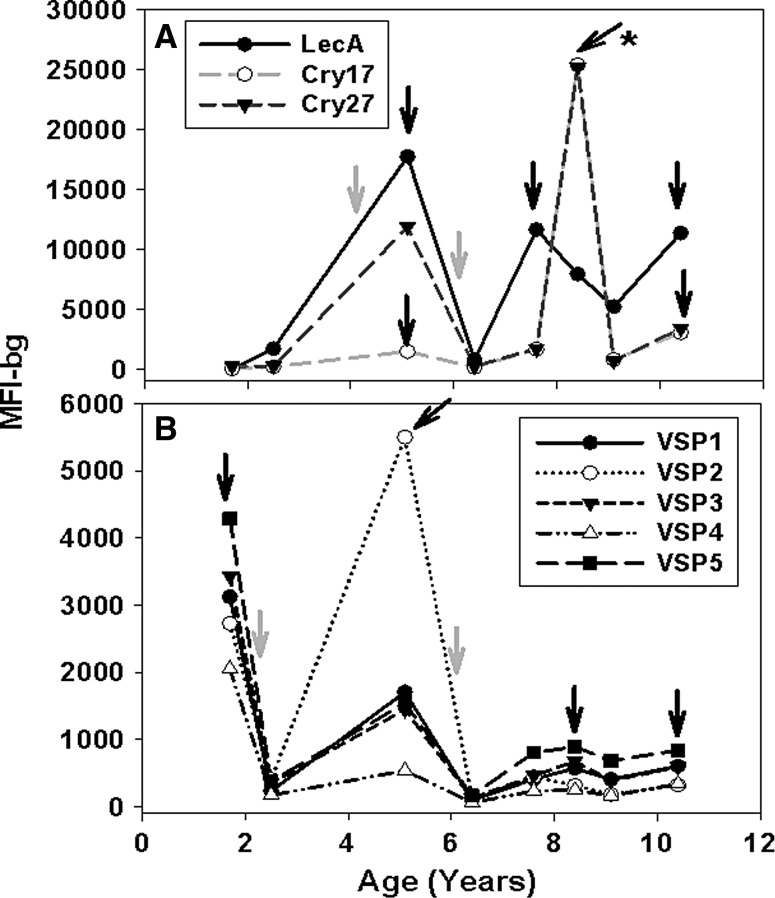
The complexities of the serological episodes to the antigens for most children are represented by one child longitudinally in Figure 1. Over a period of about 9 years, this child showed evidence of three serological episodes to the LecA antigen (dark shaded arrows) and three serological episodes to the Cry17 and Cry27 antigens (Figure 1A). A possible secondary IgG response (dark shaded arrow with asterisk) is shown to the Cry17 and Cry27 antigens. Stool episodes of EHC were identified in stool specimens at about 4 and 6 years of age (light shaded arrows). For the same child in Figure 1B, there were four serological episodes to the VSP antigens (dark shaded arrows). As was typical with most children, the IgG responses to the VSP antigens became less intense with increasing age, and no secondary IgG response to the VSP antigens was observed in this child. Stool episodes of GIC were identified at about 2 and 6 years of age (light shaded arrows).
Figure 1.
Longitudinal profiles of immunoglobulin G (IgG) reactivity to antigens from one Haitian child. Line plots by age (years) of longitudinal IgG reactivity (MFI-bg) to Entamoeba histolytica LecA and to Cryptosporidium parvum Cry17 and Cry27 (A) and to Giardia intestinalis VSP antigens (B). Dark arrows indicate serological episodes; dark arrow with asterisk indicates possible secondary IgG response to Cryptosporidium antigens; and light shaded arrows indicate stool episodes of E. histolytica cysts (EHC) or G. intestinalis cysts (GIC).
IgG responses to Entamoeba and Cryptosporidium antigens by age.
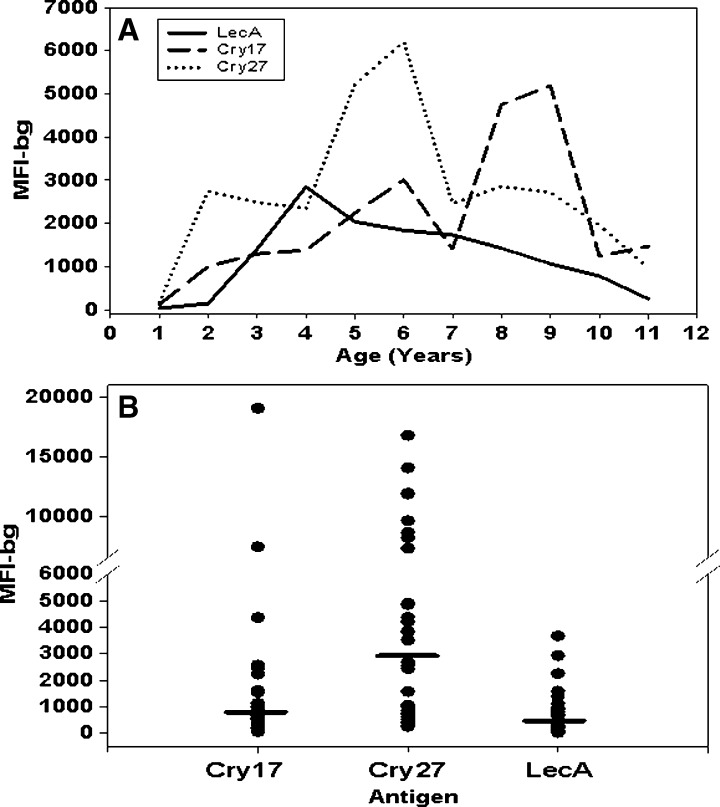
Shown in Figure 2 are median IgG responses to the LecA, Cry17, and Cry27 antigens from children by age (Figure 2A) and from the 30 Haitian adults (Figure 2B). For the children, serum specimens from all seasons were included. As expected, at 1 year of age, the median IgG responses to all antigens were relatively low, and afterward, began to increase. The median IgG responses to Cry17 and Cry27 antigens varied throughout the age range. In contrast, at 4 years of age, the median IgG responses to LecA peaked and then diminished slowly. The median IgG responses to the LecA, Cry17, and Cry27 antigens from the 30 Haitian adults (Figure 2B, bars) were similar to those of the older children. Responses of adults to LecA clustered around lower values, but responses to the Cry17 and Cry27 antigens displayed a wide range of values.
Figure 2.
Median immunoglobulin G (IgG) responses to Entamoeba and Cryptosporidium antigens from Haitian children and adults. Median IgG responses to Entamoeba histolytica LecA and Cryptosporidium parvum, Cry17 and Cry27 antigens. A, line plot by age (years) on all children; and B, scatter plot on single serum specimens collected from 30 adults. Horizontal bars in scatter plot are median IgG responses. The IgG responses determined by Luminex, the median fluorescence intensity minus the background (MFI-bg).
IgG responses to Giardia antigens by age.
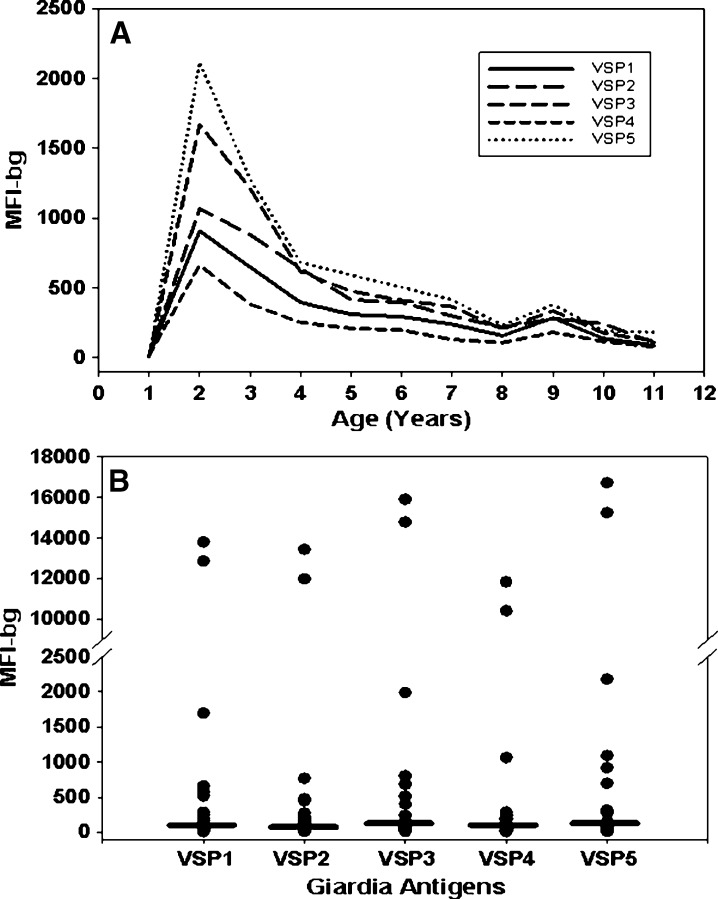
Shown in Figure 3 are median IgG responses to the VSP antigens from children by age (Figure 3A) and from the 30 Haitian adults (Figure 3B). For the children, serum specimens from all seasons were included. As expected, at 1 year of age, the median IgG responses to all antigens were relatively low, and afterward, began to increase. However, unlike the Entamoeba and Cryptosporidium antigens (Figure 2A), IgG responses to all Giardia antigens peaked at 2 years of age, dropped sharply by 4 years of age, and remained low. Median IgG responses from the 30 Haitian adults were generally low and similar to those of the older children.
Figure 3.
Median immunoglobulin G (IgG) responses to Giardia antigens from Haitian children and adults. Shown are median IgG responses to Giardia antigens, variant-specific surface proteins, VSP1, VSP2, and VSP3 from assemblage A and VSP4 and VSP5 from assemblage B. A, line plot by age (years) on all children; and B, scatter plot on single serum specimens collected from 30 adults. Horizontal bars in scatter plot are median IgG responses. The IgG responses determined by Luminex, the median fluorescence intensity minus the background (MFI-bg).
IgG responses to Entamoeba and Cryptosporidium antigens by dry and rainy seasons.
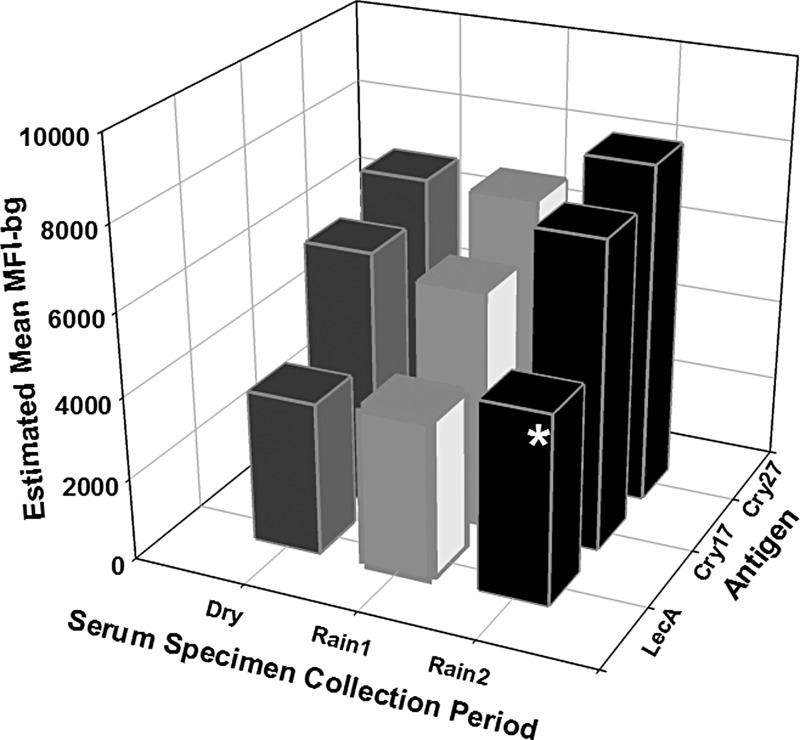
Shown in Figure 4 are estimated mean IgG responses to LecA, Cry17, and Cry27 antigens from serum specimens collected from the children in the Dry, Rain1, and Rain2 seasons. The estimated mean IgG responses to the LecA antigen was significantly higher (P = 0.04) in serum specimens collected in the Rain2 season than in serum specimens collected in the Dry season. A similar trend was observed for IgG responses to Cry17 and Cry27 antigens that approached significance (P = 0.09 and P = 0.11, respectively).
Figure 4.
Estimated mean immunoglobulin G (IgG) responses to Entamoeba and Cryptosporidium antigens from serum specimens collected from Haitian children during dry and rainy seasons. Dry is January–March, Rain1 is April–July, and Rain2 is August–October. Shown are estimated mean IgG responses (MFI-bg) to Entamoeba histolytica LecA and Cryptosporidium parvum Cry17 and Cry27 antigens. Statistical analysis was performed by the generalized estimating equation method with negative binominal distribution to account for correlations from repeated measurements on the same child. The model adjusts for age of the children at the time of submission of the serum specimen. The asterisk indicates statistically higher estimated mean IgG responses to the LecA antigen (P = 0.04) from sera collected during the Rain2 season than from sera collected during the Dry season. A similar trend existed for the Cry17 and Cry27 antigens (P = 0.09 and P = 0.1, respectively).
IgG responses to Giardia antigens by dry and rainy seasons.
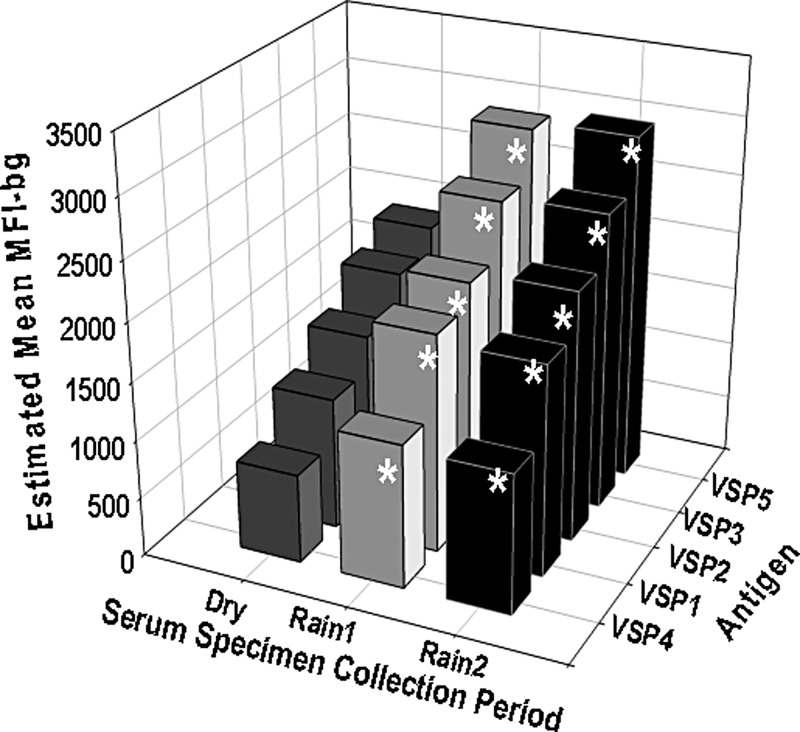
Shown in Figure 5 are estimated mean IgG responses to the VSP antigens from serum specimens collected from the children in the Dry, Rain1, and Rain2 seasons. For all Giardia antigens, the estimated mean IgG responses were significantly higher in serum specimens collected in the Rain1 (P < 0.05) and Rain2 (P < 0.01) season than in serum specimens collected in the Dry season.
Figure 5.
Estimated mean immunoglobulin G (IgG) responses to Giardia antigens from serum specimens collected from Haitian children during dry and rainy seasons. Dry is January–March, Rain1 is April–July, and Rain2 is August–October. Shown are estimated mean IgG responses (MFI-bg) to Giardia intestinalis variant-specific surface proteins, VSP1, VSP2, and VSP3 from assemblage A and VSP4 and VSP5 from assemblage B. Statistical analysis was performed by the generalized estimating equation method with negative binominal distribution to account for correlations from repeated measurements on the same child. The model adjusts for age of the children at the time of submission of the serum specimen. The asterisks indicate statistically higher estimated mean IgG responses to the VSP antigens from sera collected during the rainy seasons (Rain1 [P < 0.05] and Rain2 [P < 0.01]) than from sera collected during the Dry season.
Stool examinations by dry and rainy seasons.
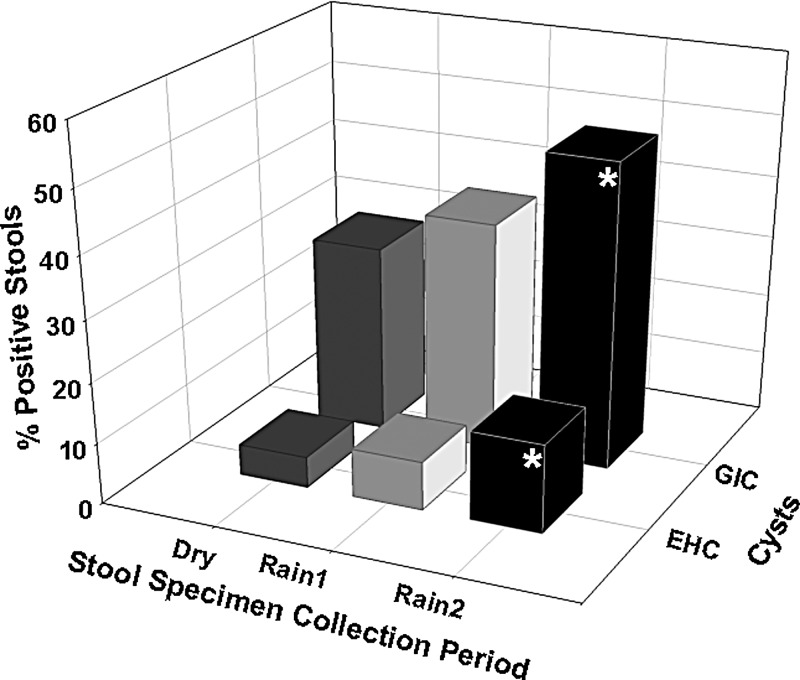
Shown in Figure 6 is the prevalence of stools positive for EHC and GIC in the Dry, Rain1, and Rain2 seasons. The pattern of the prevalence of positive stools was similar to the pattern of IgG responses to all antigens plotted against the dry and rainy seasons (Figures 4 and 5), increasing from the Dry to the Rain2 season. The percentage of stools positive for EHC and GIC was significantly higher (P < 0.019) in the stool specimens collected in the Rain2 season than in the stool specimens collected in the Dry season.
Figure 6.
Prevalence of stools positive for Giardia intestinalis cysts (GIC) and Entamoeba histolytica cysts (EHC) collected from Haitian children during dry and rainy seasons. The EHC-positive stools can include not only E. histolytica cysts but also E. histolytica-like cysts, such as E. dispar and E. moshkovskii cysts. Dry is January–March, Rain1 is April–July, and Rain2 is August–October. Shown is percentage of stools positive for EHC and GIC by season the stool specimens were collected. Statistical analyses performed by the z test. The asterisks indicate that the prevalence of positive stools for EHC and GIC were statistically higher (P < 0.019) in stools collected during the Rain2 season than in stools collected during the Dry season.
Discussion
As in any area with poor sanitary and hygiene conditions, children living in the peri-urban areas of Leogane, Haiti are at high risk of acquiring infection with E. histolytica, C. parvum, or G. intestinalis. Our serologic analyses showed that these infections were frequent and repeated. Of the 142 children studied from 1990 to 1999, 90.1%, 97.9%, and 96.5% showed at least one serological episode to E. histolytica, C. parvum, and G. intestinalis, respectively. These infection rates are high, and in dramatic contrast to the United States where only 6.9 to 8.5 Giardia infections and 1.0 to 1.3 per year Cryptosporidium infections occurred per 100,000 individuals from 1999 to 2002.24,25 Entamoeba infections are very rare in the United States and are not reported nationally.26 Our analyses also showed two important and noteworthy trends: first, a pronounced difference in antibody responses by age; and second a significant relationship between antibody responsiveness and seasonality.
The longitudinal IgG profiles to the antigens studied in these Haitian children, who were chronically exposed to Entamoeba, Cryptosporidium, and Giardia, differed as the children aged. At 1 year of age, IgG responses to all antigens (Figures 2A and 3A) and the prevalence of stools positive for cysts were relatively low (data not shown), as expected for breastfed children.27,28 Shortly after 1 year of age, the IgG responses began to increase to all antigens (Figures 2A and 3A), reflecting the increase in exposure to the waterborne pathogens. Overall, median IgG responses to the Cryptosporidium antigens showed no indication of a decline as children aged over the 10-year study. In contrast, median IgG responses to the Giardia VSP and Entamoeba LecA antigens declined after peaking at 2 and 4 years of age (Figures 2A and 3A), respectively, which is reflected in Table 3 for Giardia VSP antigens, showing lower estimated mean IgG responses in older children than in younger children. This was not the case with the LecA antigen, which also showed a decline in IgG responses in the older children but was not as steep as the decline of IgG responses to the Giardia antigens in the older children (Figures 2A and 3A). Despite the relatively slow decline of IgG responses to the LecA antigen in the older children (Figure 2A), the prevalence of children with at least one serological episode in both older and younger children (Table 2) and the estimated mean IgG responses to LecA in both older and younger children (Table 3) were similar. For all antigens, the IgG responses of older children were similar to the adults (Figures 2 and 3). In a study in Bangalore, India, increased IgG responses to crude antigen from G. intestinalis and E. histolytica trophozoites were shown by ELISA in children 13–24 and 38–47 months of age, respectively.29 In another study, 4- and 5-year-old children in an urban slum of Dhaka, Bangladesh showed higher IgG responses to LecA than in children who were younger, but older children were not tested.30 In contrast to the VSP antigens, IgG responses to the Cry17 and Cry27 antigens showed no evidence of a decrease with age. Unlike the Giardia antigens, the estimated mean IgG responses to the Cry17 and Cry27 antigens were higher in children ≥ 4.5 years of age than in children < 4.5 years of age and was significant (P = 0.048) for the Cry17 antigen (Table 3), indicating that IgG responses increase with repeated exposure to Cryptosporidium.
In an area where chronic exposure to Giardia does not exist, these same VSP antigen coupled beads were tested on serum specimens collected from adults in British Columbia, Canada during a giardiasis outbreak (data not shown).13 The median IgG responses from the Canadians were 3.7–15.5 times higher than the median IgG responses shown here from the 30 Haitian adults (Figure 3B), showing that IgG responses to the Giardia antigens in the Canadian adults living in a non-endemic area were stronger than the IgG responses in the Haitian adults.13 No clinical data was available from the Canadian adults, but many were confirmed stool positive for Giardia cysts.13 Although high infection rates of enteric protozoans occur in Haitian adults and older children, most present no overt clinical symptoms (Lammie PJ, personal observation). Further studies are required for comparison of persons with and without chronic exposures to Giardia.
Significantly (P < 0.001) fewer serological episodes to the Giardia antigens occurred in older children compared with younger children (Table 2); nonetheless, Giardia infections were common among the older children. The reasons for reduced IgG responses to the Giardia antigens in older Haitian children and adults are not known. It has been speculated that E. histolytica may be able to avoid at least some antibody responses by “capping and shedding.” A portion of the parasite membrane with bound immunoglobulin is “capped” and then “shed”, thus, reducing the effector function of the bound antibody31,32; whether this process is related to the reduction in antibody responses seen among older children and adults is not clear. It is also possible that shifts toward IgA or cell-mediated immune responses could occur over time. Whether the age at which peak IgG responses are observed reflects transmission intensity with Giardia and possibly E. histolytica is an interesting concept that requires further observation.
The risk of infection with these protozoa increases during the rainy seasons at which time increases in the prevalence of positive stools (Figure 6) and increases in IgG responses to the antigens were observed (Figures 4 and 5). This seasonal pattern of IgG responses was not observed for the mosquito-borne disease filariasis that was also included in the original 28-plex study on the 142 children, using the Brugia malayi antigens Bm14 and Bm33 (data not shown).16 Rain has been shown to be an important risk factor for waterborne diseases, particularly in settings where sanitation is sub-optimal.33–35 These data highlight the importance of controlling for seasonality in sero-epidemiologic studies of these infections.
Our study was subject to some limitations. Of the 135 children who submitted stool specimens over the 6-year collection period, 65.9% and 22.2% had microscopic evidence of G. intestinalis cysts or E. histolytica cysts (or E. histolytica-like cysts), respectively, in at least one of their stools. These estimates may be low because of the intermittent shedding of cysts in stools and the relatively infrequent collection of stool specimens. Furthermore, the number of stools positive for non-pathogenic E. dispar and E. moshkovskii and pathogenic E. histolytica is not known, because microscopy cannot distinguish this species29; we did not carry out any stool examination for Cryptosporidium and are consequently not able to comment on changes in infection prevalence with age. It is also likely that the Haitian children were infected with different species of Cryptosporidium, because the Cry17 and Cry27 antigens are conserved in many species of this genus, which has been shown in children living in poor sanitary and hygiene conditions in Lima, Peru.11 Our estimates of the number of stool and serologic episodes are almost certainly underestimated; many stool and serological episodes may have been missed because of the timing or the relatively infrequent collection of specimens as a result of unavailability of some children. Timing of the collection of serum and stool specimens is critical to show the kinetic relationship between immune responses and infection, as shown in Table 4. Of 19 and two children with GIC- and EHC-positive stools, respectively, who showed negative IgG responses to the antigens in serum specimens collected within 6 months of their positive stool specimen, all were IgG positive in serum specimens collected beyond 6 months of the positive stool specimens (Table 4). The four children who were GIC-positive but never showed a positive IgG response to the VSP antigens could be a result of poor timing of serum specimen collection; detectable levels of IgG requires some production time, at least 2–3 weeks after infection or reinfection. Nonetheless, our results do illustrate the use of the MBA for monitoring these infections.
The MBA is versatile and can provide not only information on the immune responses in children and/or adults who are chronically exposed to these protozoans but can also provide information on the impact of interventions to improve sanitary and hygiene conditions or water quality. However, if the MBA or other serological techniques are used to monitor the impacts of such programs, the seasonal shifts in antibody responses to enteric protozoans, and possibly other enteric pathogens, will require consideration to carry out accurate assessments. The MBA can provide more insight into the immune responses from persons chronically infected with Giardia, Entamoeba, and Cryptosporidium by simultaneously acquiring serum antibody and cytokine profiles on longitudinal stool extracts from persons exposed to these pathogens. The MBA is at least as sensitive as ELISA, and as shown in this study, it was sufficiently sensitive to provide valuable sero-epidemiological data, including the pronounced age-dependent downward shift in responses to the Giardia VSP antigens.36–41
ACKNOWLEDGMENTS
We express our gratitude to families and children who participated in this study over many years, the staff of Hopital Ste. Croix and the members of the filariasis research team, including David Addiss, Michael Beach, Jacky Louis Charles, Jean Marc Brissau, Dardith Desire, and the many trainees over the years who contributed to the follow-up of these children.
Disclaimer: Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Service. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Financial support: We appreciate the financial support of the National Institutes of Health (NIH), CDC, and the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (grant nos. 920528 and 940441). Katy Hamlin was supported by a CDC/APHL Emerging Infectious Disease Fellowship. Support for the production and purification of the LecA antigen was provided by NIH, grant R01 AI043596.
Authors' addresses: Delynn M. Moss, Jeffrey W. Priest, and Gordana Derado, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Waterborne Disease Prevention Branch, Atlanta, GA, E-mails: dmm3@cdc.gov, jip8@cdc.gov, and uwx8@cdc.gov. Katy Hamlin, The George Washington University School of Public Health and Health Sciences, Washington, DC, E-mail: kl.hamlin28@gmail.com. Joel Herbein, TechLab, Inc., Blacksburg, VA, E-mail: jherbein@techlab.com. William A. Petri Jr., University of Virginia, Health Sciences Center, Charlottesville, VA, E-mail: wap3q@virginia.edu. Patrick J. Lammie, Centers for Disease Control and Prevention, Division of Parasitic Diseases, Atlanta, GA, E-mail: pjl1@cdc.gov.
References
- 1.Walsh JA. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8:228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- 2.Rose JB. Environmental ecology of Cryptosporidium and public health implications. Annu Rev Public Health. 1997;18:135–161. doi: 10.1146/annurev.publhealth.18.1.135. [DOI] [PubMed] [Google Scholar]
- 3.Yason JA, Rivera WL. Genotyping of Giardia duodenalis isolates among residents of slum area in Manila, Philippines. Parasitol Res. 2007;101:681–687. doi: 10.1007/s00436-007-0533-8. [DOI] [PubMed] [Google Scholar]
- 4.Cedillo-Rivera R, Leal YA, Yepez-Mulia L, Gomez-Delgado A, Ortega-Pierres G, Tapia-Conyer R, Munoz O. Seroepidemiology of giardiasis in Mexico. Am J Trop Med Hyg. 2009;80:6–10. [PubMed] [Google Scholar]
- 5.Haque R, Mollah NU, Ali IK, Alam K, Eubanks A, Lyerly D, Petri WA., Jr Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J Clin Microbiol. 2000;38:3235–3239. doi: 10.1128/jcm.38.9.3235-3239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leo M, Haque R, Kabir M, Roy S, Lahlou RM, Mondal D, Tannich E, Petri WA., Jr Evaluation of Entamoeba histolytica antigen and antibody point-of-care tests for the rapid diagnosis of amebiasis. J Clin Microbiol. 2006;44:4569–4571. doi: 10.1128/JCM.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss DM, Bennett SN, Arrowood MJ, Wahlquist SP, Lammie PJ. Enzyme-linked immunoelectrotransfer blot analysis of a cryptosporidiosis outbreak on a United States Coast Guard cutter. Am J Trop Med Hyg. 1998;58:110–118. doi: 10.4269/ajtmh.1998.58.110. [DOI] [PubMed] [Google Scholar]
- 8.Moss DM, Chappell CL, Okhuysen PC, DuPont HL, Arrowood MJ, Hightower AW, Lammie PJ. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J Infect Dis. 1998;178:827–833. doi: 10.1086/515377. [DOI] [PubMed] [Google Scholar]
- 9.Moss DM, Montgomery JM, Newland SV, Priest JW, Lammie PJ. Detection of Cryptosporidium antibodies in sera and oral fluids using multiplex bead assay. J Parasitol. 2004;90:397–404. doi: 10.1645/GE-3267. [DOI] [PubMed] [Google Scholar]
- 10.Priest JW, Bern C, Roberts JM, Kwon JP, Lescano AG, Checkley W, Cabrera L, Moss DM, Arrowood MJ, Sterling CR, Gilman RH, Lammie PJ. Changes in serum immunoglobulin G levels as a marker for Cryptosporidium sp. infection in Peruvian children. J Clin Microbiol. 2005;43:5298–5300. doi: 10.1128/JCM.43.10.5298-5300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Priest JW, Bern C, Xiao L, Roberts JM, Kwon JP, Lescano AG, Checkley W, Cabrera L, Moss DM, Arrowood MJ, Sterling CR, Gilman RH, Lammie PJ. Longitudinal analysis of Cryptosporidium species-specific immunoglobulin G antibody responses in Peruvian children. Clin Vaccine Immunol. 2006;13:123–131. doi: 10.1128/CVI.13.1.123-131.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priest JW, Kwon JP, Moss DM, Roberts JM, Arrowood MJ, Dworkin MS, Juranek DD, Lammie PJ. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J Clin Microbiol. 1999;37:1385–1392. doi: 10.1128/jcm.37.5.1385-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Priest JW, Moss DM, Visvesvara GS, Jones CC, Li A, Isaac-Renton JL. Multiplex assay detection of immunoglobulin G antibodies that recognize Giardia intestinalis and Cryptosporidium parvum antigens. Clin Vaccine Immunol. 2010;17:1695–1707. doi: 10.1128/CVI.00160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helmy H, Weil GJ, Ellethy AS, Ahmed ES, Setouhy ME, Ramzy RM. Bancroftian filariasis: effect of repeated treatment with diethylcarbamazine and albendazole on microfilaraemia, antigenaemia and antifilarial antibodies. Trans R Soc Trop Med Hyg. 2006;100:656–662. doi: 10.1016/j.trstmh.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Lammie PJ, Reiss MD, Dimock KA, Streit TG, Roberts JM, Eberhard ML. Longitudinal analysis of the development of filarial infection and antifilarial immunity in a cohort of Haitian children. Am J Trop Med Hyg. 1998;59:217–221. doi: 10.4269/ajtmh.1998.59.217. [DOI] [PubMed] [Google Scholar]
- 16.Moss DM, Priest JW, Boyd A, Weinkopff T, Kucerova Z, Beach MJ, Lammie PJ. Multiplex bead assay for serum samples from children in Haiti enrolled in a drug study for the treatment of lymphatic filariasis. Am J Trop Med Hyg. 2011;85:229–237. doi: 10.4269/ajtmh.2011.11-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamlin KL, Moss DM, Priest JW, Roberts J, Kubofcik J, Gass K, Streit TG, Nutman TB, Eberhard ML, Lammie PJ. Longitudinal monitoring of the development of antifilarial antibodies and acquisition of Wuchereria bancrofti in a highly endemic area of Haiti. PLoS Negl Trop Dis. 2012;6:e1941. doi: 10.1371/journal.pntd.0001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Truant AL, Elliott SH, Kelly MT, Smith JH. Comparison of formalin-ethyl ether sedimentation, formalin-ethyl acetate sedimentation, and zinic sulfate flotation techniques for detection of intestinal parasites. J Clin Microbiol. 1981;13:882–884. doi: 10.1128/jcm.13.5.882-884.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haque R, Mondal D, Duggal P, Kabir M, Roy S, Farr BM, Sack RB, Petri WA., Jr Entamoeba histolytica infection in children and protection from subsequent amebiasis. Infect Immun. 2006;74:904–909. doi: 10.1128/IAI.74.2.904-909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houpt E, Barroso L, Lockhart L, Wright R, Cramer C, Lyerly D, Petri WA. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine. 2004;22:611–617. doi: 10.1016/j.vaccine.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Halstead SB, Streit TG, Lafontant JG, Putvatana R, Russell K, Sun W, Kanesa-Thasan N, Hayes CG, Watts DM. Haiti: absence of dengue hemorrhagic fever despite hyperendemic dengue virus transmission. Am J Trop Med Hyg. 2001;65:180–183. doi: 10.4269/ajtmh.2001.65.180. [DOI] [PubMed] [Google Scholar]
- 22.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 23.Zeger SL, Kun-Yee Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1998;44:1049–1060. [PubMed] [Google Scholar]
- 24.Hlavsa MC, Watson JC, Beach MJ. Cryptosporidiosis surveillance–United States 1999–2002. MMWR Surveill Summ. 2005;54:1–8. [PubMed] [Google Scholar]
- 25.Hlavsa MC, Watson JC, Beach MJ. Giardiasis surveillance–United States, 1998–2002. MMWR Surveill Summ. 2005;54:9–16. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Summary of notifiable diseases–United States, 2010. MMWR. 2012;59:1–111. [PubMed] [Google Scholar]
- 27.Hanson LA, Korotkova M. The role of breastfeeding in prevention of neonatal infection. Semin Neonatol. 2002;7:275–281. doi: 10.1016/s1084-2756(02)90124-7. [DOI] [PubMed] [Google Scholar]
- 28.Morrow AL, Rangel JM. Human milk protection against infectious diarrhea: implications for prevention and clinical care. Semin Pediatr Infect Dis. 2004;15:221–228. doi: 10.1053/j.spid.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Shetty N, Narasimha M, Elliott E, Raj IS, Macaden R. Age-specific sero-prevalence of amoebiasis and giardiasis in southern Indian infants and children. J Trop Pediatr. 1992;38:57–63. doi: 10.1093/tropej/38.2.57. [DOI] [PubMed] [Google Scholar]
- 30.Haque R, Ali IM, Petri WA., Jr Prevalence and immune response to Entamoeba histolytica infection in preschool children in Bangladesh. Am J Trop Med Hyg. 1999;60:1031–1034. doi: 10.4269/ajtmh.1999.60.1031. [DOI] [PubMed] [Google Scholar]
- 31.Calderon J, Avila EE. Antibody-induced caps in Entamoeba histolytica: isolation and electrophoretic analysis. J Infect Dis. 1986;153:927–932. doi: 10.1093/infdis/153.5.927. [DOI] [PubMed] [Google Scholar]
- 32.Espinosa-Cantellano M, Martinez-Palomo A. Entamoeba histolytica: mechanism of surface receptor capping. Exp Parasitol. 1994;79:424–435. doi: 10.1006/expr.1994.1104. [DOI] [PubMed] [Google Scholar]
- 33.Arnone RD, Walling JP. Evaluating Cryptosporidium and Giardia concentrations in combined sewer overflow. J Water Health. 2006;4:157–165. [PubMed] [Google Scholar]
- 34.Jagai JS, Castronovo DA, Monchak J, Naumova EN. Seasonality of cryptosporidiosis: a meta-analysis approach. Environ Res. 2009;109:465–478. doi: 10.1016/j.envres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols G, Lane C, Asgari N, Verlander NQ, Charlett A. Rainfall and outbreaks of drinking water related disease and in England and Wales. J Water Health. 2009;7:1–8. doi: 10.2166/wh.2009.143. [DOI] [PubMed] [Google Scholar]
- 36.Kellar KL, Mahmutovic AJ, Bandyopadhyay K. Multiplexed microsphere-based flow cytometric immunoassays. Curr Protoc Cytom. 2006 doi: 10.1002/0471142956.cy1301s35. Chapter 13: Unit 13. 1. [DOI] [PubMed] [Google Scholar]
- 37.Kellar KL, Kalwar RR, Dubois KA, Crouse D, Chafin WD, Kane BE. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry. 2001;45:27–36. doi: 10.1002/1097-0320(20010901)45:1<27::aid-cyto1141>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 38.Jung S, Oh EJ, Yang CW, Ahn WS, Kim Y, Park YJ, Han K. Comparative evaluation of ELISA and Luminex panel reactive antibody assays for HLA alloantibody screening. Korean J Lab Med. 2009;29:473–480. doi: 10.3343/kjlm.2009.29.5.473. [DOI] [PubMed] [Google Scholar]
- 39.Wagner B, Freer H. Development of a bead-based multiplex assay for simultaneous quantification of cytokines in horses. Vet Immunol Immunopathol. 2009;127:242–248. doi: 10.1016/j.vetimm.2008.10.313. [DOI] [PubMed] [Google Scholar]
- 40.Elberse KE, Tcherniaeva I, Berbers GA, Schouls LM. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pnueumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin Vaccine Immunol. 2010;17:674–682. doi: 10.1128/CVI.00408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickering JW, Martins TB, Schroder MC, Hill HR. Comparison of multiplex flow cytometric assay with enzyme-linked immunosorbent assay for quantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae Type b. Clin Diagn Lab Immunol. 2002;9:872–876. doi: 10.1128/CDLI.9.4.872-876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]