Abstract
Toxocara spp. infection and the seroconversion rate in the Amazon have been poorly investigated. This study analyzed individual and household-level risk factors for the presence of IgG antibodies to Toxocara spp. in urban Amazonian children over a period of 7 years and evaluated the seroconversion rates over a 1-year follow-up. In children < 59 months of age, the overall prevalence rate was 28.08% in 2003 and 23.35% in 2010. The 2010–2011 seroconversion rates were 13.90% for children 6–59 months of age and 12.30% for children 84–143 months of age. Multilevel logistic regression analysis identified child age, previous wheezing, and current infection with hookworm as significant associated factors for Toxocara spp. seropositivity in 2003. In 2010, age, previous helminthiasis, and having a dog were associated with seropositivity, whereas having piped water inside the household was a protective factor. Control programs mainly need to target at-risk children, water quality control, and animal deworming strategies.
Introduction
Human toxocariasis is a disease caused by infection of larvae from Toxocara canis (from dogs) or Toxocara cati (from cats). In humans, development of the parasites does not occur and infections are accidental. Human beings acquire the infection by incidental ingestion of embryonated eggs present in soil or soil-contaminated food or water,1 or less frequently, by ingesting meat from intermediate hosts.2 Once ingested, the eggs hatch in the intestine, and the larvae reach systemic circulation and are distributed throughout the whole organism.3
The clinical presentation of human toxocariasis is varied and related to both parasitic load and immunological responses. Most of the cases have a benign or asymptomatic presentation, but severe symptoms may occur caused by larval migration,4 such as fever, eosinophilia, hepatomegaly, ocular symptoms, pulmonary or cardiac symptoms, and even cerebral lesions.5 Three major syndromes are recognized: visceral larva migrans, ocular larva migrans,6,7 and covert toxocariasis,8 in which only a few symptoms predominate, mainly respiratory symptoms. Most human infections are diagnosed serologically. Enzyme-linked immunosorbent assay (ELISA) for the detection of immunoglobulin (IgG) antibodies to excretory/secretory antigens of Toxocara spp. larvae is used most frequently.6,9
In Brazil, seroepidemiological surveys have indicated positivity rates between 21.5% and 52% in children < 15 years of age,9,10 but in other countries, prevalence can reach 85–86%.11,12
Although the seroprevalence in the general Brazilian population has been well described, the seroepidemiology of Toxocara spp. infection in the Amazon has been poorly studied. There are a few studies indicating the prevalence rates and risk factors in Brazil, Peru, and Venezuela. In the Brazilian Amazon, the prevalence in urban children < 5 years of age has been reported to be 21.5%,13 increasing to 26.7% in the rural general population, and to 26.8%14 or 52% in the riverine population.9 In the Peruvian and Venezuelan Amazon, seroprevalence rates of 35.6%15 and 34.9%16 have been found, respectively. Seroconversion studies are even scarcer, and only two small studies examining incidence rates in Brazil have been published thus far, in which the incidence rates were 7.63 per 100 children-years of follow-up17 and 17.9% in the general population.18
Risk factors for Toxocara infection have been found among environmental and household variables, including contact with contaminated soil or animals19,20; such studies can help identify geographical targets for intervention, such as environmental sanitary measures and educational public policies targeted to risk groups.14,21
In this study, we analyzed individual and household-level risk factors for the presence of IgG antibodies to larval antigens of Toxocara spp. in urban Amazonian children in the city of Assis Brasil, located on the border between Brazil and Peru between 2003 and 2010, evaluated the seroconversion rates between 2010 and 2011, and discussed possible interventions to control human toxocariasis in settings similar to this one.
Material and Methods
Study area.
Assis Brasil was founded in 1976 from old established communities in areas of rubber plantations. In 2003, the population was estimated at 3,667 inhabitants, and 38% lived in rural areas. As of 2011, it had a population of 6,075 inhabitants, of which 39% resided in rural areas22; it is located 344 miles southwest of Rio Branco (Figure 1), and it borders the municipality of Brasileia to the east, the cities of Iñapari (Peru) and Bolpebra (Bolivia) to the south, and the municipality of Sena Madureira to the north. The climate is equatorial hot and humid, a subdivision of tropical climate. It features a predominantly rainy season between November and April and a predominantly dry season between May and October. The average annual temperature is 24.5°C, ranging between 20°C and 32°C. The relative humidity is 80–90% throughout the year. The annual rainfall is between 1,600 and 2,750 mm. The native vegetation is open rainforest with palm trees and tropical rain forest.23
Figure 1.
Map showing the location of Brazil, the Amazon, Acre state, and the municipality of Assis Brasil. The map inside the box shows the Interoceanic Highway connecting Brazil to the Pacific Ocean.
Study design and population.
In this population-based study, the population investigated consisted of children living in the urban areas of Assis Brasil, state of Acre, Brazil, in the years of 2003 and 2010. The study was divided into a prevalence study and a seroconversion study. The children were located using the census records of the local community health workers.
The prevalence study consisted of two censuses performed in 2003 and 2010 with children 6 to 59 months of age in each year. In 2003, the census identified 182 children 6 to 59 months of age living in 180 households. In 2010, there were 357 children 6 to 59 months of age living in 283 households. We examined 80% (146) and 77% (275) of the eligible children each year in Assis Brasil.
The seroconversion study consisted of two cohorts, with children that were selected from one or the other cross-sectional study. In 2003, there were 105 children 6 to 59 months of age with a negative serology, which were re-examined in 2010, when they were between 6 and 12 years of age. Of these, 84 children had a negative serology in 2010, and they were tested again 12 months later. These children comprised cohort 1, which was tested three times (2003, 2010, and 2011). The second cohort consisted of 165 seronegative children between 6 and 59 months of age examined in the 2010 census and retested 12 months later, in 2011. The seroconversion rates and confidence intervals were calculated for cohort 1 and cohort 2 for the period 2010–2011.
Data collection occurred in January and February 2003, 2010, and 2011, through questionnaires, to investigate sanitation and housing conditions (type of housing construction, household connection to the sewage system, source and treatment of water supply, piped water supply inside the household, intermittence of water supply, waste collection and disposal, presence of open sewage near the house, type of toilet, presence of electric power, susceptibility to flooding during rain, presence of animals in the house), family socio-economic status (possession of household and household appliances, maternal education, whether benefiting from social assistance), child demographic information (age in years, gender, skin color), access to services and child care (attending nursery or school, living with one or both biological parents, number of siblings that died before 5 years of age), characteristics of the birth and breastfeeding (birth weight and duration of exclusive breastfeeding), occurrence and treatment of previous or present intestinal parasitosis, respiratory symptoms, and other diseases. We also collected information on the place of birth and time of residence of the mother and children in the house, in the city and in the state, to analyze the frequency of internal and external migration. Children not tested in the 2003 census (36 children) had no significant differences in their characteristics from those tested. Children not tested in the 2010 census tended to be younger (55 younger than 2 years of age, P < 0.001) and to report previous intestinal parasitosis with less frequency than those tested (P < 0.001).
Antigen preparation.
Excretory–secretory larval antigen for ELISA was prepared as described by Elefant and colleagues.24 Briefly, T. canis eggs collected from the uterus of female worms were embryonated after incubation in 2% formalin for ~1 month at 28°C and artificially hatched in serum-free Eagle medium. The L2 larvae were recovered and incubated at 37°C. At weekly intervals, the culture supernatant was removed, treated with 5 μL/mL of the protease inhibitor phenyl-methyl-sulfonyl fluoride (200 mM), concentrated with Amicon Ultrafiltration units (Millipore, Danvers, MA), dialyzed against distilled water, centrifuged (18,500 × g for 60 min at 4°C), and filtered using 0.22 μm Millipore membranes. The excretory–secretory larval antigen prepared with T. canis is likely to contain both species-specific epitopes and common epitopes that are shared by T. cati,25 but no attempt was made to determine the levels of between-species cross-reactivity observed in the standard diagnostic ELISA.
Preincubation of sera with Ascaris suum adult worm extract (AWE).
To remove antibodies elicited by exposure to Ascaris, which could cross-react with Toxocara antigens,26 the test samples were preincubated with an AWE of Ascaris suum.24 Briefly, the adult worms recovered from porcine intestines were macerated in distilled water, and 1.5 M NaOH was added for a final concentration of 0.15 M. After 2 hr of incubation at room temperature, the mixture was neutralized with 6 M HCl and then centrifuged (18,500 × g for 20 min at 4°C). After removing the lipid with ether, the supernatant was filtered using 0.22 μm Millipore membranes. All test sera were preincubated for 30 min at 37°C with a solution (25 μg/mL) of AWE in 0.01 M phosphate-buffered saline (PBS, pH 7.2) containing 0.05% Tween 20 (PBS-T) before use in ELISA.
Sample collection and antibody detection.
Venous blood was collected in sterile vacuum tubes with a clot activator. Samples were centrifuged, and the sera were separated and stored in −20°C until tested. Serum samples were tested for IgG antibodies to excreted–secreted larval antigens by ELISA at a dilution of 1:320, as previously described.14,24,27 Polystyrene 96-well microplates (Corning, Costar, New York, NY) were coated for 1 hr at 37°C followed by 18 hr at 4°C with 1.9 μg/mL of solid-phase antigen dissolved in 0.06 M carbonate-bicarbonate buffer, pH 9.6 (100 μL/well) and subsequently blocked for 2 hr at 37°C with PBS-T containing 2.5% bovine serum albumin (Sigma, St. Louis, MO). After a 40-min incubation at 37°C, the serum samples were removed, and horseradish peroxidase-conjugated goat anti-human IgG (Sigma) was added at a 1:10,000 dilution (40 min at 37°C), followed by the phenylenediamine substrate (0.4 mg/mL, Sigma). The absorbance readings were made at 492 nm. The cut-off absorbance value was defined as the mean absorbance reading for 96 negative control sera plus three standard deviations. Antibody levels were expressed as reactivity indices (RIs), which were calculated as the ratio of the absorbance values of each test sample and the cut-off value. Positive samples had RIs > 1.
Stool sample examination.
To determine whether seropositivity to Toxocara spp. was associated with current exposure to common intestinal nematodes of humans, we examined stool samples for intestinal parasite eggs, cysts, and larvae according to a standard sedimentation–concentration method.28 Logistical limitations prevented the collection of more than one stool sample from each subject, and 16.43% and 12.77% of children tested for Toxocara spp. did not provide a stool sample for analysis in the 2003 and 2010 censuses, respectively. Although parasite prevalence rates derived from the examination of a single stool sample are likely to be underestimated, this bias is considered to be relatively small for most common intestinal nematodes of humans,29 except for Strongyloides stercoralis. No significant differences (P > 0.05) were detected for most demographic, socio-economic, and environmental parameters between subjects who provided a stool sample or blood and those who did not provide such samples.
Statistical analysis.
A database was created with SPSS 13.0 software (SPSS Inc., Chicago, IL). The distribution of the independent variables was identified using analysis of variance to compare the means, and χ2 or Fisher tests were used for comparing frequencies or proportions with an α = 0.05 critical level for the years 2003, 2010, and 2011. A causal model for Toxocara spp. infection was developed a priori based on a literature review of risk factors for the Toxocara parasite and other intestinal parasites with similar methods of transmission.30 In this model, five levels of possible associated risk factors were considered: socio-economic variables, domestic and peridomestic conditions, access to health services and child care, characteristics of birth and breastfeeding, and morbidities and other child factors (Table 1).
Table 1.
Conceptual model for hierarchical analysis of factors associated with a positive serology for Toxocara spp.
| DISTAL | Block 1: Socio-economic variables |
| Wealth status | |
| Receipt of benefits | |
| Possession of household | |
| Years of parental schooling | |
| Block 2: Domestic and peridomestic environment | |
| Type of household | |
| House floor | |
| Presence of electric power | |
| Source of domestic water supply | |
| Piped water supply in house | |
| Source of drinking water | |
| Treatment of drinking water | |
| Intermittence of water supply | |
| Type of toilet | |
| Domestic waste disposal | |
| Public waste collection | |
| Presence of dogs and cats | |
| Susceptibility to flooding during rain | |
| Presence of open sewage near house | |
| INTERMEDIATE | Block 3: Access to health services and child care |
| Number of siblings that died before the age of 5 years | |
| Living with one or both biological parents | |
| Attending nursery or school | |
| PROXIMAL | Block 4: Characteristics of the birth and breastfeeding |
| Low birth weight | |
| Duration of exclusive breastfeeding | |
| Block 5: Morbidity and other child factors | |
| Age | |
| Gender | |
| Skin color | |
| Time living in the house | |
| Respiratory symptoms or diseases | |
| Other recent morbidities | |
| Previous or present diagnosis of intestinal parasites |
Adapted from Prado and others, 2003.30
A household wealth index using principal component analysis for 2003 and 2010 was constructed as validated by Filmer and Pritchett for urban areas.31 This index was created based on the presence of 21 consumer goods and household appliances (television, stereo, DVD player, gas stove, refrigerator, washing machine, telephone, bicycle, blender, electric iron, car, sofa, satellite dish, mobile phone, motorcycle, computer, boat, motor boat, water well, power generator, and microwave oven) as described in previous publications/studies.32,33
Multiple logistic regression analysis using the hierarchical conceptual model, as represented in Table 1, was performed using R software version 2.14.0 (The R Foundation for Statistical Computing, Vienna, Austria). Separate multiple logistic regression models for the years 2003 and 2010 were fitted to individual- and household-level variables using manual inclusion and forward selection procedures within each conceptual block of variables, starting from the most distal to the most proximal block of the conceptual model. Because of the missing values, only 118 and 265 observations were retained in the final models for 2003 and 2010, respectively.
The overall seroconversion rate and confidence intervals (CIs) were calculated between the years 2010 and 2011, using the binomial distribution and exact probability computation in the Epicalc package for R 2.14.0 software. Age-specific seroconversion rates were also calculated for intervals of 5 months (between 6 and 11 months of age) or 12 months of age for all other ages, using the same procedures as described previously. Differences were considered statistically significant if a P < 0.05 was obtained using χ2 or Fisher tests. Because of the time between 2003 and 2010 and the possible loss of antibodies or reinfection, we did not evaluate the seroconversion for this 7-year period. For the period 2010–2011, we considered as putative risk factors the same variables analyzed at baseline in 2010 for the two cohorts.
Ethical considerations.
The study was approved by the Ethics Committee for Research with Human Beings at the Federal University of Acre. We obtained informed consent from the legal guardian of each participant before the study. Children with a positive stool examination were treated with antiparasitic drugs.
Results
Parental and child socio-economic characteristics in 2003 and 2010.
There were some important socio-economic changes in the city between 2003 and 2010, namely in housing conditions, source of domestic water and drinking water, and size and ethnic composition of the pediatric population (Table 2). Although the main house building material continued to be wood, more houses tended to have cement, brick, or ceramic tile as the predominant home floor material in 2010. Other significant changes were observed in decreased house ownership, increased number of households with electric power, decreased number of latrines, improved waste collection, and increased number of houses with open sewage systems (Table 2).
Table 2.
Distal Block: distribution of children between 6 and 59 months of age according to socioeconomic and demographic features, Assis Brasil, Acre, 2003 and 2010
| Variables | 2003 | 2010 | P value† | ||
|---|---|---|---|---|---|
| (N = 182) | (N = 357) | ||||
| N* | % | N* | % | ||
| Block 1: Socio-economic | |||||
| Receipt of benefits | < 0.001 | ||||
| No | 172 | 94.5 | 249 | 69.7 | |
| Yes | 10 | 5.5 | 108 | 30.3 | |
| Possession of household | 0.018 | ||||
| Owned | 148 | 81.3 | 260 | 72.8 | |
| Not owned | 34 | 18.7 | 97 | 27.2 | |
| Years of maternal schooling | 0.314 | ||||
| 0 | 8 | 7.6 | 17 | 4.8 | |
| 1–4 | 23 | 21.9 | 88 | 24.8 | |
| 5–8 | 31 | 29.5 | 82 | 23.1 | |
| > 8 | 43 | 41.0 | 168 | 47.3 | |
| Block 2: Domestic and peridomestic environment | |||||
| Type of household construction | 0.164 | ||||
| Brick walls | 16 | 8.8 | 43 | 12.0 | |
| Wooden walls or another materials | 165 | 91.2 | 314 | 88.0 | |
| Predominant house floor material | 0.001 | ||||
| Cement, brick ceramic tile | 30 | 16.5 | 102 | 28.7 | |
| Wood or land | 152 | 83.5 | 254 | 71.3 | |
| Presence of electric power | < 0.001 | ||||
| No | 20 | 11.0 | 10 | 2.8 | |
| Yes | 162 | 89.0 | 347 | 97.2 | |
| Source of domestic water supply | < 0.001 | ||||
| Only public water supply | 139 | 76.4 | 285 | 79.8 | |
| Only well water supply | 0 | 0.0 | 34 | 9.5 | |
| Others sources including well | 43 | 23.6 | 20 | 5.7 | |
| Well and public water supply | 0 | 0.0 | 18 | 5.0 | |
| Piped water supply | – | ||||
| No | NA | NA | 28 | 7.3 | |
| Yes, only inside the household | NA | NA | 257 | 72.0 | |
| Yes, only outside the household | NA | NA | 74 | 20.7 | |
| Source of drinking water | < 0.001 | ||||
| Only public network | 134 | 73.6 | 176 | 49.7 | |
| Mineral or/and wells | 40 | 22.0 | 162 | 45.8 | |
| Rain, lakes and pitfalls | 8 | 4.4 | 16 | 4.5 | |
| Drinking mineral water | < 0.001 | ||||
| No | 171 | 94.0 | 218 | 61.1 | |
| Yes | 11 | 6.0 | 139 | 38.9 | |
| Treatment of drinking water | < 0.001 | ||||
| Untreated | 57 | 31.3 | 123 | 34.5 | |
| Mineral untreated | 11 | 6.0 | 123 | 34.5 | |
| Boil and/or filtered and/or chlorinated | 81 | 44.5 | 79 | 22.0 | |
| Only chlorinated | 33 | 18.2 | 32 | 9.0 | |
| Intermittence of water supply | < 0.001 | ||||
| Never | 136 | 74.7 | 104 | 30.3 | |
| Often or rarely | 46 | 25.3 | 239 | 69.7 | |
| Type of toilet | < 0.001 | ||||
| Flushed toilet | 27 | 14.8 | 222 | 62.1 | |
| Latrine | 115 | 63.2 | 103 | 28.9 | |
| Without toilet | 40 | 22.0 | 32 | 9.0 | |
| Domestic waste disposal | 0.002 | ||||
| Public garbage collection | 161 | 88.5 | 343 | 96.0 | |
| Buried or burned | 8 | 4.4 | 7 | 2.0 | |
| Played in the environment (open area or river) | 13 | 7.1 | 7 | 2.0 | |
| Public waste collection | 0.001 | ||||
| No | 21 | 11.5 | 14 | 3.9 | |
| Yes | 161 | 88.5 | 343 | 96.1 | |
| Dogs in the household | – | ||||
| No | NA | NA | 210 | 60.0 | |
| Yes | NA | NA | 140 | 40.0 | |
| Cats in the household | – | ||||
| No | NA | NA | 313 | 89.4 | |
| Yes | NA | NA | 37 | 10.6 | |
| Susceptibility to flooding during rain | – | ||||
| No | NA | NA | 204 | 59.1 | |
| Yes | NA | NA | 141 | 40.9 | |
| Presence of open sewage near house | < 0.001 | ||||
| No | 147 | 81.2 | 217 | 60.8 | |
| Yes | 34 | 18.8 | 140 | 39.2 | |
Missing in some variables.
Pearson's χ2 test.
NA = not available.
In 2003, most houses used water only from the public system (76.4%), whereas the rest used water solely from other sources (rain, river, or lakes). In 2010, the number of houses using water only from the public network increased to 79.8%, with a decrease of usage from other sources. Only 9.5% used water from their own wells (9.5%) or from both the public system and their wells (5%) (Table 2). The source of drinking water also changed (Table 2). There was a reduction in the frequency of children drinking water only from public sources from 73.6% to 49.7%, and an increase in the consumption of mineral water from 6.0% in 2003 to 38.9% in 2010. The usage of drinking water from rain, lakes, and pitfalls remained the same (4.4% in 2003 and 4.5% in 2010).
No significant changes were observed in the number of schooling years of the female guardian (Table 2). Between 2003 and 2010, the mothers of 133 (38.0%) children analyzed in 2010 migrated from other cities or from rural/ riverine areas of Assis Brasil to the urban area of this city, giving birth to a child in the subsequent years. Table 3 shows some of the changes in access to parental care and health services, mainly an increase in the deaths of a child younger than 5 years of age from 4.9% to 11.7%.
Table 3.
Intermediate Block: distribution of children between 6 and 59 months of age according to access to health services and child care, Assis Brasil, Acre, 2003 and 2010
| Variables | 2003 | 2010 | P value† | ||
|---|---|---|---|---|---|
| (N = 182) | (N = 357) | ||||
| N* | % | N* | % | ||
| Block 3: Acces to health services and child care | |||||
| Living with biological parents | 0.730 | ||||
| Living with biological parents | 125 | 67.7 | 240 | 67.2 | |
| Living with biological mother only | 47 | 25.8 | 91 | 25.5 | |
| Living with biological father only or none of the parents | 10 | 5.5 | 26 | 7.3 | |
| Any sibling died before 5 years of age? | 0.029 | ||||
| No | 97 | 95.1 | 309 | 88.3 | |
| Yes | 5 | 4.9 | 41 | 11.7 | |
| Attending nursery or school | |||||
| No | NA | NA | 227 | 65.6 | |
| Yes | NA | NA | 119 | 34.4 | |
Missing in some variables.
Pearson's χ2 test.
NA = not available.
Table 4 shows the characteristics of children < 5 years of age by year of study. The distribution of the population by gender and age were similar in both periods. There was a major change in race distribution, with an increase in indigenous children living in the urban area. The proportion of children presenting with previous episodes of wheezing or asthma decreased from 40.9% to 24.0%. Most of the children had been living in the urban area since birth. Only 10.4% in 2003 and 16.9% in 2010 had not been born in the urban area of Assis Brasil or had lived part of their lives in other places (rural areas, riverine areas, or other cities).
Table 4.
Proximal Block: distribution of children between 6 and 59 months of age according to characteristics of the birth and breastfeeding and other child factors, Assis Brasil, Acre, 2003 and 2010
| Variables | 2003 | 2010 | P value† | ||
|---|---|---|---|---|---|
| (N = 182) | (N = 357) | ||||
| N* | % | N* | % | ||
| Block 4: Characteristics of the birth and breastfeeding | |||||
| Low birth weight | 0.016 | ||||
| No, > 2.500 g | 151 | 96.2 | 280 | 90.3 | |
| Yes, ≤ 2.500 g | 6 | 3.8 | 30 | 9.7 | |
| Duration of exclusive breastfeeding‡ | 0.125 | ||||
| 30 days | 105 | 64.8 | 152 | 58.7 | |
| ≤ 30 days | 57 | 35.2 | 107 | 41.3 | |
| Block 5: Morbidity and other child factors | |||||
| Gender | 0.234 | ||||
| Men | 86 | 47.3 | 182 | 51.0 | |
| Female | 96 | 52.7 | 175 | 49.0 | |
| Age in months | 0.333 | ||||
| From 6 to 11 | 17 | 9.3 | 49 | 13.8 | |
| From 12 to 23 | 45 | 24.7 | 86 | 24.2 | |
| From 24 to 35 | 42 | 23.1 | 60 | 16.8 | |
| From 36 to 47 | 42 | 23.1 | 86 | 24.2 | |
| From 48 to 59 | 36 | 19.8 | 75 | 21.0 | |
| Skin color | < 0.001 | ||||
| White | 23 | 17.2 | 81 | 23.6 | |
| Black | 9 | 6.8 | 3 | 0.9 | |
| Indigenous | 5 | 3.8 | 35 | 10.2 | |
| Brown (“Pardo”)§ | 96 | 72.2 | 224 | 65.3 | |
| Previous helminthiasis in lifetime | – | ||||
| No | NA | NA | 172 | 48.5 | |
| Yes | NA | NA | 183 | 51.5 | |
| Wheezing or asthma in the previous 12 months | < 0.001 | ||||
| No | 104 | 59.1 | 263 | 76.0 | |
| Yes | 72 | 40.9 | 83 | 24.0 | |
| Other morbidities in the previous 12 months | < 0.001 | ||||
| No | 105 | 57.5 | 149 | 41.7 | |
| Yes | 77 | 42.3 | 208 | 58.3 | |
| Treatment of intestinal parasites in the previous six months | 0.028 | ||||
| No | 79 | 43.4 | 186 | 52.7 | |
| Yes | 103 | 56.6 | 167 | 47.3 | |
| Soil-transmitted helminthes in fecal exam in the day of interview | 0.200 | ||||
| No | 128 | 86.5 | 277 | 89.6 | |
| Yes | 20 | 13.5 | 32 | 10.4 | |
Missing in some variables.
Pearson's χ2 test.
Excluding the children that only breastfed.
Brown skin color is the one observed among offspring from Black and White people.
NA = not available.
Prevalence of antibodies in children aged 6 months to 59 months.
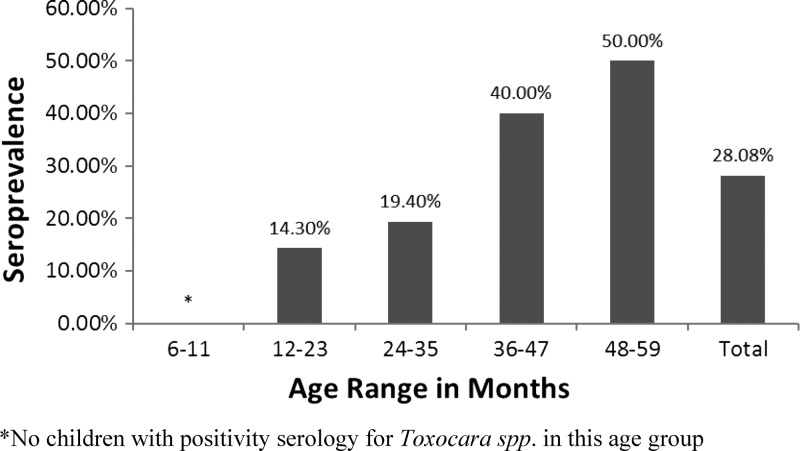
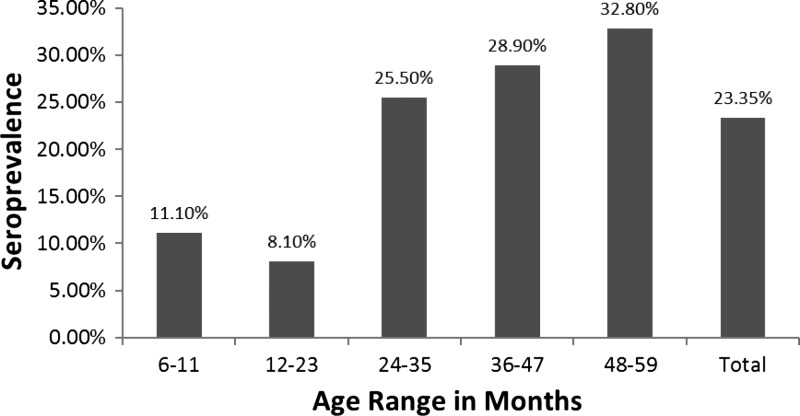
The IgG antibodies to Toxocara spp. larval antigens were detected in 41 and 65 subjects in 2003 and 2010, respectively. In 2003, the overall seroprevalence rate was 28.08% (95% CI, 21.43–35.86%), and in 2010, it was 23.35% (95% CI, 18.74–28.71%) This difference was not significant (P = 0.20). The seroprevalence rate was significantly higher among children 36–59 months of age than in younger children, both in 2003 and 2010 (P = 0.001 and 0.003, respectively, see Figures 2 and 3).
Figure 2.
Prevalence of immunoglobulin G (IgG) antibodies to excreted–secreted larval antigens of Toxocara canis according to age in Assis Brasil, 2003.
Figure 3.
Prevalence of immunoglobulin G (IgG) antibodies to excreted–secreted larval antigens of Toxocara canis according to age in Assis Brasil, 2010.
Factors associated with the presence of serum anti-Toxocara antibodies in 2003 and 2010.
In 2003 (Table 5), having positive serology was significantly associated with child age and wheezing or asthma in the past 12 months and current infection with other helminths (Ascaris lumbricoides and Trichuris trichiura in 33.3% of children).
Table 5.
Crude and adjusted odds ratio for having a positive serology for Toxocara spp. in children from 6 to 59 months of age, Assis Brasil, 2003
| Variables | Missing | Crude OR | P value* | Adjusted OR† | (CI 95%) | p value* |
|---|---|---|---|---|---|---|
| Child age | 0 | |||||
| Age in years | 2.14 | < 0.001 | 2.28 | (1.47–3.56) | < 0.001 | |
| Wheezing or asthma in the previous 12 months | 5 | |||||
| No | 1 | 1 | ||||
| Yes | 2.7 | 0.037 | 2.73 | (1.06–7.03) | 0.034 | |
| Soil-transmitted helminthes in fecal exam | 24 | |||||
| No | 1 | 1 | ||||
| Yes | 7.15 | 0.001 | 9.48 | (2.59–34.65) | 0.002 |
Wald test.
† Odds Ratio (OR) adjusted by all variables in the table and gender.
In 2010 (Table 6), age and having a previous history of intestinal parasitosis continued to be important factors associated with the presence of antibodies against Toxocara spp. On the day of blood draw, some of the seropositive children had a fecal exam positive for A. lumbricoides, T. trichiura, Ancylostoma spp., Hymenolepis nana (19.2%), or intestinal protozoa (36.5%), but no association was found between harboring intestinal parasites in the feces and positive serology. Having a dog was also associated with seropositivity, but having a cat was not. This information was not collected in 2003; therefore, we cannot compare the effect of owning a pet between the 2 years.
Table 6.
Crude and adjusted odds ratio for having a positive serology for Toxocara spp. in children from 6 to 59 months of age, Assis Brasil, 2010
| Variables | Missing | Crude OR | P value* | Adjusted OR† | (CI 95%) | P value* |
|---|---|---|---|---|---|---|
| Child age | 0 | |||||
| Age in years | 1.54 | 0.009 | 1.45 | (1.10–1.92) | 0.008 | |
| Dogs in the household | 7 | |||||
| No | 1 | 1 | ||||
| Yes | 1.86 | 0.02 | 2.11 | (1.13–3.95) | 0.019 | |
| Piped water supply inside the household | 0 | |||||
| No | 1 | 1 | ||||
| Yes | 0.29 | < 0.001 | 0.23 | (0.12–0.44) | < 0.001 | |
| Previous helminthiasis in lifetime | 2 | |||||
| No | 1 | 1 | ||||
| Yes | 2.22 | 0.049 | 1.97 | (1.00–3.88) | 0.024 |
Wald test.
Odds Ratio (OR) adjusted by all variables in the table and gender.
In 2010, having pumped piped water inside the household (either from the public system or from wells) was a protective factor compared with those that had piped water outside the household or did not have piped water at all (Table 6). Although the majority of children lived in houses with piped water inside (72.0%), ~20.7% had piped water only outside the household, coming either from the public system or wells. The remaining 7.3% extracted their water directly from rain, dams, or waterholes (Table 2). Therefore, those households without piped water inside the household were at higher risk of contamination, regardless of whether the water was coming from the public system, wells, or pitfalls.
Seroconversion rates for 2010–2011 and associated factors.
The 1-year seroconversion rate was 13.90% for children 6–59 months of age (CI 9.04–20.17%) and 12.30% for children 84–143 months of age (CI 6.37–22.45%). No age-specific differences were observed in the seroconversion rates in the follow-up (Table 7), suggesting that the observed age-prevalence pattern was caused by cumulative exposure rather than an age-related change in seroconversion rate.
Table 7.
Seroconversion rates for Toxocara spp. according to age strata, Assis Brasil, 2010–2011
| Absence of seroconversion | Presence of seroconversion | P value# | |||
|---|---|---|---|---|---|
| N* | % | N* | % | ||
| Child age in months | |||||
| Under 60 months of age* | 0.459 | ||||
| From 6 to 11 | 12 | 8.5 | 1 | 4.3 | |
| From 12 to 23 | 38 | 26.8 | 6 | 26.1 | |
| From 24 to 35 | 25 | 17.6 | 8 | 34.8 | |
| From 36 to 47 | 39 | 27.5 | 4 | 17.4 | |
| From 48 to 59 | 28 | 19.6 | 4 | 17.4 | |
| 84 months and over** | 0.438 | ||||
| From 84 to 95 | 11 | 20.0 | 1 | 12.5 | |
| From 96 to 107 | 10 | 18.2 | 3 | 37.5 | |
| From 108 to 119 | 14 | 25.5 | 2 | 25.0 | |
| From 120 to 131 | 12 | 21.8 | 0 | 0.0 | |
| From 132 to 143 | 8 | 14.5 | 2 | 25.0 | |
Fisher's exact test,
Cohort 2,
Cohort 1.
Having a dog in the household was the only factor associated with seroconversion in children 84–143 months of age (P = 0.019). In younger children (6–59 months of age), drinking water from a public source and the type of treatment of drinking water were associated with seroconversion in unadjusted analysis, but having piped water inside the house was not.
Drinking water from a public source was more frequent among those that seroconverted than in those that did not seroconvert (82.6% versus 49.3%, P = 0.002). On the other hand, the consumption of mineral water was more common among children who continued to be seronegative at the follow-up (42.3% versus 17.4%, P = 0.018). The majority of children who seroconverted drank water from the public system without any treatment (52.2%). A few of them treated the water from the public system with chlorine only (13.1%) or treated the water with multiple methods (chlorine, boiling, or filtering, 17.4%). Three children that seroconverted drank only mineral water without treatment. Among those children that remained seronegative, only 29.6% drank water from the public system without any treatment.
Neither the wealth index based on consumable items nor other variables included in the analysis were associated with Toxocara spp. seroconversion (P > 0.05).
Discussion
The prevalence of anti-Toxocara antibodies in Assis Brasil varied from 23% in 2003 to 28% in 2010 (P = 0.20, χ2 test). This result is similar to the prevalence found in other studies of children < 15 years of age, such as those from non-Amazonian Brazil (21.8–48.4%),34–37 Nigeria (29.6%),38 Iran (25–25.6%),39,40 and Argentina (23–37.9%).41,42
There are few studies published about toxocariasis epidemiology in the Brazilian Amazon. Damian and colleagues9 found a prevalence of 33% of anti-Toxocara antibodies in children 0–5 years of age living in the state of Amazonas. This prevalence increased to 50% between 6 and 10 years of age. Previous studies in Acre detected a seroprevalence of 26.8% in the general rural population, with a higher prevalence (36.6%) in children between 5 and 14 years of age.14 In other parts of the Amazon, higher prevalence has been found, such as in the Peruvian Amazon (32.4–44.92%)15,43,44 and in the Venezuelan Amazon (34.9%).16 This higher prevalence can be explained by the fact the study populations included older subjects.
There was a change of 5.0% in the seroprevalence of Toxocara spp. in Assis Brasil between 2003 and 2010, but this change was not significant. In other words, factors that may have contributed to this minor decrease in seroprevalence were not enough to decrease Toxocara spp. transmission rates as expected, according to previous publications6; this may be a result of several reasons. First, between 2003 and 2010, there was an improvement in certain living conditions, such as house floor type, house latrines, garbage disposal, and house ownership. Second, the sources of domestic and drinking water supply also improved. However, domestic water quality worsened, as more houses tended to frequently lack water, and open sewage increased significantly.
Another important factor that may have contributed to maintaining transmission levels is that the actual size of the study population increased from 182 to 357 children between 2003 and 2010. This is a combined result of population growth (increased number of children born) and internal and external migration. The fact that almost 40.0% of the families moved to the urban area of Assis Brasil after 2003 but only 16.9% of the children had not been born in the urban area indicates that early in the study period, migration was reinforced. However, as the majority of the children had been born in the urban area to migrant parents, the majority of the infections detected were most likely acquired in the urban setting. The construction of the Interoceanic Highway between Rio Branco and the Pacific Ocean, which was terminated on the Brazilian side in early 2003, may have contributed to this internal migration. However, the municipality did not have adequate urban infrastructure to deal with the consequences of economic development, resulting in increased urban illegal settlement areas with worsened living conditions.
The main factors associated with seroprevalence were having a piped water supply inside the household, ownership of a dog, child age, previous history of asthma or wheezing, and previous or present history of helminthiasis.
Our results suggest that Toxocara spp. transmission occurred in our study mainly because of the use of contaminated water in children < 60 months of age. Even with an improvement in the public sanitation system of Assis Brasil from 2003 to 2010, and more houses receiving piped water, this improvement per se was not capable of decreasing toxocariasis prevalence. Being able to pump water from the public network inside the house can protect from infection, which suggests that water contamination is occurring in the peridomestic environment after the water comes from the public system, most likely because peridomestic sanitation conditions did not improve to the same extent as the water supply. Wandering infected animals in the peridomestic environment, waste disposal, and open sewage in the house are the most likely sources of domestic and drinking water contamination by eggs. Similar results were found by Baboolal and Rawlins45 when studying 1,009 school-age children in the Caribbean. It was observed that children without access to piped water were at higher risk of infection in Trinidad45; in Turkey, not having access to piped water was also associated with toxocariasis.46
Approximately 40.0% of infected children in Assis Brasil had a dog as a pet. Damian and others9 also found that the main risk factor for anti-Toxocara antibodies in children was household contact with dogs or puppies. Mendonça and others37 reported that in a cohort with a high prevalence of seropositive children, only 15.0% of them washed their hands after contact with pets. This putative risk factor was confirmed in another study performed in the Amazon in which 95 dogs were assessed for the presence of eggs in their feces, and 18.9% of the dogs were positive for T. canis, which is the second most prevalent canine parasite.47 Several other studies also found an association between dog ownership or dog contact and toxocariasis.4,39,41,48,49 Wolfe and Wright50 analyzed fur samples from 60 dogs in Ireland and United Kingdom, and they found the presence of T. canis eggs in 25% of the samples, proving the possibility of parasite transmission because of direct contact with dogs. Other studies found prevalence between 21.56% and 95% of dogs with eggs in their fur.51–53
The seroconversion rate obtained here is slightly higher than those described in non- Amazonian areas of Brazil (7.63%)17; the seroprevalence of anti-Toxocara antibodies increased during childhood in Assis Brasil but not the seroconversion rates. In other words, no influence of behavioral or biological features that can explain the rise in seroprevalence according to age could be shown in this study, only a cumulative effect of exposure. These findings are corroborated by Agudelo and colleagues,54 who state that the differences in seroprevalence according to age are the result of the persistence of antibodies for several years after the initial infection or the result of several infections until the age of 20. Given that the factors associated with seroconversion changed with age but not the seroconversion rate per itself, it is reasonable to assume that both contaminated water and animal contact were equally efficient in maintaining Toxocara spp. transmission among this population.
The occurrence of recent wheezing was associated with the presence of antibodies against Toxocara spp. in this study. This relationship between wheezing and toxocariasis has been described by several authors. Figueiredo and colleagues48 studied 208 children between 1 and 14 years of age in a large urban city in Brazil and detected a positive association between asthma and a positive serology for Toxocara spp., mainly in children > 3 years. Bujs and colleagues55 found that seropositive kindergarten children presented with a higher prevalence of asthma and recurrent bronchitis than those that were seronegative. Chan and colleagues and Cobzaru and colleagues56,57 both showed that asthmatic children had higher titers of anti-Toxocara antibodies than non-asthmatic children.
How Toxocara spp. causes asthma remains unclear. Bujs and colleagues55 showed that Toxocara larval antigens favor the development of a Th2 response, with production of interleukin-4 (IL-4) and IL-5 cytokines and consequent production of IgE by B lymphocytes. At the same time, Toxocara larval stages can migrate into the lung, causing tissue alterations that result in coughing, wheezing, asthma-like symptoms, and pulmonary infiltrate.58 Dabrowska and others (2012)59 showed eosinophilic perivasculitis and vasculitis in mice lungs infected with T. canis, and Pinelli and others (2005)60 showed that mice infected with T. canis develop airway hyper-responsiveness, increased levels of IgE and eosinophils in bronchoalveolar lavage, and increased levels of IL-5 after experimental infection, with the presence of T. canis larvae observed even after 60 days post-infection.
In our study, some seropositive children were infected with A. lumbricoides, T. trichiura, Ancylostoma spp., and H. nana, and an association was found between anti-Toxocara antibodies and a previous history of intestinal parasitosis. Other studies have indicated the existence of an association between toxocariasis and other helminth infections, such as T. trichiura, Schistosoma mansoni, and A. lumbricoides61,62; this association can be explained because the mechanism of transmission of Toxocara spp. is similar to other helminthes, and therefore, the risk of infection is similar in areas where these parasites co-exist. At the same time, cross-reactivity, caused by the antigenic similarity between intestinal parasites, can occur in the immunoassay.63,64 Therefore, we pre-absorbed all tested sera with antigens of Ascaris suum to minimize the presence of false-positive immunological reactions with derivatives of this parasite26 in our study. This technical procedure has been described by several groups as increasing the specificity of the test because it reduces cross-reactivity to Ascaris spp.63 However, there may have been some degree of cross-reaction with T. trichiura65 that could not be properly controlled. No decrease in the specificity of the excretory antigen ELISA has been found in the literature after incubation with Ancylostoma antigens36; therefore, this possible bias is minimized in our study. Recently, Mendonça and others (2013)36 described a new in-house ELISA procedure using preincubation with Ascaris lumbricoides antigens instead of A. suum antigens, which resulted in higher Toxocara ELISA specificity. This new procedure may help reduce cross-reactivity issues in future studies. Because an association was found between seropositivity and a medical history of previous intestinal parasitosis, it is unlikely that cross-reactivity biased the findings.
This study has some limitations. The first limitation is that toxocariasis is measured by the presence of serum IgG antibodies only, and therefore, it is not possible to differentiate between acute and previous infections. However, serological diagnosis of acute human toxocariasis is still limited, and even specific IgM antibodies are reported to occur in both acute and chronic phases of toxocariasis.66,67 Lopez and others (2005)68 tried to follow 19 children that were treated for acute toxocariasis measuring IgM levels, and half of them remained with detectable IgM after 18 months of follow-up. In the same study, of 75 positive children, only three were negative for IgG.68 Elefant and others (2006)24 also attempted to follow acute cases of toxocariasis using serology, and the only good marker of acute infection was IgG, although it cannot be used for treatment follow-up. Even the detection of other classes of immunoglobulins (IgE, IgA, and subclasses of IgG) or circulating Ag is not able to discriminate between active and self-cured generalized toxocaral infections.67 The best way to evaluate acute toxocariasis remains in the use of clinical symptoms coupled with non-specific laboratory diagnosis, such as counting the number of eosinophils in a hemogram,67 which was not possible in this study.
Detection bias during follow-up may have occurred as well, as antibodies against Toxocara spp. are not present forever in the sera. Although the duration of such antibodies has been reported to be as long as 4 years,24 it is still possible that a Toxocara spp. primary infection or reinfection occurring long before 2003 or right after 2003 and 2010 may not have been detected because of a decrease in IgG levels below detectable levels. However, the extent of this bias must be small, as the prevalence rates in this study are close to the reported prevalence in other areas of the Amazon.10,14,16
Another limitation of this study is the number of missing cases in 2003 and 2010. For 2003, these limitations are mostly a result of children not being tested for the presence of antibodies. Given that no significant differences were found between tested and non-tested children, this limitation is unlikely to have biased the study. However, the small number of children tested may in fact have decreased the power of the study to detect significant associations for this year. In addition, two possible risk factors were not investigated in 2003, the presence of animals and piped water in the house, which limits the study.
For 2010, non-tested children were less likely to have had a previous intestinal parasite, therefore a selection bias may have occurred in which those mothers who perceived their children as healthy may have agreed to have them tested less frequently, which may have led to an overestimation of the association between the presence of anti-Toxocara IgG and intestinal parasitosis. However, as this variable has been shown to be associated in other studies with toxocariasis, this bias does not invalidate the present findings. Approximately 63% of eligible children < 1 year of age were not examined because of technical limitations in blood sampling. Therefore, the seroprevalence in this age stratum may have been underestimated. The lack of association between the socioeconomic index based on consumption of goods measured at the time of blood sampling and Toxocara infection may be explained by the fact that it may not be an adequate instrument to correlate with past exposure, but socioeconomic conditions were also measured in additional ways.
Finally, recall biases may have occurred for a few variables (the number of siblings that died before 5 years of age and duration of exclusive breastfeeding), which would have reduced the power of the study to detect a significant association between them and having a positive serology for Toxocara spp. The small number of children who seroconverted between 2010 and 2011 is also a limitation of the study because it was not possible to control the analysis for all confounding factors. At the same time, the small number of events may have prevented the detection of other important associations with seroconversion.
Toxocariasis is a parasitic disease that occurs at high prevalence in poor countries, and the Amazon region is vulnerable to it as well. Although associated with the quality of living conditions, the general improvement of social, economic, and environmental conditions, as has been occurring in the Amazon, does not appear to be sufficient for specifically reducing the transmission of this parasite. Even after 8 years of accelerated Amazonian regional development, such as the construction of the Pacific road, new health care units, improved waste collection, and other public policies in the Acre state, the toxocariasis prevalence remained the same.
Human toxocariasis control in the Amazon requires deeper investigation of personal hygiene habits, detection and treatment of infected individuals, and the empirical use of chemoprophylaxis in higher risk subjects, such as young children. For the successful control of animal toxocariasis in the Amazon, a study on the ownership of cats and dogs and the prevalence of Toxocara spp. eggs in their feces and in public areas such as schools and recreational places must be performed. Only the treatment of infected children and dogs and the adoption of education programs will be effective for reducing transmission.
Footnotes
Financial support: This study received financial aid from the UFAC (Brazil) and FUNTAC (Programa Pesquisa para o SUS Edital MS/CNPq/FDCT-FUNTAC/SESACRE n. 01/09) and support from the UFAC Master Program in Public Health. Research fellowships were awarded by CNPq, UFAC, DECIT, and CAPES-Reuni.
Authors' addresses: Humberto Oliart-Guzmán, Breno M. Delfino, Antonio C. Martins, Saulo A. S. Mantovani, Athos M. Braña, Thasciany M. Pereira, Fernando L. C. C. Branco, Alanderson A. Ramalho, Rhanderson G. Campos, Cristieli S. M. de Oliveira, and Pascoal T. Muniz, Universidade Federal do Acre - Centro de Ciências da Saúde e do Desporto, Rio Branco, Acre, Brazil, E-mails: hosoliart@hotmail.com, brenomd_@hotmail.com, antonio_camargo@hotmail, sauloaugustomantovani@hotmail.com, athosbrana@hotmail.com, thascy_moraes@hotmail.com, flccbranco@uol.com.br, alandersonalves@hotmail.com, doutorhanderson@hotmail.com, crisufac@hotmail.com, and pascoaltorres@uol.com.br. Pablo S. Fontoura, Instituto de Medicina Tropical - Laboratório de Soroepidemiologia e Imunobiologia, São Paulo, Brazil, E-mails: pablobiomed@yahoo.com.br. Thiago S. de Araujo, Federal University of Acre, Rio Branco, Acre, Brazil, E-mail: thiagoacre@gmail.com. Guita Rubinsky-Elefant, Institute of Tropical Medicine of São Paulo-University of São Paulo - Laboratory of Soroepidemiology and Immunobiology, São Paulo, Brazil, and Institute of Biomedical Sciences- University of São Paulo - Parasitology, São Paulo, Brazil, E-mails: guitare@usp.br or muferrei@usp.br. Cláudia T. Codeço, Oswaldo Cruz Foundation - Program for Scientific Computing, Rio de Janeiro, Brazil, E-mail: codeco@fiocruz.br. Mônica da Silva-Nunes, Centro de Ciências da Saúde e do Desporto, Universidade Federal do Acre, Acre, Brazil, E-mail: msnunes1@yahoo.com.br.
References
- 1.Santarem VA, Rubinsky-Elefant G, Ferreira MU. Soil-transmitted helminthic zoonoses in humans and associated risk factors. In: Pascucci S, editor. Soil Contamination. Rijeka, Croatia: InTech; (2011). pp. 43–66. [Google Scholar]
- 2.Choi D, Lim JH, Choi DC, Lee KS, Paik SW, Kim SH, Choi YH, Huh S. Transmission of Toxocara canis via ingestion of raw cow liver: a cross-sectional study in healthy adults. Korean J Parasitol. 2012;50:23–27. doi: 10.3347/kjp.2012.50.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor MR, Keane CT, O'Connor P, Mulvihill E, Holland C. The expanded spectrum of toxocaral disease. Lancet. 1988;1:692–695. doi: 10.1016/s0140-6736(88)91486-9. [DOI] [PubMed] [Google Scholar]
- 4.Chieffi PP, Ueda M, Camargo ED, de Souza AM, Guedes ML, Gerbi ML, Spir M, Moreira AS. Visceral larva migrans: a seroepidemiological survey in five municipalities of São Paulo state, Brazil. Rev Inst Med Trop Sao Paulo. 1990;32:204–210. doi: 10.1590/s0036-46651990000300010. [DOI] [PubMed] [Google Scholar]
- 5.Overgaauw PA. Aspects of Toxocara epidemiology: human toxocarosis. Crit Rev Microbiol. 1997;23:215–231. doi: 10.3109/10408419709115137. [DOI] [PubMed] [Google Scholar]
- 6.Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003;16:265–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlowski Z. Toxocariasis in humans: clinical expression and treatment dilemma. J Helminthol. 2001;75:299–305. doi: 10.1017/s0022149x01000464. [DOI] [PubMed] [Google Scholar]
- 8.Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalence and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010;104:3–23. doi: 10.1179/136485910X12607012373957. [DOI] [PubMed] [Google Scholar]
- 9.Damian MM, Martins M, Sardinha JF, Souza LO, Chaves A, Tavares Ade M. Frequency of the antibody anti-Toxocara canis in a community along the Uatuma river, State of Amazonas. Rev Soc Bras Med Trop. 2007;40:661–664. doi: 10.1590/s0037-86822007000600013. [DOI] [PubMed] [Google Scholar]
- 10.Matos MF, Melitão DN, Brum MA, Omais M, Quilião ME, Dorval ME, Pereira Ada C, Possi LA, Sauer L, Camargo ED, Tundisi RN. Presence of anti-Toxocara antibodies in children selected at Hospital Universitário, Campo Grande, MS, Brazil. Rev Inst Med Trop Sao Paulo. 1997;39:49–50. doi: 10.1590/s0036-46651997000100010. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi E, Tuda J, Imada M, Akao N, Fujita K. The high prevalence of asymptomatic Toxocara infection among schoolchildren in Manaso, Indonesia. Southeast Asian J Trop Med Public Health. 2005;36:1399–1406. [PubMed] [Google Scholar]
- 12.Thompson DE, Bundy DA, Cooper ES, Schantz PM. Epidemiological characteristics of Toxocara canis zoonotic infection of children in a Caribbean community. Bull World Health Organ. 1986;64:283–290. [PMC free article] [PubMed] [Google Scholar]
- 13.Rubinsky-Elefant G, da Silva-Nunes M, Malafronte RS, Muniz PT, Ferreira MU. Human toxocariasis in rural Brazilian Amazonia: seroprevalence, risk factors, and spatial distribution. Am J Trop Med Hyg. 2008;79:93–98. [PubMed] [Google Scholar]
- 14.Ferreira MU, Rubinsky-Elefant G, de Castro TG, Hoffmann EH, da Silva-Nunes M, Cardoso MA, Muniz PT. Bottle feeding and exposure to Toxocara as risk factors for wheezing illness among under-five Amazonian children: a population-based cross-sectional study. J Trop Pediatr. 2007;53:119–124. doi: 10.1093/tropej/fml083. [DOI] [PubMed] [Google Scholar]
- 15.Roldán WH, Cavero YA, Espinoza YA, Jiménez S, Gutiérrez CA. Human toxocariasis: a seroepidemiological survey in the Amazonian city of Yurimaguas, Peru. Rev Inst Med Trop Sao Paulo. 2010;52:37–42. doi: 10.1590/s0036-46652010000100006. [DOI] [PubMed] [Google Scholar]
- 16.Lynch NR, Eddy K, Hodgen AN, Lopez RI, Turner KJ. Seroprevalence of Toxocara canis infection in tropical Venezuela. Trans R Soc Trop Med Hyg. 1988;82:275–281. doi: 10.1016/0035-9203(88)90446-4. [DOI] [PubMed] [Google Scholar]
- 17.Correa CR, Bismarck CM. Toxocariasis: incidence, prevalence and the time serum remains positive in school children from Campinas, SP, Brasil. J Trop Pediatr. 2010;56:215–216. doi: 10.1093/tropej/fmp095. [DOI] [PubMed] [Google Scholar]
- 18.Anaruma Filho F, Chieffi PP, Correa CR, Camargo ED, da Silveira EP, Aranha JJ. Human toxocariasis: incidence among residents in the outskirts of Campinas, State of São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2003;45:293–294. doi: 10.1590/s0036-46652003000500010. [DOI] [PubMed] [Google Scholar]
- 19.Paquet-Durand I, Hernández J, Dolz G, Zuñiqa JJ, Schinieder T, Epe C. Prevalence on Toxocara spp., Toxoscaris leonine and ancylostomidae in public parks and beaches in different climate zones of Costa Rica. Acta Trop. 2007;104:30–37. doi: 10.1016/j.actatropica.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Capuano DM, Rocha Gde M. Environmental contamination by Toxocara spp. eggs in Riberão Preto, São Paulo, State, Brasil. Rev Inst Med Trop Sao Paulo. 2005;47:223–226. doi: 10.1590/s0036-46652005000400009. [DOI] [PubMed] [Google Scholar]
- 21.Fontanarrosa MF, Vezzani D, Basabe J, Eiras DF. An epidemiological study of gastrointestinal parasites of dogs from Southern Greater Buenos Aires (Argentina): age, gender, mixed infections, and seasonal and spatial patterns. Vet Parasitol. 2006;136:283–295. doi: 10.1016/j.vetpar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Instituto Brasileiro de Geografia e Estatística Resultados parciais do Estado do Acre no Censo 2010. (2010). http://www.ibge.gov.br/home/estatistica/populacao/censo2010/tabelas_ pdf/total_populacao_acre.pdf Available at. Accessed October 10, 2012.
- 23.Sistema Estadual de Informações Ambientais . Assis Brasil, Acre: http://www.seiam.ac.gov.br Available at. Accessed October 10, 2012. [Google Scholar]
- 24.Elefant GR, Shimizu SH, Sanchez MC, Jacob CM, Ferreira AW. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA, and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal. 2006;20:164–172. doi: 10.1002/jcla.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy MW, Maizels RM, Meghji M, Young L, Qureshi F, Smith HV. Species-specific and common epitopes on the secreted and surface antigens of Toxocara cati and Toxocara canis infective larvae. Parasite Immunol. 1987;9:407–420. doi: 10.1111/j.1365-3024.1987.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 26.Romasanta A, Romero JL, Arias M, Sánchez-Andrade R, López C, Suárez JL, Díaz P, Díez-Baños P. Diagnosis of parasitic zoonoses by immunoenzymatic assay-analysis of cross-reactivity among the excretory/secretory antigens of Fasciola hepatica, Toxocara canis, and Ascaris suum. Immunol Invest. 2003;32:131–142. doi: 10.1081/imm-120022974. [DOI] [PubMed] [Google Scholar]
- 27.Prestes-Carneiro LE, Santarém V, Zago SC, Miguel NA, Zamballi Sde F, Villas R, Vaz AJ, Rubinsky-Elefant G. Sero-epidemiology of toxocariasis in a rural settlement in São Paulo state, Brazil. Ann Trop Med Parasit. 2008;102:347–356. doi: 10.1179/136485908X278801. [DOI] [PubMed] [Google Scholar]
- 28.de Souza EA, da Silva-Nunes M, Malafronte Rdos S, Muniz PT, Cardoso MA, Ferreira MU. Prevalence and spatial distribution of intestinal parasitic infections in a rural Amazonian settlement, Acre State, Brasil. Cad Saude Publica. 2007;23:427–434. doi: 10.1590/s0102-311x2007000200019. [DOI] [PubMed] [Google Scholar]
- 29.Gyorkos TW, MacLean JD, Law CG. Absence of significant differences in intestinal parasite prevalence estimates after examination of either one or two stool specimens. Am J Epidemiol. 1989;130:976–980. doi: 10.1093/oxfordjournals.aje.a115430. [DOI] [PubMed] [Google Scholar]
- 30.Prado MS, Strina A, Barreto ML, Oliveira-Assis AM, Paz LM, Cairncross S. Risk factors for infection with Giardia duodenalis in pre-school children in the city of Salvador, Brazil. Epidemiol Infect. 2003;131:899–906. doi: 10.1017/s0950268803001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data on tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 32.Muniz PT, Castro TG, Araújo TS, Nunes NB, da Silva-Nunes M, Hoffmann EH, Ferreira MU, Cardoso MA. Child health and nutricion in the western Brazilian Amazon: population-based surveys in two counties in Acre State. Cad Saude Publica. 2007;23:1283–1293. doi: 10.1590/s0102-311x2007000600004. [DOI] [PubMed] [Google Scholar]
- 33.da Silva-Nunes M, Codeço CT, Malafronte RS, da Silva NS, Juncansen C, Muniz PT, Ferreira MU. Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg. 2008;79:624–635. [PubMed] [Google Scholar]
- 34.Campos Júnior D, Elefant GR, de Melo E, Silva EO, Gandolfi L, Jacob CM, Tofeti A, Pratesi R. Frequency of seropositivity to Toxocara canis in children of different socioeconomic strata. Rev Soc Bras Med Trop. 2003;36:509–513. [PubMed] [Google Scholar]
- 35.Muradian V, Gennari SM, Glickman LT, Pinheiro SR. Epidemiological aspects of visceral larva migrans in children living at São Remo Community, São Paulo (SP), Brazil. Vet Parasitol. 2005;134:93–97. doi: 10.1016/j.vetpar.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 36.Mendonça LR, Figueiredo CA, Esquivel R, Fiaccone RL, Pontes-de-Carvalho L, Cooper P, Barreto ML, Alcantare-Neves NM. Seroprevalence and risk factors for Toxocara infection in children from an urban large setting in northeast Brazil. Acta Trop. 2013;128:90–95. doi: 10.1016/j.actatropica.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paludo ML, Falavigna DL, Elefant GR, Gomes ML, Baggio ML, Amadei LB, Falavigna-Guilherme AL. Frequency of Toxocara infection in children attended by the health public service of Maringá, south Brazil. Rev Inst Med Trop Sao Paulo. 2007;49:343–348. doi: 10.1590/s0036-46652007000600002. [DOI] [PubMed] [Google Scholar]
- 38.Ajayi OO, Duhlinska DD, Agwale SM, Njoku SM. Frequency of human toxocariasis in Jos, Plateau State, Nigeria. Mem Inst Oswaldo Cruz. 2000;95:147–149. doi: 10.1590/S0074-02762000000200002. [DOI] [PubMed] [Google Scholar]
- 39.Sharif M, Daryani A, Barzegar G, Nasrolahei M, Khalilian A. Seroprevalence of toxocariasis in schoolchildren in northern Iran. Pak J Biol Sci. 2010;13:180–184. doi: 10.3923/pjbs.2010.180.184. [DOI] [PubMed] [Google Scholar]
- 40.Sadjjadi SM, Khosravi M, Mehrabani D, Orya A. Seroprevalence of Toxocara infection in school children in Shiraz, southern Iran. J Trop Pediatr. 2000;43:327–330. doi: 10.1093/tropej/46.6.327. [DOI] [PubMed] [Google Scholar]
- 41.Chiodo P, Basualdo J, Ciarmela L, Pezzani B, Apezteguia M, Minvielle M. Related factors to human toxocariasis in a rural community of Argentina. Mem Inst Oswaldo Cruz. 2006;101:397–400. doi: 10.1590/s0074-02762006000400009. [DOI] [PubMed] [Google Scholar]
- 42.Alonso JM, Bojanich MV, Chamorro M, Gorodner JO. Toxocara seroprevalence in children from a subtropical city in Argentina. Rev Inst Med Trop Sao Paulo. 2000;42:235–237. doi: 10.1590/s0036-46652000000400010. [DOI] [PubMed] [Google Scholar]
- 43.Espinoza Y, Roldán W, Huapaya P, Huiza A, Jiménez S. Prevalencia de anticuerpos IgG anti-Toxocara, en pobladores del distrito de Perené. Departamento de Junín. An Fac Med. 2006;67((Suppl 1)):S66. [Google Scholar]
- 44.Roldán WH, Espinoza YA, Huapaya PE, Huiza AF, Sevilla CR, Jimenéz S. Frequency of human toxocariasis in a rural population from Cajamarca, Peru determined by DOT-ELISA test. Rev Inst Med Trop Sao Paulo. 2009;51:67–71. doi: 10.1590/s0036-46652009000200002. [DOI] [PubMed] [Google Scholar]
- 45.Baboolal S, Rawlins SC. Seroprevalence of toxocariasis in schoolchildren in Trinidad. Trans R Soc Trop Med Hyg. 2002;96:139–143. doi: 10.1016/s0035-9203(02)90281-6. [DOI] [PubMed] [Google Scholar]
- 46.Dermirci M, Kaya S, Cetin E, Aridogan B, Onal S, Korkmaz M. Seroepidemiological investigation of toxocariasis in the sparta region of Turkey. Iran J Parasitol. 2010;5:52–59. [PMC free article] [PubMed] [Google Scholar]
- 47.Labruna MB, Pena HF, Souza SL, Pinter A, Silva JC, Ragozo AM, Camargo LM, Gennari SM. Prevalência de endoparasitas em cães da área urbana do município de Monte Negro, Rondônia. Arq Inst Biol (Sao Paulo) 2006;73:183–193. [Google Scholar]
- 48.Figueiredo SD, Taddei JA, Menezes JJ, Novo NF, Silva EO, Cristóvão HL, Cury MC. Clinical-epidemiological study of toxocariasis in a pediatric population. J Pediatr (Rio J) 2005;81:126–132. [PubMed] [Google Scholar]
- 49.Teixeira CR, Chieffi PP, Lescano SA, de Melo Silva EO, Fux B, Cury MC. Frequency and risk factors for toxocariasis in children from pediatric outpatient center in southeastern Brazil. Rev Inst Med Trop Sao Paulo. 2006;48:251–255. doi: 10.1590/s0036-46652006000500003. [DOI] [PubMed] [Google Scholar]
- 50.Wolfe A, Wright IP. Human toxocariasis and direct contact with dogs. Vet Rec. 2003;152:419–422. doi: 10.1136/vr.152.14.419. [DOI] [PubMed] [Google Scholar]
- 51.Aydenizöz-Ozkayhan M, Yagci BB, Erat S. The investigation of Toxocara canis eggs in coats of different dog breeds as a potential transmission route in human toxocariasis. Vet Parasitol. 2008;152:94–100. doi: 10.1016/j.vetpar.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Roddie G, Stafford P, Holland C, Wolfe A. Contamination of dog hair with eggs of Toxocara canis. Vet Parasitol. 2008;152:85–93. doi: 10.1016/j.vetpar.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Overgaauw PA, van Zutphen L, Hoek D, Yaya FO, Roelfsema J, Pinelli E, van Knapen F, Kortbeek LM. Zoonotic parasites in fecal samples and fur from dogs and cats in The Netherlands. Vet Parasitol. 2009;163:115–122. doi: 10.1016/j.vetpar.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 54.Agudelo C, Villareal E, Cáceres E, López C, Elijach J, Ramírez N, Hernández C, Corredor A. Human and dogs Toxocara canis infection in a poor neighborhood in Bogota. Mem Inst Oswaldo Cruz. 1990;85:75–81. doi: 10.1590/s0074-02761990000100012. [DOI] [PubMed] [Google Scholar]
- 55.Buijs J, Borsboom G, Renting M, Hilgersom WJ, van Wieringen JC, Jansen G, Neijens J. Relationship between allergic manifestations and Toxocara seropositivity: a cross-sectional study among elementary school children. Eur Respir J. 1997;10:1467–1475. doi: 10.1183/09031936.97.10071467. [DOI] [PubMed] [Google Scholar]
- 56.Chan PW, Anuar AK, Fong MY, Debruyne JA, Ibrahim J. Toxocara seroprevalence and childhood asthma among Malaysian children. Pediatr Int. 2001;43:350–353. doi: 10.1046/j.1442-200x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- 57.Cobzaru RG, Rîpâ C, Leon MM, Luca MC, Ivan A, Luca M. Correlation between asthma and Toxocara canis infection. Rev Med Chir Soc Med Nat Iasi. 2012;116:727–730. [PubMed] [Google Scholar]
- 58.Hotez PJ, Wilkins PP. Toxocariasis: America's most common neglected infection of poverty and a helminthiasis of global importance? PLoS Negl Trop Dis. 2009;3:e400. doi: 10.1371/journal.pntd.0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dabrowska J, Walski M, Dybicz M, Doligalska M. Comparative ultrastructural studies of the alterations to muse lung parenchyma during Trichinella spiralis or Toxocara canis infection. Parasite Immunol. 2012;34:455–463. doi: 10.1111/j.1365-3024.2012.01381.x. [DOI] [PubMed] [Google Scholar]
- 60.Pinelli E, Withagen C, Fonville M, Verlaan A, Dormans J, van Loveren H, Nicoll G, Maizels RM, van der Giessen J. Persistent airway hyper-responsiveness and inflammation of Toxocara canis-infected BALB/c mice. Clin Exp Allergy. 2005;35:826–832. doi: 10.1111/j.1365-2222.2005.02250.x. [DOI] [PubMed] [Google Scholar]
- 61.Mendonça LR, Veiga RV, Dattoli VC, Figueiredo CA, Fiaccone R, Santos J, Curz ÁA, Rodrigues LC, Cooper PJ, Pontes-de-Carvalho LC, Barreto ML, Alcantara-Neves NM. Toxocara seropositivity, atopy and wheezing in children living in poor neighborhoods in urban Latin American. PLoS Negl Trop Dis. 2012;6:e1886. doi: 10.1371/journal.pntd.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sariego I, Kanobana K, Junco R, Vereecken K, Nuñez FA, Polman K, Bonet M, Rojas L. Frequency of antibodies to Toxocara in Cuban schoolchildren. Trop Med Int Health. 2012;17:711–714. doi: 10.1111/j.1365-3156.2012.02996.x. [DOI] [PubMed] [Google Scholar]
- 63.Ishida MM, Rubinsky-Elefant G, Ferreira AW, Hoshino-Shimizu S, Vaz AJ. Helminth antigens (Taenia solium, Taenia crassiceps, Toxocara canis, Schistosoma mansoni and Echinococcus granulosus) and cross-reactivities in human infections and immunized animals. Acta Trop. 2003;89:73–84. doi: 10.1016/j.actatropica.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991;29:1831–1835. doi: 10.1128/jcm.29.9.1831-1835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chieffi PP, Santos SV, Queiroz ML, Lescano SA. Human toxocariasis: contribution by Brazilian researchers. Rev Inst Med Trop Sao Paulo. 2009;51:301–308. doi: 10.1590/s0036-46652009000600001. [DOI] [PubMed] [Google Scholar]
- 66.Smith HV. Antibody reactivity in human Toxocariasis. In: Lewis JW, Maizels RM, editors. Toxocara and Toxocariasis: Clinical, Epidemiological and Molecular Perspectives. London: British Society for Parasitology, Inc.; 1993. pp. 91–109. [Google Scholar]
- 67.Fillaux J, Magnaval JF. Laboratory diagnosis of human toxocariasis. Vet Parasitol. 2013;193:327–336. doi: 10.1016/j.vetpar.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 68.Lopez Mde L, Martin G, Chamorro Mdel C, Mario Alonso J. Toxocariasis in children from a subtropical region. Medicina (B Aires) 2005;65:226–230. [PubMed] [Google Scholar]