Abstract
This study investigated the distribution of parasites as main contaminants in water environments of peninsular Malaysia (October 2011–December 2011) and the southeastern coast of Thailand (June 2012). Sixty-four water samples, 33 from Malaysia and 31 from Thailand, of various water types were examined according to U.S. Environmental Protection Agency guidelines. Drinking or household water types from both countries were free from parasitic contamination. The recreational/environmental (except a swimming pool in Malaysia) and effluent water types from these two countries were contaminated with waterborne parasites: Giardia (0.04–4 cysts/L), Cryptosporidium (0.06–2.33 oocysts/L), hookworm (6.67–350 ova/L), Ascaris (0.33–33.33 ova/L), and Schistosoma (9.25–13.33 ova/L). The most contaminated sites were recreational lake garden 3 in Malaysia and river 2 in Thailand. Higher concentrations of Giardia, Cryptosporidium, and hookworm were found in samples from Malaysia than in samples from Thailand. The presence of Giardia cysts showed a significant association with the presence of Cryptosporidium oocysts (P < 0.005).
Introduction
Water shortages and limited access to clean water sources caused by water contamination with pathogenic bacteria, viruses, protozoa,1 and helminths2 represent a major human health hazard. These contaminations occurred mainly because of improper management of water supplies, reuse of wastewaters, poor sanitation systems, and lack of awareness and poor hygienic behavior among human populations.1,3–5 In addition, parasite contamination of open water resources, such as reservoirs, lakes, and rivers, may also have been contributed by domestic and wild animals inhabiting these surroundings.3,5,6
These parasites can be transmitted to humans through accidental consumption of contaminated waters (i.e., Giardia, Cryptosporidium and Ascaris ova) or via direct skin penetration (i.e., miracidia of Schistosoma).2 The clinical symptoms for parasitic infections (i.e., giardiasis and cryptosporidiosis) may include loss of appetite, nausea, vomiting, diarrhea, loose or watery stool, malabsorption, dehydration, and stomach cramps.6 For helminthiasis, symptoms such as intestinal bleeding, abdominal pains, anemia, severe diarrhea and malnutrition are observed in humans.2,7
To obtain and maintain parasite-free waters, various eradication methods, such as free chlorination have been applied to reduce parasite contamination in water resources.8 However, this chemical inactivation method, which is effective and safe at low dosages, could not completely eliminate waterborne protozoa, particularly Giardia and Cryptosporidium because of resistant characteristics of these contamination agents during their transmissive stages.8
Approximately 536 protozoa-related waterborne outbreaks have been reported worldwide since 1948–early 2012; 207 cases of Giardia lamblia infection and 293 cases of Cryptosporidium parvum infection.5,9–15 A recent outbreak of Cryptosporidium occurred in public swimming pools in Iowa, United States during June–August 2013; 528 cases were reported.12 However, during 1948–2012, only scanty data for outbreaks could be obtained from countries in Southeast Asia because of lack of documentation and recorded cases, especially in rural areas.9 There has been a slight increase in these infections during the past few years in Malaysia and Thailand.
In Malaysia, extensive studies on parasites detection in water environments have been conducted since 2004. Giardia was isolated from water bodies in zoos, recreational lakes, and rivers; Cryptosporidium was isolated from recreational lakes and rivers; Blastocystis was isolated from drinking water and sewage samples; free-living amoeba were isolated from swimming pools and recreational lakes; and helminths were isolated from recreational lakes.16–21 In Thailand, extensive water studies related to parasites have been conducted since 2006. Giardia and/or Cryptosporidium were isolated from wastewaters, rivers and coastal areas; Blastocystis was isolated from drinking water and freshwater; free-living amoeba were isolated from freshwater and hot springs; and liver flukes were isolated from the Mekong River basin.2,5,22,23 However, in other countries in Southeast Asia, less data are available for parasite detection directly from water compared with data for the prevalence of parasites in humans.20,21
The present study was conducted to obtain current data on the distribution of waterborne protozoa and helminths in various water types in western peninsular Malaysia and along the southeastern coast of Thailand. This information would provide a better understanding of parasite contamination in water in the tropical regions of Southeast Asia, especially in Malaysia and Thailand.
Materials and Methods
Sampling sites and sample collection.
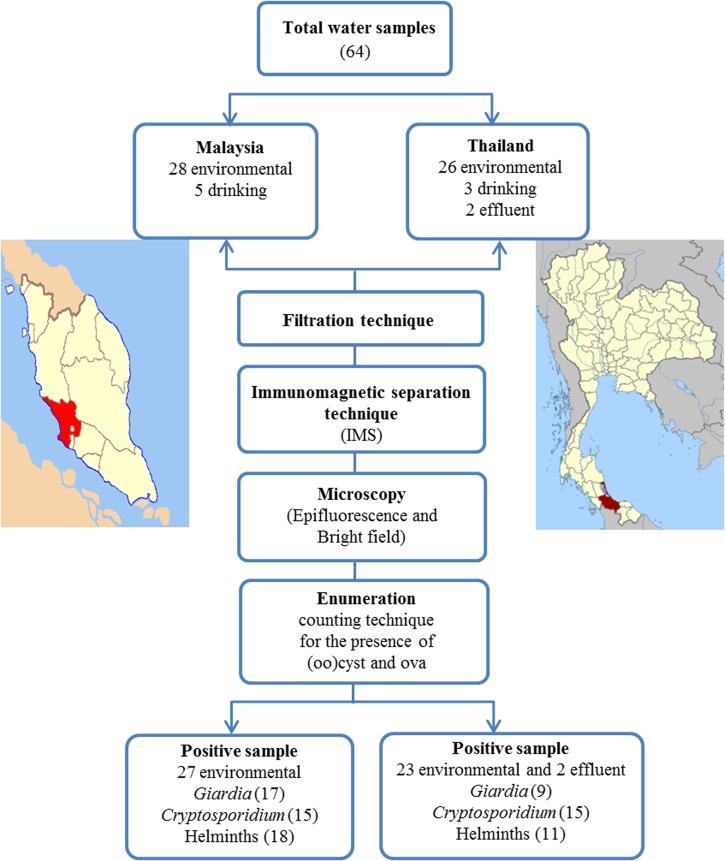
Water samples (10 liters from each sampling station) were collected in Petaling Jaya (101.61°E, 3.10°N) and Puchong (101.61°E, 3.00°N) areas in Selangor State, Malaysia during October–December 2011 and in Songkhla (100.61°E, 7.16°N) on the southeastern coast of Thailand in June 2012 (Figure 1). Malaysia and Thailand are neighboring countries and both have similar cultural and climate characteristics. The climate in peninsular Malaysia and southern Thailand is generally hot and humid. Water sampling was conducted during the rainy season in both countries.
Figure 1.
Study sites in Malaysia and Thailand. IMS = immunomagnetic separation.
A total of 33 water samples were obtained in Malaysia and 31 water samples were obtained in Thailand. These water samples were grouped into three main categories: drinking or household water, recreational or environmental water, and waste or effluent water. For reservoirs, ponds, and lakes, water samples were obtained at a depth of 10 meter or from a mixture of surface and bottom water in shallow areas. For rivers and rainwater, water samples were obtained from surface water and open spaces, respectively.
Laboratory investigations.
Filtration of water samples and purification of parasites.
Collected water samples were filtered by using a flat-bed membrane filtration technique (EMD Millipore Corp., Billerica, MA). Oocysts and/or ova in soil sediments and debris from water were trapped on the surface of a nitrocellulose membrane (1.2 μm pore size, 142 mm diameter; EMD Millipore Corp.), scrapped off the membrane, and collected in 0.1% Tween 80. This eluate was concentrated to a volume of 10 mL by centrifugation (Kubota Corporation, Osaka, Japan) at 1,050 × g for 10 minutes at room temperature before being transferred to a 10-mL Leighton tube (L tube). Samples were analyzed by using an immunomagnetic separation (IMS) technique according to Method 1622/1623 (Environmental Protection Agency, Washington, DC [EPA], 2005) for Cryptosporidium (Chemical Abstracts Service Registry no. 137259-50-8) and Giardia (Chemical Abstracts Service Registry no. 137259-49-5) as per registered by the U.S. Government.
Subsequently, SL buffers and antibody-coated magnetic beads (Dynabeads® Giardia-Combo and Dynabeads® Crypto-Combo; Life Technologies Corp., Grand Island, NY) were added to L tubes and mixed by using a Dynal rotator (Life Technologies Corp.) for 1 hour. The Dynabeads–oocysts were purified from the supernatant (sediment and debris) by using a magnetic particle concentrator (MPC®-1; Life Technologies Corp.). The supernatant was decanted into a Falcon (Brookings, SD) tube, stored at 4°C, and used for detection of parasites other than Giardia and Cryptosporidium. The (oo)cysts were separated from the Dynabeads by the addition of 50 μL of 0.1N HCI.
Detection of Giardia cysts and Cryptosporidium oocysts.
After the IMS technique, 50 μL of sample (presumably containing oocysts) was applied to single-well microscope slides (Invitrogen Dynal AS, Oslo, Norway) and stained with fluorescein isothiocyanate–conjugated Giardia/Cryptosporidium monoclonal antibody reagent (Cellabs, Brookvale, New South Wales, Australia) and 4′,6′-diamidino-2-phenylindole solution (Sigma-Aldrich, St. Louis, MO). Fixed slides were examined by using epifluorescence microscope (Model BX51 microscope; Olympus, Tokyo, Japan). Positive controls were included in a kit provided by Cellabs. Negative controls were prepared by using phosphate-buffered saline, pH 7.4. Putative Giardia cysts and Cryptosporidium oocysts showed a typical fluorescent appearance (based on size, shape, and internal structures (e.g., round to ovoid Giardia cysts 8–18 μm × 5–15 μm and ovoid or spherical Cryptosporidium oocysts 4–6 μm diameter; Method 1622/1623; EPA, 2005).
Detection of other parasites.
Supernatants obtained during the IMS technique (stored at 4 °C) were re-suspended by using Pasteur pipette to ensure that all sediments were mixed well with eluate. Approximately 50 μL of eluted sediments (from 10 mL) was applied onto a plain slide and mounted with Lugol's iodine or normal saline (1:2) and examined by using a bright-field light microscope (Model-CX40; Olympus). A negative control slide was prepared and examined with every 15–20 sample slides.
Enumeration of parasites.
(Oo)cysts or ova were counted at least two times per slide and counts were adjusted to a volume of 1 liter water to minimize error. For example, if 10 cysts/L of Giardia were detected in a volume of 50 μL, which is equivalent to 10 liters, this finding indicates that 1 cyst was detected in a 1-liter water sample. For other parasites, if 2 hookworm ova found in 50 μL of slide (from 10ml supernatant equivalent to 10 L), it would be 400 ova in 10 L of water sample and subsequently 40 ova in 1 L of water sample.
Measurements of physical parameters and meteorologic information.
Five physical parameters (temperature, pH, dissolved oxygen [DO], salinity, and turbidity) were simultaneously measured in situ at all sampling sites by using a handheld multi-parameter instrument (model 556; YSI Inc., Yellow Springs, OH) and turbidity parameter instrument (HACH-Model 2100P; ICM, Oregon). In addition, monthly rainfall and relative humidity for each sampling day was obtained from the nearest weather station (Malaysian Meteorological Department, Petaling Jaya Station; Thai Meteorological Department, Songkhla Station; and Norwegian Meteorological Institute and World Weather Online API, WeatherSpark.com).
Statistical analysis.
Statistical analysis was performed by using Microsoft (Redmond, WA) Excel, Pearson's correlation (version 1.0.6) in Free Statistics Software,24 and SPSS version 21.0 software (IBM, Armonk, NY) to analyze correlations between enumerated parasites and their physical parameters. Statistically significant results had an α value < 0.005.
Results
Parasitic contamination in water.
Of 33 samples from Malaysia, 5 were from drinking or household water (mineral, drinking, tap, and rain) and 28 samples were from recreational or environmental water (swimming pool, recreational lakes, rivers, and a waterfall). Of 31 samples of Thailand, 3 were from drinking or household water (mineral, drinking and rain), 26 were from recreational or environmental water (ponds, lakes, reservoirs, rivers, and a waterfall), and 2 were from waste or effluent water from hospitals.
Numbers of parasites found in water samples are shown in Table 1. There was no parasite contamination of drinking or household water in Malaysia and Thailand. A selected swimming pool in Malaysia was also found to be free of parasites. However, other untreated environmental water in both countries and effluent water in Thailand were contaminated with waterborne parasites. The main waterborne parasites found in both countries were Giardia, Cryptosporidium, hookworm, Ascaris, and Schistosoma. All parasites were detected in recreational lake garden 3 (LG3) in Malaysia and river 2 in Thailand. River 1 in Thailand was the least contaminated site (only one parasite, Cryptosporidium oocysts). In the water sites in Malaysia, at least 2 types of waterborne parasites were detected (e.g., Giardia and Cryptosporidium in LG2 and a waterfall). Malaysia had a higher concentration of Giardia cysts (4 cysts/L) and Cryptosporidium oocysts (2.33 oocysts/L) in river water and a high hookworm ova concentration (70.33 ova/L) in LG3. However, a higher concentration of helminths was found in Thailand, particularly Ascaris ova (33.33 ova/L) in a waterfall and Schistosoma ova (13.33 ova/L) in pond 1.
Table 1.
Parasites found in water samples, Malaysia and Thailand*
| Water sample | No. | No. positive | Mean ± SD | ||||
|---|---|---|---|---|---|---|---|
| Giardia | Cryptosporidium | Hookworm | Ascaris | Schistosoma | |||
| Malaysia | |||||||
| Drinking/household water | |||||||
| Mineral water (MM) | 1 | 0 | ND | ND | ND | ND | ND |
| Drinking water (MD) | 1 | 0 | ND | ND | ND | ND | ND |
| Tap water (MT) | 2 | 0 | ND | ND | ND | ND | ND |
| Rain water (MR) | 1 | 0 | ND | ND | ND | ND | ND |
| Recreational/environmental water | |||||||
| Treated | |||||||
| Swimming pool (SP) | 1 | 0 | ND | ND | ND | ND | ND |
| Untreated | |||||||
| Lake Garden 1 (LG1) | 7 | 7 | 0.14 ± 0.10 | 0.23 ± 0.21 | 28.54 ± 37.84 | 19.03 ± 23.73 | ND |
| Lake Garden 2 (LG2) | 2 | 2 | 0.20 ± 0.28 | 0.10 ± 0.14 | ND | ND | ND |
| Lake Garden 3 (LG3) | 12 | 12 | 2.58 ± 4.37 | 1.51 ± 3.43 | 70.30 ± 55.06 | 9.25 ± 14.84 | 9.25 ± 19.99 |
| River (MR) | 3 | 3 | 4 ± 0.87 | 2.33 ± 3.62 | ND | 0.33 ± 0.58 | ND |
| Waterfall (MW) | 3 | 3 | 0.17 ± 0.29 | 0.83 ± 0.29 | ND | ND | ND |
| Total | 33 | 27 | |||||
| Thailand | |||||||
| Drinking/household water | |||||||
| Mineral water (TM) | 1 | 0 | ND | ND | ND | ND | ND |
| Drinking water (TD) | 1 | 0 | ND | ND | ND | ND | ND |
| Rain water (TR) | 1 | 0 | ND | ND | ND | ND | ND |
| Recreational/environmental water | |||||||
| Pond 1 (TP1) | 2 | 2 | ND | ND | ND | 20 ± 28.28 | 10 ± 14.14 |
| Pond 2 (TP2) | 2 | 2 | 0.22 ± 0.31 | 0.45 ± 0.63 | 30 ± 42.42 | ND | ND |
| Natural lake (TNL) | 7 | 7 | 0.21 ± 0.33 | 0.73 ± 1.33 | ND | ND | ND |
| Reservoir lake (TRL) | 6 | 5 | 0.04 ± 0.10 | 0.26 ± 0.49 | ND | 3.33 ± 8.16 | ND |
| River 1 (TR1) | 3 | 2 | ND | 0.30 ± 0.29 | ND | ND | ND |
| River 2 (TR2) | 3 | 3 | 0.52 ± 0.50 | 1.29 ± 1.54 | 6.67 ± 11.55 | 20 ± 34.64 | 13.33 ± 11.55 |
| Waterfall (TW) | 3 | 2 | 0.11 ± 0.19 | 0.06 ± 0.10 | ND | 33.33 ± 30.55 | ND |
| Waste/effluent water | |||||||
| Treatment plant (TTP) | 2 | 2 | 0.22 | 0.89 ± 0.31 | 350 ± 494.97 | ND | ND |
| Total | 31 | 25 | |||||
ND = not determined. Other detected parasites with negligible counts (1 or 2 per 25 microscopy slides examination) were Blastocystis cysts (LG1, TNL, TRL, TR2, TW, and TTP), Entamoeba cysts (TP1), Enterobius ova (TW), Hymenolepis ova (TP2 and TTP), tapeworm ova (TTP), Toxocara ova (LG3, TNL and TRL), Toxoplasma oocysts (LG1), ectoparasites of the orders Anastroca (LG2, LG3 and TTP) and Cladocera (LG1, LG2, TW, and TP2), copepods (LG3 and TR2), lice (LG1 and LG3), and Brachinecta organisms (LG3).
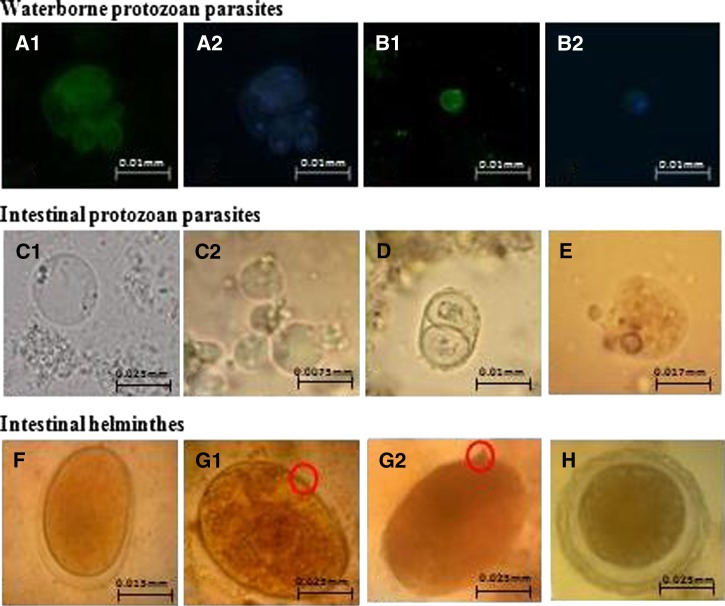
Other waterborne parasites, such as Blastocystis cysts, Entamoeba cysts, Enterobius ova, Hymenolepis ova, tapeworm ova, Toxocara ova, Toxoplasma oocysts, were also detected in these water samples (1 or 2 per 25 microscopic slides), as shown in Figure 2. In addition, we also found ectoparasites, such as members of the orders Anastroca and Cladocera, copepods, lice, and a Brachinecta sp., as indicated in Table 1.
Figure 2.
Photomicrograph of parasites detected in water samples from Malaysia and Thailand, 400 × magnification: Giardia (A), Cryptosporidium (B), Blastocystis (C), Toxoplasma (D), Entamoeba (E), Hookworm (F), Schistosoma (G), and Ascaris (H).
Physical parameters and meteorologic data.
Monthly rainfall at Petaling Jaya station, Malaysia, was 250–300 mm in October 2011, 300–400 mm in November 2011, and 250–350 mm in December 2011. At Songkhla station in Thailand, low rainfall (50–100 mm) was reported in June 2012. In Malaysia, there was no rain on sampling days in all recreational lakes except for rivers. It rained on previous sampling days at LG1, LG3, and a waterfall. In Thailand, it rained on the sampling day at a natural lake but it did not rain at other sites during sampling. However, it rained heavily on the day before sampling at river 1.
Physical parameters and meteorologic data for each sampling location in peninsular Malaysia and along the southeastern coast of Thailand are shown in Table 2. For the pipeline distribution system of tap water and for rain water, high humidity was reported in both countries. However, lower humidity (60–72%) was noted at sampling sites in Malaysia than at sampling sites in Thailand (68.2–87.25%). Water temperature was high during sampling in Malaysia (≤ 32.11°C) or Thailand (≤ 31.64°C). Turbidity was low in drinking or household water from Malaysia (0.13–2.01 nephelometric turbidity unit [NTUs]) and Thailand (0.16–1.37 NTU). Higher turbidity was observed for recreational or environmental water from Thailand (1.04–88.71 NTU) compared with water from Malaysia (1.02–24.64 NTU). Lower salinity was found in environmental water samples from Malaysia (0.02–0.23 ppt) than in samples from Thailand (0.02–1.68 ppt). Low levels (0.06–0.12 g/L) of total dissolved solids (TDS) were found in drinking and environmental water in both countries. However, TDS level in a swimming pool was 0.23 g/L and in a treatment plant was 0.25g/L. Predictably, a high salinity (13.53 ppt) and TDS (14.48 g/L) was detected in a natural lake, which is also an estuary, in Thailand.
Table 2.
Physical parameters and meteorologic data of each sampling location in peninsular Malaysia and southeastern coast of Thailand*
| Location | Water sample (no.) | Baseline data of water samples on sampling day (mean ± SD) | |||||
|---|---|---|---|---|---|---|---|
| Humidity (%) | Temperature (°C) | Turbidity (NTU) | Salinity (ppt) | Others | |||
| Drinking/household water | |||||||
| Malaysia | Mineral water (1) | – | 19.20 | 0.13 | 0.07 | pH | 6.93 ± 0.06 |
| Drinking water (1) | – | 17.70 | 0.18 | 0.08 | DO | 5.7 ± 3.03 | |
| Tap water (2) | 84.67 ± 12.1 | 24.79 ± 0.69 | 2.01 ± 1.53 | 0.04 ± 0.01 | TDS | 0.06 ± 0.04 | |
| Rain water (1) | 88.67 ± 6.43 | 21.11 | 1.66 | 0 | |||
| Thailand | Mineral water (1) | – | 25.47 | 0.16 | 0.24 | pH | 6.85 ± 0.36 |
| Drinking water (1) | – | 28.28 | 0.27 | 0.06 | DO | 4.07 ± 2.74 | |
| Rain water (1) | 84 | 25.2 | 1.37 | 0 | TDS | 0.12 ± 0.14 | |
| Recreational/environmental water | |||||||
| Malaysia | Treated: swimming pool (1) | 72 ± 4.24 | 23.62 | 1.02 | 0.17 | pH | 6.98 |
| DO | 16.1 | ||||||
| Untreated: Lake Garden 1 (7) | 67.33 ± 4.51 | 31.55 ± 0.65 | 24.64 ± 2.62 | 0.05 ± 0.01 | TDS | 0.23 | |
| Lake Garden 2 (2) | 60 | 32.11 ± 1.48 | 9.99 | 0.03 | pH | 6.34 ± 1.16 | |
| Lake Garden 3 (12) | 69.8 ± 4.38 | 29.06 ± 0.48 | 15.45 ± 6.66 | 0.06 ± 0.01 | DO | 10.68 ± 3.77 | |
| River (3) | 68 ± 3.6 | 28.7 | 22.27 | 0.23 ± 0.003 | TDS | 0.07 ± 0.02 | |
| Waterfall (3) | 71 ± 5.44 | 27.8 | 1.07 | 0.02 ± 0.001 | |||
| Thailand | Natural lake (7) | 87.25 ± 2.36 | 27.58 ± 0.6 | 88.71 ± 61.11 | 13.53 ± 6.55 | ||
| Reservoir lake (6) | 68.2 ± 12 | 30.85 ± 0.46 | 9.86 ± 1.89 | 0.02 | pH | 6.54 ± 0.92 | |
| River 1(3) | 79 ± 4.58 | 28.63 ± 057 | 61.57 ± 44.07 | 0.05 | DO | 13.68 ± 6.25 | |
| River 2 (3) | 71 ± 5.66 | 30.58 ± 0.48 | 15.3 ± 2.4 | 0.32 ± 0.36 | TDS | 0.1 ± 0.21 | |
| Waterfall (3) | 85 ± 1.41 | 24.86 ± 0.3 | 4.93 ± 0.06 | 1.68 ± 2.89 | (except natural lake: 14.48 ± 6.57 g/L) | ||
| Pond 1 (2) | 69 ± 2.83 | 29.80 ± 0.05 | 1.04 ± 0.03 | 0.05 | |||
| Pond 2 (2) | 78.5 ± 0.71 | 30.34 ± 0.35 | 54.7 ± 11.03 | 0.05 | |||
| Waste or effluent water | pH | 7 ± 0.04 | |||||
| DO | 7.05 ± 0.92 | ||||||
| Thailand | Treatment plant (2) | 73 ± 2.83 | 31.64 ± 1.32 | 38.1 ± 18.1 | 0.18 | TDS | 0.25 ± 0.01 |
NTU = nephelometric turbidity units; DO = dissolved oxygen (mg/L); TDS = total dissolved oxygen (g/L) of total dissolved solids.
Correlation analysis of waterborne parasites.
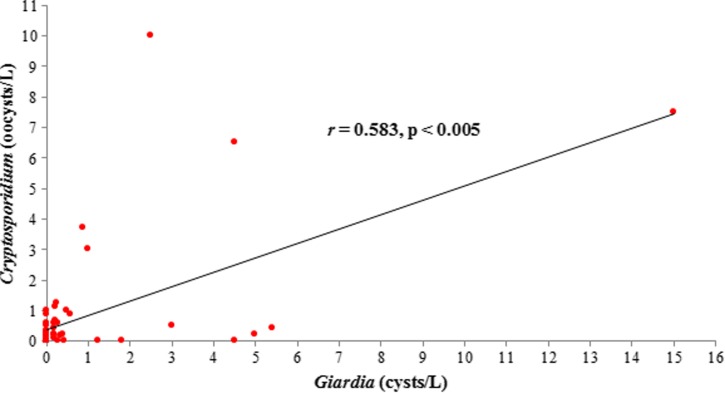
A significant positive correlation (r = 0.583) was observed between the presence of Giardia cysts and Cryptosporidium oocysts in recreational or environmental water (Figure 3). Otherwise, there was no significant correlation between each parasite or between parasites and physical parameters.
Figure 3.
Positive correlation (r = 0.583) between presence of Giardia cysts and Cryptosporidium oocysts in recreational or environmental water type, Malaysia and Thailand.
Discussion
In our study, no parasites were detected in drinking or household water from Malaysia and Thailand. Thus, filtered or treated water was safe for drinking or domestic purposes. This study is the first to report on safe drinking water from the perspective of parasite contamination in Southeast Asia.
For recreational or environmental water, only a swimming pool was free from parasite contamination and this finding might have been the result of proper chlorination treatment at this site.17 For other sites of recreational or environmental water, five parasites were identified: Giardia, Cryptosporidium, hookworm, Ascaris, and Schistosoma. These parasites were detected on the basis of identification of (oo)cysts or ova. Because ova of Ancylostoma and Necator (and most other hookworm species) are indistinguishable, additional laboratory tests (culture of fecal samples) are needed to distinguish these two genera. Malaysia had high contamination with Giardia, Cryptosporidium, and hookworm, and Thailand had high contamination with Ascaris and Schistosoma. These findings could be the result of the fact that samples from Malaysia were obtained from restricted-flow water, such as recreational lakes, whereas most samples from Thailand were obtained from free-flow water, such as rivers and a waterfall.
The most contaminated sites were LG3 in Malaysia and river 2 in Thailand; all five parasites were found at these sites. LG3 in Malaysia is situated in a city and it controls flash floods by functioning as a water storage area during heavy rains. Water from a row of shops (i.e., butchers, wet markets, and restaurants) drains directly into LG3, which probably plays an important role in parasite contamination found in our study.21 If lake water levels become too high, water would be channeled into a large drainage system. Furthermore, this recreational lake is mostly use for fishing activities. Thus, parasite contamination may contribute to human infections by accidental contact.
River 2 in Thailand is a confluence of two upstream rivers (from a mountainous area and a city area), flows through a small town, and ends in an area that contains remote villages. Samples from this river was obtained from a floating market (upstream), inside town temple (midstream), and village fishing docks (downstream). Contamination sources in this river might have been the floating market and the villages. In contrast, river 1 is located in a city area, and this river has a dense flow during rainy season.22 The day before sampling of this river, it rained heavily, which might explain why there was less contamination with parasites because of a dilution effect that might have washed away most contaminants.25 Most environmental water in Thailand was used directly by villagers. This could lead to signs and symptoms, such as diarrhea, related to parasitic infections in affected persons.
Our study showed that the level of turbidity in drinking water was safe and conformed with standard regulations of the World Health Organization,26 the Malaysia National Standard of Drinking Water Quality formulated by the Ministry of Health,27 and the Thailand Ministry of Natural Resources and Environment.28 River 1 and the natural lake had high turbidity and exceeded the U.S. EPA Ambient Water Quality Standard. This finding is also supported by fishing activity in surface water near banks and contaminated aquatic ecosystems at the shore and in shallow areas,29 which results in parasite contamination at these water sites. Also, low-velocity settling characteristics of waterborne protozoan parasites (Giardia = 0.84 μm/second and Cryptosporidium = 0.35 μm/second) can prolong their survival by attachment to suspended particles and decrease the mobilization rate of (oo)cysts in water.30
The presence of waterborne protozoa in a natural lake (an estuary near a sea waterway) that has high salinity and distinct high TDS helps explain the tolerance adaptation of these parasites in adverse conditions, which has affected ecosystem diversity.31–33 There are many rivers, including river 1 and river 2, which join to become one river, and flow towards this natural lake. Although this lake contains brackish water, contaminants and pollutants from rivers indirectly affected the environment of this lake. No waterborne parasites, especially protozoan parasites, have been identified in the brackish water of this estuary, with the exception of related studies on seawater fish helminths31 and in situ studies of Cryptosporidium in shellfish or salty water.33,34 To the best of our knowledge, this is the first study reporting on parasitic contamination with Giardia and Cryptosporidium in the brackish water of this estuary. No helminths were found in high-salinity natural lake water because of intolerance to hypertonic water, which would cause the helminths to burst.21,25
A positive correlation between concentrations of Giardia cysts and Cryptosporidium oocysts in recreational or environmental water was found in our study. The average concentration of Giardia cysts was two times higher than that of Cryptosporidium oocysts in samples from recreational or environmental water from Malaysia and Thailand. This finding is consistent with data of the U.S. EPA, which reported that Giardia and Cryptosporidium are primary sources of water contamination.9 Our data was also supported by a study in Thailand, which reported a significant positive correlation between these two protozoan parasites in tropical water environments.5 In addition, the hot and humid climates with seasonal rainfall may sustain increasing occurrence of Giardia cysts and Cryptosporidium oocysts in these tropical water environments. However, studies reported that seasonal and climate changes (during the four seasons) in certain countries do not contribute to the correlation between these two waterborne parasites.35,36
One limitation of this study was the use of the IMS technique. This technique was chosen because it has high sensitivity, is commercially available, and it effectively purifies waterborne protozoans from other debris. However, the IMS is not suitable for detecting other waterborne parasites, such as free-living amoeba cysts (i.e., Acanthamoeba and Naegleria).37,38 Our study focused on a simple method for identification of parasites contaminating water environments. Thus, species identification and obtaining of pathogenicity data by molecular analysis were limited. Based on these findings, studies with larger sample sizes are needed, and global positioning systems and geographic information systems should be included to assess the distribution of waterborne parasites in this region.
In conclusion, waterborne protozoa parasites and helminths ova were found mostly in environmental water samples from Malaysia and Thailand, a finding that corresponds to human activities and local maintenance systems in the affected areas. Parasite contamination, especially with Giardia and Cryptosporidium, of various water types in these two neighboring countries is a serious health concern. On the basis of the data obtained, we make the following recommendations. First, recreational or environmental water should be treated by government and non-government sectors to eliminate parasites and effectively control these emerging pathogens to reduce waterborne parasite transmission. Second, procedures (storage, treatment, and distribution) are urgently needed to prevent release of waste into water environments and encourage use of biological filters in drainage systems before water is diverted into storages areas, such as recreational lakes. Third, rivers should be monitored regularly by conducting water analysis, including tests for the presence of waterborne parasites. In addition, persons involved in water activities should be provided with information about health education, waterborne health hazards, and primary behavior practices. Fourth, epidemiologic studies, microbiologic evaluations of water and food products, and quantitative microbial risk assessment should be conducted26,38 to provide better control strategies against these waterborne pathogenic parasites.
ACKNOWLEDGMENTS
We thank Wan Hafiz Wan Ismail, and Redzuan Ahmad Naziri (Parasites: Southeast Asian Diagnostic Laboratory Diagnostic Unit) for assistance.
Footnotes
Financial support: This study was supported by University of Malaya High Impact Research Grant UM-MOHE UM.C/625/1/HIR/MOHE/MED/18 from the Ministry of Higher Education Malaysia, PV 049/2011B, PV 014/2012A, PG117-2012B, UMRG 374/11HTM, UMRG 488/12HTM and the Faculty of Science, Prince of Songkla University Research Fund, Thailand.
Disclosure: Part of this work have been presented in 5th ASEAN Congress of Tropical Medicine and Parasitology (ACTMP), Manila, The Philippines, 15–17 May, 2012.
Authors' addresses: Thulasi Kumar, Subashini Onichandran, Yvonne A. L. Lim, Init Ithoi, Hemah Andiappan, Tan Tian Chye, Wan Y. W. Sulaiman, Yee Ling Lau, and Veeranoot Nissapatorn, Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: thulasi_asi@yahoo.com, subashinionichandran@yahoo.com, limailian@um.edu.my, init@um.edu.my, april_hem@yahoo.com, tantianchye@um.edu.my, wanyus@um.edu.my, lauyeeling@um.edu.my, and nissapat@gmail.com or thulasi_asi@yahoo.com. Nongyao Sawangjaroen, Department of Microbiology, Faculty of Science, Prince of Songkla University, Hat Yai, Thailand, E-mail: nongyao.s@psu.ac.th. Cristina C. Salibay, Biological Science Department, De La Salle University-Dasmarinas, Dasmarinas, The Philippines, E-mail: cgcsaibay@yahoo.com. Julieta Z. Dungca, School of Science and Technology, Centro Escolar University, Manila, The Philippines, E-mail: julie_dungca@yahoo.com.
References
- 1.Ferrer A, Nguyen-Viet H, Zinsstag J. Quantification of diarrhea risk related to wastewater contact in Thailand. EcoHealth. 2012;9:49–59. doi: 10.1007/s10393-012-0746-x. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty P. Helminths and nematodes: general features and classification. Textbook of Medical Parasitology. Second edition. Kolkata, India: New Central Book Agency; 2005. pp. 131–138. [Google Scholar]
- 3.Ostan I, Kilimcioğlu AA, Girginkardeşler N, Ozyurt BC, Limoncu ME, Ok UZ. Health inequities: lower socio-economic conditions and higher incidences of intestinal parasites. BMC Public Health. 2007;7:342. doi: 10.1186/1471-2458-7-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pampiglione S, Visconti S, Pezzino G. Human intestinal parasites in sub-Saharan Africa. II. Sao Tomé and Principe. Parassitologia. 1987;29:15–25. [PubMed] [Google Scholar]
- 5.Srisuphanunt M, Karanis P, Charoenca N, Boonkhao N, Ongerth JE. Cryptosporidium and Giardia detection in environmental waters of southwest coastal areas of Thailand. Parasitol Res. 2010;106:1299–1306. doi: 10.1007/s00436-010-1795-0. [DOI] [PubMed] [Google Scholar]
- 6.Pettoello MM, Guandalini S, Ecuba P, Corvino C, di Martino L. Lactose malabsorption in children with symptomatic Giardia lamblia infection: feasibility of yogurt supplementation. J Pediatr Gastroenterol Nutr. 1989;9:295–300. doi: 10.1097/00005176-198910000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Hall A, Hewitt G, Tuffrey V, de Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4:118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernando WJ. Theoretical considerations and modeling of chemical inactivation of microorganisms: inactivation of Giardia cysts by free chlorine. J Theor Biol. 2009;259:297–303. doi: 10.1016/j.jtbi.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks: an update 2004–2010. Water Res. 2011;45:6603–6614. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Outbreak of Cryptosporidiosis associated with a firefighting response–Indiana and Michigan, June 2011. MMWR Morb Mortal Wkly Rep. 2012;61:153–156. [PubMed] [Google Scholar]
- 11.Disease Control and Epidemiology . Giardiasis. Seattle, WA: Seattle and King County, Public Health; 2013. [Google Scholar]
- 12.Anonymous . Global Dispatch: Iowa Cryptosporidium Outbreak Tops 500 Since June 2013. 2013. http://www.theglobaldispatch.com/iowa-cryptosporidium-outbreak-tops-500-since-june-24902/ Available at. Accessed September 24, 2013. [Google Scholar]
- 13.Karanis P, Kourenti C, Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health. 2007;5:1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- 14.Robbins A. Infectious Disease Epidemiology Report: Cryptosporidiosis and Giardiasis Surveillance Report–Maine 2012. 2013 MeCDC, DID: Surveillance Report 3. [Google Scholar]
- 15.Serdarevic F, Jones RC, Weaver KN, Black SR, Ritger KA, Guichard F, Dombroski P, Emanuel BP, Miller L, Gerber SI. Multi-pathogen waterborne disease outbreak associated with a dinner cruise on Lake Michigan. Epidemiol Infect. 2012;140:621–625. doi: 10.1017/S0950268811000896. [DOI] [PubMed] [Google Scholar]
- 16.Azman J, Init I, Wan Yusoff WS. Occurrence of Giardia and Cryptosporidium oocysts in the river water of two recreational areas in Selangor, Malaysia. Trop Biomed. 2009;26:289–302. [PubMed] [Google Scholar]
- 17.Init I, Lau YL, Arin Fadzlun A, Foead AI, Neilson RS, Nissapatorn V. Detection of free living amoebae, Acanthamoeba and Naegleria, in swimming pools, Malaysia. Trop Biomed. 2010;27:566–577. [PubMed] [Google Scholar]
- 18.Lim YA, Aahmad RA. Occurrence of Giardia cysts and Cryptosporidium oocysts in the Temuan Orang Asli (aborigine) River System. Southeast Asian J Trop Med Public Health. 2004;35:801–810. [PubMed] [Google Scholar]
- 19.Lim YA, Ramasame SD, Mahdy MA, Sulaiman WY, Smith HV. Detection and molecular characterization of Giardia isolated from recreational lake water in Malaysia. Parasitol Res. 2009;106:289–291. doi: 10.1007/s00436-009-1602-y. [DOI] [PubMed] [Google Scholar]
- 20.Lee SC, Ngui R, Tan TK, Roslan MA, Ithoi I, Lim YA. Aquatic biomonitoring of Giardia cysts and Cryptosporidium oocysts in peninsular Malaysia. Environ Sci Pollut Res Int. 2014;21:445–453. doi: 10.1007/s11356-013-1925-1. [DOI] [PubMed] [Google Scholar]
- 21.Onichandran S, Kumar T, Lim YA, Sawangjaroen N, Andiappan H, Salibay CC, Chye TT, Ithoi I, Dungca JZ, Sulaiman WY, Ling LY, Nissapatorn V. Waterborne parasites and physico-chemical assessment of selected lakes in Malaysia. Parasitol Res. 2013;112:185–191. doi: 10.1007/s00436-013-3610-1. [DOI] [PubMed] [Google Scholar]
- 22.Diallo MB, Anceno AJ, Tawatsupa B, Tripathi NK, Wangsuphachart V, Shipin OV. GIS-based analysis of the fate of waste-related pathogens Cryptosporidium parvum, Giardia lamblia and Escherichia coli in a tropical canal network. J Water Health. 2009;7:133–143. doi: 10.2166/wh.2009.010. [DOI] [PubMed] [Google Scholar]
- 23.Sutthikornchai C, Jantanavivat C, Thongrungkiat S, Harnroongroj T, Sukthana Y. Protozoal contamination of water used in Thai frozen food industry. Southeast Asian J Trop Med Public Health. 2005;36:41–45. [PubMed] [Google Scholar]
- 24.Wessa P. Pearson Correlation (v1.0.6) in Free Statistics Software (v1.1.23-r7) Office for Research Development and Education; 2012. http://www.wessa.net/rwasp_correlation.wasp/ Available at. Accessed March 20, 2013. [Google Scholar]
- 25.Martinez J, Merino S. Host-parasite interactions under extreme climatic conditions. Curr Zool. 2011;57:390–405. [Google Scholar]
- 26.World Health Organization . Acceptability Aspects: Taste, Odour and Appearance. Guidelines for Drinking Water Quality. Fourth edition. Geneva: World Health Organization; 2011. pp. 221–230. [Google Scholar]
- 27.Ministry of Health . National Standard of Drinking Water Quality Malaysia. Kuala Lumpur. Malaysia: Engineering Service Division; 2004. [Google Scholar]
- 28.Notification of the Ministry of Industry Industrial Products Standards Act No. 322 BE 2511, 1968 as Cited in Ministry of Natural Resources and Environment, Drinking Water Quality Standard in Thailand-WEPA. Royal Gazette. 1978;95:68. [Google Scholar]
- 29.Chesapeake Bay Field Office of Northeast Region . The Conowingo Dam and Chesapeake Bay Water Quality. Annapolis, MD; US Fish and Wildlife Service: 2013. pp. 1–4. [Google Scholar]
- 30.Dumetre A, Aubert D, Puech PH, Hohweyer J, Azas N, Villena I. Interaction forces drive the environmental transmission of pathogenic protozoa. Appl Environ Microbiol. 2012;78:905–912. doi: 10.1128/AEM.06488-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culurgioni J, Sabatini A, De Murtas R, Mattiucci S, Figus V. Helminth parasites of fish and shellfish from the Santa Gilla Lagoon in southern Sardinia, Italy. J Helminthol. 2013;21:1–10. doi: 10.1017/S0022149X13000461. [DOI] [PubMed] [Google Scholar]
- 32.Dunlop J, McGregor G, Horrigan N. In: Characterisation of impacts and a discussion of regional target setting for riverine ecosystems in Queensland. Potential Impacts of Salinity and Turbidity in Riverine Ecosystems. Dunlop J, McGregor G, Horrigan N, editors. Brisbane, Queensland, Australia: The National Action Plan for Salinity and Water Quality; 2005. pp. 18–42. [Google Scholar]
- 33.Nasser AM, Zaruk N, Tenenbaum L, Netzan Y. Comparative survival of Cryptosporidium, coxsackievirus A9 and Escherichia coli in stream, brackish and sea waters. Water Sci Technol. 2003;47:91–96. [PubMed] [Google Scholar]
- 34.Izumi T, Yagita K, Endo T, Ohyama T. Detection system of Cryptosporidium parvum oocysts by brackish water benthic shellfish (Corbicula japonica) as a biological indicator in river water. Arch Environ Contam Toxicol. 2006;51:559–566. doi: 10.1007/s00244-005-0174-9. [DOI] [PubMed] [Google Scholar]
- 35.Helmi K, Skraber S, Burnet JB, Leblanc L, Hoffmann L, Cauchie HM. Two-year monitoring of Cryptosporidium parvum and Giardia lamblia occurrence in a recreational and drinking water reservoir using standard microscopic and molecular biology techniques. Environ Monit Assess. 2011;179:163–175. doi: 10.1007/s10661-010-1726-7. [DOI] [PubMed] [Google Scholar]
- 36.Van Dyke MI, Ong CS, Prystajecky NA, Isaac-Renton JL, Huck PM. Identifying host sources, human health risk and indicators of Cryptosporidium and Giardia in a Canadian watershed influenced by urban and rural activities. J Water Health. 2012;10:311–323. doi: 10.2166/wh.2012.131. [DOI] [PubMed] [Google Scholar]
- 37.Ashbolt NJ. The significance of HPCs for water quality and human health. In: Bartram J, Cortruvo J, Exner M, Fricker C, Glasmacher A, editors. Heterotrophic Plate Counts and Drinking-Water Safety. Cornwall, UK: IWA Publishing; 2003. pp. 146–177. [Google Scholar]
- 38.Cupples AC, Rose JB, Xagoraraki I, McNamara CJ, Konkol N, Mitchell R. New molecular methods for detection of waterborne pathogen and microbial deterioration of cultural heritage materials. In: Mitchell R, Dong-Gu J, editors. Environmental Microbiology. Second Edition. New York: Wiley-Blackwell; 2010. pp. 57–153. [Google Scholar]