Abstract
This study aimed to investigate the association between meteorological-related risk factors and bacillary dysentery in a subtropical inland Chinese area: Changsha City. The cross-correlation analysis and the Autoregressive Integrated Moving Average with Exogenous Variables (ARIMAX) model were used to quantify the relationship between meteorological factors and the incidence of bacillary dysentery. Monthly mean temperature, mean relative humidity, mean air pressure, mean maximum temperature, and mean minimum temperature were significantly correlated with the number of bacillary dysentery cases with a 1-month lagged effect. The ARIMAX models suggested that a 1°C rise in mean temperature, mean maximum temperature, and mean minimum temperature might lead to 14.8%, 12.9%, and 15.5% increases in the incidence of bacillary dysentery disease, respectively. Temperature could be used as a forecast factor for the increase of bacillary dysentery in Changsha. More public health actions should be taken to prevent the increase of bacillary dysentery disease with consideration of local climate conditions, especially temperature.
Introduction
Bacillary dysentery, a diarrheal disease caused by different species of Shigella bacteria, including S. dysenteriae, S. flexneri, S. boydii, and S. sonnei,1 is a major public health issue in many countries in the world. Typical clinical characteristics of bacillary dysentery include fever, stomachache, and uncontrolled loose or watery stools containing visible red blood.2 The transmission of bacillary dysentery is fecal–oral, which may involve polluted food, water, daily contact, and flies. Despite the fact that the incidence of intestinal infectious diseases has declined considerably in recent years worldwide, the incidence of bacillary dysentery remains high in developing countries.3–6 The epidemics of bacillary dysentery are most common in overcrowded populations with inadequate sanitation.
As a fecal–oral disease, bacillary dysentery can be affected by changes in ambient environment. For example, higher temperature in summer may increase the reproduction of the bacteria along the food chain and water supply.7,8 Rainfall, relative humidity, air pressure, and maximum speed of wind may also affect the reproduction of pathogens and the contamination of drinking water, which may increase the incidence of bacillary dysentery.2,7,9,10 However, the mechanism of the association between meteorological variables and bacillary dysentery is still far from clear, with very few studies examining the quantitative relationship between meteorological factors and bacillary dysentery.
Bacillary dysentery is one of the major public health issues in China, with 237,930 new notified cases in 2011, and it is ranked fourth among the national notifiable diseases from the Chinese National Notifiable Disease Report. The incidence of bacillary dysentery in China is higher than developed countries. Although the Chinese government has made efforts in the prevention and control of bacillary dysentery, there have been challenges in reducing the incidence of bacillary dysentery in a changing environment. Hunan Province is one of the most seriously affected provinces in the Yangtze River Region, with 213,048 non-fatal notified cases and 66 fatal notified cases from 2004 to 2010. Changsha City is one of the most seriously affected areas, and it had the highest incidence in Hunan Province from 2004 to 2010, with the highest incidence of 43.05/100,000 in 2006. The number of cases in Changsha City ranked first in Hunan Province from 2004 to 2006. It is still a big public health problem in Changsha City. There have been very few studies of the impacts of climate variations on bacillary dysentery in China.
This study aimed to examine the current situation of bacillary dysentery disease in Changsha City and explore the possible consequences caused by climate variations. We used the Autoregressive Integrated Moving Average with Exogenous Variables (ARIMAX) model to quantify the association between multiple meteorological factors and bacillary dysentery in Changsha City, a subtropical area of inland China, to provide more evidence to support decision-making for the prevention and control of bacillary dysentery in a changing climate.
Materials and Methods
Study area.
The study area was Changsha City, the capital city of Hunan Province. It is located between longitude 111°53′ and 114°15′ E and latitude 27°51′ and 28°41′ N (Figure 1) along the Yangtze River watershed (southeast) of China. The city is compromised of nine counties, with a total land area of 11,800 km2 and a population of 3,849,090 in 2010.11 Changsha City has a typical subtropical monsoon climate, with hot and humid summers and dry and mild winters. The monthly average temperature ranges from 5°C in the winter to 38.5°C in the summer. Rainfall is typically between 30 mm in the winter to 250 mm in the summer.12 As the capital of Hunan Province, Changsha City has a high population density of about 600 people/km2 in 2010.2,7,13–17 Changsha City is a more developed city than other cities in Hunan. The public health infrastructure was well-developed. The typical humid subtropical monsoon climate and abundant rivers and lakes lead to the frequent high temperatures, rainy weather, and floods. All the meteorological and demographic conditions above are optimum for bacillary dysentery transmission.
Figure 1.
Location of the study area of Changsha City in Hunan Province, China.
Bacillary dysentery is the most serious diarrhea disease in Changsha City, with 9,006 new notified cases from 2004 to 2010, which is the highest incidence in Hunan Province.
Data collection.
Monthly total number of bacillary dysentery cases in Changsha City from January of 2004 to December of 2010 and the annual population were obtained from the National Notifiable Disease Surveillance System. All bacillary dysentery cases were diagnosed by clinical symptoms and serological tests. According to the National Communicable Disease Control Act, physicians in hospitals must report every case of bacillary dysentery to the local health authority. Then, the local health authority must report these cases to the next level of the Health Organization within 24 hours.18 Therefore, it is believed that the degree of compliance in disease notification over the study period is consistent.
Monthly meteorological data over the same period were collected from the China Meteorological Data Sharing Service System (http://cdc.cma.gov.cn/home.do). One of the aims of the analysis was to explore potential climatic risk factors that may increase the risk of bacillary dysentery in the study area. Therefore, we intended to include all meteorological variables that may be related to the health outcome, even if their association has not been clarified by previous studies. Based on literature review and possible biological mechanisms of the causal relationship, the following variables were included in our analysis: temperatures, relative humidity, rainfall, air pressure, and wind speed. The reproduction of the parasites, such as flies, increases during hot and humid days. Higher ambient temperature may lead to elevated consumption of raw foods and increased outdoor recreational activity.15 These factors contribute to a higher risk of bacillary dysentery bacteria exposure. Moreover, given that different temperature measures represent different climate variations, which may result in different impacts on the health outcome, we selected all temperature- and humidity-related factors, including monthly mean temperature calculated by daily mean temperature (MeanT), mean maximum temperature (MeanMaxT) calculated by daily maximum temperature, mean minimum temperature (MeanMinT) calculated by daily minimum temperature, and mean relative humidity (MeanH). The impact of rainfall on the transmission of bacillary dysentery has not been consistently reported. Low rainfall can be expected to interrupt water supply and contribute to poor hygiene.10 A likely initial effect of high rainfall is to flush fecal contaminants from dwellings into water supplies, but continued rain could lead to a subsequent improvement in water quality.10 A dose–response relationship may exist between rainfall and bacillary dysentery. Therefore, monthly total rainfall (RF) was also collected. Mean air pressure (MeanP) and maximum speed of wind (MaxW) were selected to explore whether they would affect the transmission of bacillary dysentery, because these factors have been studied before in other regions.7,9
Methods.
Descriptive analysis for the temporal trend of the number of bacillary dysentery cases.
For this study, we used sequence charts to describe the temporal trend and the distribution of the number of bacillary dysentery cases over the study period of 7 years.
Univariate analysis.
Cross-correlation analyses with the consideration of potential lagged effects were applied before the multivariate regression analysis. Cross-correlation function (CCF) was performed to examine the correlation between each meteorological variable and incidence of bacillary dysentery with various lag values.19,20 The lag value with the maximum correlation coefficient for each meteorological factor was selected for inclusion in subsequent time series regression analysis. Up to 1 month lagged effect was examined based on results from previous studies and the biological plausibility that it may take 1 month from the time of ingestion of polluted food and water for the intestinal infectious disease to occur, diagnosis to be received, and disease notifications to be sent.2,10,21,22 Only those variables showing a significant result in the CCF analysis were selected for additional multivariate regression analysis.
Multivariate time series regression analysis.
The ARIMAX models were used to quantify the relationship between meteorological factors and the incidence of bacillary dysentery. The ARIMAX model is the combination of multiple regression analysis and time series analysis, and it increases the precision of the forecast.23 It is an extension of the Autoregressive Integrated Moving Average (ARIMA) model24 and can examine the relationship between multiple meteorological factors and bacillary dysentery concurrently. More information can be saved by the ARIMAX model than the ARIMA model. When choosing variables to be included, there are fewer restrictions on the characteristics of the data.
The process of the model development included test of the stationarity, estimation of coefficients, and post-model evaluation, including distribution of the model residuals and goodness of fit. The model does not require the stabilization of inputted series if cointegration between meteorological variables and cases exists.25 If the cointegration did not exist, the spurious regression might influence the analysis. Therefore, the series should be stabled,26 and the stationarity of the data series was tested by the Augmented Dickey–Fuller Unit Root (ADF) test.23 The coefficients of the meteorological variables with lagged effects were estimated by the ARIMAX model.27 The model residuals were tested by the ADF test. The white noise test of residuals was also performed according to the autocorrelation plot residuals graph.28 The forecasting ability of the model was tested by the goodness of fit between the observed and expected numbers of bacillary dysentery cases23,28,29 using the mean standard error (MSE),30 which was calculated as
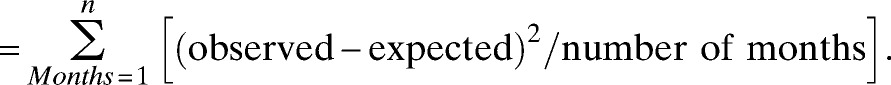 |
Smaller MSE values show that expected incidences from the models have a better fit for the observed incidences.
Because of the high correlation between MeanT, MeanMaxT, and MeanMinT, three separated models with MeanT, MeanMaxT, and MeanMinT were set up, respectively, to avoid multicollinearity in the models. All analyses were performed by SAS, version 9.1.3 (SAS Institute Inc., USA). Variables with P < 0.05 were considered to be significant in the ARIMAX models.
Ethical review.
The present study was fully reviewed by the human research ethical committee of Shandong University. We were notified that the use of deidentified disease surveillance data and meteorological data did not require the oversight by an ethics committee.
Results
Descriptive analysis of the temporal trend of the number of bacillary dysentery cases.
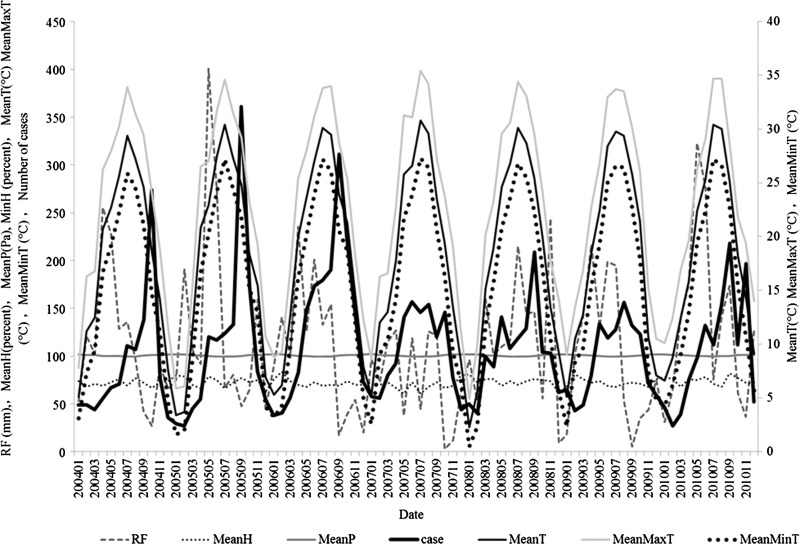
In total, 9,006 live cases and no deceased cases were notified during the study period, with a mean monthly incidence rate of 2.79 per 100,000. As Figure 2 shows, in Changsha City over the period from 2004 to 2010, monthly numbers of bacillary dysentery cases varied from 27 in 2010 to 361 in 2005. The time series of the number of cases and the meteorological variables are shown in Figure 2. There were distinctive seasonal variations, with most cases occurring from June to October (summer and autumn) and peaking in September.
Figure 2.
Sequence of meteorological variables and bacillary dysentery in Changsha City, China from 2004 to 2010.
Cross-correlation analysis with lagged effects.
Correlation coefficients with up to 1-month lag are presented in Table 1. A 1-month lagged MeanT (CCF = 0.714, P < 0.05), MeanMaxT (CCF = 0.709, P < 0.05), and MeanMinT (CCF = 0.716, P < 0.05) were positively associated with the number of bacillary dysentery cases. The 1-month lagged effects of MeanH (CCF = −0.186, P < 0.05) and MeanP (CCF = −0.674, P < 0.05) on bacillary dysentery were negative. RF and MaxW did not have a significant correlation with the number of bacillary dysentery.
Table 1.
Correlation coefficients between the incidence of bacillary dysentery disease and meteorological variables in Changsha City from 2004 to 2010
| Climate variables | Lag (months) | Coefficient | P value |
|---|---|---|---|
| RF (mm) | 0 | −0.080 | > 0.05 |
| RF (mm) | 1 | 0.116 | > 0.05 |
| MeanT (°C) | 0 | 0.630 | < 0.05 |
| MeanT (°C) | 1 | 0.714 | < 0.05 |
| MeanH (%) | 0 | −0.141 | > 0.05 |
| MeanH (%) | 1 | −0.186 | < 0.05 |
| MeanP (Pa) | 0 | −0.465 | < 0.05 |
| MeanP (Pa) | 1 | −0.674 | < 0.05 |
| MeanMaxT (°C) | 0 | 0.631 | < 0.05 |
| MeanMaxT (°C) | 1 | 0.709 | < 0.05 |
| MeanMinT (°C) | 0 | 0.631 | < 0.05 |
| MeanMinT (°C) | 1 | 0.716 | < 0.05 |
| MaxW (m/s) | 0 | 0.027 | > 0.05 |
| MaxW (m/s) | 1 | 0.163 | > 0.05 |
Multivariate time series regression analysis.
The parameters of three regression models are shown in Table 2. One-month lagged effects of MeanT, MeanMaxT, and MeanMinT were included in models 1, 2, and 3, respectively. The ARIMAX models suggested that a 1°C rise in MeanT, MeanMaxT, and MeanMinT might relate to a 14.8%, 12.9%, and 15.5% increase, respectively, in the incidence of bacillary dysentery disease. The other three meteorological variables were not significantly included in these models.
Table 2.
Parameters estimated by ARIMAX models for the relationship between bacillary dysentery and meteorological variables in Changsha City from 2004 to 2010
| Meteorological variables | Lag months | Coefficients | Standard error | t | P value | MSE |
|---|---|---|---|---|---|---|
| Model 1 | 1.297 | |||||
| Constant | 0 | −10.007 | 53.416 | −0.19 | 0.8514 | |
| MeanT (°C) | 1 | 0.148 | 0.050 | 2.97 | 0.0030 | |
| MeanH (%) | 1 | 0.005 | 0.030 | 0.16 | 0.869 | |
| MeanP (Pa) | 1 | 0.097 | 0.516 | 0.19 | 0.851 | |
| Model 2 | 1.302 | |||||
| Constant | 0 | 6.005 | 51.282 | 0.12 | 0.907 | |
| MeanMaxT (°C) | 1 | 0.129 | 0.046 | 2.78 | 0.006 | |
| MeanH (%) | 1 | 0.013 | 0.031 | 0.41 | 0.680 | |
| MeanP (Pa) | 1 | −0.070 | 0.491 | −0.14 | 0.887 | |
| Model 3 | 1.288 | |||||
| Constant | 0 | −13.176 | 53.404 | −0.25 | 0.805 | |
| MeanMinT (°C) | 1 | 0.155 | 0.051 | 3.03 | 0.003 | |
| MeanH (%) | 1 | −0.005 | 0.029 | −0.19 | 0.853 | |
| MeanP (Pa) | 1 | 0.139 | 0.519 | 0.27 | 0.789 |
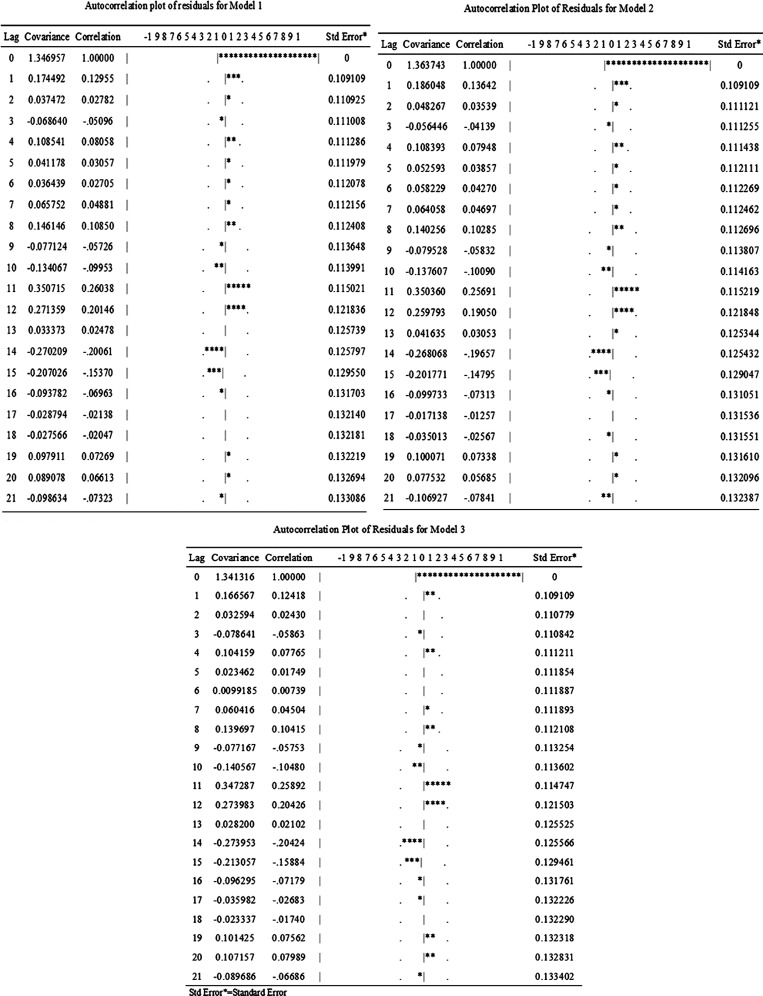
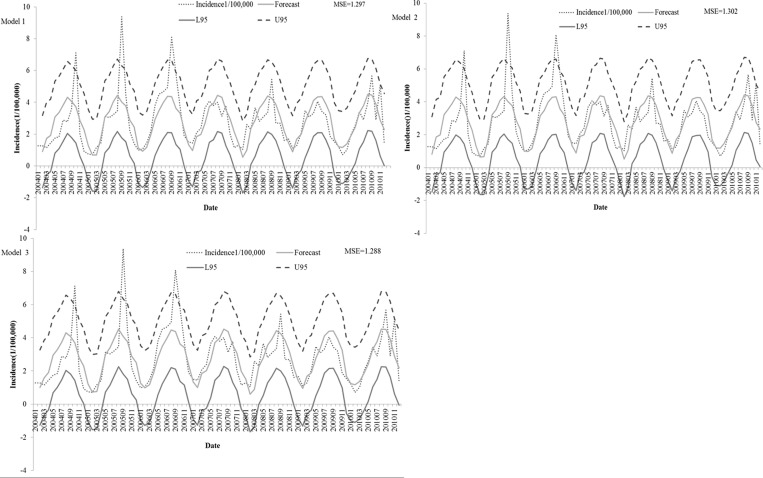
Figure 3 shows that almost all of the covariances are limited in two times standard error. An autocorrelation check of residuals showed randomly distributed residuals with no autocorrelation among them (Figure 3). The observed incidences of bacillary dysentery and the predicted incidences from models 1–3 had an excellent goodness of fit, which is shown in Figure 4, with an MSE of 1.297, 1.302, and 1.288, respectively.
Figure 3.
Autocorrelation check of residuals for three models. *Autocorrelation coefficient.
Figure 4.
Reported and expected cases of bacillary dysentery from three ARIMAX models in Changsha City from 2004 to 2010. L = The lower limit of 95% confidence interval; U = The upper limit of 95% confidence interval.
Discussion
Our study confirms that the temporal distribution of bacillary dysentery in Changsha City has varied over time. Most cases occurred in summer and autumn. To our knowledge, this study is the first epidemiological study to examine the impacts of meteorological factors on bacillary dysentery disease in a subtropical inland area of China using the ARIMAX model. Meanwhile, the results indicate that MeanT, MeanMaxT, and MeanMinT are key factors that contribute to the transmission of bacillary dysentery in Changsha City.
Evidence shows that, as the temperature increases, there is a corresponding increase in bacillary dysentery cases.2,7,13,16,17 This finding is consistent with our results, showing that temperatures have positive effects on bacillary dysentery disease in Changsha City. Temperature may affect the transmission of bacillary dysentery through several pathways.31–33 First, increasing temperature can increase the survival and replication of the pathogens in the environment. The optimum temperature for the growth of bacillus dysenteriae is 37°C.15 The reproduction of the parasites, such as flies, also increases during hot days.15 Second, ambient temperature can increase the chance of contamination of food sources through behavior changes during hot temperatures, such as elevated consumption of raw foods in summers.34–36 Third, higher temperatures may increase the chance of person-to-person contact, resulting in more people exposed to bacillary dysentery pathogens.37 All these factors can contribute to more mortality and morbidity caused by bacillary dysentery and similar food-borne diseases.
The temperature in the previous 1 month has the greatest significant association with the number of monthly bacillary dysentery disease cases. The ARIMAX model suggests that temperatures have already affected the transmission of bacillary dysentery in Changsha City, and a 1°C increase in temperatures may cause more than a 12% increase in the incidence. A 1-month time lag of the temperatures suggests that temperature could be used as an early forecasting factor of bacillary dysentery. This result is consistent with many other studies on meteorological variables and intestinal infectious diseases that have found an average lagged effect of 1 month.2,7,10,22,38 The lagged period of 1 month in our study is biologically plausible and includes growth of the bacteria in an optimum environment, transmission through polluted food and water, time period from the onset of the intestinal infectious disease to the visit to the healthcare facility, laboratory diagnosis, and notification to the system.21
We could not detect a clear association between bacillary dysentery and other climatic variables, including RF, MeanP, and MeanH, by the ARIMAX model, although the associations were statistically significant in the correlation analysis. This finding is similar to findings in Denmark, Australia, China, and Bangladesh,2,10,38–40 in that RF cannot affect bacillary dysentery directly. However, some studies of Pacific Islands report a dose–response relationship between RF and bacillary dysentery, suggesting that extremely high and low values of RF may lead to an increase in the number of cases. Low RF can be expected to interrupt water supply and contribute to poor environmental hygiene.10 Other results in New Zealand and Vietnam also show that RF is a positive factor for bacillary dysentery.14,41,42 MeanH, MeanP, and MaxW may affect the transmission of bacillary dysentery by influencing the reproduction and growth of Shigella bacteria.7 They may also affect the contamination of drinking water, which affects the incidence of diseases.9 Our single-factor correlation analysis has shown that MeanH and MeanP may be correlated with bacillary dysentery disease in Changsha City. This result is in partial concordance with the results from other cities in China.7,22 However, the association is not confirmed by the ARIMAX model. Given the interaction among the meteorological factors, it may be inappropriate to analyze them independently. There are very few studies examining the relationship between these two climate variables and food-borne diseases. More research is called for a better understanding of the association.
The ARIMAX model is a useful tool for dealing with the relationship between multiple meteorological factors and bacillary dysentery. Compared with the ARIMA model, the ARIMAX model has fewer limitations in the prerequisite of the selection of variables, and it is superior in controlling confounders, such as secular trends and seasonality, which makes the analysis and forecast more appropriate. It can efficiently examine the relationship between multiple meteorological factors and bacillary dysentery, allowing the analysis of the interaction between meteorological factors. The excellent goodness of fit of the regression models indicates that ARIMAX model could be an appropriate tool to quantify the relationship between multiple meteorological factors and bacillary dysentery.
Some limitations of the study should be acknowledged. Meteorological variables are components of the causal network of bacillary dysentery, and they are neither necessary nor sufficient for the transmission of the infection. Socioeconomic factors (e.g., human behavior, preventive actions taken by the government, economics, and sanitary environment) could not be analyzed by this study because of a lack of data availability. In addition, we used monthly meteorological data in the analysis, which may diminish the temporal trend of climate factors and the relationship between meteorological data and bacillary dysentery, particularly in detecting the lagged effect. Daily or weekly data could be used in future studies if data are available. Although uncertainties exist, our study has provided evidence to support a positive association between temperature and numbers of cases of bacillary dysentery in a Chinese setting, which is consistent with the increasing diarrheal diseases associated with rising temperatures.43
In conclusion, our study indicates that bacillary dysentery is mainly related to the temperatures in the study region in China. Increasing temperature may bring more diarrheal disease burden in the tropical and subtropical areas in China. There are some public health implications of this study that practitioners and policymakers can use in response to the prevention and control of infectious diseases in a warming climate. In particular, consideration of local temperature variations should be integrated into the development of strategies or early warning systems for the prevention and control of bacillary dysentery to reduce the burden of disease related to rising temperatures.
ACKNOWLEDGMENTS
The authors thank the Chinese Center for Disease Control and Prevention, the National Meteorological Information Center of China, and the Data Center for the Institute of Geographic Sciences and Natural Resources Research of China for sharing with us the data needed for this study.
Footnotes
Financial support: This study was supported by National Basic Research Program of China (973 Program) Grant 2012CB955502.
Authors' addresses: Lu Gao, Xiujun Li, and Baofa Jiang, Department of Epidemiology and Health Statistics, School of Public Health, Shandong University, Jinan City, Shandong Province, People's Republic of China, E-mails: gaolusdu@sina.com, xjli@sdu.edu.cn, and bjiang@sdu.edu.cn. Ying Zhang, School of Public Health, China Studies Centre, The University of Sydney, New South Wales, Australia and School of Population Health, University of Adelaide, Adelaide, Australia, E-mail: ying.zhang@sydney.edu.au. Guoyong Ding, Department of Occupational and Environmental Health, School of Public Health, Taishan Medical College, Taian City, Shandong Province, People's Republic of China, E-mail: dgy153@126.com. Qiyong Liu, State Key Laboratory for Infectious Diseases Prevention and Control, National Institute for Communicable Disease Control and Prevention, China Centers for Disease Control of Prevention, Beijing City, People's Republic of China, E-mail: liuqiyong@icdc.cn. Maigeng Zhou, National Center for Chronic and Noncommunicable Disease Control and Prevention, China Centers for Disease Control of Prevention, Beijing City, People's Republic of China, E-mail: maigengzhou@126.com.
References
- 1.Niyogi SK. Shigellosis. J Microbiol. 2005;43:133–143. [PubMed] [Google Scholar]
- 2.Patrick ME, Christiansen LE, Waino M, Ethelberg S, Madsen H, Wegener HC. Effects of climate on incidence of Campylobacter spp. in humans and prevalence in broiler flocks in Denmark. Appl Environ Microbiol. 2004;70:7474–7480. doi: 10.1128/AEM.70.12.7474-7480.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, Canh do G, Chaicumpa W, Agtini MD, Hossain A, Bhutta ZA, Mason C, Sethabutr O, Talukder K, Nair GB, Deen JL, Kotloff K, Clemens J. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 2006;3:e353. doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang XY, Du L, Von Seidlein L, Xu ZY, Zhang YL, Hao ZY, Han OP, Ma JC, Lee HJ, Ali M, Han CQ, Xing ZC, Chen JC, Clemens J. Occurrence of shigellosis in the young and elderly in rural China: results of a 12-month population-based surveillance study. Am J Trop Med Hyg. 2005;73:416–422. [PubMed] [Google Scholar]
- 5.Wang XY, Tao F, Xiao D, Lee H, Deen J, Gong J, Zhao Y, Zhou W, Li W, Shen B, Song Y, Ma J, Li ZM, Wang Z, Su PY, Chang N, Xu JH, Ouyang PY, von Seidlein L, Xu ZY, Clemens JD. Trend and disease burden of bacillary dysentery in China (1991–2000) Bull World Health Organ. 2006;84:561–568. doi: 10.2471/blt.05.023853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 7.Guan P, Huang D, Guo J, Wang P, Zhou B. Bacillary dysentery and meteorological factors in northeastern China: a historical review based on classification and regression trees. Jpn J Infect Dis. 2008;61:356–360. [PubMed] [Google Scholar]
- 8.Kovats RS, Edwards SJ, Hajat S, Armstrong BG, Ebi KL, Menne B. The effect of temperature on food poisoning: a time-series analysis of salmonellosis in ten European countries. Epidemiol Infect. 2004;132:443–453. doi: 10.1017/s0950268804001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma SL, Tang QL, Liu HW, He J, Gao SH. Correlation analysis for the attack of bacillary dysentery and meteorological factors based on the Chinese medicine theory of Yunqi and the medical-meteorological forecast model. Chin J Integr Med. 2012;19:182–186. doi: 10.1007/s11655-012-1239-z. [DOI] [PubMed] [Google Scholar]
- 10.Singh RB, Hales S, de Wet N, Raj R, Hearnden M, Weinstein P. The influence of climate variation and change on diarrheal disease in the Pacific Islands. Environ Health Perspect. 2001;109:155–159. doi: 10.1289/ehp.01109155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Census Office of the National Bureau of Statistics of Population and Employment Statistics Division . Tabulation on the 2010 Population Census of the People's Republic of China. 2010. http://219.235.129.58/reportYearQuery.do?id=0200&r=0.5453362756536959 Available at. Accessed March 28, 2013. [Google Scholar]
- 12.China Meteorological Data Sharing Service System . The Surface Meteorological Data. 2013. http://cdc.cma.gov.cn/home.do Available at. Accessed February 1, 2013. [Google Scholar]
- 13.Checkley W, Epstein LD, Gilman RH, Figueroa D, Cama RI, Patz JA, Black RE. Effect of El Niño and ambient temperature on hospital admissions for diarrhoeal diseases in Peruvian children. Lancet. 2000;355:442–450. doi: 10.1016/s0140-6736(00)82010-3. [DOI] [PubMed] [Google Scholar]
- 14.Kelly-Hope LA, Alonso WJ, Thiem VD, Anh DD, Canh do G, Lee H, Smith DL, Miller MA. Geographical distribution and risk factors associated with enteric diseases in Vietnam. Am J Trop Med Hyg. 2007;76:706–712. [PubMed] [Google Scholar]
- 15.Lake IR, Gillespie IA, Bentham G, Nichols GL, Lane C, Adak GK, Threlfall EJ. A re-evaluation of the impact of temperature and climate change on foodborne illness. Epidemiol Infect. 2009;137:1538–1547. doi: 10.1017/S0950268809002477. [DOI] [PubMed] [Google Scholar]
- 16.Tam CC, Rodrigues LC, O'Brien SJ, Hajat S. Temperature dependence of reported Campylobacter infection in England, 1989–1999. Epidemiol Infect. 2006;134:119–125. doi: 10.1017/S0950268805004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas CJ, Davies G, Dunn CE. Mixed picture for changes in stable malaria distribution with future climate in Africa. Trends Parasitol. 2004;20:216–220. doi: 10.1016/j.pt.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health of the People's Republic of China . Emergency Events and Regulation of the Notifiable Disease Surveillance System. 2006. http://www.gov.cn/zwgk/2006-09/08/content_382018.htm Available at. Accessed February 1, 2013. [Google Scholar]
- 19.Akanda AS, Jutla AS, Islam S. Dual peak cholera transmission in Bengal Delta: a hydroclimatological explanation. Geophys Res Lett. 2009;36:L19401. [Google Scholar]
- 20.Akanda AS, Jutla AS, de Magny GC, Alam M, Siddique AK, Sack RB, Huq A, Colwell RR, Islam S. Hydroclimatic influences on seasonal and spatial cholera transmission cycles: implications for public health intervention in the Bengal Delta. Water Resour Res. 2011;47:W00H07. [Google Scholar]
- 21.Neimann J, Engberg J, Molbak K, Wegener HC. A case-control study of risk factors for sporadic Campylobacter infections in Denmark. Epidemiol Infect. 2003;130:353–366. doi: 10.1017/s0950268803008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Bi P, Hiller JE, Sun Y, Ryan P. Climate variations and bacillary dysentery in northern and southern cities of China. J Infect. 2007;55:194–200. doi: 10.1016/j.jinf.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Granger CWJ, Swanson N. Future development in the study of cointegrated variables. Oxf Bull Econ Stat. 1996;58:537–553. [Google Scholar]
- 24.Wangdi K, Singhasivanon P, Silawan T, Lawpoolsri S, White NJ, Kaewkungwal J. Development of temporal modelling for forecasting and prediction of malaria infections using time-series and ARIMAX analyses: a case study in endemic districts of Bhutan. Malar J. 2010;9:251. doi: 10.1186/1475-2875-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornelsen L, Normand C. Impact of the smoking ban on the volume of bar sales in Ireland: evidence from time series analysis. Health Econ. 2012;21:551–561. doi: 10.1002/hec.1728. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Jiang B, Gu W, Liu Q. Temporal trend and climate factors of hemorrhagic fever with renal syndrome epidemic in Shenyang City, China. BMC Infect Dis. 2011;11:331. doi: 10.1186/1471-2334-11-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chadsuthi S, Modchang C, Lenbury Y, Iamsirithaworn S, Triampo W. Modeling seasonal leptospirosis transmission and its association with rainfall and temperature in Thailand using time–series and ARIMAX analyses. Asian Pac J Trop Med. 2012;5:539–546. doi: 10.1016/S1995-7645(12)60095-9. [DOI] [PubMed] [Google Scholar]
- 28.Box GEP, Jenkins GM. Time Series Analysis: Forecasting and Control. San Francisco, CA: Holden Day; 1976. pp. 181–218. [Google Scholar]
- 29.Lee HS, Her M, Levine M, Moore GE. Time series analysis of human and bovine brucellosis in South Korea from 2005 to 2010. Prev Vet Med. 2012;3296:1–8. doi: 10.1016/j.prevetmed.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Bi P, Hiller JE. Climate variations and salmonellosis transmission in Adelaide, South Australia: a comparison between regression models. Int J Biometeorol. 2008;52:179–187. doi: 10.1007/s00484-007-0109-4. [DOI] [PubMed] [Google Scholar]
- 31.Tiradoa MC, Clarkeb R, Jaykusc LA, McQuatters-Gollopd A, Franke JM. Climate change and food safety: a review. Food Res Int. 2010;43:1745–1765. [Google Scholar]
- 32.Carcavallo RU, Curto de Casas S. Some health impacts of global warming in South America: vector-borne diseases. J Epidemiol. 1996;6:S153–S157. [Google Scholar]
- 33.Asin S, Catala S. Development of Trypanosoma cruzi in Triatoma infestans-influence of temperature and blood consumption. J Parasitol. 1995;81:1–7. [PubMed] [Google Scholar]
- 34.Hall GV, D'Souza RM, Kirk MD. Foodborne disease in the new millennium: out of the frying pan and into the fire? Med J Aust. 2002;177:614–618. doi: 10.5694/j.1326-5377.2002.tb04984.x. [DOI] [PubMed] [Google Scholar]
- 35.Lehane L. Ciguatera update. Med J Aust. 2000;172:176–179. doi: 10.5694/j.1326-5377.2000.tb125546.x. [DOI] [PubMed] [Google Scholar]
- 36.Ashbolt R, Givney R, Gregory JE, Hall G, Hundy R, Kirk M, McKay I, Meuleners L, Millard G, Raupach J, Roche P, Prasopa-Plaizier N, Sama MK, Stafford R, Tomaska N, Unicomb L, Williams C. OzFoodNet Working Group Enhancing foodborne disease surveillance across Australia in 2001: the OzFoodNet Working Group. Commun Dis Intell Q Rep. 2002;26:375–406. doi: 10.33321/cdi.2002.26.32. [DOI] [PubMed] [Google Scholar]
- 37.El Saadi O, Esterman A, Cameron S, Roder D. Murray River water, raised cyanobacterial cell counts, and gastrointestinal and dermatological symptoms. Med J Aust. 1995;162:122–125. doi: 10.5694/j.1326-5377.1995.tb138473.x. [DOI] [PubMed] [Google Scholar]
- 38.D'Souza RM, Becker NG, Hall G, Moodie KB. Does ambient temperature affect foodborne disease? Epidemiology. 2004;15:86–92. doi: 10.1097/01.ede.0000101021.03453.3e. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Bi P, Hiller JE. Weather and the transmission of bacillary dysentery in Jinan, Northern China: a time-series analysis. Public Health Rep. 2008;123:61–66. doi: 10.1177/003335490812300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. Refractory periods and climate forcing in cholera dynamics. Nature. 2005;436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- 41.Weinstein P, Woodward A. Ecology, Climate and Campylobacteriosis in New Zealand. Integration of Public Health with Adaptation to Climate Change. Leiden, The Netherlands: Taylor and Francis; 2005. pp. 60–71. [Google Scholar]
- 42.Lei J, Xinyu L, Guirong L, Yuan L. Analysis of the association between meteorological factors and incidence of dysentery in Beijing. Mod Prev Med. 2007;34:2470–2471. [Google Scholar]
- 43.Kolstad EW, Johansson KA. Uncertainties associated with quantifying climate change impacts on human health: a case study for diarrhea. Environ Health Perspect. 2011;119:299–305. doi: 10.1289/ehp.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]