Abstract
Diarrheal disease remains a leading cause of morbidity in areas with limited access to safe water and sanitation. As water and sanitation interventions continue to be implemented, it will be important to understand the ecological context in which they can prevent diarrhea. We conducted six serial case control studies in Ecuador to estimate the risk of diarrhea from unimproved water and sanitation and the potential for effect modification by rainfall. Unimproved water source and unimproved sanitation increased the adjusted odds of diarrhea (odds ratio [OR] = 3.6, 95% confidence interval [95% CI] = 1.7–7.8 and OR = 1.7, 95% CI = 1.2–2.5, respectively). The OR associated with an unimproved water source was highest after maximum rainfall (OR = 6.8, 95% CI = 1.9–24.5), whereas the OR associated with unimproved sanitation was highest after minimal rainfall (OR = 2.9, 95% CI = 1.3–6.6). Our finding that use of safe water sources and improved sanitation facilities are most protective under opposing rainfall conditions highlights the need for integrated interventions to reduce the burden of diarrheal disease.
Introduction
Diarrheal disease continues to be a leading cause of mortality in children younger than 5 years. Nearly 1.9 million children die annually from diarrheal disease, accounting for 19% of all deaths in this age group.1 This global burden of diarrheal disease disproportionately affects developing regions, largely because of the lack of safe water and basic sanitation.2–4 The United Nations Millennium Development Goals aim to cut in half the proportion of those people without access to safe water and basic sanitation by 2015. Although we have surpassed the target for water, we will likely fall short of the target for basic sanitation.5 An estimated 2.5 billion people still lack access to improved sanitation facilities, and 1.1 billion people defecate in the open.5 As we aim to improve coverage of basic sanitation, it will be important to have a broader understanding of the environmental context in which improved sanitation can prevent exposure to enteric pathogens.
Enteric pathogens are shed by infected individuals into the environment.6 These pathogens can flow throughout the community through various environmental pathways that are influenced by water and sanitation infrastructure as well as the ecological environment. For example, inadequate sanitation in the community can cause the release of pathogens into the soil or promote the transport of pathogens through flies.7 Water runoff, governed by ecological factors such as rain and soil moisture content, may facilitate the spread of these pathogens throughout a community, resulting in contamination of primary water sources.8,9 Drinking untreated water from these primary sources may result in infection and additional transmission. Thus, inadequate sanitation, unsafe water sources, and rainfall may have interdependent effects on diarrhea.
The literature on rainfall and diarrhea pays little attention to the use of improved water and sanitation. Additionally, there is large variability in the way that rainfall has been captured. It may be for these reasons that the relationship between rainfall and diarrhea remains unclear. Recent studies have shown positive,10–12,41 negative,10,41 and threshold-type associations between rainfall and diarrheal disease.13,14 In these studies, rainfall indicators included total, average, or number of extreme rainfall events defined by some threshold over a period of time (referred to as a lag period). Lag periods in which rainfall was summarized ranged from 0 days to 4 months before diarrhea episodes. In one study, changing the lag period led to a significant change in the direction of rainfall effects.10
Developing a better understanding of how rainfall directs pathogen transport and potentially modifies the effects of improved water and sanitation is important to the design of effective interventions for diarrhea. In the present study, we use a serial community-based case control design to capture diarrhea events in Borbón, a developing and urban community in northwestern Ecuador, over a 6-month period covering the rainy season. We estimate the associations between diarrhea and household water and sanitation practices, the interaction between water and sanitation, and the potential for effect modification by extreme rainfall events.
Methods
Study site.
Borbón is located on the northwestern coast of Ecuador in the province of Esmeraldas. Borbón has a population of approximately 5,000 people; it has 1,175 houses and spans a geographic area of 1.3 km2. Although it was once considered remote, this area is now connected to the coast on the west and the Andes to the east by a paved road. The new road has encouraged in-migration, creating potential for new settlements on the outer edges of the town where water and sanitation infrastructure may not reach. A water treatment plant was established in 2004, but it does not supply water to all households, and the supply is unreliable, forcing some to use well or river water for consumption and hygiene. Sanitation options in the town are mixed and include flush toilets, latrines, and open pits. At the time of the study, there was no functioning sewage system in place, although one was being constructed.
Cohort and census.
In November of 2008, all houses in Borbón were mapped and enumerated using global positioning system technology. Two distinct cohorts of approximately 200 households were randomly selected and recruited into the study. The only requirement for study inclusion was that the selected house was occupied during the 2-week recruitment period. During recruitment, we collected census data and obtained oral consent from a household representative. Institutional Review Board (IRB) committees at the University of Michigan and Universidad San Francisco de Quito approved all protocols.
Serial case control.
A 15-day case control study was conducted each month between December of 2008 and May of 2009, which spanned a typical rainy season in the region. The six case control studies were nested within one of two distinct cohorts described above. We alternated between the two cohorts, allowing us to follow a larger sample of households over time. Two days before the case control period, the census was updated, cross-sectional surveys were conducted on household water and sanitation practices, and observations of hygiene were recorded. During the case control period, daily visits were made to each of 200 houses in the cohort to identify cases of diarrhea. Cases were defined as having three or more loose stools in a 24-hour period. Census, survey, and case data were collected from the head of the household if present; otherwise, data were collected from any available adult in the household.
Rainfall measurement.
Daily precipitation was collected using a Hoboware Data Logging Rain Gauge (RG3; Onset Computer Corporation, Bourne, MA) installed in Borbón from November of 2008 to May of 2009. Rainfall was summarized as the number of days of extreme rainfall. Extreme rainfall days were those days exceeding the 90th percentile of the observed daily rainfall totals, a threshold selected based on previous studies.8,12 Extreme rainfall days were summarized over lag periods of 3, 4, and 5 weeks before the end of the 15-day case control period. These lag periods were chosen to reflect an average incubation period of 1–2 weeks among pathogens commonly found in the region15,16 and allow for an additional 2–3 weeks, during which time secondary transmission events may have taken place. Although cumulative, these lag periods are comparable with others found in the literature.10,13,14 Pearson's correlation coefficients were used to measure the strength of the relationship between rainfall and each lag period. The lag period with the strongest correlation with diarrhea prevalence was chosen for additional analysis. P values are not reported here, because the strength (not statistical significance) of the correlation was used to select the most appropriate lag period.
Household-level risk factors.
Ownership, used as a proxy for socioeconomic status, was assumed to be static between November of 2008 and May of 2009, and therefore, it was only measured one time at the beginning of the study. Households were assigned a total score scaled from zero to one calculated by summing an individual score (s) assigned to the following household assets: house and car (s = 4/39); business, farm, motorcycle, and motorboat (s = 3/39); canoe, chainsaw, television, computer, mobile phone, cooking stove, and refrigerator (s = 2/39); and solar panel, sewing machine, stereo, blender, and bicycle (s = 1/39). Other household-level factors were expected to vary over time and measured monthly during the study period: (1) household demographics (i.e., residents of the household, age distribution, and education level), (2) hygiene score ranging from zero to one, (3) drinking water-related factors (such as source, storage, and treatment), and (4) sanitation facility and garbage disposal. Hygiene was scored based on a set of observations of household cleanliness. Each observation was assigned a zero or one to indicate improved or unimproved. These scores were summed to form a hygiene index. Observations were made of visible fecal matter, loose garbage, improper storage of food and drinking water, and indicators of handwashing, such as the presence of soap and water. We considered uncovered wells and river water as unimproved water sources. Covered wells and piped, bottled, and rainwater were categorized as improved water sources. Open pit latrines (rudimentary holes), open defecation, and use of temporary vessels were considered unimproved sanitation, whereas flush toilets and pit latrines with raised platforms, slabs, or seats were considered improved.17 Waste disposal was categorized as unimproved (disposed of in a field, ditch, or river) or improved (collected or burned).
Logistic regression models.
Given the high probability of secondary infections within a household and our interest in household-level factors, we assigned the household as the unit of analysis for diarrhea. We defined a case household as any household with at least one case of diarrhea in the 15-day case control period. Control households were defined as all non-case households in the cohort. Six months of household-level data were pooled for analyses with univariable and multivariable logistic regression models. Robust z statistics18 were used to allow unbiased inference in the presence of repeated measurements on households. The multivariable regression model included predictors of primary interest, such as water source, sanitation, and extreme rainfall. Age and ownership, both well-documented risk factors,3,16,19–23 were added to the multivariable model. Additionally, we expected that, as the number of household residents increased, the probability of finding a case of diarrhea in the household would also increase, and therefore, we added household population size to the model as well. Four models were run. Model 1 included only main effects, model 2 included an interaction term between unimproved water and unimproved sanitation, and models 3 and 4 included the interaction effects of rainfall on unimproved water and sanitation, respectively.
Using models 2–4, we estimated multiplicative interactions between water source and sanitation (model 2), extreme rainfall and water source (model 3), and extreme rainfall and sanitation (model 4). To quantify the interactive effect, we calculated the adjusted odds ratios (ORs) between water or sanitation and diarrhea under different values of rainfall. Because these calculations involved contrasts of regression coefficients, their SEs required knowledge of the covariance between the coefficient estimates. To address this complication, we used the naïve bootstrap to obtain 95% confidence intervals (95% CIs) for these ORs. Bootstrap estimates were obtained by sampling with replacement from the original dataset a number of observations equal to the original sample size. This sampling was repeated 1,000 times. All statistical analyses were carried out using R (v.2.11.1).
Results
Cohort characteristics.
We followed 433 different households in Borbón between December of 2008 and May of 2009. The age distribution of the total cohort of 2,295 individuals had a median of 17 years and ranged between 0 and 93 years. During the six 15-day case control periods, we made 1,035 household observations, identifying 204 case households and 831 control households (Table 1). Approximately 48% of 1,035 households had one or more children younger than 5 years. The median household population size was five persons (range = 1–25), and the median household ownership score was 0.33 (range = 0–1), with widespread ownership of homes, television sets, and DVD players but limited ownership of vehicles, businesses, and farms. There were little to no differences between the two distinctly sampled cohorts for all variables shown in Table 1.
Table 1.
Characteristics of case and control households from a series of six community-based case control studies in Borbón, Ecuador (from December of 2008 to May of 2009)
| Case households, N = 204 (%) | Control households, N = 831 (%) | |
|---|---|---|
| Age | ||
| One or more children < 5 years of age | 157 (77.0) | 335 (40.3) |
| Household population | ||
| ≥ 5 people | 157 (77.0) | 400 (48.1) |
| Ownership score (0–1) | ||
| < 0.3 | 94 (46.1) | 319 (38.4) |
| ≥ 0.3 to < 0.6 | 107 (52.5) | 470 (56.6) |
| ≥ 0.6 | 3 (1.5) | 42 (5.1) |
| Education | ||
| ≥ 1 household member completed high school | 64 (31.4) | 339 (40.8) |
| Drinking water source | ||
| Improved | ||
| Piped water | 160 (78.4) | 593 (71.4) |
| Bottled water | 16 (7.8) | 149 (17.9) |
| Rain water | 8 (3.9) | 36 (4.3) |
| Covered well | 2 (1.0) | 9 (1.1) |
| Unimproved | ||
| Uncovered well | 9 (4.4) | 11 (1.3) |
| River | 7 (3.4) | 9 (1.1) |
| Unknown | 2 (1.0) | 24 (2.9) |
| Point-of-use water treatment | ||
| Yes | 56 (27.5) | 250 (30.1) |
| No | 146 (71.6) | 556 (66.9) |
| Unknown | 2 (1.0) | 25 (3.0) |
| Water storage vessel mouth size | ||
| Small (< 8 cm) | 128 (62.7) | 556 (66.9) |
| Large (≥ 8 cm) | 74 (36.3) | 251 (30.2) |
| Unknown | 2 (1.0) | 24 (2.9) |
| Type of sanitation facility | ||
| Improved | ||
| Flush toilet | 112 (54.9) | 558 (67.1) |
| Pit latrine with raised platform | 16 (7.8) | 69 (8.3) |
| Unimproved | ||
| Open pit latrine | 50 (24.5) | 116 (14.0) |
| Open field | 12 (5.9) | 21 (2.5) |
| River | 4 (2.0) | 12 (1.4) |
| In a bag, newspaper, or basin | 8 (3.9) | 30 (3.6) |
| Unknown | 2 (1.0) | 25 (3.0) |
| Number of families sharing a toilet or latrine | ||
| 1 | 112 (54.9) | 527 (63.4) |
| ≥ 2 | 63 (30.9) | 204 (24.5) |
| Not applicable | 24 (11.8) | 63 (7.6) |
| Unknown | 5 (2.5) | 37 (4.5) |
| Household hygiene score (0–1) | ||
| < 0.6 | 41 (20.1) | 105 (12.6) |
| ≥ 0.6 to < 0.8 | 79 (38.7) | 273 (32.9) |
| ≥ 0.8 | 75 (36.8) | 416 (50.1) |
| Unknown | 9 (4.4) | 37 (4.5) |
| Waste disposal | ||
| Improved | ||
| Collected by the city | 134 (65.7) | 601 (72.3) |
| Burned at home | 13 (6.4) | 47 (5.7) |
| Unimproved | ||
| Field, ditch, or river | 55 (27.0) | 159 (19.1) |
| Unknown | 2 (1.0) | 24 (2.9) |
Diarrhea prevalence and rainfall.
The 15-day period prevalence of diarrhea was highest in December of 2008 (just before the rainy season began; 7.2 cases per 100 persons) and lowest in January of 2009 (at the beginning of the rainy season; 3.3 cases per 100 persons) (Figure 1). The number of extreme rainfall days (> 18-mm rainfall) was negatively correlated with diarrhea using 3-, 4-, and 5-week lag periods. Extreme rainfall days summarized over 5 weeks had a higher correlation with diarrhea prevalence (R2 = 0.22) than extreme rainfall days summarized over 3 (R2 = 0.12) and 4 weeks (R2 = 0.05). When summarized over 5 weeks, the number of extreme rainfall days ranged from 1 to 5 days.
Figure 1.
Daily rainfall and the 15-day period prevalence of diarrhea from a series of six 15-day community-based case control studies (horizontal bars) in Borbón, Ecuador (from November of 2008 to May of 2009).
Risk factors for diarrhea.
An unadjusted analysis of predictors for diarrhea revealed significant associations with household demographic factors, socioeconomic factors, water, sanitation, hygiene, and extreme rainfall (Table 2). Factors selected a priori for the adjusted analysis retained their associations with diarrhea (Table 2, multivariable model 1). For example, after adjusting for children under 5 years, household size, ownership, extreme rainfall, and either unimproved water source or unimproved sanitation, both unimproved water source and unimproved sanitation were associated with increased odds of diarrhea (OR = 3.6, 95% CI = 1.7–7.8 and OR = 1.7, 95% CI = 1.2–2.5, respectively). Adjusting for these same predictors, each additional day of extreme rainfall in the past 5 weeks lowered the odds of diarrhea by a factor of 0.8 (95% CI = 0.7–0.9).
Table 2.
OR and robust 95% CIs for household-level diarrhea from a series of six community-based case control studies in Borbón, Ecuador (from December of 2008 to May of 2009)
| Predictor | Unadjusted estimates | Multivariable model 1 | Multivariable model 2 | Multivariable model 3 | Multivariable model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Household with child < 5 years of age | 4.95 | 3.38–7.24 | 3.75 | 2.40–5.86 | 3.60 | 2.31–5.61 | 3.73 | 2.39–5.82 | 3.78 | 2.41–5.92 |
| Household population | 1.25 | 1.18–1.33 | 1.16 | 1.08–1.25 | 1.17 | 1.08–1,25 | 1.16 | 1.09–1.25 | 1.17 | 1.09–1.25 |
| Minimal ownership (score = 0) | 7.84 | 2.25–27.29 | 7.82 | 1.98–30.95 | 7.58 | 1.91–30.18 | 8.00 | 2.02–31.72 | 8.60 | 2.17–34.06 |
| High school education | 0.66 | 0.46–0.96 | − | − | − | − | − | − | ||
| Unimproved water source | 3.39 | 1.67–6.87 | 3.60 | 1.66–7.84 | 6.81 | 2.33–19.90 | 0.90 | 0.14–5.83 | 3.48 | 1.59–7.62 |
| Water treatment | 0.85 | 0.60–1.22 | − | − | − | − | − | − | ||
| Small-mouthed water storage vessel | 0.78 | 0.55–1.10 | − | − | − | − | − | − | ||
| Unimproved sanitation | 2.01 | 1.42–2.85 | 1.71 | 1.16–2.51 | 1.88 | 1.27–2.80 | 1.73 | 1.18–2.55 | 3.61 | 1.29–10.10 |
| Number of families sharing sanitation | 0.99 | 0.88–1.12 | − | − | − | − | − | − | ||
| Improved hygiene (score = 1) | 0.16 | 0.06–0.47 | − | − | − | − | − | − | ||
| Improved waste disposal | 0.66 | 0.44–0.98 | − | − | − | − | − | − | ||
| Extreme rainfall days* | 0.88 | 0.80–0.98 | 0.83 | 0.74–0.93 | 0.82 | 0.73–0.93 | 0.81 | 0.72–0.91 | 0.88 | 0.77–1.01 |
| Water source × sanitation | 0.24 | 0.06–0.91 | ||||||||
| Water source × extreme rainfall days* | − | − | − | − | 1.50 | 0.93–2.41 | − | − | ||
| Sanitation × extreme rainfall days* | − | − | − | − | − | − | 0.81 | 0.63–1.06 | ||
Extreme rainfall days are defined using a threshold equivalent to the 90th percentile of the daily rainfall distribution (> 18 mm) summed over the 5 weeks before the end of the 15-day case control period.
Effect modification by water and sanitation.
Water source and sanitation significantly modified each other's association with diarrhea (Table 2, multivariable model 2). Holding all other variables in this model constant, the predicted OR of diarrhea associated with an unimproved water source was statistically significant only in households with improved sanitation (OR = 7.1, 95% CI = 2.4–21.3) (Table 3). Likewise, the OR of diarrhea associated with unimproved sanitation was statistically significant only in households using improved water sources (OR = 1.9, 95% CI = 1.2–2.9) (Table 3).
Table 3.
Predicted OR's for diarrhea and bootstrap 95% CIs associated with unimproved water and sanitation (from December of 2008 to May of 2009)
| Unimproved Water Source | Unimproved Sanitation | ||||
|---|---|---|---|---|---|
| ORa | 95% CI | ORb | 95% CI | ||
| Households with unimproved sanitation | 1.39 | 0.30, 5.27 | Households with an unimproved water source | 0.37 | 0.06, 2.03 |
| Households with improved sanitation | 7.14 | 2.42, 21.28 | Households with an improved water source | 1.89 | 1.23, 2.85 |
Adjusted for children under five years, household population, household ownership, extreme rainfall and sanitation
Adjusted for children under five years, household population, household ownership, extreme rainfall and water source
Effect modification by rainfall.
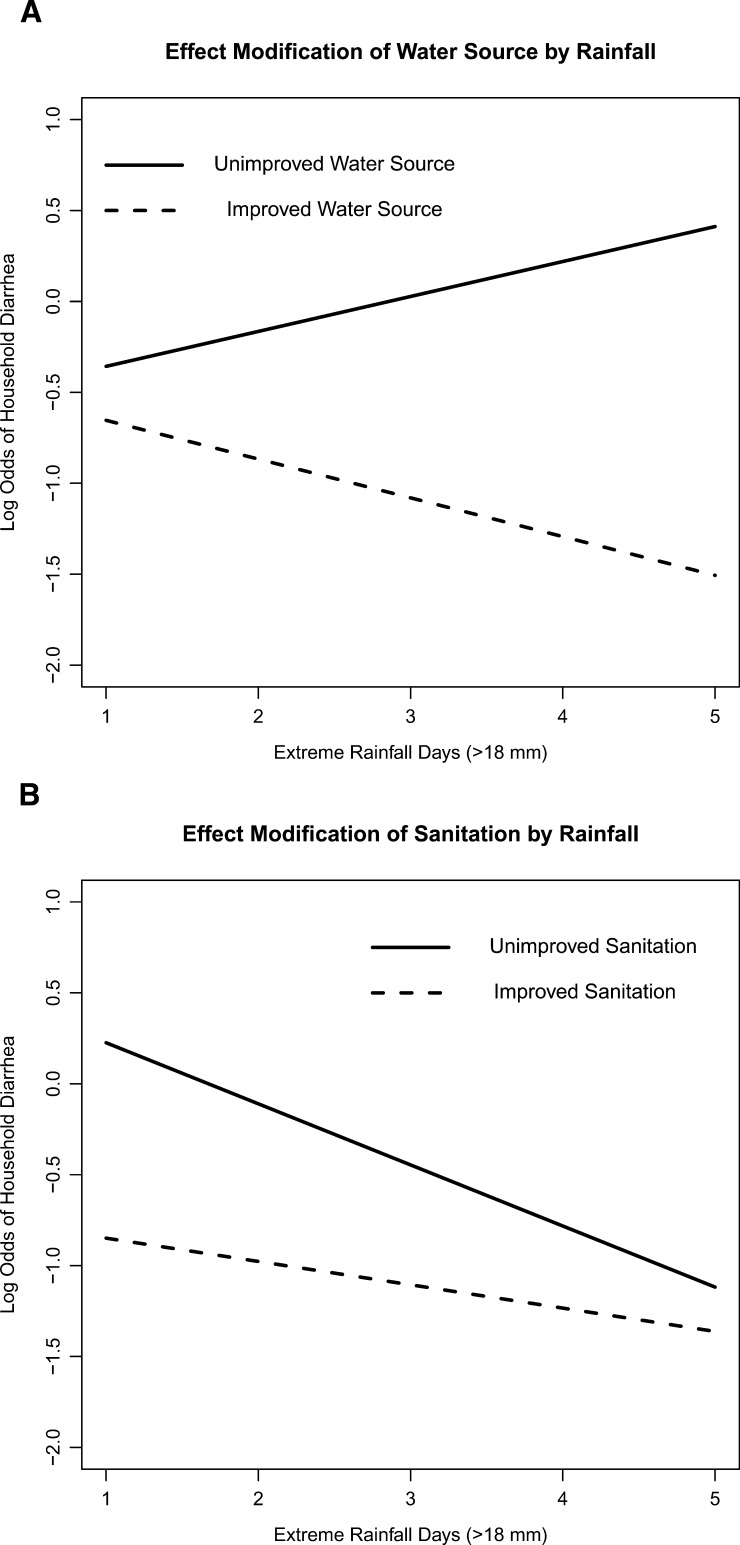
Extreme rainfall in the past 5 weeks modified the associations between unimproved water source, sanitation, and diarrhea (Table 2, multivariable models 3 and 4). Although these effect modifications were not statistically significant across the range of extreme rainfall days, the direction of effects at 1 and 5 days of rainfall is worth noting. Here, we use 1 and 5 days of rainfall combined with coefficient estimates from models 3 and 4 to estimate ORs of diarrhea. We assumed a median household size and ownership, at least one child under the age of 5 years, and an improved water source (model 3) or improved sanitation (model 4). After 5 days of rainfall, unimproved water significantly increased the odds of diarrhea (OR = 6.8, 95% CI = 1.9–24.5) (Table 4). After only 1 day of rainfall, this effect disappeared (OR = 1.4, 95% CI = 0.1–5.1). In contrast, after 1 day of rainfall, unimproved sanitation significantly increased the odds of diarrhea (OR = 2.9, 95% CI = 1.3–6.6), whereas after 5 days, the effect was insignificant (OR = 1.3, 95% CI = 0.7–2.2). Results were qualitatively similar when we assumed an unimproved water source or unimproved sanitation. These trends are shown in Figure 2 .
Table 4.
Predicted ORs for diarrhea and bootstrap 95% CIs associated with unimproved water and sanitation at rainfall endpoints (from December of 2008 to May of 2009)
| 1 Day of extreme rainfall* | 5 Days of extreme rainfall* | |||
|---|---|---|---|---|
| OR† | 95% CI | OR† | 95% CI | |
| Households with an unimproved water source | 1.35 | 0.13–5.09 | 6.81 | 1.87–24.51 |
| Households with unimproved sanitation | 2.93 | 1.30–6.64 | 1.28 | 0.74–2.16 |
Extreme rainfall days are defined using a threshold equivalent to the 90th percentile of the daily rainfall distribution (> 18 mm) and summed over the 5 weeks before the end of the 15-day case control period.
Adjusted for children under 5 years, household population, household ownership, and improved sanitation or water source.
Figure 2.
(A) The predicted probability of household diarrhea on the log odds scale (y axis) for a household with median household size and ownership, at least one child under the age of 5 years, improved sanitation, and either an improved (dashed line) or unimproved (solid line) water source at each value of extreme rainfall days in the 5 weeks before the end of the 15-day case control period in Borbón, Ecuador (from December of 2008 to May of 2009). (B) The predicted probability of household diarrhea on the log odds scale (y axis) for a household with median household size and ownership, at least one child under the age of 5 years, an improved water source, and either improved (dashed line) or unimproved (solid line) sanitation at each value of extreme rainfall days (x axis) in the 5 weeks before the end of the 15-day case control period in Borbón, Ecuador (from December of 2008 to May of 2009).
Discussion
Use of an unimproved water source, unimproved sanitation, and extreme rainfall events during the rainy season were each associated with diarrhea. However, we found that risk stems from a complex interaction between pairwise combinations of these three factors. For example, an unimproved water source was only a risk factor in households with improved sanitation, and unimproved sanitation was only a risk factor in households with an improved water source. These interdependent effects, previously described in the literature,24,25 suggest that enteric pathogens exploit multiple environmental pathways to cause diarrhea. Our results also show that these environmental pathways are modified by extreme rainfall. Our finding that extreme rainfall was protective for diarrhea is consistent with some studies10 and inconsistent with others.11,13,14,26 The reasons for this discrepancy in the literature may be because of the fact that the context in each study is different (i.e., factors that are protective under some circumstances are often a risk under other circumstances). With regards to the relationship between rainfall and diarrhea, the distribution of improved water and sanitation facilities, the patterns of rainfall, and the distribution of pathogens causing diarrhea may be important defining contexts to consider. We discuss each of these contexts in turn with respect to the results of our study.
First, the distribution of unimproved water and sanitation facilities may affect the relationship between rainfall and diarrhea. Extreme rainfall events may reduce risk by flushing enteric pathogens from the local environment. Our finding that unimproved household sanitation was only a risk factor for diarrhea in dry conditions but not wet conditions supports this flushing hypothesis. After 5 days of extreme rainfall, many enteric pathogens may already be flushed out of unimproved sanitation facilities, reducing the level of risk that they pose to a household. At the same time, extreme rainfall may flush enteric pathogens into unimproved sources of water, increasing risk for diarrhea. Our finding that unimproved water sources were only a risk factor after heavy rainfall supports the latter hypothesis.
Elevated risk in households using unimproved water sources may arise because of increased microbial contamination of sources, such as the river and uncovered wells, after heavy rainfall. In a previous study conducted in a village 15 km southeast of Borbón, significantly higher Escherichia coli counts in surface and stored water were found during the rainy season compared with the dry season. Surface and shallow groundwater may be more contaminated after heavy rainfall because of both the flushing of human and animal fecal material and the transport of soil-resident bacteria from the land environment into the water. Levy and others27 also reported higher microbial contamination of river water at sites located downstream of the village compared with those sites located upstream of and along the village, linking human settlement to fecal contamination of river water. Other water quality studies have linked higher concentrations of fecal indicator bacteria in shallow groundwater with rainfall, solid waste, and pit latrines in the environment.28,29 During our study, illegal use of the sewage pipes still under construction may have resulted in sewage overflow after heavy rainfall, sending fecal matter up into the streets. Additionally, given that sanitation practices in Borbón included the use of open pit latrines, the river, and open fields and the disposal of fecal matter in temporary vessels along with household solid waste, there may have been a variety of fecal sources in the environment, leading to high levels of source water contamination after heavy rainfall.
We estimate that 4% of households in Borbón use unsafe water sources, which compared with other study sites such as Dhaka, Bangladesh30 and rural areas of the Pacific Islands,14 is very low. Given our finding that use of unimproved water sources increases risk of diarrhea in wet conditions, we would expect that, in populations with more prevalent reliance on unsafe water, extreme rainfall events would promote diarrheal disease.
Our observation that unimproved sanitation facilities were no longer a risk factor in wet conditions is broadly consistent with the work by Hashizume and others,30 which reported no effect of unimproved sanitation during a flood period but found a positive effect up to 6 months post-flooding. The most common unimproved sanitation facilities in Borbón are open pit latrines. Open pit latrines, unlike flush toilets, are located outdoors and unlike latrines with raised platforms, are often poorly constructed, facilitating the movement of pathogens into the surrounding environment. As described by Cronin and others,29 pathogen movement can be subsurface from dug latrines or through surface runoff from flooded latrines. Surface runoff was recently discussed in the work by Knappett and others,31 which found evidence for the movement of E. coli from unsanitary latrines (defined as open pits or visible effluent) to nearby ponds at distances ranging from 15 to 80 m.
Second, inconsistent associations between rainfall and diarrheal disease seen in the literature may be explained by a difference in rainfall patterns between sites. For example, both Borbón and Dhaka are situated along a river and characterized by a tropical monsoon climate. However, total weekly rainfall in Dhaka peaked to over 200 mm 11 times between 1996 and 2003,13 whereas in Borbón, the same was observed only 3 times between 2003 and 2011 (unpublished data). Furthermore, monsoon rains and coastal storm surges cause intense flooding in Bangladesh32 but not coastal Ecuador. Therefore, compared with this site, Borbón experiences less severe flooding. Rainfall patterns in Borbón may be enough to flush pathogens from the local environment but insufficient to cause the flood-associated stagnant water shown to increase risk of diarrhea.30
Third, differences in the effects of rainfall on diarrhea found in previous studies and our study may also be attributed to the varying etiology of diarrhea between sites. The major pathogens in our region are rotavirus, pathogenic E. coli, and Shigella and in the context of coinfections, Plesiomonas shigelloides and Giardia.16,33 Other studies have reported, in addition to these pathogens, a high prevalence of Vibrio cholerae, Aeromonas spp., and Cryptosporidium spp.,34–36 which are rarely found in our study region. Given differences in pathogen prevalence and that pathogens may respond differently to rainfall,37 the combined effects of rainfall on pathogen-associated diarrhea are likely to differ between sites. The variability in pathogen prevalence between regions may also explain the wide range of lag periods associated with diarrhea across studies.10–14 At our study site, a 5-week rainfall period was most strongly correlated with diarrhea. This correlation may be influenced by the environmental persistence of rotavirus and Giardia cysts in water, sewage, and soil for up to several weeks.38,39 The 5-week period is also several times longer than the incubation periods of rotavirus, E. coli, and often, Giardia, allowing enough time for secondary transmission events to noticeably affect diarrhea prevalence.15,40
This study was carried out over a 6-month period (from the beginning to the end of a rainy season). It is possible that the overall decline in diarrhea from the beginning to the end of the rainy season was the result of a reporting bias. Diarrhea reporting took place during daily household visits over 15-day periods every other month. Although daily visits are likely to reduce recall bias, they may increase respondent fatigue. However, we believe respondent fatigue to be minimal, because these household visits made by our study staff limited the effort required by study participants. It is also possible that water, sanitation, and hygiene practices were altered in response to heavy rainfall, but our household surveys, which were repeated every 2 months, indicated minimal change to drinking water source and sanitation throughout the rainy season.
In addition to the protective effect of rainfall on diarrhea, we observed modification of this effect by household water and sanitation during the rainy season. Although the effect modifications were not statistically significant, we believe that this result may have been a power issue because of the small sample size. Effect modifications were consistent in direction and strength when a rainfall period of 4 weeks instead of 5 weeks was used and when extreme rainfall was defined according to the 95th percentile instead of the 90th percentile of the daily rainfall distribution. Furthermore, although effect modifications were not significant across the range of observed extreme rainfall days, we did find significant effects at the extreme rainfall endpoints (days 1 and 5). Our analysis shows that water and sanitation were associated with diarrhea risk at opposing levels of extreme rainfall. Although there are other ways to summarize rainfall, such as total or average rainfall, these indicators may capture a different mechanism of pathogen movement. For example, high total rainfall may have a dilution effect, decreasing the concentration of enteric pathogens in unimproved water sources, whereas pulses of extreme rainfall may increase the concentration by flushing enteric pathogens into these same water sources. Recently, Carlton and others41 highlighted this difference on a regional scale by showing the potential for increased pathogen flushing after heavy rainfall following relatively dry periods compared with wet periods, which were defined by total rainfall. In the same study region as the region in the study by Carlton and others41 but focused on a single urbanized community, our results support the idea of pathogen flushing on a local scale during relatively wet periods. Additionally, our study has several advantages over others, including a local measurement of rainfall, a community-based design that followed participants of all ages, sampling of both mild and severe cases of diarrhea, and repeated measurements of water and sanitation practices. How water and sanitation modify the climate–disease relationship can have important public health implications.
In some regions, such as Borbón, Ecuador, heavy rainfall may be protective for diarrheal disease. Where rainfall is not protective however, the risk for diarrheal disease may be reduced through use of improved water sources. In contrast, during and just after a period of drought, risk of diarrhea may be addressed through improved sanitation. Our findings that the use of improved water sources and improved sanitation facilities are most protective when the other factor is also improved and under opposing ecological conditions highlight the need for integrated intervention strategies to reduce the burden of diarrheal disease.
ACKNOWLEDGMENTS
We acknowledge Geovanny Hurtado Borja, Patricia Bravo Gordillo, Leonar Javier Hurtado Borja, and Jessica Renteria as well as the Ecología, Desarrollo, Salud y Sociedad (EcoDESS) project field team (administered out of the Universidad San Francisco de Quito) for their invaluable contributions to specimen and data collection.
Footnotes
Financial support: This work was supported by National Institutes of Health Grant R01-AI050038 and the University of Michigan Interdisciplinary Training Program in Infectious Diseases funded by National Institute of Allergy and Infectious Diseases Grant T32AI 049816.
Authors' addresses: Darlene Bhavnani, Jason E. Goldstick, and Joseph N. S. Eisenberg, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, MI, E-mails: dbhavnan@umich.edu, jasoneg@med.umich.edu, and jnse@umich.edu. William Cevallos, Centro de Biomedicina, Universidad Central de Ecuador, Quito, Ecuador, E-mail: wcevallos@uce.edu.ec. Gabriel Trueba, Microbiology Institute, Universidad San Francisco de Quito, Quito, Ecuador, E-mail: gtrueba@usfq.edu.ec.
References
- 1.Boschi-Pinto C, Velebit L, Shibuya K. Estimating child mortality due to diarrhoea in developing countries. Bull World Health Organ. 2008;86:710–717. doi: 10.2471/BLT.07.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink G, Gunther I, Hill K. The effect of water and sanitation on child health: evidence from the demographic and health surveys 1986–2007. Int J Epidemiol. 2011;40:1196–1204. doi: 10.1093/ije/dyr102. [DOI] [PubMed] [Google Scholar]
- 3.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 4.Pruss A, Kay D, Fewtrell L, Bartram J. Estimating the burden of disease from water, sanitation, and hygiene at a global level. Environ Health Perspect. 2002;110:537–542. doi: 10.1289/ehp.110-1240845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UN United Nations, The Millennium Development Goals Report 2011. 2011. http://www.un.org/millenniumgoals/pdf/(2011_E)%20MDG%20Report%202011_Book%20LR.pdf Available at. Accessed November 17, 2011.
- 6.Cairncross S, Blumenthal U, Kolsky P, Moraes L, Tayeh A. The public and domestic domains in the transmission of disease. Trop Med Int Health. 1996;1:27–34. doi: 10.1046/j.1365-3156.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 7.Nmorsi OP, Agbozele G, Ukwandu NC. Some aspects of epidemiology of filth flies: Musca domestica, Musca domestica vicina, Drosophilia melanogaster and associated bacteria pathogens in Ekpoma, Nigeria. Vector Borne Zoonotic Dis. 2007;7:107–117. doi: 10.1089/vbz.2006.0539. [DOI] [PubMed] [Google Scholar]
- 8.Thomas KM, Charron DF, Waltner-Toews D, Schuster C, Maarouf AR, Holt JD. A role of high impact weather events in waterborne disease outbreaks in Canada, 1975–2001. Int J Environ Health Res. 2006;16:167–180. doi: 10.1080/09603120600641326. [DOI] [PubMed] [Google Scholar]
- 9.Auld H, MacIver D, Klaassen J. Heavy rainfall and waterborne disease outbreaks: the Walkerton example. J Toxicol Environ Health A. 2004;67:1879–1887. doi: 10.1080/15287390490493475. [DOI] [PubMed] [Google Scholar]
- 10.Chou WC, Wu JL, Wang YC, Huang H, Sung FC, Chuang CY. Modeling the impact of climate variability on diarrhea-associated diseases in Taiwan (1996–2007) Sci Total Environ. 2010;409:43–51. doi: 10.1016/j.scitotenv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Drayna P, McLellan SL, Simpson P, Li SH, Gorelick MH. Association between rainfall and pediatric emergency department visits for acute gastrointestinal illness. Environ Health Perspect. 2010;118:1439–1443. doi: 10.1289/ehp.0901671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curriero FC, Patz JA, Rose JB, Lele S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am J Public Health. 2001;91:1194–1199. doi: 10.2105/ajph.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashizume M, Armstrong B, Hajat S, Wagatsuma Y, Faruque AS, Hayashi T, Sack DA. Association between climate variability and hospital visits for non-cholera diarrhoea in Bangladesh: effects and vulnerable groups. Int J Epidemiol. 2007;36:1030–1037. doi: 10.1093/ije/dym148. [DOI] [PubMed] [Google Scholar]
- 14.Singh RB, Hales S, de Wet N, Raj R, Hearnden M, Weinstein P. The influence of climate variation and change on diarrheal disease in the Pacific Islands. Environ Health Perspect. 2001;109:155–159. doi: 10.1289/ehp.01109155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gascon J. Epidemiology, etiology and pathophysiology of traveler's diarrhea. Digestion. 2006;73((Suppl 1)):102–108. doi: 10.1159/000089785. [DOI] [PubMed] [Google Scholar]
- 16.Bhavnani D, Goldstick JE, Cevallos W, Trueba G, Eisenberg JN. Synergistic effects between rotavirus and coinfecting pathogens on diarrheal disease: evidence from a community-based study in northwestern Ecuador. Am J Epidemiol. 2012;176:387–395. doi: 10.1093/aje/kws220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO/UNICEF WHO/UNICEF Joint Monitoring Programme (JMP) for Water Supply and Sanitation. Types of Drinking-Water Sources and Sanitation. 2011. http://www.wssinfo.org/definitions-methods/watsan-categories/ Available at. Accessed November 17, 2011.
- 18.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 19.Ochoa TJ, Ecker L, Barletta F, Mispireta ML, Gil AI, Contreras C, Molina M, Amemiya I, Verastegui H, Hall ER, Cleary TG, Lanata CF. Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from Periurban areas in Lima, Peru. Clin Infect Dis. 2009;49:1694–1702. doi: 10.1086/648069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeager BA, Lanata CF, Lazo F, Verastegui H, Black RE. Transmission factors and socioeconomic status as determinants of diarrhoeal incidence in Lima, Peru. J Diarrhoeal Dis Res. 1991;9:186–193. [PubMed] [Google Scholar]
- 22.Strina A, Rodrigues LC, Cairncross S, Ferrer SR, Fialho AM, Leite JP, Ribeiro HC, Jr, Barreto ML. Factors associated with rotavirus diarrhoea in children living in a socially diverse urban centre in Brazil. Trans R Soc Trop Med Hyg. 2012;106:445–451. doi: 10.1016/j.trstmh.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Nundy S, Gilman RH, Xiao L, Cabrera L, Cama R, Ortega YR, Kahn G, Cama VA. Wealth and its associations with enteric parasitic infections in a low-income community in Peru: use of principal component analysis. Am J Trop Med Hyg. 2011;84:38–42. doi: 10.4269/ajtmh.2011.10-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenberg JN, Scott JC, Porco T. Integrating disease control strategies: balancing water sanitation and hygiene interventions to reduce diarrheal disease burden. Am J Public Health. 2007;97:846–852. doi: 10.2105/AJPH.2006.086207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanDerslice J, Briscoe J. Environmental interventions in developing countries: interactions and their implications. Am J Epidemiol. 1995;141:135–144. doi: 10.1093/oxfordjournals.aje.a117401. [DOI] [PubMed] [Google Scholar]
- 26.Saidi SM, Iijima Y, Sang WK, Mwangudza AK, Oundo JO, Taga K, Aihara M, Nagayama K, Yamamoto H, Waiyaki PG, Honda T. Epidemiological study on infectious diarrheal diseases in children in a coastal rural area of Kenya. Microbiol Immunol. 1997;41:773–778. doi: 10.1111/j.1348-0421.1997.tb01925.x. [DOI] [PubMed] [Google Scholar]
- 27.Levy K, Hubbard AE, Nelson KL, Eisenberg JN. Drivers of water quality variability in northern coastal Ecuador. Environ Sci Technol. 2009;43:1788–1797. doi: 10.1021/es8022545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard G, Pedley S, Barrett M, Nalubega M, Johal K. Risk factors contributing to microbiological contamination of shallow groundwater in Kampala, Uganda. Water Res. 2003;37:3421–3429. doi: 10.1016/S0043-1354(03)00235-5. [DOI] [PubMed] [Google Scholar]
- 29.Cronin AA, Breslin N, Gibson J, Pedley S. Monitoring source and domestic water quality in parallel with sanitary risk identification in northern Mozambique to prioritise protection interventions. J Water Health. 2006;4:333–345. doi: 10.2166/wh.2006.029. [DOI] [PubMed] [Google Scholar]
- 30.Hashizume M, Wagatsuma Y, Faruque AS, Hayashi T, Hunter PR, Armstrong B, Sack DA. Factors determining vulnerability to diarrhoea during and after severe floods in Bangladesh. J Water Health. 2008;6:323–332. doi: 10.2166/wh.2008.062. [DOI] [PubMed] [Google Scholar]
- 31.Knappett PS, Escamilla V, Layton A, McKay LD, Emch M, Williams DE, Huq R, Alam J, Farhana L, Mailloux BJ, Ferguson A, Sayler GS, Ahmed KM, van Geen A. Impact of population and latrines on fecal contamination of ponds in rural Bangladesh. Sci Total Environ. 2011;409:3174–3182. doi: 10.1016/j.scitotenv.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan AE, Xun WW, Ahsan H, Vineis P. Climate change, sea-level rise, and health impacts in Bangladesh. Environment: Sci Policy Sustain Dev. 2011;53:18–33. [Google Scholar]
- 33.Escobar JC, Bhavnani D, Trueba G, Ponce K, Cevallos W, Eisenberg J. Plesiomonas shigelloides infection, Ecuador, 2004–2008. Emerg Infect Dis. 2012;18:322–324. doi: 10.3201/eid1802.110562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert MJ, Faruque AS, Faruque SM, Sack RB, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol. 1999;37:3458–3464. doi: 10.1128/jcm.37.11.3458-3464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasan KZ, Pathela P, Alam K, Podder G, Faruque SM, Roy E, Haque AK, Haque R, Albert MJ, Siddique AK, Sack RB. Aetiology of diarrhoea in a birth cohort of children aged 0–2 year(s) in rural Mirzapur, Bangladesh. J Health Popul Nutr. 2006;24:25–35. [PubMed] [Google Scholar]
- 36.Mukherjee AK, Chowdhury P, Bhattacharya MK, Ghosh M, Rajendran K, Ganguly S. Hospital-based surveillance of enteric parasites in Kolkata. BMC Res Notes. 2009;2:110. doi: 10.1186/1756-0500-2-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris AM, Chowdhury F, Begum YA, Khan AI, Faruque AS, Svennerholm AM, Harris JB, Ryan ET, Cravioto A, Calderwood SB, Qadri F. Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. Am J Trop Med Hyg. 2008;79:708–714. [PMC free article] [PubMed] [Google Scholar]
- 38.deRegnier DP, Cole L, Schupp DG, Erlandsen SL. Viability of Giardia cysts suspended in lake, river, and tap water. Appl Environ Microbiol. 1989;55:1223–1229. doi: 10.1128/aem.55.5.1223-1229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ansari SA, Springthorpe VS, Sattar SA. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev Infect Dis. 1991;13:448–461. doi: 10.1093/clinids/13.3.448. [DOI] [PubMed] [Google Scholar]
- 40.Atchison CJ, Tam CC, Hajat S, van Pelt W, Cowden JM, Lopman BA. Temperature-dependent transmission of rotavirus in Great Britain and The Netherlands. Proc Biol Sci. 2010;277:933–942. doi: 10.1098/rspb.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlton EJ, Eisenberg JN, Goldstick J, Cevallos W, Trostle J, Levy K. Heavy rainfall events and diarrhea incidence: the role of social and environmental factors. Am J Epidemiol. 2014;179:344–352. doi: 10.1093/aje/kwt279. [DOI] [PMC free article] [PubMed] [Google Scholar]