Abstract
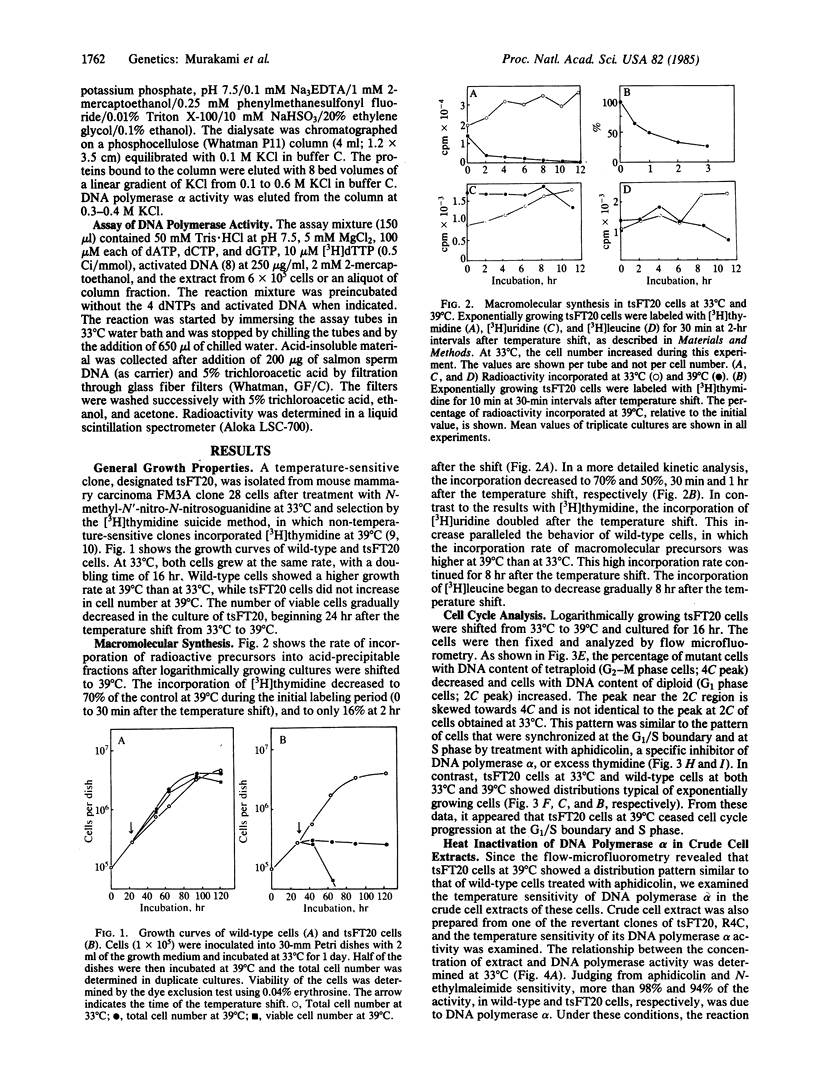
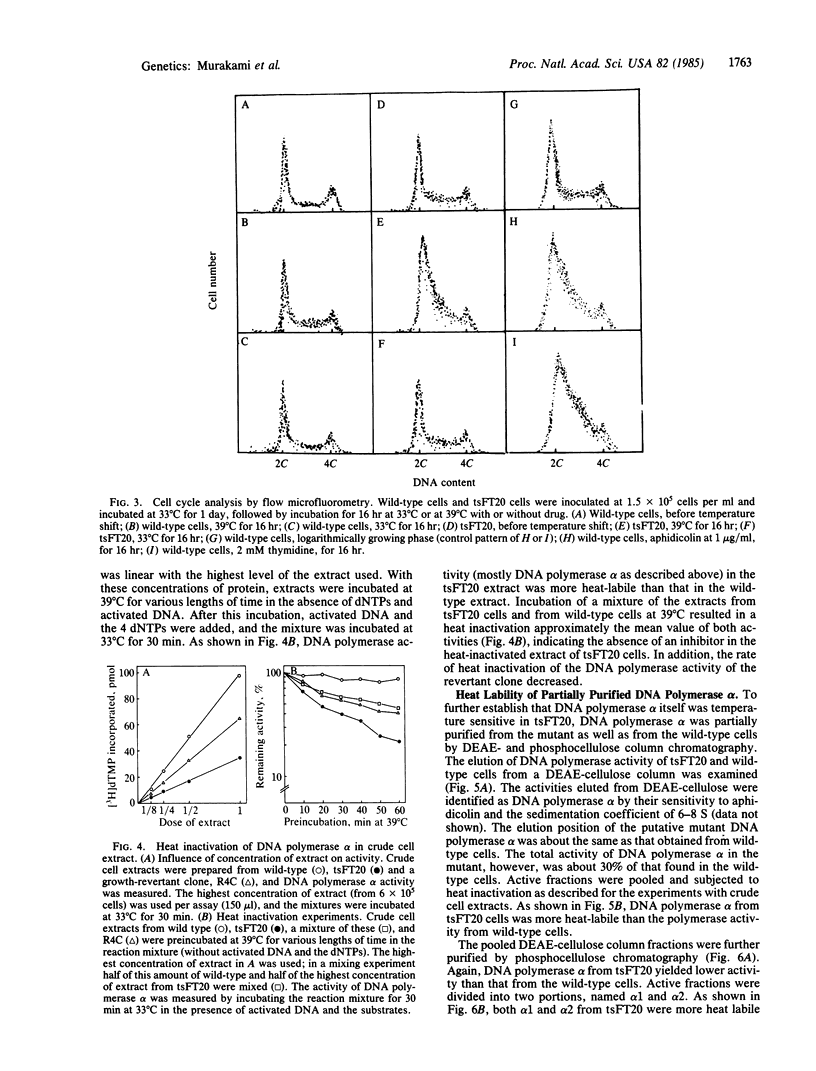
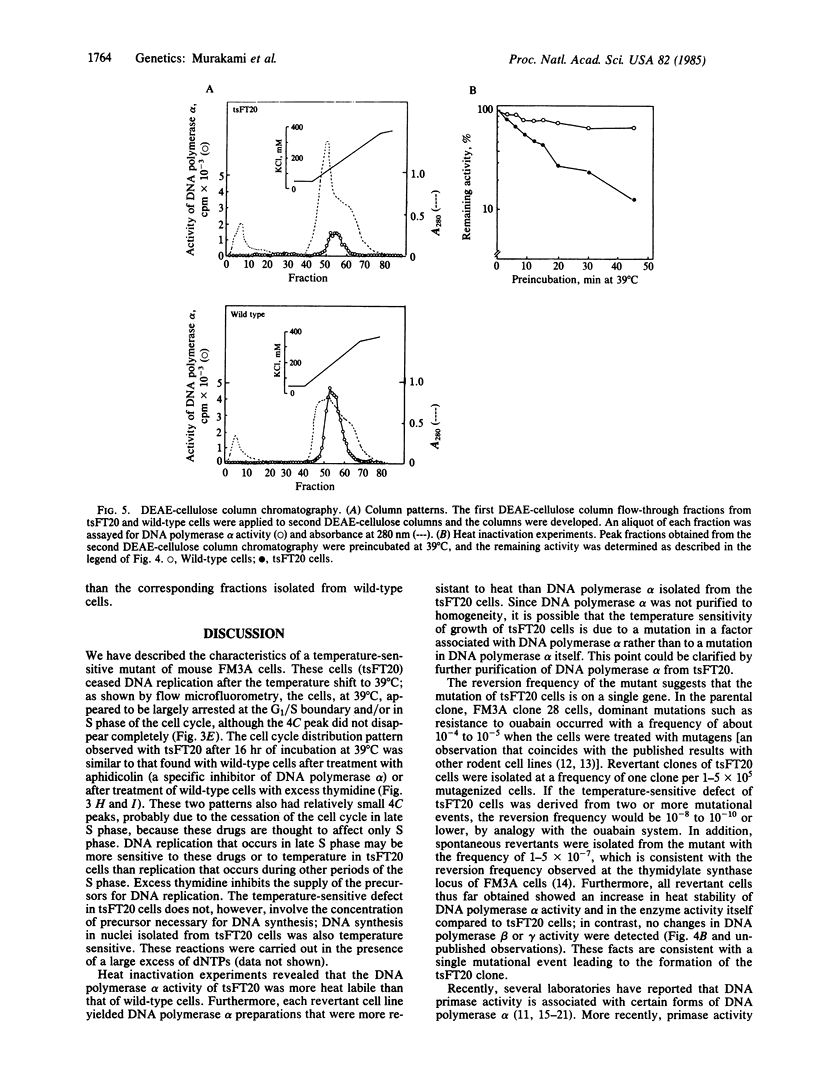
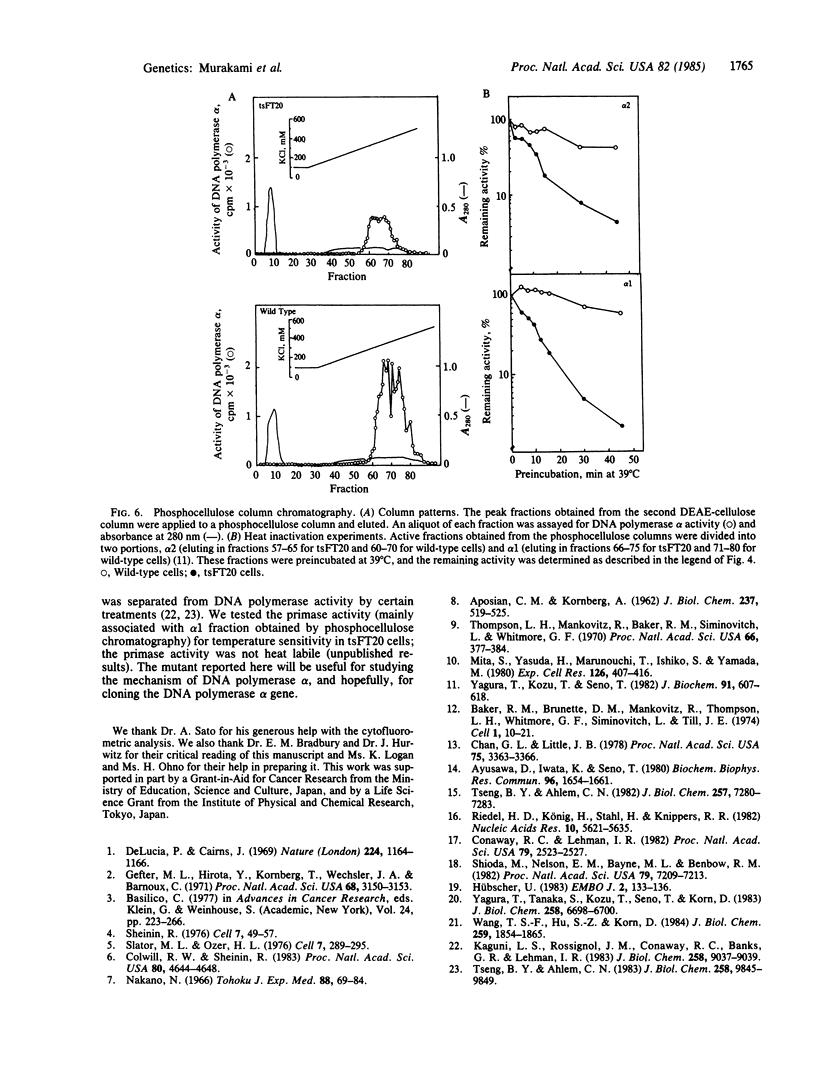
The characterization of a temperature-sensitive mutant (tsFT20 strain, dnats) of mouse FM3A cells is reported. After incubation of tsFT20 cells at the nonpermissive temperature (39 degrees C), DNA synthesis ceased with little change in either RNA or protein synthesis. Flow-microfluorometric analysis revealed that the cell cycle of tsFT20 cells grown at 39 degrees C for 16 hr was similar to that of wild-type cells that were synchronized at the G1/S boundary and at S phase by treatment with aphidicolin, a specific inhibitor of DNA polymerase alpha. The DNA polymerase alpha activity of tsFT20 cells measured in crude cell extracts or in purified preparations was inactivated more rapidly at 39 degrees C than the activity of wild-type cells. In the growth revertants of the tsFT20 cell strain, the heat lability of DNA polymerase alpha decreased. These data suggest that tsFT20 is a temperature-sensitive mutant of DNA polymerase alpha or of a factor associated with DNA polymerase alpha that is essential for its activity.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Ayusawa D., Iwata K., Seno T. Isolation of mouse FM3A cell mutants with thermolabile thymidylate synthetase by resistance to aphidicolin. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1654–1661. doi: 10.1016/0006-291x(80)91364-9. [DOI] [PubMed] [Google Scholar]
- Chan G. L., Little J. B. Induction of ouabain-resistant mutations in C3H 10T1/2 mouse cells by ultraviolet light. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3363–3366. doi: 10.1073/pnas.75.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill R. W., Sheinin R. ts A1S9 locus in mouse L cells may encode a novobiocin binding protein that is required for DNA topoisomerase II activity. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4644–4648. doi: 10.1073/pnas.80.15.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U. The mammalian primase is part of a high molecular weight DNA polymerase alpha polypeptide. EMBO J. 1983;2(1):133–136. doi: 10.1002/j.1460-2075.1983.tb01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni L. S., Rossignol J. M., Conaway R. C., Banks G. R., Lehman I. R. Association of DNA primase with the beta/gamma subunits of DNA polymerase alpha from Drosophila melanogaster embryos. J Biol Chem. 1983 Aug 10;258(15):9037–9039. [PubMed] [Google Scholar]
- Mita S., Yasuda H., Marunouchi T., Ishiko S., Yamada M. A temperature-sensitive mutant of cultured mouse cells defective in chromosome condensation. Exp Cell Res. 1980 Apr;126(2):407–416. doi: 10.1016/0014-4827(80)90280-3. [DOI] [PubMed] [Google Scholar]
- Nakano N. Establishment of cell lines in vitro from a mammary ascites tumor of mouse and biological properties of the established lines in a serum containing medium. Tohoku J Exp Med. 1966 Jan 25;88(1):69–84. doi: 10.1620/tjem.88.69. [DOI] [PubMed] [Google Scholar]
- Riedel H. D., König H., Stahl H., Knippers R. Circular single stranded phage M13-DNA as a template for DNA synthesis in protein extracts from Xenopus laevis eggs: evidence for a eukaryotic DNA priming activity. Nucleic Acids Res. 1982 Sep 25;10(18):5621–5635. doi: 10.1093/nar/10.18.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin R. Preliminary characterization of the temperature-sensitive defect in DNA replication in a mutant mouse L cell. Cell. 1976 Jan;7(1):49–57. doi: 10.1016/0092-8674(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Shioda M., Nelson E. M., Bayne M. L., Benbow R. M. DNA primase activity associated with DNA polymerase alpha from Xenopus laevis ovaries. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7209–7213. doi: 10.1073/pnas.79.23.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. L., Ozer H. L. Temperature-sensitive mutants of Balb/3T3 cells: description of a mutant affected in cellular and polyoma virus DNA synthesis. Cell. 1976 Feb;7(2):289–295. doi: 10.1016/0092-8674(76)90028-3. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mankovitz R., Baker R. M., Till J. E., Siminovitch L., Whitmore G. F. Isolation of temperature-sensitive mutants of L-cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):377–384. doi: 10.1073/pnas.66.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. A DNA primase from mouse cells. Purification and partial characterization. J Biol Chem. 1983 Aug 25;258(16):9845–9849. [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. DNA primase activity from human lymphocytes. Synthesis of oligoribonucleotides that prime DNA synthesis. J Biol Chem. 1982 Jul 10;257(13):7280–7283. [PubMed] [Google Scholar]
- Wang T. S., Hu S. Z., Korn D. DNA primase from KB cells. Characterization of a primase activity tightly associated with immunoaffinity-purified DNA polymerase-alpha. J Biol Chem. 1984 Feb 10;259(3):1854–1865. [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA polymerase accompanied by a novel RNA polymerase activity: purification and partial characterization. J Biochem. 1982 Feb;91(2):607–618. doi: 10.1093/oxfordjournals.jbchem.a133732. [DOI] [PubMed] [Google Scholar]
- Yagura T., Tanaka S., Kozu T., Seno T., Korn D. Tight association of DNA primase with a subspecies of mouse DNA polymerase alpha. J Biol Chem. 1983 Jun 10;258(11):6698–6700. [PubMed] [Google Scholar]


