Abstract
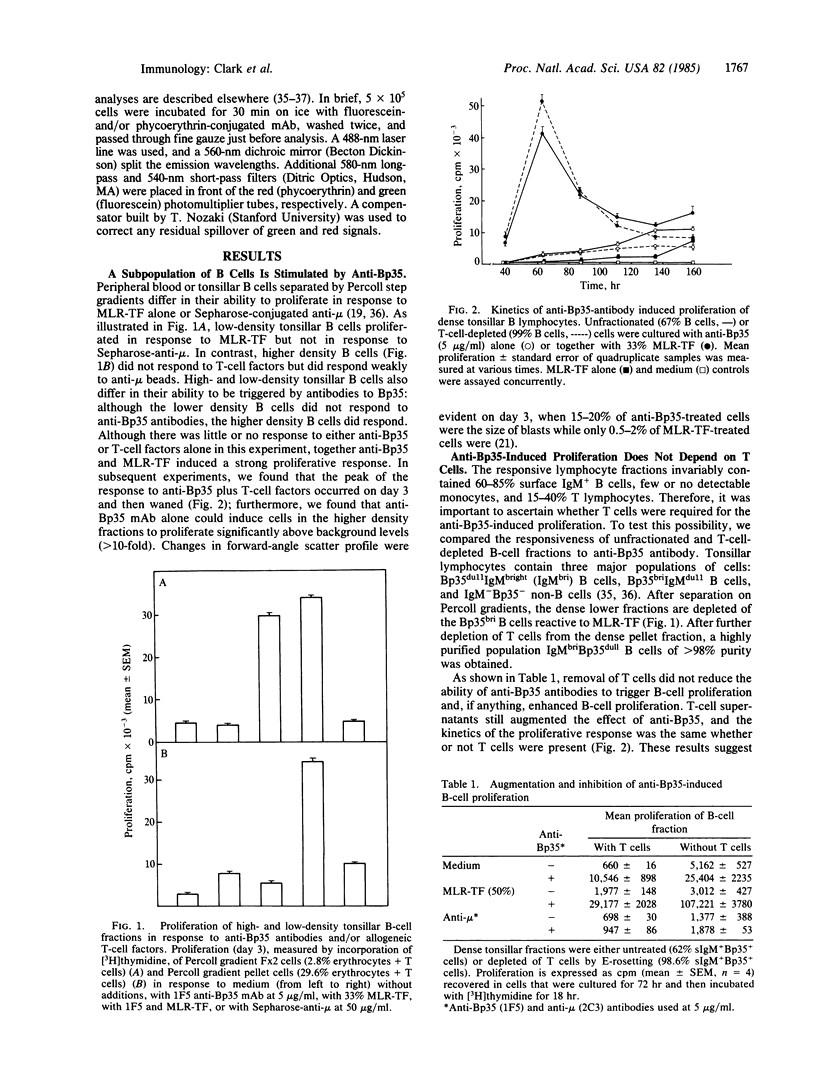
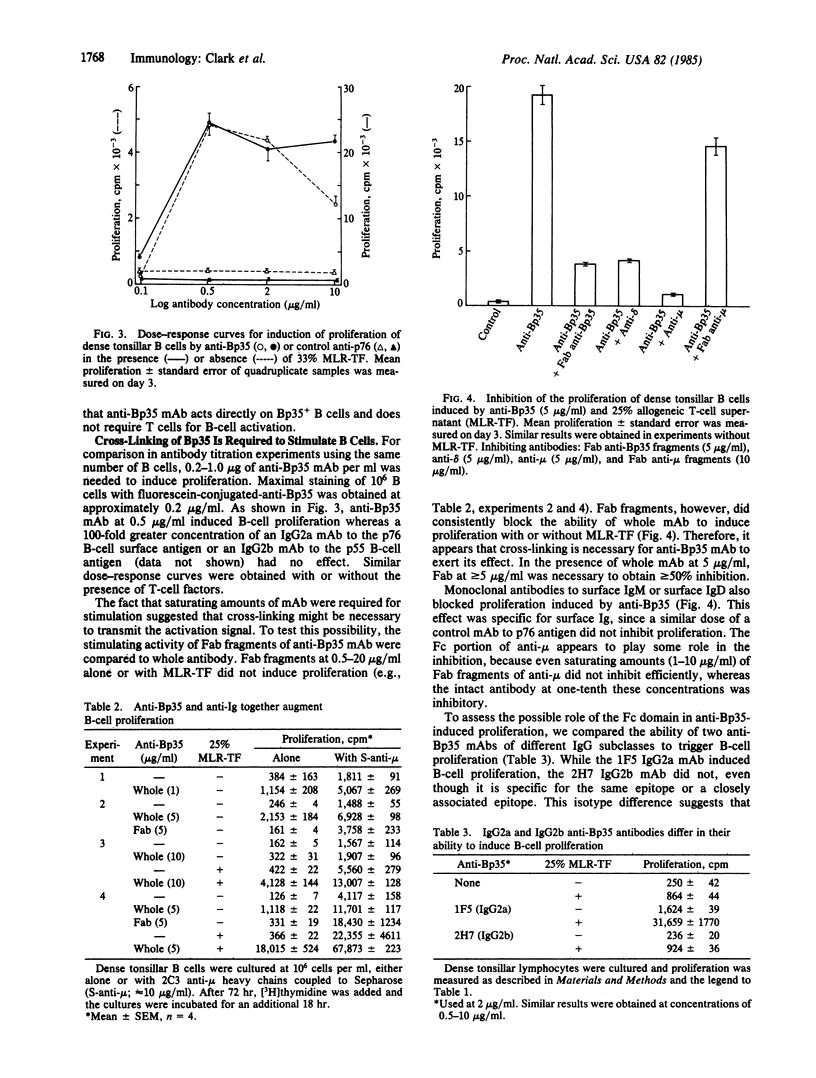
A 35-kDa polypeptide, Bp35, expressed on the surface of all B cells, plays a role in B-cell activation. Monoclonal antibodies to Bp35 stimulate human tonsillar B cells to proliferate. The activation induced by anti-Bp35 is similar to anti-Ig-mediated in several ways: the activation does not require T cells but is augmented by T-cell-derived allogeneic factors; monovalent Fab fragments to Bp35 do not trigger proliferation but instead block activation by whole antibody, indicating that cross-linking is required; and induction by anti-Bp35, like the induction by anti-Ig, is inhibited by monoclonal anti-IgM via an Fc domain-dependent mechanism. However, several features of anti-Bp35-mediated proliferation are clearly different from activation by anti-Ig: anti-Bp35 monoclonal antibodies do not require attachment to beads to function, the proliferation induced by anti-Bp35 and anti-Ig is additive, and Fab fragments of anti-Bp35 augment proliferation induced by anti-Ig. Models for the possible function of the Bp35 polypeptide as either a "bridge" or a "second signal" with surface Ig in B-cell activation are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Bullock W. W., Melchers F. Inhibition of mitogenic stimulation of mouse lymphocytes by anti-mouse immunoglobulin antibodies. I. Mode of action. Eur J Immunol. 1974 Nov;4(11):715–722. doi: 10.1002/eji.1830041103. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Nadler L. M., Stashenko P., McCluskey R. T., Schlossman S. F. Stages of B cell differentiation in human lymphoid tissue. J Exp Med. 1981 Sep 1;154(3):737–749. doi: 10.1084/jem.154.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Unanue E. R. B lymphocyte biology studied with anti-Ig antibodies. Immunol Rev. 1980;52:3–28. doi: 10.1111/j.1600-065x.1980.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Chiorazzi N., Fu S. M., Kunkel H. G. Induction of polyclonal antibody synthesis by human allogeneic and autologous helper factors. J Exp Med. 1979 Jun 1;149(6):1543–1548. doi: 10.1084/jem.149.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorazzi N., Fu S. M., Kunkel H. G. Stimulation of human B lymphocytes by antibodies to IgM and IgG: functional evidence for the expression of IgG on B-lymphocyte surface membranes. Clin Immunol Immunopathol. 1980 Mar;15(3):301–313. doi: 10.1016/0090-1229(80)90042-2. [DOI] [PubMed] [Google Scholar]
- Corbel C., Melchers F. The synergism of accessory cells and of soluble alpha-factors derived from them in the activation of B cells to proliferation. Immunol Rev. 1984 Apr;78:51–74. doi: 10.1111/j.1600-065x.1984.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Falkoff R. J., Muraguchi A., Hong J. X., Butler J. L., Dinarello C. A., Fauci A. S. The effects of interleukin 1 on human B cell activation and proliferation. J Immunol. 1983 Aug;131(2):801–805. [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Iida K., Nadler L., Nussenzweig V. Identification of the membrane receptor for the complement fragment C3d by means of a monoclonal antibody. J Exp Med. 1983 Oct 1;158(4):1021–1033. doi: 10.1084/jem.158.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Butler J. L., Falkoff R. J., Fauci A. S. Human B cell activation, proliferation and differentiation. Immunol Rev. 1984 Apr;78:75–96. doi: 10.1111/j.1600-065x.1984.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Differential expression of cell activation markers after stimulation of resting human B lymphocytes. J Immunol. 1984 Jun;132(6):2857–2861. [PubMed] [Google Scholar]
- Kehry M., Ewald S., Douglas R., Sibley C., Raschke W., Fambrough D., Hood L. The immunoglobulin mu chains of membrane-bound and secreted IgM molecules differ in their C-terminal segments. Cell. 1980 Sep;21(2):393–406. doi: 10.1016/0092-8674(80)90476-6. [DOI] [PubMed] [Google Scholar]
- Kuritani T., Cooper M. D. Human B cell differentiation. IV. Effect of monoclonal anti-immunoglobulin M and D antibodies on B cell proliferation and differentiation induced by T cell factors. J Immunol. 1983 Sep;131(3):1306–1311. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Monroe J. G., Niedel J. E., Cambier J. C. B cell activation. IV. Induction of cell membrane depolarization and hyper-I-A expression by phorbol diesters suggests a role for protein kinase C in murine B lymphocyte activation. J Immunol. 1984 Mar;132(3):1472–1478. [PubMed] [Google Scholar]
- Oettgen H. C., Bayard P. J., Van Ewijk W., Nadler L. M., Terhorst C. P. Further biochemical studies of the human B-cell differentiation antigens B1 and B2. Hybridoma. 1983;2(1):17–28. doi: 10.1089/hyb.1983.2.17. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Glazer A. N., Stryer L. Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J Cell Biol. 1982 Jun;93(3):981–986. doi: 10.1083/jcb.93.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. C. Induction and suppression of polyclonal antibody responses by anti-Ig reagents and antigen-nonspecific helper factors: a comparison of the effects of anti-Fab, anti-IgM, and anti IgD on murine B cells. Immunol Rev. 1980;52:115–139. doi: 10.1111/j.1600-065x.1980.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Phillips N. E., Parker D. C. Cross-linking of B lymphocyte Fc gamma receptors and membrane immunoglobulin inhibits anti-immunoglobulin-induced blastogenesis. J Immunol. 1984 Feb;132(2):627–632. [PubMed] [Google Scholar]
- Phillips N. E., Parker D. C. Fc-dependent inhibition of mouse B cell activation by whole anti-mu antibodies. J Immunol. 1983 Feb;130(2):602–606. [PubMed] [Google Scholar]
- SELL S., GELL P. G. STUDIES ON RABBIT LYMPHOCYTES IN VITRO. I. STIMULATION OF BLAST TRANSFORMATION WITH AN ANTIALLOTYPE SERUM. J Exp Med. 1965 Aug 1;122:423–440. doi: 10.1084/jem.122.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman C. L., Unanue E. R. Control of B-lymphocyte function. I. Inactivation of mitogenesis by interactions with surface immunoglobulin and Fc-receptor molecules. J Exp Med. 1976 Oct 1;144(4):882–896. doi: 10.1084/jem.144.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieckmann D. G., Asofsky R., Mosier D. E., Zitron I. M., Paul W. E. Activation of mouse lymphocytes by anti-immunoglobulin. I. Parameters of the proliferative response. J Exp Med. 1978 Mar 1;147(3):814–829. doi: 10.1084/jem.147.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Van Wauwe J. P., Goossens J. G., Beverley P. C. Human T lymphocyte activation by monoclonal antibodies; OKT3, but not UCHT1, triggers mitogenesis via an interleukin 2-dependent mechanism. J Immunol. 1984 Jul;133(1):129–132. [PubMed] [Google Scholar]
- Yoshizaki K., Nakagawa T., Kaieda T., Muraguchi A., Yamamura Y., Kishimoto T. Induction of proliferation and Ig production in human B leukemic cells by anti-immunoglobulins and T cell factors. J Immunol. 1982 Mar;128(3):1296–1301. [PubMed] [Google Scholar]



