SUMMARY
Respiratory syncytial virus infections are a major burden in infants less than 3 months of age. Newborns and infants express a distinct immune system that is largely dependent on innate immunity and passive immunity from maternal antibodies. Antibodies can regulate immune responses against viruses through interaction with Fc gamma receptors leading to enhancement or neutralization of viral infections. The mechanisms underlying the immunomodulatory effect of Fc gamma receptors on viral infections have yet to be elucidated in infants. Herein, we will discuss current knowledge of the effects of antibodies and Fc gamma receptors on infant innate immunity to RSV. A better understanding of the pathogenesis of RSV infections in young infants may provide insight into novel therapeutic strategies like vaccination.
Keywords: respiratory syncytial virus, fc gamma receptor, maternal antibodies, innate immunity
INTRODUCTION
Respiratory syncytial virus can cause bronchiolitis that is a major burden in infants because almost all children will have had an RSV infection by the age of two years [1, 2]. The severity of RSV infection ranges from mild upper respiratory symptoms to severe lower respiratory tract infection resulting in mechanical ventilation and admission to an intensive care unit. Strikingly, severe infections occur predominantly in infants below 6 months of age and usually involve primary infections. Risk factors such as prematurity, lung disease and congenital heart disease only account for ~50% of the severe cases [1]. It is currently unknown which other factors may account for the remaining 50% of patients with severe RSV infections.
Control of RSV infection in early life
Infants below 6 months of age are largely dependent on innate immunity and the presence of maternal antibodies (matAbs) during infectious diseases. As severe RSV cases involve primary infections, innate immunity and maternal Abs are likely to have an important role. This raises the question why severe RSV infections are highly prevalent in a population having matAbs. This lack of protection might be due to the matAb properties and/or inefficient interaction between these matAbs and the innate immune system.
Innate immunity comprises particular cells and mechanisms that are the first line of defense against infections after the physical barrier has been breached. It is comprised of innate immune cells and soluble components such as the complement system, antimicrobial proteins and peptides. Cells such as monocytes, macrophages, dendritic cells, NK cells and granulocytes contain specific pathogen-recognition molecules, e.g. Toll-like receptors (TLRs), which induce the production of cytokines and activate the adaptive immune response. The innate immune response is supported by a soluble biochemical host defense cascade known as the complement system. Upon activation, this system complements the ability of Abs and phagocytic cells to clear pathogens. Due to an immature adaptive immune system and limited antigen (Ag) encounter in utero, neonates and infants below 6 months of age rely particularly on their still maturing innate immune system to defend themselves against infectious diseases [3]. The importance of innate immune receptors at this age is illustrated by the fact that deficiencies in TLR signaling increase susceptibility to infections with Streptococcus spp., Listeria monocytogenes, respiratory syncytial virus and Toxoplasma gondii in the first years of life [4].
MatAbs are produced by the mother after infection or vaccination. The induction of high affinity Ag-specific Abs is called affinity maturation and leads to high avidity, neutralizing Abs. During pregnancy, matAbs are transferred across the placenta to the fetus and remain in the serum of infants during the first months of life. Immunoglobulins, mainly IgG1, IgG3 and IgG4, cross the placenta actively and are the most important maternal antibodies [5]. IgM is a molecule too large to be transported across the placenta and IgA is transferred to the neonate in small amounts through breast milk [6]. The importance of matAbs is illustrated in newborns with a genetic inability to produce Abs such as agammaglobulinemia. These patients are usually protected against invasive bacterial infections up to 6 months when matAbs are still present [7].
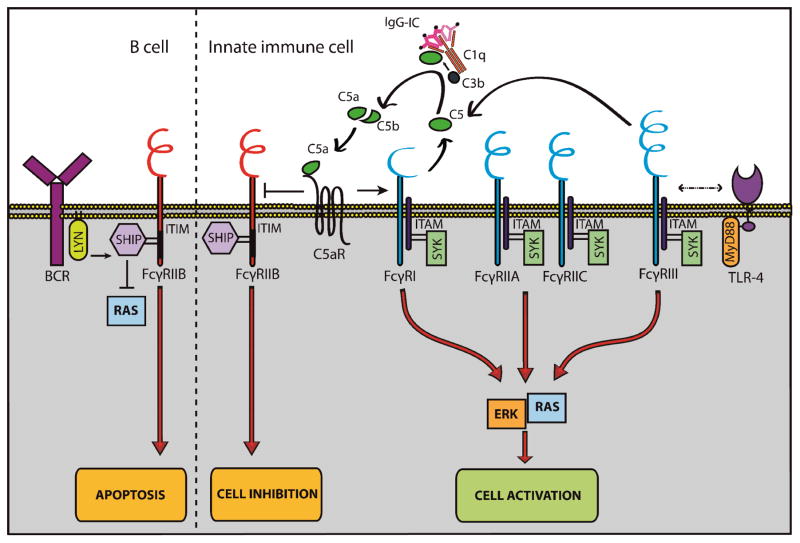
Fc gamma receptors (FcγRs) are essential for the recognition of IgG and internalization of immune complexes to induce an immune response. FcγRs can be divided into either activating or inhibitory receptors and all innate immune cells contain their own specific set of FcγRs. B cells only express the inhibitory FcγRIIB (Table 1). The balance between activating and inhibitory FcγRs together with the avidity of this binding determines the threshold to immune activation [8]. Interaction between FcγRs and pathogen-recognition receptors and the complement system as components of the innate immune system has been described and the role of IgG in this cross-talk is currently being elucidated [9–11] (Figure 1.)
Table 1. Expression of different types of FcγR on innate cells and B cells and its proposed effect in the immune response against pathogens.
The right hand column, separated by a dated vertical line, indicates inhibitory receptors.
| Type | FcγRI | FcγRIIA | FcγRIIC | FcγRIIIA | FcγRIIIB | FcγRIIB |
|---|---|---|---|---|---|---|
| Expression | Monocyte Macrophage Dendritic cell Neutrophil Eosinophil Mast cell1 |
Monocyte Macrophage Dendritic cell Neutrophil Basophil Eosinophil |
NKcell | Monocyte Macrophage Dendritic cell NK cell Mast cell |
Neutrophil Eosinophil1 |
Monocyte Macrophage Dendritic cell Neutrophil Basophil Mast cell NK cell B cell |
| Effect | Phagocytosis Cytokine release ADE2 Degranulation3 Complement activation4 |
Phagocytosis Cytokine release ADE2 ADCC5 |
Cytokine release ADCC5 6 |
Phagocytosis Cytokine release Cross-talk TLR-4 Complement activation4 ADCC5 |
Phagocytosis Chemoattractants Cross-talk TLR-4 Degranulation3 ADCC |
Reduced phagocytosis Reduced TLR sigaling Reduced proliferation Apoptosis7 |
Upon stimulation with IFN-γ;
Antibody-dependent enhancement of disease;
Mast cells;
In mice models;
Antibody-dependent cellular toxicity;
Interaction with FcγRIII;
B cells
Figure 1. Interplay between FcγRs and other receptors on innate immune cells and B cells.
FcγRs are expressed on APCs, NK cells, granulocytes and B cells. Depending on the ITAM or ITIM motif, FcγRs can be divided in activating (blue) or inhibitory (red) receptors. Activating receptors are able to initiate cell activation and induce phagocytosis, ADCC and the oxidative burst. Cross-talk with TLR-4 has been suggested for a proper immune response. The inhibitory FcγR, FcγRIIB, induces cell inhibition.
Cross-talk between the complement system and activating FcγRs creates a positive feedback loop. Activating FcγRs (FcγRI and FcγRIII) promote the complement system to generate C5a. C5a binds C5aR which is co-expressed on the cell. This binding induces increased expression levels of activating FcγRs and decreased levels of inhibitory FcγRs.
B cells only express the inhibitory FcγR, FcγRIIB. Engagement of FcγRIIB to BCR leads to inhibition of cellular proliferation and induces apoptosis. (BCR: B cell receptor; C5aR: complement 5a receptor; ERK: extracellular-signal-regulated kinases; FcγR: Fc gamma receptor; IgG-IC; immune complex; ITAM: immunoreceptor tyrosine-based activation motif; ITIM: immunoreceptor tyrosine-based inhibition motif; LYN: member of src-related family of protein-tyrosine kinases; MyD88: myeloid differentiation primary response gene 88; RAS: member of small GTPase proteins; SHIP: SH2 domain containing inositol-5 phosphatase; Syk: spleen tyrosine kinase)
In this review, we will discuss the presence and the properties of RSV-specific Abs in infants and elaborate on the interaction between Abs, FcγRs and the innate immune system, both in general and in RSV infections.
RSV SPECIFIC ANTIBODIES IN INFANTS
RSV-specific matAbs are present during the first 6 months of life and have an estimated half-life of 1.5 months [12–15]. IgG1 (66%) and IgG3 (5.3%) are the predominant subtypes present in infants [16–20]. These titers are highest among infants < 6 months and infants > 24 months, possibly indicating matAbs and “self made antibodies” respectively. Furthermore, RSV-specific IgG2 and IgG4 are not detected in the sera [20].
The clinical relevance of RSV-specific IgG is unknown at this moment and conflicting evidence is present regarding the effects of matAbs. Several studies observed that matAbs do not have a clinically beneficial effect and high anti-RSV Ab levels are associated with an increased risk of recurrent wheezing [21–23]. Other studies indicate that high titers of maternal IgG are associated with protection against RSV infection [23–27]. These studies calculated the amount of IgG by investigating the neutralizing effect of the sera via plaque reduction or the reduction of the RSV-induced cytopathic effect in in vitro cell culture systems. The amount and effect of non-neutralizing Abs are not measured by these methods. An EIA without pre-selecting neutralizing Abs might more accurately represent the total amount of RSV-specific IgG. By using an EIA, a marginal increased risk of hospitalization has been associated with moderate levels of matAbs compared to low levels [28]. RSV infected infants have comparable amounts but significantly lower avidity of RSV-specific IgG1 compared to non-RSV infected infants. These data indicate that Ab properties, like avidity, might play a role in RSV infections [20].
A more pronounced Ab response after RSV infection in individuals with low titers of matAbs has been observed, indicating that maternal Abs can inhibit the B-cell response and the production of Abs [29, 30]. Further, passive transfer of RSV-specific Abs in mice attenuates the titer and the neutralizing activity of Abs against F and G-proteins expressed by vaccinia virus recombinants [31–33]. All together, these data suggest that matAbs alter the humoral response against RSV resulting in a non-protective immune response.
Besides Ab properties, another important aspect to consider is the different epitopes that Abs can be directed to. RSV has several epitopes recognized by the immune system; the F protein, a surface glycoprotein, causes immune cell membranes to merge and mediates cell entry. The G protein is either membrane bound (mG) or secreted in a soluble form (sG) and has been implicated in immune evasion. The role of other viral proteins, like the small hydrophobic (SH) protein and the non-structural proteins (NS1 and NS2), are less well defined and currently being investigated.
The protective role of RSV-specific Abs is demonstrated by the introduction of palivizumab. Palivizumab is a mAb against the F protein of RSV and reduces hospitalization and decreases the total number of wheezing days in high-risk groups and preterm infants [34, 35]. In mice, mAbs against the RSV G protein offer protection against respiratory disease. Both neutralizing and non-neutralizing anti-RSV G protein mAbs induce protection by inhibition of replication or reduction of pulmonary inflammation [36, 37]. Given a half-life of 20 days, mAbs offer short-term protection and should be administered monthly. Therefore, the induction of a persistent protective immune response against RSV through vaccination has been a topic of interest for decades. The failure of a vaccine trial with formalin-inactivated RSV shows the complexity of inducing a proper immune response against RSV. Proper affinity maturation and the induction of neutralizing Abs are important for an adequate immune response against RSV [38, 39]. Formalin-inactivated RSV induces non-neutralizing Abs and thereby causes an enhanced respiratory disease after concurrent RSV infection [39]. This process is mediated through insufficient stimulation of TLR on B cells and is dependent on the formation of immune complexes. Inactivation of RSV with formalin induces reactive carbonyl groups that may alter immunogenicity and favor a Th2 response possibly resulting in pulmonary eosinophilia and a deleterious immune responses [40]. An important regulatory role of CD8 T cells has been suggested because these cells inhibit RSV vaccine-enhanced disease and eosinophilia [41]. Consequently a novel vaccine should induce a balanced CD4 and CD8 T cell response to minimize immunopathology.
Overall, the clinical and immunological effects of RSV-specific Abs on RSV infection are still unclear. Studies have reported either a protective or deleterious effect of matAbs (Table 2). Future research on the specific properties of matAbs is needed to interpret this contradictory data.
Table 2. Clinical relevance of IgG in RSV infections in young infants.
RSV-specific IgG can be divided into IgG1, IgG2, IgG3, IgG4 and mAb IgG1. Contradictory data is present regarding the protective effects of total maternal IgG. Low avidity maternal IgG1 has been associated with an increased susceptibility to RSV infections. Palivizumab (mAb IgG1) has a protective effect against severe RSV infection in high-risk infants. IgG2-4 are either undetectable or only detected in the minority of young infants below the age of 6 months.
| Type | Total IgG | IgG1 | mAb IgG1 (Palivizumab) | IgG2 | IgG3 | IgG4 |
|---|---|---|---|---|---|---|
| Clinical relevance | High titer: |
Low avidity:
|
Monthly administration:
|
Not detectable | Detectable in the minority of young infants | Not detectable |
Amount of IgG measured by plaque reduction or reduction of RSV-induced cytopathic effect in in vtiro cell culture system;
Amount of IgG measured by EIA;
Avidity measured by using an antibody eluting agent (sodium thiocyanate);
Administered to high-risk and preterm infants
THE EFFECT OF FC GAMMA RECEPTORS ON INNATE IMMUNITY
Fc gamma receptors and monocytes, macrophages and dendritic cells
FcγRI, FcγRIIA and FcγRIIIA are involved in Ab-mediated phagocytosis of pathogens by monocytic cells like monocytes, macrophages and dendritic cells. Phagocytosis is important for the activation of Syk kinase and the induction of the immune response by release of inflammatory cytokines [42]. The down-side of Ab-mediated phagocytosis is shown in the context of several viral infections and is called Ab-dependent enhancement (ADE). ADE has been observed in secondary dengue infections of both monocytes and dendritic cells in which Abs increase infectivity via FcγRI and FcγRIIA [43]. Furthermore, the ligation of dengue virus particles with Abs reduces the expression of TLR and shifts the formation of cytokines towards anti-inflammatory cytokines [44, 45]. The same principle has been observed in the context of HIV infection and is dependent on FcγRs [46, 47]. These data suggest a mechanism by which ADE in the context of viral infections increases infectivity and impairs the innate immune response [43, 48]. A comprehensive review has been published concerning the different mechanisms of ADE in viral infections after secondary infections or vaccination [49].
Beside initiating phagocytosis, FcγRIII has been implicated in cross-talk with TLRs. The presence of immune complexes results in the association between TLR4 and FcγRIII. Furthermore, the activation of FcγR requires the presence and integrity of TLR4 because both FcγRIII-deficient mice and TLR4-deficient mice were unable to elicit an immune response upon stimulation of macrophages with IgG [11].
FcγRIIB is known as an inhibitory receptor resulting in immunosuppression and decreased phagocytosis by monocytic cells [50]. Pre-treatment of macrophages with IgG1 inhibits the TLR4 response induced by lipopolysaccharide, a process dependent on the activation of FcγRIIB. [51]. In dendritic cells, blockage of FcγRIIB leads to dendritic cell maturation, up-regulation of the type I interferon genes and increased amounts of CCL-5 (chemokine C-C motif ligand 5) which underlines the inhibitory effects of FcγRIIB on the immune response [52, 53].
The role of Fc gamma receptors expressed on monocytes, dendritic cells and macrophages in RSV infections
In animal studies, macrophages are essential for the restriction of RSV replication in the lungs. ADE after vaccination has been observed in RSV infections in vitro with both human and a mouse monocyte-like cell line and macrophages. Monoclonal Abs can have a neutralizing effect, a disease enhancing effect or both [54]. The concentration of IgG and the simultaneous presence of different mAbs determine whether the Abs are neutralizing or disease enhancing [54, 55]. The enhancing activity of RSV-specific Ab levels is confined to sera from infants aged 0 to 6 months and acts via FcγR [56]. This suggests that matAbs may contribute to ADE of RSV infection ADE has not yet been investigated in the context of disease severity of RSV infections. Dendritic cells are the most important antigen presenting cells and function as a bridge between innate and adaptive immunity. RSV can infect DCs but reduces T cell activation by impairment of the immunological synapse between DCs and T cells [57]. RSV-specific T cell activation and release of IFN-γ are enhanced when mouse DCs are exposed to RSV immune complexes. The use of FcγR-deficient mice showed that this process is dependent on activating FcγRs. The authors conclude that the presence of antibodies might induce a more efficient T cell response through the phagocytosis of opsonized virus particles by DCs [58].
RSV has evolved different mechanisms to alter or counteract the antiviral effect of monocytic cells. RSV evades Ab neutralization by the production of sG which acts as a decoy antigen. The use of FcγR-deficient mice shows that the production of sG decreases the antiviral effect of cells bearing FcγRs underlining the role of Abs in this process [59, 60]. Other structural and non-structural proteins alter the antiviral immune response through direct interaction with recognition receptors [61–65]. All experiments in the context of human cells were performed in a serum-free environment. Therefore, the role of antibodies in the immune evasion mechanisms of RSV is unknown.
Fc gamma receptors and NK cells
NK cells are activated by virus-infected cells directly through activation receptors or through immune complexes. FcγRIIIA is the most important FcγR present on NK cells [66]. In the context of Abs, CD56dim NK cells are of interest considering their abundant presence in peripheral blood and their expression of FcγRIIIA. IgG is able to enhance the production of cytokines by CD56dim NK cells upon encountering target cells [67]. Binding of immune complexes to FcγRIIIA induces antibody-dependent cellular cytoxicity (ADCC) through CD56dim NK cells by degranulation and perforin-dependent targeted cell lysis [68, 69]. In the absence of a pathogen, IgG is able to inhibit NK cell activity [70]. ADCC has extensively been studied in the context of HIV infections in which the interaction of anti-HIV Abs with NK cells offers protection and even predicts viral load and clinical outcome [71, 72]. ADCC against herpes simplex virus and HIV is decreased in neonates compared to adults possibly reflecting, in part, deficient interaction of matAbs and NK cells in the neonatal period [73–75].
FcγRIII is aided in its cytotoxic effect by FcγRIIC. Not all individuals express FcγRIIC on their NK cells. The FcγRIIC gene originates from the unequal crossover between IIA and IIB genes, which results in an FcγR that is homologous to FcγRIIB extracellularly and to FcγRIIA intracellularly [76]. When FcγRIIC is expressed on NK cells, it mediates IFN-γ production and aids FcγRIII in its cytotoxic effect [68, 77]. Finally, the inhibitory FcγRIIB was detected in only a few individuals and was able to suppress NK cell function [76, 78].
The role of Fc gamma receptors expressed on NK cells in RSV infections
In RSV infected mice, NK cells accumulate in the lung upon RSV infection and cause acute lung injury through the production of IFN-γ [79, 80]. RSV infection induces ADCC via NK cells as shown by the cytotoxic effect of NK cells on a RSV-infected human cell line. This process is already present in young infants, implying a role of matAbs [81, 82]. Recent studies are unraveling the specific subsets of NK cells responsible for the RSV-induced ADCC. Lung tissues from fatal RSV infections showed a near absence of NK cells [83]. These low amounts of NK cells, however might represent the end-stage values of NK cells considering the use of lung tissue samples after the patients died. In the acute phase of RSV infection, increased amounts of NK cells are found. CD56+/FcγRIIIA+ NK cells and IFN-γ production are increased in the acute phase of hospitalized RSV-infections compared to control patients without RSV-infection [84]. The increased presence of FcγRIIIA+ NK cells, IFN-γ and lung damage in severe RSV infections, raises the possibility that IgG, as a ligand for FcγRIIIA, negatively influences the immune response in RSV infections.
Fc gamma receptors and granulocytes
Neutrophils
Resting neutrophils express FcγRIIA in a low-avidity state which is functional inactive [85, 86]. fMLP and PMA are two well-known neutrophil activators that regulate the functionality of FcγRIIA in an opposing manner. Whereas fMLP increases the binding of IgG-coated particles, PMA suppresses this process and thereby the functionality of FcγRIIA. These data indicate that the functionality of FcγRIIA is not merely dependent on the activation of neutrophils, but that a stimulus-specific signal determines whether FcγRIIA on activated neutrophils becomes functionally active. [87]. Future research investigating the effects of specific infectious agents, such as RSV, on the functionality of FcγRIIA is needed to translate these findings to understand its role in the pathophysiology of infectious diseases. The activation of FcγRIIIB leads to increased phagocytosis and the recruitment of neutrophils to inflammatory sites [88–90]. As stated before in the context of macrophages, stimulation of neutrophils with IgG results in the association between TLR4 and FcγRIII. PMN from TLR4-deficient mice are unable to elicit an immune response upon stimulation with IgG. However, the effect of immune complexes on PMN from FcγRIII-deficient mice was not investigated [11]. The precise role of the inhibitory FcγRIIB in human neutrophils is as yet unknown. Resting human neutrophils express FCGR2B2 mRNA, but express low levels of FcγRIIB on the cell surface. FcγRIIB might inhibit phagocytosis, superoxide production and neutrophil adhesion [91].
The role of Fc gamma receptors expressed on neutrophils in RSV infections
Neutrophils play an important role in the development of lung pathology in severe primary RSV infections in infants below 6 months [92]. Neither RSV alone nor specific RSV-Abs alone are able to activate neutrophils. The presence of RSV and RSV-specific Abs results in the formation of RSV immune complexes [93]. After phagocytosis of the immune complexes, profound activation of neutrophils and increased amounts of interleukin 8, oxygen radicals and thromboxane B2 are observed. These products cause immune complex-mediated lung pathology and bronchoconstriction in RSV infections [93–95].
Mast cells
IgG Abs have been known to activate mast cells, long before the currently established correlation between IgE, mast cells and allergic reactions [96]. Upon stimulation with IFN-γ, FcγRI expression on mast cells is upregulated and IgG has an activating effect. The increased expression of FcγRI allows IgG, and particularly IgG1, to cause degranulation and prolonged survival of mast cells [97–99].
FcγRIIIA expression is present on mast cells and induces IgG-mediated degranulation [100, 101]. Inhibition of Kit-induced mast cell proliferation upon activation of FcγRIIB has been observed and bone marrow-derived mast cells do not respond to IgG unless they are FcγRIIB-deficient [102]. These data suggest that IgG does bind to bone marrow-derived mast cells, but has an inhibitory effect through FcγRIIB that was confirmed by the increased sensitivity of mast cells from FcγRII-deficient mice upon stimulation with IgG [96, 103].
Currently, there is compelling evidence of a role for mast cells in the immune response against viral infections [104]. Studies observed that viral infection of mast cells leads to the recruitment of monocytes, NK cells and T lymphocytes [105]. The addition of dengue-specific Abs to dengue infected mast cells leads to increased expression of RIG-I and MDA-5 and induces a profound and possibly harmful release of cytokines and chemokines. It has been concluded that FcγRIIA plays a role in the ADE of dengue infections in mast cells [106–108].
The role of Fc gamma receptors expressed on mast cells in RSV infections
Mast cells express TLR4 suggesting direct recognition of pathogens including RSV. RSV infection of rodents and cows has been associated with mast cell activation. In humans, RSV bronchiolitis is associated with an increased number of mast cells [109]. Pre-treatment of mast cells with purified human total IgG results in increased amounts of chemokines compared to stimulation with RSV alone [110].
Eosinophils
Although eosinophils are mostly involved in allergic reactions, they also possess antimicrobial properties [111–113]. FcγRII is responsible for Ab-mediating killing of tumor cells and antibodies are able to enhance the killing ability and activate FcγRII-dependent degranulation of eosinophils against a variety of pathogens like Toxoplasma gondii and Candida albicans [111, 114, 115]. Abs, particularly IgG1 and IgG3 but not IgE, activate eosinophils resulting in their degranulation and causing bronchial hyperreactivity as seen in asthma patients, a process dependent on FcγRII [116]. Interestingly, immobilized IgG induces death of eosinophils and soluble IgG is able to prolong survival of eosinophils [117].
After activation with chemoattractants or IFN-γ, FcγRI and FcγRII become membrane-expressed on eosinophils. FcγRIII is mainly present intracellular in resting eosinophils. Upon activation however, FcγRIII becomes membrane-expressed transiently before secretion of the receptor takes place [118, 119]. The exact role of FcγRIII in eosinophils is yet to be determined.
The role of Fc gamma receptors expressed on eosinophils in RSV infections
Increased amounts of eosinophils are observed in nasopharyngeal aspirates of RSV infected infants compared to non-infected infants [120]. RSV replication in eosinophils results in the release of infectious virions and the pro-inflammatory mediator interleukin 6 [121]. Eosinophils inactivate RSV by the release of a specific protein called eosinophil cationic protein (ECP) [122, 123]. Infants with elevated levels of ECP during a primary RSV infection are ten times more likely to develop wheezing later in childhood [124]. Pulmonary eosinophilia attracted in response to primary RSV infection is particularly evident in the youngest human infants and in neonatal mouse models [125, 126]. Currently no data are available concerning the effect of Abs or immune complexes on RSV-infected eosinophils. In the context of RSV vaccination, Polack et al. showed that IgG mediates increased activation of eosinophils and enhanced respiratory disease after challenge with formalin inactivated RSV [38]. Therefore, abundant eosinophilic acitivation or ECP release might play a role in the immunopathology of RSV infections.
Basophils
Basophils are a sparse subset in the population of leukocytes and they are involved in allergic reactions. Only recently, the role of FcγR on basophils is being elucidated. IgG is not able to activate basophils probably due to the high expression of the inhibitory FcγRIIB in this cell type. A low degree of basophil activation is achieved by selectively activating FcγRIIA and thereby bypassing FcγRIIB [127–130].
The role of Fc gamma receptors expressed on basophils in RSV infections
RSV induces basophil accumulation in a mouse model. Furthermore, basophils were the only cells responsible for the release of interleukin 4, a cytokine which might contribute to the pulmonary pathogenesis of RSV infections [131]. No published data are available on the role of Abs and basophils in the context of RSV.
FC GAMMA RECEPTORS AND B CELLS
The B cell compartment is predominantly known for its adaptive aspects in the immune defense. However, the expression of TLRs and the internalization of viruses upon binding with the B cell receptor (BCR) suggest that B cells are involved in directly sensing infectious agents [132]. A specific B cell population, called B1 cells, is already present during the fetal period. A progressive loss of B1 cells was shown with a fourfold difference between cord blood and adults above 20 years of age that indicates a specific role of B1 cells in childhood [133]. Natural Abs are spontaneously produced by B1 cells without prior infection or immunization and are a major recognition molecule of innate immunity [134–136]. In mice, natural Abs activate the complement system, facilitate antigen uptake and protect against S. pneumoniae, Listeria monocytogenes and influenza virus [134, 137–140].
B cells only express the inhibitory FcγRIIB and the expression of FcγRIIB varies among the different subtypes of B cells. Engagement of FcγRIIB to BCR leads to inhibition of cellular proliferation and induces apoptosis. B cells that express high-affinity BCR will receive signals from both BCR and FcγRIIB, whereas B cells that have low-affinity BCR will predominantly receive signals via FcγRIIB resulting in apoptosis. Plasma cells express high levels of FcγRIIB and are therefore susceptible to the induction of apoptosis by Abs [141]. Thus, overall, FcγRIIB acts as a regulator of humoral immunity.
The role of Fc gamma receptors expressed on B cells in RSV infections
B cell responses may contribute to the protective and/or pathological effect of primary RSV infections and are increased in the acute and convalescent phase of RSV infection [142, 143]. Some characteristics of RSV-specific B cells from RSV infected individuals have been determined. B cells from RSV infected infants younger than 3 months express fewer somatic mutations after both a primary and a secondary infection compared to individuals older than 3 months. Somatic mutation is an important property of B cells in order to produce neutralizing Abs upon encountering a pathogen [144]. These data suggest that inefficient somatic mutation underlies the poor Ab response in neonates against RSV infections. As mentioned before, high levels of matAbs reduce the Ab response of infants against RSV [30]. Although B cells are the main source of Abs, no study so far has investigated the effect of antibodies on the humoral response in natural RSV infections. The insufficient TLR stimulation on B cells resulting in the lack of a protective Ab response upon formalin-inactived RSV vaccine highlights the importance of adequate activation of B cells [39].
FC GAMMA RECEPTORS AND THE COMPLEMENT SYSTEM
The complement system is a cascade that aids Abs in the clearance of pathogens. IgG1, IgG2 and IgG3 are capable of activating the classical pathway of complement. In this cascade, the formation of complement factors C3a and C5a allows induction of phagocytosis and pro-inflammatory cytokines through complement receptors (C3aR and C5aR) [145]. The interplay between complement and FcγR is essential in this process (Figure 1). Pre-treatment with IgG1 is able to shift this balance by selectively activating FcγRIIB and thereby limiting the inflammatory response [146, 147]
The role of Fc gamma receptors and complement in RSV infections
The complement system is important in the pathogenesis of RSV infections. RSV activates the complement system and induces the production of C3a and C5a [148]. C3aR-deficient mice show decreased viral replication suggesting a non-protective role of C3aR. The disease enhancing effect of C3aR has been confirmed in the context of the formalin-inactivated RSV vaccine. Vaccinated C5-deficient mice express an upregulation of C3aR and enhanced respiratory disease upon infection with RSV [149]. Moreover, C3 is increased in enhanced respiratory disease caused by vaccination with formalin-inactivated RSV [38]. Both studies conclude that C3aR might contribute to the pathogenesis of RSV infection [150]. Indeed, increased complement activation has been observed in patients who died from RSV bronchiolitis [38]. The effects of pre-existent RSV-specific Abs has been studied by injecting naïve mice with RSV-specific Abs to mimic infants having matAbs. The following Ab-mediated viral restriction is dependent on the complement system [60]. IgG1 is the predominant RSV-specific Ab present in infants with RSV-infections and suppresses complement-mediated inflammation. This predominant interaction of IgG1 with FcγRIIB as stated by Karsten et al. is yet to be investigated in the context of RSV infections [147]. Studies on the effect of Abs on the adaptive immune response against RSV show that FcγR and complement contribute to the Ab-mediated induction of CD4+ T cells resulting in a high ratio of CD4+/CD8+ T cells [58]. The balance between CD4+ and CD8+ T cells might be important considering patients with severe RSV infections have relatively low amounts of CD4+ T cells compared to CD8+ T cells [72, 151]. A role of complement and the induction of neutralizing versus non-neutralizing Abs in shaping the CD4+/CD8+ ratio and disease severity has been suggested [58].
CONCLUSIONS
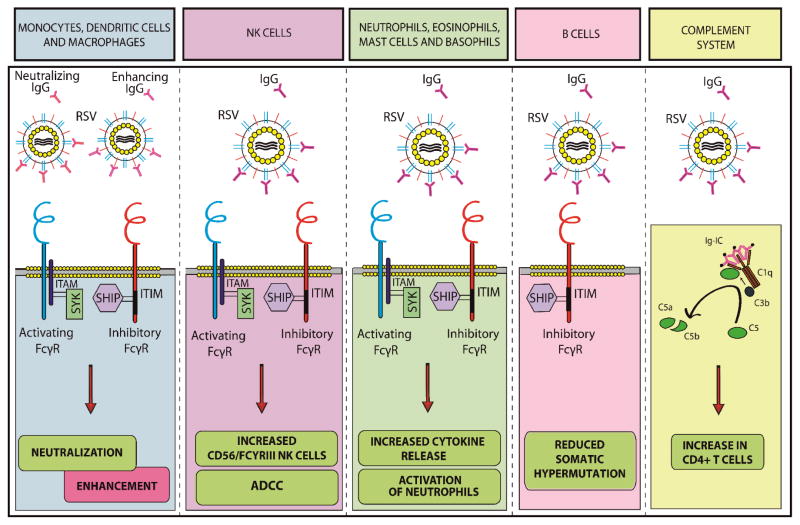
This review has presented the extensive evidence for the immunomodulatory effect of Abs and FcγRs on innate immunity. All cells of the innate immune system express FcγRs and are affected by the presence of antibodies. Circulating matAbs should be taken into account when contemplating disease pathogenesis of infectious diseases in young infants. Most of these mechanisms are present in the context of RSV (Figure 2). Therefore, it is likely that these pathways play a significant role in the development of severe RSV infections. Currently, clinical evidence is lacking and future research is necessary to investigate how the balance between activating and inhibitory FcγRs determines the resulting immune response during severe RSV infections. Elucidating the Ab properties, like the neutralizing capacity, the avidity and the titers of matAbs against RSV in young infants could explain why matAbs are either protective or disease enhancing. In vitro experiments using primary cells represent an important approach to studying these pathways. Characterizing the effect of Abs and activated FcγRs on neutralization of RSV in vitro, cytokine production and the adaptive immune response could advance our knowledge on this subject. Cross-talk between Abs and innate immune receptors, such as TLRs will be of particular interest in the context of an infection that frequently strikes young infants. Studies of FcγR-deficient mice can be used to study the effect of FcγRs in vivo. Although immune evasion mechanisms of RSV are currently being unraveled, the presence of Abs has not been investigated when contemplating clinical relevance. These mechanisms will give more insight into the effects of matAbs on the immune response and disease severity during RSV infections in infants. Secondly, it will provide essential knowledge relevant to the induction of protective Abs during maternal or neonatal immunization. Therefore, characterizing matAbs and the role of FcγRs will provide new insights in the pathogenesis of RSV infections and the development of novel preventive strategies.
Figure 2. Summary of the interaction between RSV, antibodies, FcγRs and the innate immune system.
In monocytic cells, the interaction between Abs and FcγRs has a neutralizing or a disease enhancing effect on RSV. FcγRIIIA+ NK cells are responsible for ADCC, induce higher amounts of cytokines in the presence of Abs and increased amounts of FcγRIIIA+ NK cells are observed in severe RSV infections. Profound activation of neutrophils and increased cytokine release against RSV are induced in the presence of RSV-specific Abs. B cells from RSV infected infants younger than 3 months of age express fewer somatic mutations compared to older individuals. The complement system is involved in the Ab-mediated induction of CD4+ T cells upon RSV infection.
Acknowledgments
JJ is supported by a research grant of the Nijmegen Institute for Infection, Inflammation and Immunity (N4i). GF and MV are supported by the VIRGO consortium, an Innovative Cluster approved by the Netherlands Genomics Initiative and partially funded by the Dutch Government (BSIK 03012). OL’s laboratory is supported by Global Health (OPPGH5284) and Grand Challenges Explorations (OPP1035192) awards from the Bill & Melinda Gates Foundation and by NIH grant 1R01AI100135-01
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- ADE
antibody-dependent enhancement
- BCR
B cell receptor
- C3aR
complement 3a receptor
- CCL-5
chemokine c-c motif ligand 5
- ECP
eosinophil cationic protein
- FcγR
Fc gamma receptor
- fMLP
formyl-methionyl-leucyl-phenylalanine
- matAbs
maternal antibodies
- mG
membrane bound G protein
- NS protein
non structural protein
- PMA
phorbol 12,13-dibutyrate
- sG
soluble G protein
- SH protein
small hydrophobic protein
- TLR
Toll-like receptor
Footnotes
CONFLICTS OF INTEREST
None
References
- 1.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 4.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clin Microbiol Rev. 2011;24:490–497. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He W, Ladinsky MS, Huey-Tubman KE, Jensen GJ, McIntosh JR, Bjorkman PJ. FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature. 2008;455:542–546. doi: 10.1038/nature07255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver LT, Arthur HM, Bunn JE, Thomas JE. Human milk IgA concentrations during the first year of lactation. Arch Dis Child. 1998;78:235–239. doi: 10.1136/adc.78.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkelstein JA, Marino MC, Lederman HM, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 8.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 9.van Lent PL, Blom AB, Grevers L, Sloetjes A, van den Berg WB. Toll-like receptor 4 induced FcgammaR expression potentiates early onset of joint inflammation and cartilage destruction during immune complex arthritis: Toll-like receptor 4 largely regulates FcgammaR expression by interleukin 10. Ann Rheum Dis. 2007;66:334–340. doi: 10.1136/ard.2006.057471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banas MC, Banas B, Hudkins KL, et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704–713. doi: 10.1681/ASN.2007040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittirsch D, Flierl MA, Day DE, et al. Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS Pathog. 2009;5:e1000464. doi: 10.1371/journal.ppat.1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suara RO, Piedra PA, Glezen WP, et al. Prevalence of neutralizing antibody to respiratory syncytial virus in sera from mothers and newborns residing in the Gambia and in The United States. Clin Diagn Lab Immunol. 1996;3:477–479. doi: 10.1128/cdli.3.4.477-479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacimustafaoglu M, Celebi S, Aynaci E, et al. The progression of maternal RSV antibodies in the offspring. Arch Dis Child. 2004;89:52–53. doi: 10.1136/adc.2002.017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus epidemiology in a birth cohort from Kilifi district, Kenya: infection during the first year of life. J Infect Dis. 2004;190:1828–1832. doi: 10.1086/425040. [DOI] [PubMed] [Google Scholar]
- 15.Brandenburg AH, Groen J, van Steensel-Moll HA, et al. Respiratory syncytial virus specific serum antibodies in infants under six months of age: limited serological response upon infection. J Med Virol. 1997;52:97–104. doi: 10.1002/(sici)1096-9071(199705)52:1<97::aid-jmv16>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Welliver RC, Kaul TN, Putnam TI, Sun M, Riddlesberger K, Ogra PL. The antibody response to primary and secondary infection with respiratory syncytial virus: kinetics of class-specific responses. J Pediatr. 1980;96:808–813. doi: 10.1016/s0022-3476(80)80547-6. [DOI] [PubMed] [Google Scholar]
- 17.Hornsleth A, Bech-Thomsen N, Friis B. Detection by ELISA of IgG-subclass-specific antibodies in primary respiratory syncytial (RS) virus infections. J Med Virol. 1985;16:321–328. doi: 10.1002/jmv.1890160404. [DOI] [PubMed] [Google Scholar]
- 18.Wilczynski J, Lukasik B, Torbicka E, Tranda I, Brzozowska-Binda A. Respiratory syncytial virus (RSV) antibodies in different immunoglobulin classes in small children. Acta Microbiol Pol. 1994;43:359–368. [PubMed] [Google Scholar]
- 19.Queiroz DA, Durigon EL, Botosso VF, et al. Immune response to respiratory syncytial virus in young Brazilian children. Braz J Med Biol Res. 2002;35:1183–1193. doi: 10.1590/s0100-879x2002001000011. [DOI] [PubMed] [Google Scholar]
- 20.Freitas GR, Silva DA, Yokosawa J, et al. Antibody response and avidity of respiratory syncytial virus-specific total IgG, IgG1, and IgG3 in young children. J Med Virol. 2011;83:1826–1833. doi: 10.1002/jmv.22134. [DOI] [PubMed] [Google Scholar]
- 21.Parrott RH, Kim HW, Arrobio JO, et al. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973;98:289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- 22.Bulkow LR, Singleton RJ, Karron RA, Harrison LH, Alaska RSVSG. Risk factors for severe respiratory syncytial virus infection among Alaska native children. Pediatrics. 2002;109:210–216. doi: 10.1542/peds.109.2.210. [DOI] [PubMed] [Google Scholar]
- 23.Stensballe LG, Ravn H, Kristensen K, et al. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J Allergy Clin Immunol. 2009;123:398–403. doi: 10.1016/j.jaci.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 24.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 25.Ogilvie MM, Vathenen AS, Radford M, Codd J, Key S. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol. 1981;7:263–271. doi: 10.1002/jmv.1890070403. [DOI] [PubMed] [Google Scholar]
- 26.Kasel JA, Walsh EE, Frank AL, Baxter BD, Taber LH, Glezen WP. Relation of serum antibody to glycoproteins of respiratory syncytial virus with immunity to infection in children. Viral Immunol. 1987;1:199–205. doi: 10.1089/vim.1987.1.199. [DOI] [PubMed] [Google Scholar]
- 27.Eick A, Karron R, Shaw J, et al. The role of neutralizing antibodies in protection of American Indian infants against respiratory syncytial virus disease. Pediatr Infect Dis J. 2008;27:207–212. doi: 10.1097/INF.0b013e31815ac585. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen HE, Siersma V, Andersen S, et al. Respiratory syncytial virus infection--risk factors for hospital admission: a case-control study. Acta Paediatr. 2003;92:1314–1321. [PubMed] [Google Scholar]
- 29.Murphy BR, Alling DW, Snyder MH, et al. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986;24:894–898. doi: 10.1128/jcm.24.5.894-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinoff JJ, O’Brien KL, Thumar B, et al. Young infants can develop protective levels of neutralizing antibody after infection with respiratory syncytial virus. J Infect Dis. 2008;198:1007–1015. doi: 10.1086/591460. [DOI] [PubMed] [Google Scholar]
- 31.Murphy BR, Collins PL, Lawrence L, Zubak J, Chanock RM, Prince GA. Immunosuppression of the antibody response to respiratory syncytial virus (RSV) by pre-existing serum antibodies: partial prevention by topical infection of the respiratory tract with vaccinia virus-RSV recombinants. J Gen Virol. 1989;70 (Pt 8):2185–2190. doi: 10.1099/0022-1317-70-8-2185. [DOI] [PubMed] [Google Scholar]
- 32.Crowe JE., Jr Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin Infect Dis. 2001;33:1720–1727. doi: 10.1086/322971. [DOI] [PubMed] [Google Scholar]
- 33.Vieira SE, Gilio AE, Durigon EL, Ejzenberg B. Lower respiratory tract infection caused by respiratory syncytial virus in infants: the role played by specific antibodies. Clinics (Sao Paulo) 2007;62:709–716. doi: 10.1590/s1807-59322007000600009. [DOI] [PubMed] [Google Scholar]
- 34.Committee on Infectious D. From the American Academy of Pediatrics: Policy statements--Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–1701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 35.Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 36.Boyoglu-Barnum S, Gaston KA, Todd SO, et al. A respiratory syncytial virus (RSV) anti-G protein F(ab′)2 monoclonal antibody suppresses mucous production and breathing effort in RSV rA2-line19F-infected Balb/c mice. J Virol. 2013 doi: 10.1128/JVI.01164-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caidi H, Harcourt JL, Tripp RA, Anderson LJ, Haynes LM. Combination therapy using monoclonal antibodies against respiratory syncytial virus (RSV) G glycoprotein protects from RSV disease in BALB/c mice. PLoS One. 2012;7:e51485. doi: 10.1371/journal.pone.0051485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polack FP, Teng MN, Collins PL, et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med. 2002;196:859–865. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moghaddam A, Olszewska W, Wang B, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12:905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 41.Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunologic research. 2007;39:225–239. doi: 10.1007/s12026-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 42.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 43.Ubol S, Phuklia W, Kalayanarooj S, Modhiran N. Mechanisms of immune evasion induced by a complex of dengue virus and preexisting enhancing antibodies. J Infect Dis. 2010;201:923–935. doi: 10.1086/651018. [DOI] [PubMed] [Google Scholar]
- 44.Modhiran N, Kalayanarooj S, Ubol S. Subversion of innate defenses by the interplay between DENV and pre-existing enhancing antibodies: TLRs signaling collapse. PLoS Negl Trop Dis. 2010;4:e924. doi: 10.1371/journal.pntd.0000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boonnak K, Slike BM, Burgess TH, et al. Role of dendritic cells in antibody-dependent enhancement of dengue virus infection. J Virol. 2008;82:3939–3951. doi: 10.1128/JVI.02484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laurence J, Saunders A, Early E, Salmon JE. Human immunodeficiency virus infection of monocytes: relationship to Fc-gamma receptors and antibody-dependent viral enhancement. Immunology. 1990;70:338–343. [PMC free article] [PubMed] [Google Scholar]
- 47.Takeda A, Sweet RW, Ennis FA. Two receptors are required for antibody-dependent enhancement of human immunodeficiency virus type 1 infection: CD4 and Fc gamma R. J Virol. 1990;64:5605–5610. doi: 10.1128/jvi.64.11.5605-5610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chareonsirisuthigul T, Kalayanarooj S, Ubol S. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J Gen Virol. 2007;88:365–375. doi: 10.1099/vir.0.82537-0. [DOI] [PubMed] [Google Scholar]
- 49.Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect Dis. 2010;10:712–722. doi: 10.1016/S1473-3099(10)70166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL. Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. J Biol Chem. 2002;277:5082–5089. doi: 10.1074/jbc.M110277200. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Liu S, Liu J, et al. Immune complex/Ig negatively regulate TLR4-triggered inflammatory response in macrophages through Fc gamma RIIb-dependent PGE2 production. J Immunol. 2009;182:554–562. doi: 10.4049/jimmunol.182.1.554. [DOI] [PubMed] [Google Scholar]
- 52.Dhodapkar KM, Kaufman JL, Ehlers M, et al. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci U S A. 2005;102:2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhodapkar KM, Banerjee D, Connolly J, et al. Selective blockade of the inhibitory Fcgamma receptor (FcgammaRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J Exp Med. 2007;204:1359–1369. doi: 10.1084/jem.20062545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gimenez HB, Chisholm S, Dornan J, Cash P. Neutralizing and enhancing activities of human respiratory syncytial virus-specific antibodies. Clin Diagn Lab Immunol. 1996;3:280–286. doi: 10.1128/cdli.3.3.280-286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gimenez HB, Keir HM, Cash P. In vitro enhancement of respiratory syncytial virus infection of U937 cells by human sera. J Gen Virol. 1989;70 (Pt 1):89–96. doi: 10.1099/0022-1317-70-1-89. [DOI] [PubMed] [Google Scholar]
- 56.Osiowy C, Horne D, Anderson R. Antibody-dependent enhancement of respiratory syncytial virus infection by sera from young infants. Clin Diagn Lab Immunol. 1994;1:670–677. doi: 10.1128/cdli.1.6.670-677.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez PA, Bueno SM, Carreno LJ, Riedel CA, Kalergis AM. Respiratory syncytial virus infection and immunity. Rev Med Virol. 2012;22:230–244. doi: 10.1002/rmv.1704. [DOI] [PubMed] [Google Scholar]
- 58.Kruijsen D, Bakkers MJ, van Uden NO, et al. Serum antibodies critically affect virus-specific CD4+/CD8+ T cell balance during respiratory syncytial virus infections. The Journal of Immunology. 2010;185:6489–6498. doi: 10.4049/jimmunol.1002645. [DOI] [PubMed] [Google Scholar]
- 59.Bukreyev A, Yang L, Fricke J, et al. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol. 2008;82:12191–12204. doi: 10.1128/JVI.01604-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bukreyev A, Yang L, Collins PL. The secreted G protein of human respiratory syncytial virus antagonizes antibody-mediated restriction of replication involving macrophages and complement. J Virol. 2012;86:10880–10884. doi: 10.1128/JVI.01162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuentes S, Tran KC, Luthra P, Teng MN, He B. Function of the respiratory syncytial virus small hydrophobic protein. J Virol. 2007;81:8361–8366. doi: 10.1128/JVI.02717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected] J Virol. 2004;78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Senft AP, Taylor RH, Lei W, et al. Respiratory syncytial virus impairs macrophage IFN-alpha/beta- and IFN-gamma-stimulated transcription by distinct mechanisms. Am J Respir Cell Mol Biol. 2010;42:404–414. doi: 10.1165/rcmb.2008-0229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jie Z, Dinwiddie DL, Senft AP, Harrod KS. Regulation of STAT signaling in mouse bone marrow derived dendritic cells by respiratory syncytial virus. Virus Res. 2011;156:127–133. doi: 10.1016/j.virusres.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perussia B. Fc receptors on natural killer cells. Curr Top Microbiol Immunol. 1998;230:63–88. doi: 10.1007/978-3-642-46859-9_6. [DOI] [PubMed] [Google Scholar]
- 67.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sulica A, Morel P, Metes D, Herberman RB. Ig-binding receptors on human NK cells as effector and regulatory surface molecules. Int Rev Immunol. 2001;20:371–414. doi: 10.3109/08830180109054414. [DOI] [PubMed] [Google Scholar]
- 69.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 70.Sulica A, Galatiuc C, Manciulea M, et al. Regulation of human natural cytotoxicity by IgG. IV. Association between binding of monomeric IgG to the Fc receptors on large granular lymphocytes and inhibition of natural killer (NK) cell activity. Cell Immunol. 1993;147:397–410. doi: 10.1006/cimm.1993.1079. [DOI] [PubMed] [Google Scholar]
- 71.Ahmad A, Menezes J. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 1996;10:258–266. doi: 10.1096/fasebj.10.2.8641559. [DOI] [PubMed] [Google Scholar]
- 72.Ahmad R, Sindhu ST, Toma E, et al. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol. 2001;21:227–233. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- 73.Kohl S. Defective infant antiviral cytotoxicity to herpes simplex virus-infected cells. J Pediatr. 1983;102:885–888. doi: 10.1016/s0022-3476(83)80019-5. [DOI] [PubMed] [Google Scholar]
- 74.Jenkins M, Mills J, Kohl S. Natural killer cytotoxicity and antibody-dependent cellular cytotoxicity of human immunodeficiency virus-infected cells by leukocytes from human neonates and adults. Pediatr Res. 1993;33:469–474. doi: 10.1203/00006450-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Landers DV, Smith JP, Walker CK, Milam T, Sanchez-Pescador L, Kohl S. Human fetal antibody-dependent cellular cytotoxicity to herpes simplex virus-infected cells. Pediatr Res. 1994;35:289–292. doi: 10.1203/00006450-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 76.Ernst LK, Metes D, Herberman RB, Morel PA. Allelic polymorphisms in the FcgammaRIIC gene can influence its function on normal human natural killer cells. J Mol Med (Berl) 2002;80:248–257. doi: 10.1007/s00109-001-0294-2. [DOI] [PubMed] [Google Scholar]
- 77.Morel PA, Ernst LK, Metes D. Functional CD32 molecules on human NK cells. Leuk Lymphoma. 1999;35:47–56. doi: 10.3109/10428199909145704. [DOI] [PubMed] [Google Scholar]
- 78.Dutertre CA, Bonnin-Gelize E, Pulford K, Bourel D, Fridman WH, Teillaud JL. A novel subset of NK cells expressing high levels of inhibitory FcgammaRIIB modulating antibody-dependent function. J Leukoc Biol. 2008;84:1511–1520. doi: 10.1189/jlb.0608343. [DOI] [PubMed] [Google Scholar]
- 79.Hussell T, Openshaw PJ. Intracellular IFN-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J Gen Virol. 1998;79 (Pt 11):2593–2601. doi: 10.1099/0022-1317-79-11-2593. [DOI] [PubMed] [Google Scholar]
- 80.Li F, Zhu H, Sun R, Wei H, Tian Z. Natural killer cells are involved in acute lung immune injury caused by respiratory syncytial virus infection. J Virol. 2012;86:2251–2258. doi: 10.1128/JVI.06209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scott R, de Landazuri MO, Gardner PS, Owen JJ. Human antibody-dependent cell-mediated cytotoxicity against target cells infected with respiratory syncytial virus. Clin Exp Immunol. 1977;28:19–26. [PMC free article] [PubMed] [Google Scholar]
- 82.Okabe N, Hashimoto G, Abo T, Wright PF, Karzon DT. Characterization of the human peripheral blood effector cells mediating antibody-dependent cell-mediated cytotoxicity against respiratory syncytial virus. Clin Immunol Immunopathol. 1983;27:200–209. doi: 10.1016/0090-1229(83)90070-3. [DOI] [PubMed] [Google Scholar]
- 83.Welliver TP, Garofalo RP, Hosakote Y, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tripp RA, Moore D, Barskey At, et al. Peripheral blood mononuclear cells from infants hospitalized because of respiratory syncytial virus infection express T helper-1 and T helper-2 cytokines and CC chemokine messenger RNA. J Infect Dis. 2002;185:1388–1394. doi: 10.1086/340505. [DOI] [PubMed] [Google Scholar]
- 85.Perussia B, Dayton ET, Lazarus R, Fanning V, Trinchieri G. Immune interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983;158:1092–1113. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Selvaraj P, Fifadara N, Nagarajan S, Cimino A, Wang G. Functional regulation of human neutrophil Fc gamma receptors. Immunol Res. 2004;29:219–230. doi: 10.1385/IR:29:1-3:219. [DOI] [PubMed] [Google Scholar]
- 87.Nagarajan S, Fifadara NH, Selvaraj P. Signal-specific activation and regulation of human neutrophil Fc gamma receptors. J Immunol. 2005;174:5423–5432. doi: 10.4049/jimmunol.174.9.5423. [DOI] [PubMed] [Google Scholar]
- 88.Huizinga TW, Roos D, von dem Borne AE. Neutrophil Fc-gamma receptors: a two-way bridge in the immune system. Blood. 1990;75:1211–1214. [PubMed] [Google Scholar]
- 89.Coxon A, Cullere X, Knight S, et al. Fc gamma RIII mediates neutrophil recruitment to immune complexes. a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001;14:693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 90.Marois L, Pare G, Vaillancourt M, Rollet-Labelle E, Naccache PH. Fc gammaRIIIb triggers raft-dependent calcium influx in IgG-mediated responses in human neutrophils. J Biol Chem. 2011;286:3509–3519. doi: 10.1074/jbc.M110.169516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lukens MV, van de Pol AC, Coenjaerts FE, et al. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol. 2010;84:2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaul TN, Faden H, Ogra PL. Effect of respiratory syncytial virus and virus-antibody complexes on the oxidative metabolism of human neutrophils. Infect Immun. 1981;32:649–654. doi: 10.1128/iai.32.2.649-654.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Faden H, Kaul TN, Ogra PL. Activation of oxidative and arachidonic acid metabolism in neutrophils by respiratory syncytial virus antibody complexes: possible role in disease. J Infect Dis. 1983;148:110–116. doi: 10.1093/infdis/148.1.110. [DOI] [PubMed] [Google Scholar]
- 95.Arnold R, Werner F, Humbert B, Werchau H, Konig W. Effect of respiratory syncytial virus-antibody complexes on cytokine (IL-8, IL-6, TNF-alpha) release and respiratory burst in human granulocytes. Immunology. 1994;82:184–191. [PMC free article] [PubMed] [Google Scholar]
- 96.Malbec O, Daeron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol Rev. 2007;217:206–221. doi: 10.1111/j.1600-065X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 97.Okayama Y, Kirshenbaum AS, Metcalfe DD. Expression of a functional high-affinity IgG receptor, Fc gamma RI, on human mast cells: Up-regulation by IFN-gamma. J Immunol. 2000;164:4332–4339. doi: 10.4049/jimmunol.164.8.4332. [DOI] [PubMed] [Google Scholar]
- 98.Woolhiser MR, Okayama Y, Gilfillan AM, Metcalfe DD. IgG-dependent activation of human mast cells following up-regulation of FcgammaRI by IFN-gamma. Eur J Immunol. 2001;31:3298–3307. doi: 10.1002/1521-4141(200111)31:11<3298::AID-IMMU3298>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 99.Woolhiser MR, Brockow K, Metcalfe DD. Activation of human mast cells by aggregated IgG through FcgammaRI: additive effects of C3a. Clin Immunol. 2004;110:172–180. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 100.Hazenbos WL, Gessner JE, Hofhuis FM, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 101.Okayama Y, Hagaman DD, Woolhiser M, Metcalfe DD. Further characterization of FcgammaRII and FcgammaRIII expression by cultured human mast cells. Int Arch Allergy Immunol. 2001;124:155–157. doi: 10.1159/000053696. 53696. [DOI] [PubMed] [Google Scholar]
- 102.Malbec O, Attal JP, Fridman WH, Daeron M. Negative regulation of mast cell proliferation by FcgammaRIIB. Mol Immunol. 2002;38:1295–1299. doi: 10.1016/s0161-5890(02)00078-0. [DOI] [PubMed] [Google Scholar]
- 103.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 104.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 105.Burke SM, Issekutz TB, Mohan K, Lee PW, Shmulevitz M, Marshall JS. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467–5476. doi: 10.1182/blood-2007-10-118547. [DOI] [PubMed] [Google Scholar]
- 106.King CA, Anderson R, Marshall JS. Dengue virus selectively induces human mast cell chemokine production. J Virol. 2002;76:8408–8419. doi: 10.1128/JVI.76.16.8408-8419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brown MG, King CA, Sherren C, Marshall JS, Anderson R. A dominant role for FcgammaRII in antibody-enhanced dengue virus infection of human mast cells and associated CCL5 release. J Leukoc Biol. 2006;80:1242–1250. doi: 10.1189/jlb.0805441. [DOI] [PubMed] [Google Scholar]
- 108.Brown MG, McAlpine SM, Huang YY, et al. RNA sensors enable human mast cell antiviral chemokine production and IFN-mediated protection in response to antibody-enhanced dengue virus infection. PLoS One. 2012;7:e34055. doi: 10.1371/journal.pone.0034055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Everard ML, Fox G, Walls AF, et al. Tryptase and IgE concentrations in the respiratory tract of infants with acute bronchiolitis. Arch Dis Child. 1995;72:64–69. doi: 10.1136/adc.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Al-Afif A. Human mast cell responses to respiratory syncytial virus. Halifax: Novia Scotia; 2011. [Google Scholar]
- 111.Graziano RF, Looney RJ, Shen L, Fanger MW. Fc gamma R-mediated killing by eosinophils. J Immunol. 1989;142:230–235. [PubMed] [Google Scholar]
- 112.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999;190:1465–1478. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosenberg HF, Dyer KD, Domachowske JB. Eosinophils and their interactions with respiratory virus pathogens. Immunol Res. 2009;43:128–137. doi: 10.1007/s12026-008-8058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Erbe DV, Pfefferkorn ER, Fanger MW. Functions of the various IgG Fc receptors in mediating killing of Toxoplasma gondii. J Immunol. 1991;146:3145–3151. [PubMed] [Google Scholar]
- 115.Ikeda Y, Mita H, Kudo M, Hasegawa M, Akiyama K. Degranulation of eosinophils by IgG antibody to Candida antigen. Arerugi. 1999;48:546–553. [PubMed] [Google Scholar]
- 116.Kaneko M, Swanson MC, Gleich GJ, Kita H. Allergen-specific IgG1 and IgG3 through Fc gamma RII induce eosinophil degranulation. J Clin Invest. 1995;95:2813–2821. doi: 10.1172/JCI117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim JT, Schimming AW, Kita H. Ligation of Fc gamma RII (CD32) pivotally regulates survival of human eosinophils. J Immunol. 1999;162:4253–4259. [PubMed] [Google Scholar]
- 118.Hartnell A, Kay AB, Wardlaw AJ. IFN-gamma induces expression of Fc gamma RIII (CD16) on human eosinophils. J Immunol. 1992;148:1471–1478. [PubMed] [Google Scholar]
- 119.Zhu X, Hamann KJ, Munoz NM, et al. Intracellular expression of Fc gamma RIII (CD16) and its mobilization by chemoattractants in human eosinophils. J Immunol. 1998;161:2574–2579. [PubMed] [Google Scholar]
- 120.Okamoto N, Ikeda M, Okuda M, et al. Increased eosinophilic cationic protein in nasal fluid in hospitalized wheezy infants with RSV infection. Allergol Int. 2011;60:467–472. doi: 10.2332/allergolint.10-OA-0263. [DOI] [PubMed] [Google Scholar]
- 121.Dyer KD, Percopo CM, Fischer ER, Gabryszewski SJ, Rosenberg HF. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood. 2009;114:2649–2656. doi: 10.1182/blood-2009-01-199497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Soukup JM, Becker S. Role of monocytes and eosinophils in human respiratory syncytial virus infection in vitro. Clin Immunol. 2003;107:178–185. doi: 10.1016/s1521-6616(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 123.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- 124.Pifferi M, Ragazzo V, Caramella D, Baldini G. Eosinophil cationic protein in infants with respiratory syncytial virus bronchiolitis: predictive value for subsequent development of persistent wheezing. Pediatr Pulmonol. 2001;31:419–424. doi: 10.1002/ppul.1069. [DOI] [PubMed] [Google Scholar]
- 125.Johnson TR, Graham BS. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23:S46–57. doi: 10.1097/01.inf.0000108192.94692.d2. [DOI] [PubMed] [Google Scholar]
- 126.Rosenberg HF, Dyer KD, Domachowske JB. Respiratory viruses and eosinophils: exploring the connections. Antiviral Res. 2009;83:1–9. doi: 10.1016/j.antiviral.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anselmino LM, Perussia B, Thomas LL. Human basophils selectively express the Fc gamma RII (CDw32) subtype of IgG receptor. J Allergy Clin Immunol. 1989;84:907–914. doi: 10.1016/0091-6749(89)90388-6. [DOI] [PubMed] [Google Scholar]
- 128.Takahashi K, Takada M. Detection of IgG receptor subtype on basophils using two-color FCM. Nihon Rinsho. 1992;50:2455–2459. [PubMed] [Google Scholar]
- 129.Cady CT, Powell MS, Harbeck RJ, et al. IgG antibodies produced during subcutaneous allergen immunotherapy mediate inhibition of basophil activation via a mechanism involving both FcgammaRIIA and FcgammaRIIB. Immunol Lett. 2010;130:57–65. doi: 10.1016/j.imlet.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cassard L, Jonsson F, Arnaud S, Daeron M. Fcgamma receptors inhibit mouse and human basophil activation. J Immunol. 2012;189:2995–3006. doi: 10.4049/jimmunol.1200968. [DOI] [PubMed] [Google Scholar]
- 131.Moore ML, Newcomb DC, Parekh VV, et al. STAT1 negatively regulates lung basophil IL-4 expression induced by respiratory syncytial virus infection. J Immunol. 2009;183:2016–2026. doi: 10.4049/jimmunol.0803167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Browne EP. Regulation of B-cell responses by Toll-like receptors. Immunology. 2012;136:370–379. doi: 10.1111/j.1365-2567.2012.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 135.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 136.Capolunghi F, Cascioli S, Giorda E, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 137.Avrameas S. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol Today. 1991;12:154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- 138.Thornton BP, Vetvicka V, Ross GD. Natural antibody and complement-mediated antigen processing and presentation by B lymphocytes. J Immunol. 1994;152:1727–1737. [PubMed] [Google Scholar]
- 139.Ochsenbein AF, Fehr T, Lutz C, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 140.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 142.Raes M, Peeters V, Alliet P, et al. Peripheral blood T and B lymphocyte subpopulations in infants with acute respiratory syncytial virus brochiolitis. Pediatr Allergy Immunol. 1997;8:97–102. doi: 10.1111/j.1399-3038.1997.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 143.Reed JL, Welliver TP, Sims GP, et al. Innate immune signals modulate antiviral and polyreactive antibody responses during severe respiratory syncytial virus infection. J Infect Dis. 2009;199:1128–1138. doi: 10.1086/597386. [DOI] [PubMed] [Google Scholar]
- 144.Williams JV, Weitkamp JH, Blum DL, LaFleur BJ, Crowe JE., Jr The human neonatal B cell response to respiratory syncytial virus uses a biased antibody variable gene repertoire that lacks somatic mutations. Mol Immunol. 2009;47:407–414. doi: 10.1016/j.molimm.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ricklin D, Reis ES, Lambris JD. A sweet spot to control complement-induced inflammation. Nat Med. 2012;18:1340–1341. doi: 10.1038/nm.2916. [DOI] [PubMed] [Google Scholar]
- 147.Karsten CM, Pandey MK, Figge J, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 2012;18:1401–1406. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smith TF, McIntosh K, Fishaut M, Henson PM. Activation of complement by cells infected with respiratory syncytial virus. Infect Immun. 1981;33:43–48. doi: 10.1128/iai.33.1.43-48.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Melendi GA, Hoffman SJ, Karron RA, et al. C5 modulates airway hyperreactivity and pulmonary eosinophilia during enhanced respiratory syncytial virus disease by decreasing C3a receptor expression. J Virol. 2007;81:991–999. doi: 10.1128/JVI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bera MM, Lu B, Martin TR, et al. Th17 cytokines are critical for respiratory syncytial virus-associated airway hyperreponsiveness through regulation by complement C3a and tachykinins. J Immunol. 2011;187:4245–4255. doi: 10.4049/jimmunol.1101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brand HK, Ferwerda G, Preijers F, et al. CD4+ T-cell counts and interleukin-8 and CCL-5 plasma concentrations discriminate disease severity in children with RSV infection. Pediatr Res. 2013;73:187–193. doi: 10.1038/pr.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]