Abstract
Recently, it has been demonstrated that the opportunistic fungal pathogen Cryptococcus neoformans can synthesize authentic immunomodulatory prostaglandins. The mechanism by which this takes place is unclear since there is no cyclooxygenase homolog in the cryptococcal genome. In this study, we show that cryptococcal production of both PGE2 and PGF2α can be chemically inhibited by caffeic acid, resveratrol and nordihydroguaiaretic acid. These polyphenolic molecules are frequently used as inhibitors of lipoxygenase enzymes; however, BLAST searches of the cryptococcal genome were unable to identify any homologs of mammalian, plant or fungal lipoxygenases. Next we investigated cryptococcal laccase, an enzyme known to bind polyphenols, and found that either antibody depletion or genetic deletion of the primary cryptococcal laccase (lac1Δ) resulted in a loss of cryptococcal prostaglandin production. To determine how laccase is involved, we tested recombinant laccase activity on the prostaglandin precursors, arachidonic acid (AA), PGG2 and PGH2. Using mass spectroscopy we determined that recombinant Lac1 does not modify AA or PGH2, but does have a marked activity toward PGG2 converting it to PGE2 and 15-keto-PGE2. This data demonstrates a critical role for laccase in cryptococcal prostaglandin production, and provides insight into a new and unique fungal prostaglandin pathway.
Keywords: oxylipin, fungi, polyphenol, eicosanoid, laccase
Introduction
The production of oxidized fatty acids (oxylipins) from single celled eukaryotes is an emerging area of research that has defined new mechanisms by which these organisms sense environment and community structure, signal morphological differentiation, and regulate virulence (Erb-Downward & Huffnagle, 2006, Noverr et al., 2003b, Shea & Del Poeta, 2006, Tsitsigiannis & Keller, 2007). Among these discoveries is evidence that many fungi are capable of producing 20-carbon oxylipins known as prostaglandins (Noverr et al., 2002, Tsitsigiannis et al., 2005a). In particular, Cryptococcus neoformans and Candida albicans have been identified as capable of producing authentic prostaglandin-E2 (PGE2) (Erb-Downward & Huffnagle, 2007, Erb-Downward & Noverr, 2007), a potent signaling molecule that can regulate inflammation in higher eukaryotes (Harris et al., 2002).
Prostaglandins are 20-carbon fatty acid metabolites of arachidonic acid (AA). In higher eukaryotes, the synthesis of prostaglandins is dependent upon the action of cyclooxygenase enzymes which first generate the common precursor of prostaglandin synthesis, PGH2 (Smith et al., 2000). This precursor is then converted to the various prostaglandins by the action of prostaglandin synthases. The absolute dependence of prostaglandin synthesis in higher eukaryotes upon a cyclooxygenase has led to a biochemical paradox in that there is no cyclooxygenase homolog in either the C. neoformans or C. albicans genome (Erb-Downward & Huffnagle, 2007, Erb-Downward & Noverr, 2007, Tsitsigiannis et al., 2005b) and classical inhibitors of prostaglandin synthesis, which are known to broadly inhibit cyclooxygenases, do not inhibit prostaglandin synthesis (Erb-Downward & Huffnagle, 2007, Erb-Downward & Noverr, 2007). Recently, a family of fatty acid dioxygenase enzymes (PpoA, PpoB, and PpoC) which possess homology to the catalytic domains of mammalian cyclooxygenases were identified in Aspergillus nidulans and Aspergillus fumagatis (Tsitsigiannis et al., 2005b). Further analysis showed that the cultures produced prostaglandins (as detected by immunoassay) and ppo mutants produced less (Tsitsigiannis et al., 2005a). But these enzymes do not exist in the C. neoformans genome (Tsitsigiannis et al., 2005b). The current work details our investigation into the enzymes involved in cryptococcal prostaglandin synthesis, and the discovery that a cryptococcal laccase is required for prostaglandin synthesis in C. neoformans.
Results
Chemical inhibition of cryptococcal prostaglandin synthesis
To examine the effects of chemical inhibition on cryptococcal prostaglandin production we first established a lysate system by which multiple species of prostaglandins could be measured. Briefly, lysates were generated through mechanical disruption of stationary phase C. neoformans cells followed by a 2 hr. incubation with AA. Figure 1 demonstrates that multiple prostaglandin species can be efficiently generated using this method including PGF2α and PGE2. These also can be measured using a pan-specific prostaglandin immunoassay that detects multiple prostaglandin species (Fig. 1A). In this lysate system, proportionally more PGF2α is synthesized (Fig. 1C) than PGE2 (Fig. 1B). Furthermore, production of prostaglandins is an enzymatic process (i.e. heat denaturable), because boiling of the lysates prior to incubation with AA results in a significant reduction in prostaglandin production.
Figure 1. Prostaglandin production in cryptococcal lysates.
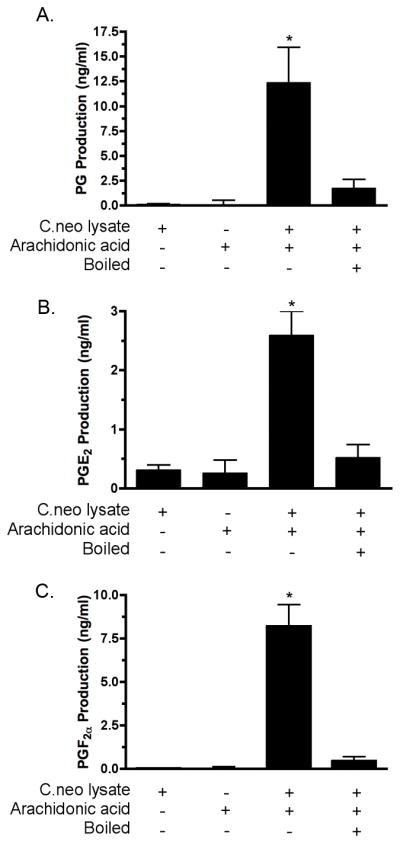
C. neoformans cells were lysed and the lysates incubated ± AA for 2 hr. at 37°C. C. neoformans lysates were incubated with AA and total prostaglandins were measured using a prostaglandin screening EIA (Panel A (n = 11; * = p < 0.001)); PGE2 were levels assayed using a PGE2 monoclonal EIA (Panel B (n = 7; * = p < 0.001)); and PGF2α levels were assayed using a PGF2α monoclonal EIA (Panel C (n = 5; * = p < 0.001)).
Since we have previously demonstrated that cyclooxygenase enzymes are not present in C. neoformans (Erb-Downward & Huffnagle, 2007), we investigated whether other classes of oxygenases could be involved by using the inhibitors resveratrol, caffeic acid and nordihydroguaiaretic acid (NDGA) to inhibit prostaglandin synthesis in cell-free lysates. Caffeic acid (Fig. 2B) and NDGA (Fig. 2A) are well characterized for their ability to inhibit lipoxygenase enzymes (Koshihara et al., 1984, Salari et al., 1984, Tanaka et al., 2003, Whitman et al., 2002) whereas resveratrol (Fig. 2A), which shares a similar structure, inhibits both cyclooxygenase and lipoxygenase enzymes (Kimura et al., 1985, Kimura et al., 1995, Tanaka et al., 2003, Pinto et al., 1999). All of the polyphenols tested significantly decreased the total prostaglandin production (Fig. 2). For resveratrol and NDGA, the inhibition was equivalent to boiling the lysates prior to the addition of AA. These findings indicate that polyphenols are capable of inhibiting cryptococcal prostaglandin production.
Figure 2. The effect of inhibitors on total prostaglandin production from C. neoformans lysates.
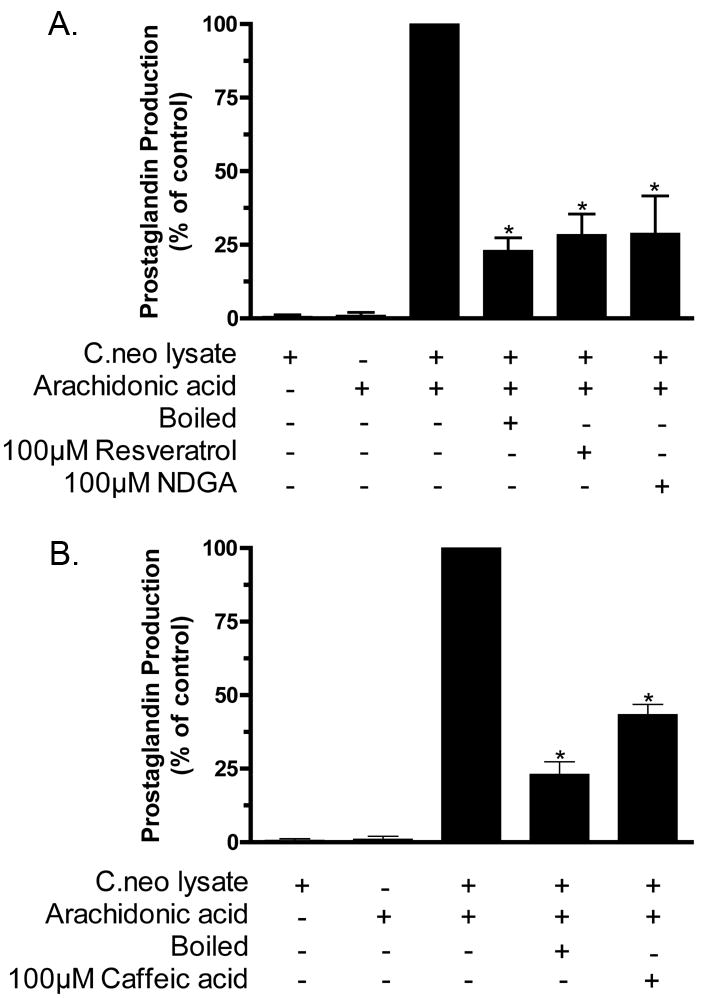
C. neoformans cells were lysed, the lysates incubated ± AA for 2 hr. at 37°C and total prostaglandin production was measured using a prostaglandin screening EIA. Panels A and B document the effect of various polyphenols on cryptococcal prostaglandin production. The results are normalized as a percent inhibition (where the lysate + AA was set to 100%). (Panel A n = 5, and Panel B n = 2 ; * = p <0.001).
BLAST searches of the cryptococcal genome for homologs of 5, 12, or 15-lipoxygenases from humans, mice, or rats did not reveal a lipoxygenase homolog (data not shown). Similarly, searches for homologs of plant lipoxygenases (1-LO from soy-beans, and 13-LO from Arabidopsis thaliana) did not yield any protein with significant homology. Even the 1-lipoxygenase found in the fungus Neurospora crassa does not have a homolog in C. neoformans (data not shown). Thus, we shifted our focus from lipoxygenases and cyclooxygenases to other enzymes known to interact with polyphenols (Li et al., 2005).
A role for laccase in cryptococcal prostaglandin production
Cryptococcal laccase (lac1) is a multi-copper oxido-reductase having a broad specificity that includes Fe (II), aminophenols, and polyphenols (Liu et al., 1999, Williamson, 1994). Additionally, we have previously noted that growth conditions that promote prostaglandin expression also result in increased levels of prostaglandin production (Noverr et al., 2001). To test whether this polyphenol-binding enzyme plays a role in cryptococcal prostaglandin synthesis, lysates were depleted of laccase prior to incubation with AA by the addition of a monoclonal antibody (G3P4D3) raised against the N-terminal portion of cryptococcal laccase. The laccase-depleted supernatants were then incubated with arachidonic acid, and prostaglandin levels were measured by EIA (Fig. 3A). Lysates treated with control antibody produced prostaglandins at the same levels as untreated lysates. However, immunoprecipitation of laccase from cryptococcal lysates significantly diminished the ability of the lysates to produce prostaglandins from arachidonic acid.
Figure 3. The effect of the removal of laccase on cryptococcal prostaglandin production.
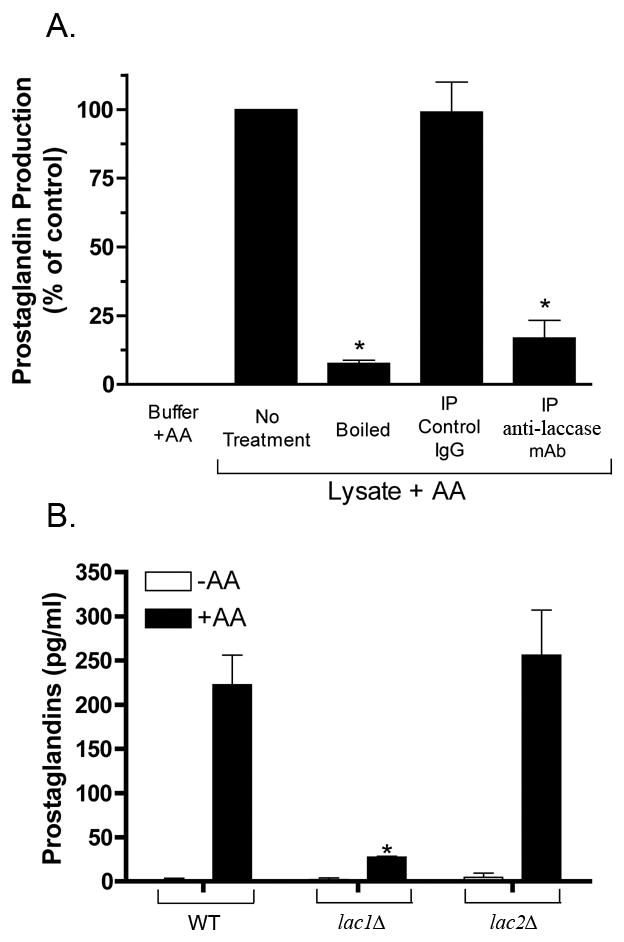
Panel A details the effect of the immunoprecipitation (IP) of laccase from cryptococcal lysates prior to the addition of AA. Inhibitory effect is displayed as a percent of control (the non-immunodepleted lysate + AA), which was set to 100 % (n = 4 ; * = p <0.001). Panel B demonstrates the effect of deleting the cryptococcal laccase genes on prostaglandin production into culture (when incubated with AA). Culture supernatants were taken from wild-type C. neoformans, lac1Δ, or lac2Δ strains that had been incubated for 2 hr. at 37°C in the presence of AA. Fungal prostaglandins were extracted from the culture supernatant into ethyl acetate and purified using RP-HPLC. Total prostaglandins were measured by EIA (n = 2; * = p <0.05).
Recently, a second laccase gene (LAC2) was identified that shares 65% identity with LAC1 at the nucleotide level (Zhu & Williamson, 2004). Sequence analysis suggests that the mAb directed against the protein product of LAC1 might also recognize the product of LAC2. To assess the relative contribution of each of these enzymes to cryptococcal prostaglandin production, LAC1 and LAC2 deletion strains were incubated in the presence of exogenous AA, the prostaglandins purified from the supernatants using organic extraction followed by RP-HPLC fractionation, and the levels assayed by EIA (Fig. 3B). The LAC2 deficient strain produced the same levels of prostaglandins as the wild-type strain upon the addition of AA. However, consistent with the lysate immunodepletion experiments, the LAC1 deficient strain of C. neoformans produced significantly lower levels of prostaglandins. Thus, loss of LAC1, but not LAC2, decreases cryptococcal prostaglandin production
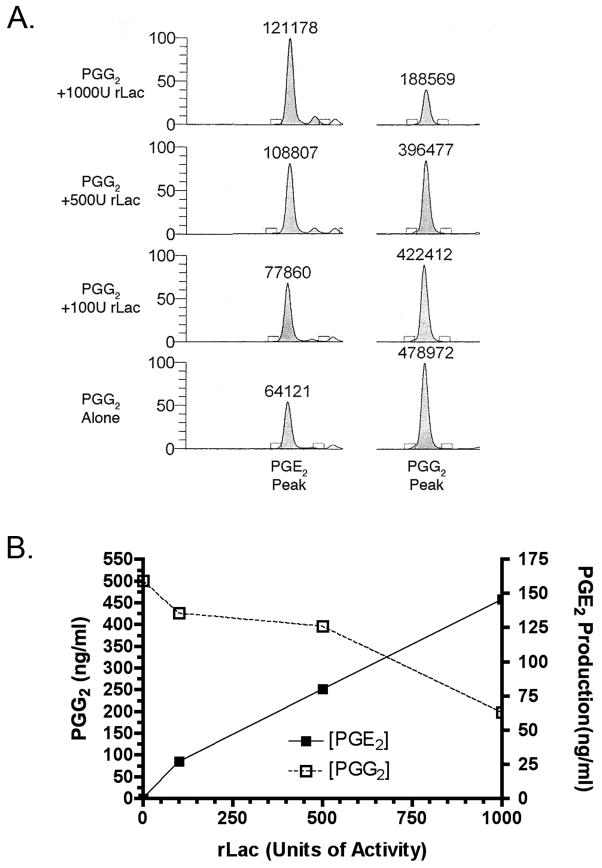
We next investigated how Lac1 is involved in prostaglandin synthesis. To address the question, recombinant Lac1 was tested for whether prostaglandins or intermediates in prostaglandin synthesis could be generated from AA, PGG2, or PGH2. If Lac1 functions as a cyclooxygenase, PGG2 and PGH2 should be generated from AA. Alternatively, if the activity of Lac1 is similar to a prostaglandin synthase, then Lac1 should convert PGH2 to individual prostaglandin species such as PGE2 or PGF2α. To this end, increasing concentrations of recombinant Lac1 (rLac) were incubated with 50μM of substrate (AA, PGG2 or PGH2) at 37°C for 3 hr. The products were then extracted into ethyl acetate, dried down and analyzed by tandem liquid chromatography-mass spectroscopy. Incubation of rLac with either AA or PGH2 alone did not result in the generation of new prostaglandins (data not shown). However, increasing amounts of rLac incubated with 50μM PGG2 resulted in a dose-dependent decrease in PGG2 and a concomitant increase in PGE2 (Fig. 4). Thus, Lac1 does not carry out the cyclooxygenase reaction or convert PGH2 to a prostaglandin like a prostaglandin synthase, but it does possess a unique activity catalyzing the conversion of PGG2 to PGE2.
Figure 4. The effect of recombinant Lac1 on PGG2 to PGE2 conversion.
Increasing concentrations of rLac1 were incubated with PGG2, and the products were separated and analyzed using LC-MS as described in Materials and Methods. Panel A is the extracted ion chromatograms of PGE2 (m/z range = 351–352 [M–H]− ion) and PGG2 (m/z range = 367–368 [M–H]− ion) with increasing concentrations of rLac1. The numbers above each peak denote the area under the peak. Panel B demonstrates the increase in PGE2 production over background levels and the decrease in PGG2.
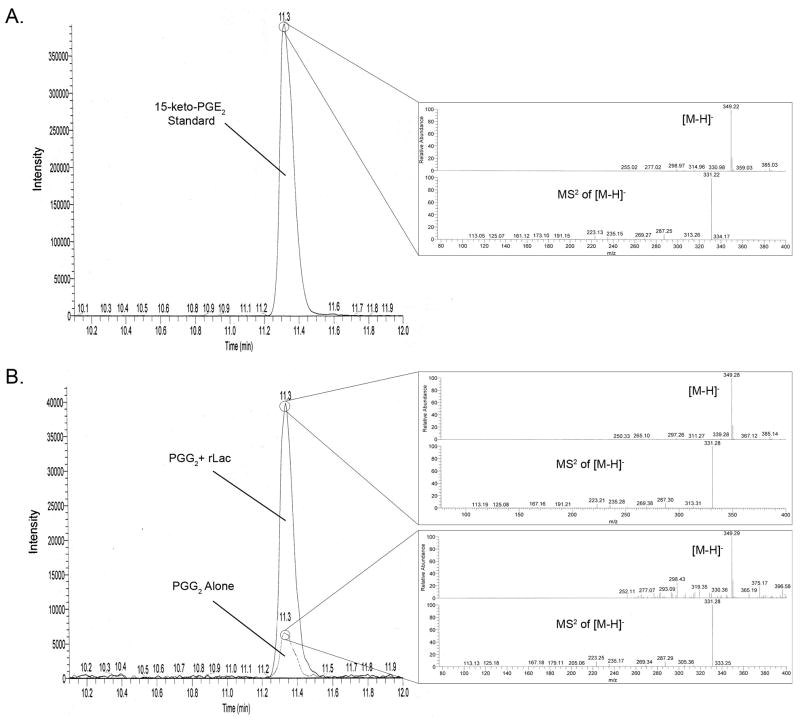
In addition to PGE2, several other peaks within the m/z range of 340–370 were notably different in the samples that contained PGG2 + rLac when compared to those that contained PGG2 alone. The most significant of these occurred with an elution time of 11.3 min. and possessed a m/z = 349 [M–H]−. Using R-package XCMS (Smith et al., 2006) and the METLIN database (http://metlin.scripps.edu/) we were able to narrow down the possible related compounds to PGE3, 15-keto-PGE2 and PGK2. Commercial standards of these compounds were obtained and assessed by LC-MS. Based on retention times, MS, and MS2 this peak was identified as the PGE2 metabolite 15-keto-PGE2 (Fig. 5). Thus, while Lac1 did not form the endocyclic peroxide that a cyclooxygenase generates, Lac1 carries out the unique conversion of PGG2 to PGE2 and 15-keto-PGE2 which clearly distinguishes it from a prostaglandin synthase.
Figure 5. The effect of recombinant Lac1 on 15-keto-PGE2 levels.
Chromatograms of samples containing 15-keto-PGE2 standard (Panel A) or PGG2 ± 1000 U recombinant Lac1 (Panel B) focusing on the m/z range = 349.1–349.5. Panel insets indicate the parent [M–H]− ion mass and MS2 of this ion.
Discussion
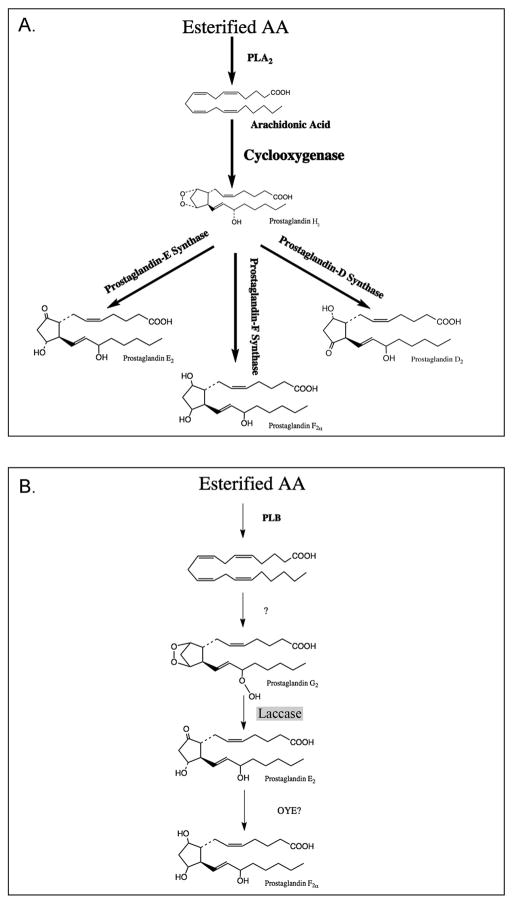
Cryptococcus neoformans can synthesize authentic PGE2 that is identical to mammalian PGE2; however, this process proceeds via unique enzymatic pathways which do not involve a cyclooxygenase enzyme (Erb-Downward & Huffnagle, 2007). We have identified the C. neoformans laccase Lac1 as a critical enzyme in this new pathway based on evidence that either the depletion or disruption of Lac1 all but eliminates specific cryptococcal prostaglandin production, and that the recombinant enzyme can convert PGG2 to PGE2 and 15-keto-PGE2. In addition to Lac1, these studies demonstrate that there are likely at least two other enzymes in the cryptococcal prostaglandin pathway: one upstream of Lac1 activities and the other downstream. The evidence for the existence of the upstream enzyme is that cryptococcal lysates can produce prostaglandins from exogenous AA, but Lac1 can only produce prostaglandins from PGG2, an oxidized product of AA. Similarly, the lysate data indicates that in addition to PGE2, cryptococcal lysates supplemented with exogenous AA synthesize PGF2α, but if Lac1 is removed from the system the enzymatic production of PGE2 and PGF2α ceases. However, Lac1 does not synthesize PGF2α, indicating that another enzyme must act on the products of Lac1 to synthesize PGF2α. Together, these data indicate that the cryptococcal prostaglandin pathway is unique not only in the enzymes involved, but also the structure of the pathway, which the evidence suggests is linear. This clearly differentiates it from the common precursor model that is seen in cyclooxygenase-dependent prostaglandin production (Fig. 6).
Figure 6. The common precursor vs. a linear model of cryptococcal prostaglandin synthesis.
Panel A depicts the common precursor model of prostaglandin synthesis where cyclooxygenase enzymes act on free arachidonic acid to produce PGH2, which then serves as the common substrate for the individual prostaglandin synthases. Panel B depicts the proposed linear model of cryptococcal prostaglandin synthesis, where each step is dependent upon the product of the step before. Dashed arrows indicate other possible synthetic routes.
The demonstration that cryptococcal laccase is critical to the production of cryptococcal prostaglandins provides the first association between a laccase and prostaglandin synthesis. Classically, laccases are thought of as enzymes involved in the oxidation of polyphenols or the degradation of plant lignin (Leonowicz et al., 2001). In C. neoformans, laccase catalyzes the formation of a polymer melanin from 2,4- or 3,5-dihydroxy polyphenolic precursors. Melanin, in turn, enhances the ability of the organism to survive the oxidative burst within a macrophage and ultraviolet radiation induced damage out in the environment (Gomez & Nosanchuk, 2003). However, recent lines of study have begun to explore the action of this class of enzymes on non-polyphenolic molecules (Casa et al., 2003). In one report investigators studied the ability of a mushroom laccase to degrade olive oil mill waste effluents, a mixture of polyphenols, fatty acids and other compounds. They found that in addition to polyphenols, fatty acids were also oxidized by this enzyme (Karlsson et al., 2001). Further study revealed that the oxidation of long-chain poly-unsaturated fatty acids by the mushroom laccase was specific and proceeded via a mechanism that was remarkably similar to that of a lipoxygenase (Zhang et al., 2002). A recent study comparing enzymes from different organisms that, based on DNA sequence homology, are considered laccases, found that many members of the family do not possess the ability to degrade lignin or oxidize polyphenols (Valderrama et al., 2003). This suggests that alternative activities for this enzyme might be more common than was previously believed. Our studies provide additional support for the activity of laccase enzymes beyond the oxidation of polyphenolic compounds and lignin degradation: the generation of oxylipins from polyunsaturated fatty acids.
In recent years much has been discovered about the critical importance of lipid mediators in single celled eukaryotic pathogens. These effects include the stimulation or inhibition of sexual development (depending on the mediator), quorum sensing, and the regulation of virulence (Erb-Downward & Noverr, 2007, Noverr & Huffnagle, 2004, Tsitsigiannis et al., 2005a, Tsitsigiannis & Keller, 2006, Tsitsigiannis & Keller, 2007, Angeli et al., 2001). Some of the prostaglandin production in these organisms is attributable to the presence of a cyclooxygenase homolog (Tsitsigiannis et al., 2005a). However, there also exists a body of literature relating to prostaglandin production in non-vertebrate organisms that has attempted to fit, unsuccessfully, what is found in these organisms into model of cyclooxygenase dependent prostaglandin production (reviewed (Lamacka & Sajbidor, 1995, Noverr et al., 2003b)). Recent studies have examined Trypanosoma brucei, Trypanosoma cruzii as well as Plasmodium falciparum, in the absence of a host, and found that they produce prostaglandins that are not inhibited by aspirin or indomethacin (Kubata et al., 2002, Kubata et al., 2000, Kilunga Kubata et al., 1998). This production also occurs in the absence of an identifiable cyclooxygenase enzyme. In these organisms, a PGF2α− synthase has been identified which is homologous to the Old Yellow enzyme (OYE) found in Saccharomyces cerevisiae (Kubata et al., 2002, Kubata et al., 2000). It has also been reported that the parasite-derived prostaglandins of Schistosoma mansoni actively suppress the immune system to facilitate infection of the host (Herve et al., 2003). PGE2 is well known for its ability to suppress inflammation (Harris et al., 2002), and 15-keto-PGE2 has been recently identified as a ligand for PPARγ (Chou et al., 2007), a nuclear receptor involved in the regulation of macrophage activation. Thus, it is tempting to speculate that cryptococcal production of prostaglandins may help down-regulate local inflammatory responses. The recent finding that cryptococcus carries out its sexual cycle in association with plants (Xue et al., 2007) is another area where these oxylipins may be involved since oxylipin regulation of sexual cycles of other fungi has been well established (Tsitsigiannis & Keller, 2006, Tsitsigiannis & Keller, 2007, Tsitsigiannis et al., 2005b). To date only two enzymes involved in cryptococcal prostaglandin synthesis have been identified: (phopholipase B (PLB) (Noverr et al., 2003a, Cox et al., 2001) and now laccase (Lac1). Both are known virulence factors and the currently proposed mechanisms of virulence do not account for their role in prostaglandin synthesis. It is our belief that as additional enzymes involved in cryptococcal prostaglandin synthesis are discovered, the contribution of prostaglandins to the pathogenesis of C. neoformans will likely become clear.
Experimental Procedures
Strains and Culture Conditions
Throughout this study, C. neoformans strain 24067 was used except where C. neoformans deletion mutants were used. For these latter studies, the LAC1 deletion mutant (lac1 ), the LAC2 deletion mutant (lac2Δ), and the parent strain (H99) were utilized (Zhu & Williamson, 2004). All strains were grown shaking at ~37°C in 30 ml Sabouraud dextrose broth (1% Neopeptone and 2% dextrose; Difco, Detroit, Mich.) until stationary phase was reached (72 hr or until the cells reached a concentration > 2×108/ml).
C. neoformans Lysis Protocol
1.2×109 cells were transferred to a 50 ml conical tube, spun down at 3000 rpm, and washed 2× with 40 ml sterile saline. Next, 1.2 ml of cold phosphate buffer (pH 7.1) was added to the tube without disturbing the pellet. This was followed by the addition of 0.5μm glass beads until the beads just began to dry. The tube was then subjected to a vigorous regimen of vortexing 5× for 1 min. followed by 1 min. on ice. The liquid was then transferred to another tube, and the beads washed 2× with 600μl cold phosphate buffer, vortexing and transferring the liquid portion with each wash. This crude lysate was then spun down at 13,000 rpm for 45 min. to remove debris and unlysed cells. From these tubes the supernatant (lysate) was transferred to a fresh 15 ml conical and kept on ice.
Prostaglandin Production and Measurement
Prostaglandins were generated from cryptococcal lysates by incubating 150 μl of lysate in a 1.5 ml Eppendorf tube with 500 μM AA (Cayman Chemical) at 37°C for 2 hr. The requirement for functional enzymes was tested by boiling a portion of C. neoformans lysate for 10 min. followed by spinning down the precipitated proteins prior to the addition of AA. The lipoxygenase inhibitors caffeic acid and nordihydroguaiaretic acid (NDGA) (Cayman Chemical) and resveratrol (Sigma Chemical) were dissolved in DMSO. Inhibition studies were performed by adding inhibitor or an equivalent amount of DMSO to lysate samples just prior to incubation with AA.
Prostaglandins were generated in whole cell supernatants by incubating 1×107 cells in 1 ml phosphate buffer, pH 7.1, with 500 μM AA or control at 37°C for 2 hr. Following incubation, the cells were spun down and the supernatant removed and assayed for prostaglandin content.
Prostaglandins were measured utilizing PGE2 specific, PGF2α specific or the Prostaglandin Screening enzyme immunoassay (EIA) (Cayman Chemical) according to manufacturer’s protocols. EIA plates were read at 405 nm, and the concentrations of prostaglandin were calculated based on a bound vs. free (B/B0) measurement. Background values in the absence of lysate were subtracted from each corresponding sample condition to obtain a specific measurement of prostaglandin production due to the conversion of AA.
Organic Extraction and Reverse-Phase HPLC
To assay the organic compounds produced in cryptococcal supernatants, samples were transferred to a silianized 12 × 75 cm glass test tube, the organic components extracted twice into 500 μl HPLC grade ethyl acetate (EtOAc) (Aldrich Chemical), and the organic phase transferred to a silianized conical screw-top vial. The contents of the vial were dried down in a water bath under a stream of grade 5 nitrogen.
Reverse-phase high performance liquid chromatography (RP-HPLC) analysis was carried out using a Waters 600 HPLC system, with a 5 μl sample loop and a Waters Symmetry 2.1×150 mm analytical column. The compounds were separated using a gradient elution starting at 75:25:0.1 (water:acetonitrile:acetic acid) for 10 min. followed by a linear shift to 0:100:0.1 (water:acetonitrile:acetic acid) over 75 min. The dry sample was brought up in 25 μl HPLC grade EtOH, and 12.5 μl was (5 μl) injected. The remainder was dried down under nitrogen and stored at −20°C for not longer than 1 week. Fractions were collected, and the organic compounds were once again extracted twice into 500 μl EtOAc, followed by drying down under nitrogen. These samples were then brought up in 150 μl prostaglandin EIA buffer (Cayman Chemical) and assayed according to manufacturer’s protocols as above.
The elution times of cyclooxygenase-derived prostaglandin standards (Cayman Chemical) were obtained using the same elution gradient.
Purified Enzyme Studies
The recombinant Lac1 used in these studies was generated as previously described (Zhu et al., 2001). Lyophilized recombinant Lac1 (rLac) was reconstituted in 50 mM phosphate buffer pH 6.5. Enzyme activity was determined by adding 1 μl of rLac to 1 ml of 1 mM epinephrine bitartrate for 30 min. at 37°C and measuring the absorbance at 475 nm. One unit of enzyme activity was equal to change of 0.001 AU. To test rLac activity on prostaglandin relevant substrates 100, 500 or 1000U of rLac was added to 50 μM AA or PGG2 in a 30 μl reaction volume for 3 hr. at 37°C. Reactions were stopped by the addition of 150μl EtOAc, the organic phase was removed and dried down under N2.
LC-MS/MS was carried out using a ThermoFinnigan Surveyor HPLC (San Jose, CA) interfaced directly to the electrospray ionization source of a ThermoFinnigan LTQ linear ion-trap mass spectrometer (San Jose, CA). Samples were resuspended in a methanol:water solution (1:1, v/v) and 20 μL aliquots were injected onto a Phenomenex Luna 2.00 × 150mm 3μm C-18(2) column (Torrence, CA). Mobile phase solvents were water + 0.1% acetic acid (A) and acetonitrile + 0.1% acetic acid (B). Compounds were separated and eluted from the analytical column with a linear gradient of 25% to 100% B over 40 min. at a flow rate of 0.3 mL/min. The sample tray was cooled to 4 °C throughout the analysis. Concentrations of compounds were calculated by taking the ratio of area of peak to the area of the internal standard peak (15-deoxy-Δ12,14–PGJ2) and multiplying by the response factor. The response factor was calculated as a ratio of the peak areas of known concentrations of commercial standards of PGG2, 15- keto-PGE2, and PGE2 to the peak area of a 1μg/ml 15-deoxyΔ12,14-PGJ2.
Immunoprecipitation
Immunoprecipitation of laccase from cryptococcal lysates was carried out by incubating 600 μl lysates on ice with 15 μg/ml anti-laccase mouse monoclonal antibody (clone G3P4D3) raised against full length recombinant laccase enzyme or control mouse IgG, for 2 hr. on ice prior to the addition of 50 μl of protein-A beads (Sigma). Tubes were set to rock overnight at 4°C and the following day, beads were spun down at 13,000 rpm for 1 min. and the supernatant transferred to a fresh tube. This depleted lysate was then treated as above to generate prostaglandins.
Statistical Analysis
Statistical analysis of results was performed using Prism 4.0 (GraphPad Software, Inc.). Statistical significance was determined using either a One-Way ANOVA with Bonferroni’s Multiple Comparison post test. For the purposes of comparing the degree of inhibition of the various inhibitors, the data was represented as a % of control (Lysate + AA).
Acknowledgments
We wish to thank Dr. Arturo Casadevall of Albert Einstein College of Medicine, Yeshiva University for providing the anti-laccase mAb. We also want to thank the other members of the Huffnagle lab for their insight and evaluation and Dr. Galen Toews for his ever thought-provoking discussions. Also, we wish to thank Kate Noon at the University of Michigan Department of Pharmacology’s Biomedical Mass Spectrometry Facility for all her help and mass spectrometry expertise. This work was supported by funding from NIAID Grant 1R01AI059201 and NIH-NHLBI T32 HL007749-11.
Abbreviations used
- PG
prostaglandin
- AA
arachidonic acid
- RP-HPLC
reverse-phase high performance liquid chromatography
- EtOAc
ethyl acetate
- rLac
recombinant laccase (Lac1)
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2α
- C.neo
C. neoformans
- NDGA
nordihydroguaiaretic acid
- PLB
phospholipase B
- OYE
Old Yellow Enzyme
References
- Angeli V, Faveeuw C, Roye O, Fontaine J, Teissier E, Capron A, Wolowczuk I, Capron M, Trottein F. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J Exp Med. 2001;193:1135–1147. doi: 10.1084/jem.193.10.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casa R, D'Annibale A, Pieruccetti F, Stazi SR, Giovannozzi Sermanni G, Lo Cascio B. Reduction of the phenolic components in olive-mill wastewater by an enzymatic treatment and its impact on durum wheat (Triticum durum Desf.) germinability. Chemosphere. 2003;50:959–966. doi: 10.1016/s0045-6535(02)00707-5. [DOI] [PubMed] [Google Scholar]
- Chou WL, Chuang LM, Chou CC, Wang AH, Lawson JA, FitzGerald GA, Chang ZF. Identification of a novel prostaglandin reductase reveals the involvement of prostaglandin E2 catabolism in regulation of peroxisome proliferator-activated receptor gamma activation. J Biol Chem. 2007;282:18162–18172. doi: 10.1074/jbc.M702289200. [DOI] [PubMed] [Google Scholar]
- Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC, Sorrell TC, Leidich SD, Casadevall A, Ghannoum MA, Perfect JR. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol. 2001;39:166–175. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- Erb-Downward JR, Huffnagle GB. Role of oxylipins and other lipid mediators in fungal pathogenesis. Future Microbiol. 2006;1:219–227. doi: 10.2217/17460913.1.2.219. [DOI] [PubMed] [Google Scholar]
- Erb-Downward JR, Huffnagle GB. Cryptococcus neoformans produces authentic prostaglandin E2 without a cyclooxygenase. Eukaryot Cell. 2007;6:346–350. doi: 10.1128/EC.00336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb-Downward JR, Noverr MC. Characterization of prostaglandin E2 production by Candida albicans. Infect Immun. 2007;75:3498–3505. doi: 10.1128/IAI.00232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez BL, Nosanchuk JD. Melanin and fungi. Curr Opin Infect Dis. 2003;16:91–96. doi: 10.1097/00001432-200304000-00005. [DOI] [PubMed] [Google Scholar]
- Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Herve M, Angeli V, Pinzar E, Wintjens R, Faveeuw C, Narumiya S, Capron A, Urade Y, Capron M, Riveau G, Trottein F. Pivotal roles of the parasite PGD2 synthase and of the host D prostanoid receptor 1 in schistosome immune evasion. Eur J Immunol. 2003;33:2764–2772. doi: 10.1002/eji.200324143. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Holmbom B, Spetz P, Mustranta A, Buchert J. Reactivity of Trametes laccases with fatty and resin acids. Appl Microbiol Biotechnol. 2001;55:317–320. doi: 10.1007/s002530000532. [DOI] [PubMed] [Google Scholar]
- Kilunga Kubata B, Eguchi N, Urade Y, Yamashita K, Mitamura T, Tai K, Hayaishi O, Horii T. Plasmodium falciparum produces prostaglandins that are pyrogenic, somnogenic, and immunosuppressive substances in humans. J Exp Med. 1998;188:1197–1202. doi: 10.1084/jem.188.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Okuda H, Arichi S. Effects of stilbenes on arachidonate metabolism in leukocytes. Biochim Biophys Acta. 1985;834:275–278. [PubMed] [Google Scholar]
- Kimura Y, Okuda H, Kubo M. Effects of stilbenes isolated from medicinal plants on arachidonate metabolism and degranulation in human polymorphonuclear leukocytes. J Ethnopharmacol. 1995;45:131–139. doi: 10.1016/0378-8741(94)01206-f. [DOI] [PubMed] [Google Scholar]
- Koshihara Y, Neichi T, Murota S, Lao A, Fujimoto Y, Tatsuno T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim Biophys Acta. 1984;792:92–97. [PubMed] [Google Scholar]
- Kubata BK, Duszenko M, Kabututu Z, Rawer M, Szallies A, Fujimori K, Inui T, Nozaki T, Yamashita K, Horii T, Urade Y, Hayaishi O. Identification of a novel prostaglandin (f2alpha) synthase in Trypanosoma brucei. J Exp Med. 2000;192:1327–1338. doi: 10.1084/jem.192.9.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubata BK, Kabututu Z, Nozaki T, Munday CJ, Fukuzumi S, Ohkubo K, Lazarus M, Maruyama T, Martin SK, Duszenko M, Urade Y. A key role for old yellow enzyme in the metabolism of drugs by Trypanosoma cruzi. J Exp Med. 2002;196:1241–1251. doi: 10.1084/jem.20020885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamacka M, Sajbidor J. The occurrence of prostaglandins and related compounds in lower organisms. Prostaglandins Leukot Essent Fatty Acids. 1995;52:357–364. doi: 10.1016/0952-3278(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Leonowicz A, Cho NS, Luterek J, Wilkolazka A, Wojtas-Wasilewska M, Matuszewska A, Hofrichter M, Wesenberg D, Rogalski J. Fungal laccase: properties and activity on lignin. J Basic Microbiol. 2001;41:185–227. doi: 10.1002/1521-4028(200107)41:3/4<185::aid-jobm185>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Li BH, Ma XF, Wang Y, Tian WX. Structure-activity relationship of polyphenols that inhibit Fatty Acid synthase. J Biochem (Tokyo) 2005;138:679–685. doi: 10.1093/jb/mvi171. [DOI] [PubMed] [Google Scholar]
- Liu L, Tewari RP, Williamson PR. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect Immun. 1999;67:6034–6039. doi: 10.1128/iai.67.11.6034-6039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Cox GM, Perfect JR, Huffnagle GB. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun. 2003a;71:1538–1547. doi: 10.1128/IAI.71.3.1538-1547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Erb-Downward JR, Huffnagle GB. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin Microbiol Rev. 2003b;16:517–533. doi: 10.1128/CMR.16.3.517-533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Huffnagle GB. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect Immun. 2004;72:6206–6210. doi: 10.1128/IAI.72.11.6206-6210.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69:2957–2963. doi: 10.1128/IAI.69.5.2957-2963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Toews GB, Huffnagle GB. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect Immun. 2002;70:400–402. doi: 10.1128/IAI.70.1.400-402.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto MC, Garcia-Barrado JA, Macias P. Resveratrol Is a Potent Inhibitor of the Dioxygenase Activity of Lipoxygenase. J Agric Food Chem. 1999;47:4842–4846. doi: 10.1021/jf990448n. [DOI] [PubMed] [Google Scholar]
- Salari H, Braquet P, Borgeat P. Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med. 1984;13:53–60. doi: 10.1016/0262-1746(84)90102-1. [DOI] [PubMed] [Google Scholar]
- Shea JM, Del Poeta M. Lipid signaling in pathogenic fungi. Curr Opin Microbiol. 2006;9:352–358. doi: 10.1016/j.mib.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Niiyama S, Sato S, Yamada A, Higashi H. Arachidonic acid metabolites contribute to the irreversible depolarization induced by in vitro ischemia. J Neurophysiol. 2003;90:3213–3223. doi: 10.1152/jn.00542.2003. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Bok JW, Andes D, Nielsen KF, Frisvad JC, Keller NP. Aspergillus cyclooxygenase-like enzymes are associated with prostaglandin production and virulence. Infect Immun. 2005a;73:4548–4559. doi: 10.1128/IAI.73.8.4548-4559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Keller NP. Oxylipins act as determinants of natural product biosynthesis and seed colonization in Aspergillus nidulans. Mol Microbiol. 2006;59:882–892. doi: 10.1111/j.1365-2958.2005.05000.x. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Keller NP. Oxylipins as developmental and host-fungal communication signals. Trends Microbiol. 2007;15:109–118. doi: 10.1016/j.tim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Kowieski TM, Zarnowski R, Keller NP. Three putative oxylipin biosynthetic genes integrate sexual and asexual development in Aspergillus nidulans. Microbiology. 2005b;151:1809–1821. doi: 10.1099/mic.0.27880-0. [DOI] [PubMed] [Google Scholar]
- Valderrama B, Oliver P, Medrano-Soto A, Vazquez-Duhalt R. Evolutionary and structural diversity of fungal laccases. Antonie Van Leeuwenhoek. 2003;84:289–299. doi: 10.1023/a:1026070122451. [DOI] [PubMed] [Google Scholar]
- Whitman S, Gezginci M, Timmermann BN, Holman TR. Structure-activity relationship studies of nordihydroguaiaretic acid inhibitors toward soybean, 12-human, and 15-human lipoxygenase. J Med Chem. 2002;45:2659–2661. doi: 10.1021/jm0201262. [DOI] [PubMed] [Google Scholar]
- Williamson PR. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol. 1994;176:656–664. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C, Tada Y, Dong X, Heitman J. The Human Fungal Pathogen Cryptococcus Can Complete Its Sexual Cycle during a Pathogenic Association with Plants. Cell Host and Microbe. 2007;1:263–273. doi: 10.1016/j.chom.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Zhang X, Eigendorf G, Stebbing DW, Mansfield SD, Saddler JN. Degradation of trilinolein by laccase enzymes. Arch Biochem Biophys. 2002;405:44–54. doi: 10.1016/s0003-9861(02)00331-4. [DOI] [PubMed] [Google Scholar]
- Zhu X, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun. 2001;69:5589–5596. doi: 10.1128/IAI.69.9.5589-5596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Williamson PR. Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res. 2004;5:1–10. doi: 10.1016/j.femsyr.2004.04.004. [DOI] [PubMed] [Google Scholar]