Abstract
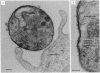
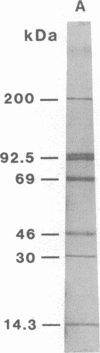
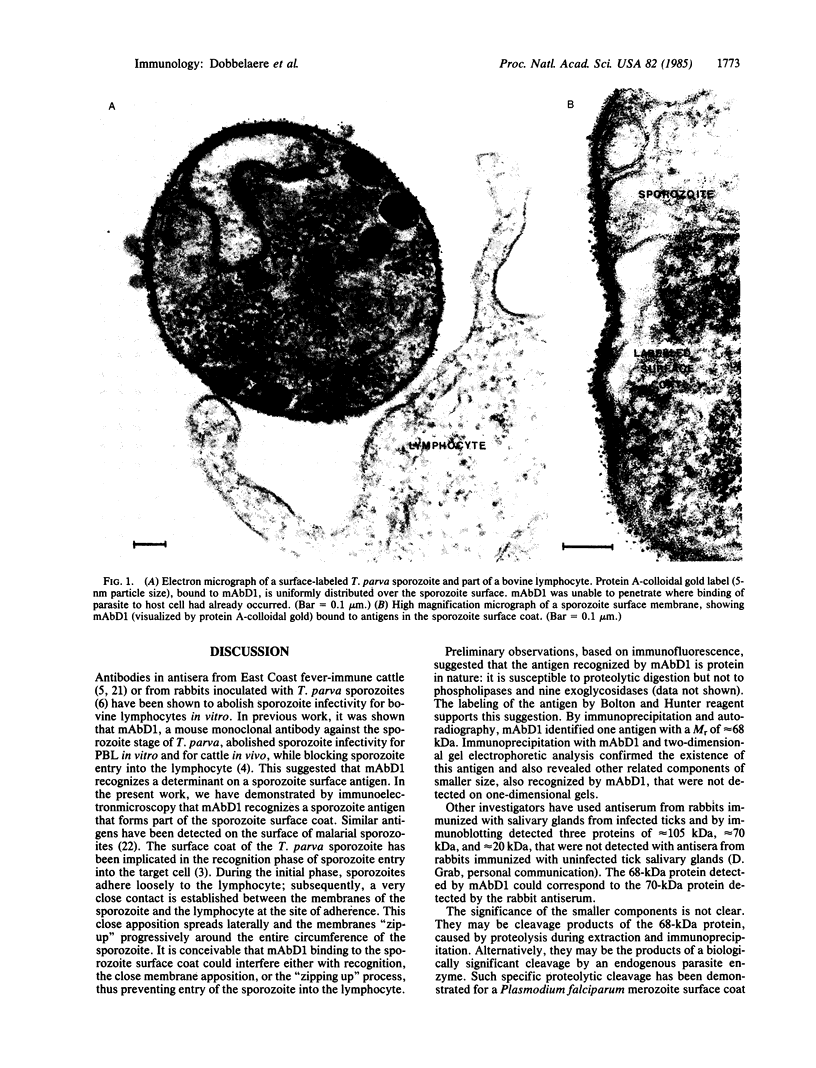
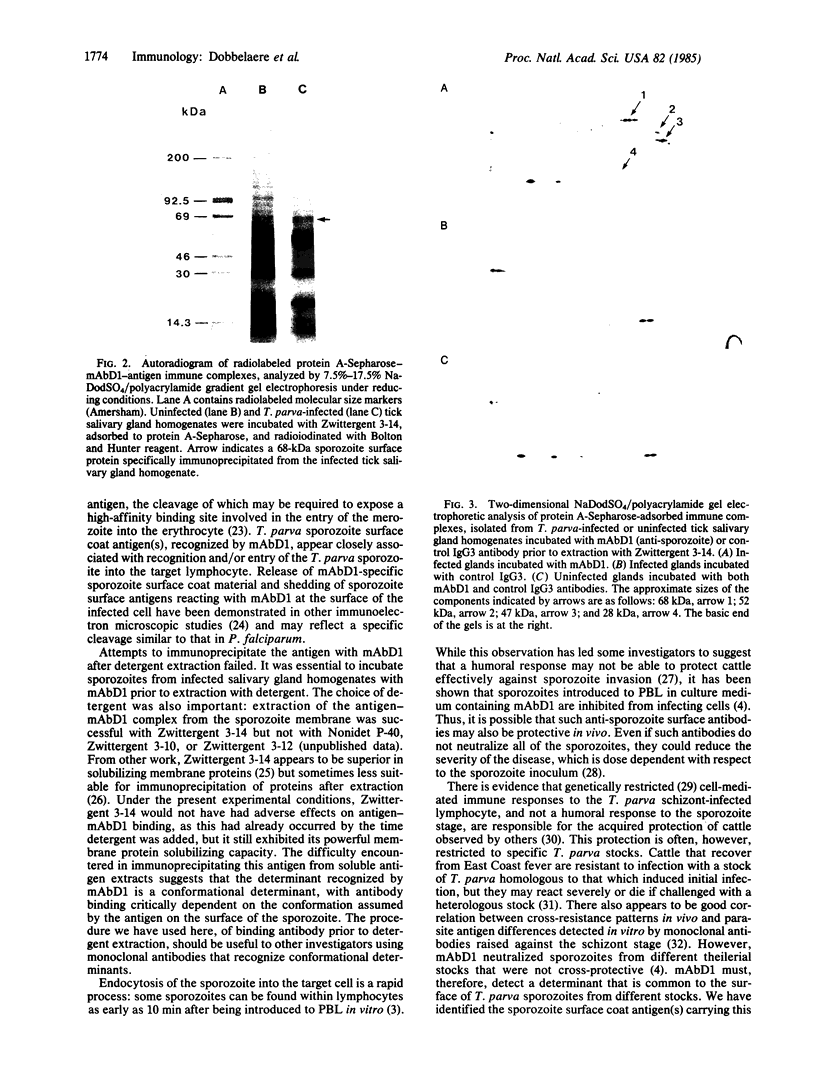
A mouse monoclonal antibody (mAbD1) that neutralizes sporozoites of different stocks of the protozoan parasite Theileria parva has been used to localize and identify a sporozoite antigen. Protein A-colloidal gold was used to localize bound mAbD1 in immunoelectron microscopic studies. mAbD1 bound to sporozoite antigen, which was evenly spread over the surface of all sporozoites. Immune complexes were obtained by incubation of sporozoite suspensions with mAbD1 followed by Zwittergent 3-14 extraction and precipitation with protein A-Sepharose. One- and two-dimensional NaDodSO4/polyacrylamide gel electrophoretic analyses were performed on these complexes, and a major protein with a molecular size of 68 kDa was identified. Other related components of 52 kDa, 47 kDa, and 28 kDa were also detected. Since antibody to this antigen(s) neutralizes T. parva sporozoites from different stocks, the results could be of relevance to the development of a broad spectrum vaccine against the cattle disease East Coast fever, which is caused by T. parva.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. P., Brown C. G., Burridge M. J., Purnell R. E. Cryopreservation of infective particles of Theileria parva. Int J Parasitol. 1973 Sep;3(5):583–587. doi: 10.1016/0020-7519(73)90082-9. [DOI] [PubMed] [Google Scholar]
- Dobbelaere D. A., Spooner P. R., Barry W. C., Irvin A. D. Monoclonal antibody neutralizes the sporozoite stage of different Theileria parva stocks. Parasite Immunol. 1984 Jul;6(4):361–370. doi: 10.1111/j.1365-3024.1984.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Dolan T. T., Young A. S., Losos G. J., McMilian I., Minder C. E., Soulsby K. Dose dependent responses of cattle to Theileria parva stabilate. Int J Parasitol. 1984 Feb;14(1):89–95. doi: 10.1016/0020-7519(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Emery D. L., Eugui E. M., Nelson R. T., Tenywa T. Cell-mediated immune responses to Theileria parva (East Coast fever) during immunization and lethal infections in cattle. Immunology. 1981 Jun;43(2):323–336. [PMC free article] [PubMed] [Google Scholar]
- Eugui E. M., Emery D. L. Genetically restricted cell-mediated cytotoxicity in cattle immune to Theileria parva. Nature. 1981 Mar 19;290(5803):251–254. doi: 10.1038/290251a0. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Büscher G., Doxsey S. Salivary gland of the tick vector of East Coast fever. III. The ultrastructure of sporogony in Theileria parva. Tissue Cell. 1982;14(1):183–206. doi: 10.1016/0040-8166(82)90017-9. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Doxsey S., Stagg D. A., Young A. S. The entry of sporozoites of Theileria parva into bovine lymphocytes in vitro. Electron microscopic observations. Eur J Cell Biol. 1982 Apr;27(1):10–21. [PubMed] [Google Scholar]
- Fine E., Aikawa M., Cochrane A. H., Nussenzweig R. S. Immuno-electron microscopic observations on Plasmodium knowlesi sporozoites: localization of protective antigen and its precursors. Am J Trop Med Hyg. 1984 Mar;33(2):220–226. doi: 10.4269/ajtmh.1984.33.220. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Gonenne A., Ernst R. Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants. Anal Biochem. 1978 Jun 15;87(1):28–38. doi: 10.1016/0003-2697(78)90565-1. [DOI] [PubMed] [Google Scholar]
- Hall R., Osland A., Hyde J. E., Simmons D. L., Hope I. A., Scaife J. G. Processing, polymorphism, and biological significance of P190, a major surface antigen of the erythrocytic forms of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Apr;11:61–80. doi: 10.1016/0166-6851(84)90055-0. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Barnwell J. W. Solubilization and immunoprecipitation of 125I-labelled antigens from Plasmodium knowlesi schizont-infected erythrocytes using non-ionic, anionic and zwitterionic detergents. Parasitology. 1984 Feb;88(Pt 1):27–36. doi: 10.1017/s0031182000054317. [DOI] [PubMed] [Google Scholar]
- Irvin A. D., Boarer C. D., Dobbelaere D. A., Mahan S. M., Masake R., Ocama J. G., Ocama J. G. Monitoring Theileria parva infection in adult Rhipicephalus appendiculatus ticks. Parasitology. 1981 Feb;82(1):137–147. doi: 10.1017/s0031182000041949. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTIN H. M., BARNETT S. F., VIDLER B. O. CYCLIC DEVELOPMENT AND LONGEVITY OF THEILERIA PARVA IN THE TICK RHIPICEPHALUS APPENDICULATUS. Exp Parasitol. 1964 Dec;15:527–555. doi: 10.1016/0014-4894(64)90045-1. [DOI] [PubMed] [Google Scholar]
- Minami T., Spooner P. R., Irvin A. D., Ocama J. G., Dobbelaere D. A., Fujinaga T. Characterisation of stocks of Theileria parva by monoclonal antibody profiles. Res Vet Sci. 1983 Nov;35(3):334–340. [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Buscher G., Masake R. A., Otim B. Bovine immune response to Theileria parva: neutralizing antibodies to sporozoites. Immunology. 1982 Apr;45(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Rurangirwa F. R., Buscher G. Evidence for a common protective antigenic determinant on sporozoites of several Theileria parva strains. Immunology. 1984 Jun;52(2):231–238. [PMC free article] [PubMed] [Google Scholar]
- Seppälä I., Sarvas H., Péterfy F., Mäkelä O. The four subclasses of IgG can be isolated from mouse serum by using Protein A-Sepharose. Scand J Immunol. 1981 Oct;14(4):335–342. doi: 10.1111/j.1365-3083.1981.tb00573.x. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]