Abstract
This work is the first attempt to quantify the overall effects of a 13-valent pneumococcal conjugate vaccine (PCV13) vaccination programme in the Dutch population taking into account all the direct and indirect effects of the vaccine on invasive pneumococcal disease. Using available Dutch data, a dynamic transmission model for the spread of pneumococci and potential subsequent invasive pneumococcal disease has been adapted to the Dutch setting. Overall, invasive pneumococcal disease cases in the Netherlands are predicted to decrease from a pre-vaccination level of 2623 cases annually to 2475, 2289, 2185, 2179, and 2178 cases annually 5-, 10-, 20-, 30-, and 40-years, respectively, post-vaccination. Therefore, vaccination with PCV13 in the Netherlands is predicted to lower invasive pneumococcal disease cases per year by up to 445 cases in the medium- to long-term. The results are quite robust for the sensitivity analyses performed on the parameters that regulate herd immunity and competition between vaccine and non-vaccine types.
Introduction
Streptococcus pneumoniae (pneumococcus) is the main cause of pneumonia, acute otitis media, bloodstream infections (bacteraemia) and severe forms of meningitis [1]. The burden of disease related to the bacterium S. pneumoniae is highly relevant from a public health perspective [2]–[4]. In nature, >90 immunologically different pneumococcal serotypes have been identified, which differ in the chemical compositions of their polysaccharide capsules. Each serotype behaves differently in respect to several properties, including invasiveness, affinity towards age groups, disease manifestation, carriage, and outbreaks. Preventing pneumococcal disease caused by specific serotypes is possible through vaccination.
The 7-valent pneumococcal conjugate vaccine (PCV7), which became available in 2000, contains serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. The 10-valent (PCV10) and 13-valent (PCV13) pneumococcal conjugate vaccines (PCVs) subsequently became available in 2009 and 2010, respectively. PCV10 includes the serotypes contained in PCV7 and three additional serotypes: serotypes 1, 5, and 7A. PCV13 contains the serotypes of PCV10 and another three additional serotypes: serotypes 3, 6A, and 19A. Conjugated vaccines administered to paediatric populations have provided direct protection in many settings [5], [6].
An additional benefit of PCV7 and PCV13 paediatric programs has been the reduction of pneumococcal disease in unvaccinated cohorts due to herd protective effects [7]–[13]. Among vaccinated children, reduction of nasopharyngeal carriage and the subsequent transmission of vaccine serotypes [14]–[19] as well as disease caused by vaccine serotypes [20], [21] has been observed. Evidence has been published on pneumococcal disease reduction in non-vaccinated populations in the US [22], UK [8], Australia [23], Netherlands [11], and other countries. Whereas herd protection would generally be expected in populations with sufficient paediatric vaccination uptake, it is in fact difficult to predict the exact epidemiological effect of broad scale use of PCVs in new settings [24], due to variation in the circulating serotypes and the inherent differences among them, as well as programmatic differences including the level of uptake and intensity of catch-up programs. Furthermore, although overall invasive pneumococcal disease (IPD) rates decreased after the introduction of PCV7, there has simultaneously been an increase of cases due to non-PCV7-vaccine serotypes. Many clinical studies have shown that, at the level of the nasopharynx, the use of PCV7 is followed by a shift from colonisation with vaccine types (VTs) to predominantly non-vaccine types (NVTs) [25]. Recent research mitigates the notion that serotype replacement would be of a scale potentially offsetting the direct and herd protection effects of the PCV. In particular, Miller et al (2011) [8] claim that further reductions might be achievable by use of higher-valent vaccines.
This being said, however, a great deal of uncertainty remains regarding major drivers such as long-term replacement disease and herd protection among unvaccinated individuals. Understanding the epidemiologic shifts following the introduction of PCV7 is relevant for the introduction of the newer higher-valent vaccines. Multiple factors affect serotype patterns. The vaccine could be the cause of replacement, but antibiotic use and resistance, as well as other biological characteristics of the individual serotypes, which indeed present considerable variations, may also influence serotype distributions. Previous epidemiological and health-economic analyses of higher-valent PCVs exclusively utilised static models [26]. However, currently it is well recognised that to model the changes in pneumococcal epidemiology after vaccination a dynamic model is required, which could take into account all these indirect effects. Only recently, van Hoek et al (2012) [27] evaluated the cost-effectiveness of the introduction of PCV13 in England and Wales using a deterministic transmission model to generate epidemiological projections of vaccine impacts. The importance of using dynamic designs, as opposed to static approaches, to explicitly model the spread of bacteria and viruses has previously been illustrated [28]–[30]. The use of different modelling approaches has shown huge differences when analysing screening for Chlamydia trachomatis [28] and pertussis vaccination strategies [29], [30]. To fully capture the value of infant PCV programs, it is necessary to develop a dynamic model that incorporates such indirect effects. The development of such models fits into the priority of designing innovations in health technology assessments, but requires input from mathematical, medical, epidemiological, and immunological fields, and scientific applications of such rigorous undertakings are few.
Melegaro et al (2010) [31] were the first to develop an age-structured transmission dynamic model that retrospectively studied the impact of PCV7 on IPD considering serotype replacement. A Susceptible-Infected-Susceptible (SIS) model was developed that considered reinfection and co-infection with NVTs and VTs. Carriage prevalence data and surveillance of IPD came from England and Wales, while degree and duration of vaccine protection and competition were derived using available US data. This study found that PCV7 vaccination could eradicate VTs, but increased the NVTs with the consequent possible increase of IPD. The authors claimed, however, that their results were sensitive to changes in protection effects and level of competition.
In the UK, a more rapid replacement of PCV7 IPD cases by non-PCV7 IPD cases was observed compared with the US. Consequently, in recent work by Choi et al (2011) [32], the model of Melagaro et al (2010) [31] was re-parameterised using vaccine coverage and IPD data from England and Wales, as well as European Union social contact mixing patterns [33]. The authors concluded that PCV7 vaccination could result in a decrease in overall IPD, mostly in children, even with a rapid replacement by NVTs within 5 years of vaccine introduction at high coverage. They also found that stopping vaccination could result in a resurgence of VT disease to pre-vaccination levels.
In the Netherlands, vaccination with PCV7 was introduced for all infants born after March 31, 2006. The schedule of vaccinations was 3 infant doses and 1 toddler dose (i.e. 3+1) at ages 2, 3, 4, and 11 months, with no catch-up campaign. As of 2011, PCV10 replaced PCV7 in the infant pneumococcal vaccination schedule. Rozenbaum et al (2010) [34] have found, using a static model, that the Dutch infant vaccination program of 4 doses of PCV7 is no longer cost-effective because of increases in IPD caused by NVTs. They claim that PCV13 could provide better benefits because of an increase in herd immunity and less replacement disease. The aim of this paper is to evaluate the potential effectiveness of PCV13 on IPD in a Dutch infant national immunisation program setting, using a dynamic model that takes into account disease transmission dynamics.
Methods
The age-structured transmission dynamic model was adapted from Melegaro et al (2010) [31], in order to evaluate the effectiveness of PCV13 in the Netherlands. Similarly to Melegaro et al (2010) [31], the model here is composed of two parts, a pre-vaccination model and a transmission dynamic model, to explore the effects of the vaccination program.
Part I: Pre-Vaccination Model
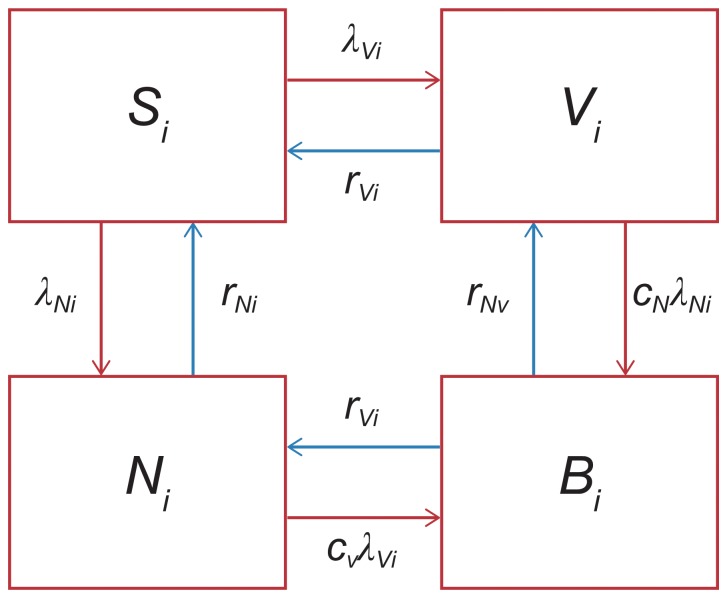
The forces of infection for VT and NVT were first estimated from the pre-PCV carriage data (λVi, λNi), with i reflecting age groups, V the VT, and N the NVT. The flowchart in Figure 1 represents the SIS structure used to consider pneumococcal carriage. Briefly, the characteristics of the SIS model were:
Figure 1. Susceptible-Infected-Susceptible (SIS) model structure.
SIS model structure for estimating the forces of infection for vaccine types and non-vaccine types. Abbreviations: λN, age-dependent forces of infection for non-vaccine types; λV, age-dependent forces of infection for vaccine types; B, both vaccine types and non-vaccine types co-colonised; cN, competition parameters that determined the relative risk of acquiring non-vaccine types if already colonised by vaccine types; cV, competition parameters that determined the relative risk of acquiring vaccine types if already colonised by non-vaccine types; i, age group; N, non-vaccine types carriers; rN, recovery rate for non-vaccine types; rV, recovery rate for vaccine types; S, susceptible; V, vaccine type carriers;.
4 compartments: S = susceptible; V = VT carriers; N = NVT carriers; and B = both VT and NVT co-colonised
Age-dependent forces of infection for VT and NVT (λVi, λNi)
Competition parameters that determined the relative risk of acquiring VT (NVT) if already colonised by NVT (VT) (cV, cN)
Recovery rates for VT and NVT
The forces of infection were assumed as step functions, with a constant value in each of the following age groups: 0–1, 2–4, 5–9, 10–19, 20–29, and ≥30 years. The recovery rate was assumed to be equal to the inverse of the duration of carriage as in Cauchemez et al (2006) [35] and Melegaro et al (2007) [36]. Competition parameters were based on point estimates and lower and upper bounds of confidence intervals derived in Melegaro et al (2007) [36] and Choi et al (2011) [32]. In particular, cV was fixed at 0.5, whereas cN took the value 0.04 [32]. Different values of cN (0 and 0.37) have been used and are reported in the sensitivity analysis, as described below. A low value of cN indicates strong competition between the VT and the NVT group, i.e. carriers of one type are less likely to acquire the other type compared with susceptible individuals. The recovery rates were assumed age-dependent but equal among the two groups (rVi = rNi).
A multinomial model was used to fit force-of-infection parameters to carriage data. The co-colonised individuals were grouped within the VT compartment because they were assumed to be detected as VT carriers. The multinomial model is as follows:
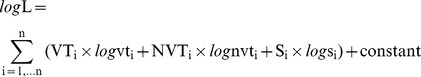 |
where i = age group, VT = observed number of VT carriers, vt = predicted prevalence of VT carriers, NVT = observed number of NVT carriers, nvt = predicted prevalence of NVT carriers, S = observed number of susceptibles, and s = predicted prevalence of susceptibles.
The model was solved for the combination of parameter values that produced the best fit.
Part II: Dynamic Model to Evaluate Vaccination Policies
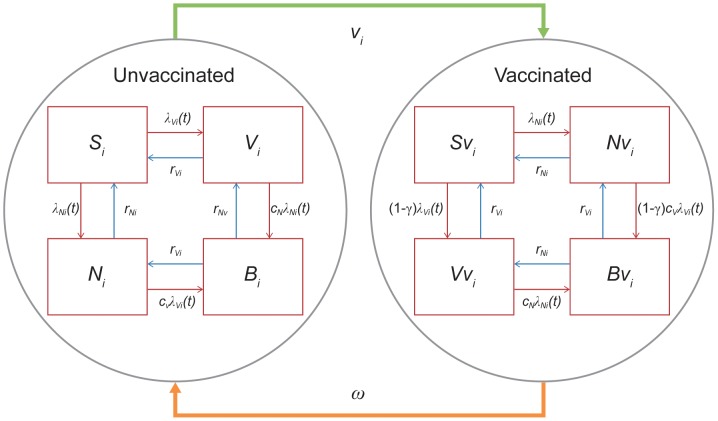
The second part of the model consisted of a dynamic model for S. pneumoniae, comprising a set of ordinary differential equations (see Melegaro et al [2010] for details [31]). The flow chart in Figure 2 shows the natural history of carriage with and without vaccination. The crucial characteristics of the model are as follows:
Figure 2. Dynamic transmission model.
Flowchart representing the dynamic mathematical model used for the transmission. Abbreviations:γi, degree of protection; λN, age-dependent forces of infection for non-vaccine types; λV, age-dependent forces of infection for vaccine types; ωi, the waning rate; B, both vaccine types and non-vaccine types co-colonised; Bv, both vaccine types and non-vaccine types co-colonised with vaccination; cN, competition parameters that determined the relative risk of acquiring non-vaccine types if already colonised by vaccine types; cV, competition parameters that determined the relative risk of acquiring vaccine types if already colonised by non-vaccine types; i, age group; N, non-vaccine types carriers; Nv, non-vaccine types carriers with vaccination; rN, recovery rate for non-vaccine types; rV, recovery rate for vaccine types; S, susceptible; Sv, susceptible with vaccination; v, vaccination coverage; V, vaccine type carriers; Vv, vaccine types carriers with vaccination.
The model was age-structured with 100 cohorts of individuals that age every year
Each individual was born at age 0 and was susceptible
The model comprised 4 compartments without vaccination: S = susceptible; V = VT carriers; N = NVT carriers; and B = both VT and NVT co-colonised
The model comprised 4 compartments with vaccination: Sv = susceptible; Vv = VT carriers; Nv = NVT carriers; and Bv = both VT and NVT co-colonised
Age-dependent forces of infection for VT and NVT (λVi, λNi)
Competition parameters that determined the relative risk of acquiring VT (NVT) if already colonised by NVT (VT) (cV, cN)
Recovery rates for VT and NVT were assumed to be equal (rVi = rNi)
Vaccination coverage was reflected by vi, the degree of protection by γi, and the waning rate by ωi
The model was parameterised as reported in Table 1 [32], [35]–[40], where the initial conditions were the pre-vaccination carriage prevalences for VT and NVT as estimated in Part I. The mixing parameter (ε) was estimated by fitting the transmission dynamic model to the age-specific IPD incidence rates pre- and post-PCV7 introduction, by minimising the Poisson deviance (data not shown). We assumed that the pre-PCV13 vaccination years corresponded with the pre-PCV7 vaccination years; therefore, we used carriage data estimated by van Gils et al (2009) [38] and an average of the IPD cases in the 2-years pre-PCV7 (June 2004–June 2006), taking the 13 serotypes included in PCV13 explicitly into account.
Table 1. Model parameters and ranges for the analysis of PCV13 in the Netherlands.
| Parameters | Definition | Values | Ranges in sensitivity analysis | Sources [Ref.] |
| υ | Coverage | 95% | RIVM [37] | |
| cN | Competition VT | 0.04 | 0–0.37 | Choi et al (2011) [32] |
| cV | Competition NVT | 0.50 | Melegaro et al (2007) [36] | |
| ε | Assortativeness | 0.85 | 0.50–1.00 | Estimated using pre- and post-PCV7 data [38], [39] |
| γ | Degree of protection | 0.52a | Choi et al (2012) [40] | |
| δ | Duration of protection | 5 years | Choi et al (2012) [40] | |
| rvi | Recovery rate for VT | 1/duration carriage | Cauchemez et al (2006) [35] Melegaro et al (2007) [36] | |
| rni | Recovery rate for NVT | 1/duration carriage | Cauchemez et al (2006) [35] Melegaro et al (2007) [36] |
We used the same degree of protection for PCV13 that was estimated by Choi et al (2011) [32] for PCV7. Sensitivity analysis was done on this value.
Abbreviations: NVT, non-vaccine types; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; RIVM, National Institute for Public Health and the Environment, Ministry of Health, Welfare and Sport; VT, vaccine types.
Inputs
Vaccination program
The model considered routine vaccination with PCV13 of infants at 2, 3, 4, and 11 months of age (3+1), without considering any catch-up campaign.
Carriage prevalence
Pre-vaccination nasopharyngeal carriage rates of S. pneumoniae were derived from a longitudinal, randomised controlled trial implemented in the Netherlands from July 2005 and February 2006 (ClinicalTrials.gov identifier: NCT00189020). In this trial, 1003 healthy newborns and 1 of their parents were enrolled to examine the effects of a 2-dose and 2+1 dose schedule with PCV7 [38]; serotype information data were also reported in Spijkerman et al (2011) [39]. As data were not available for all age groups, a quadratic function was used to predict pre-vaccination carriage prevalence for missing age groups.
The above data were also used, together with recent data from a cross-sectional observational study by Spijkerman et al (2011) [39] on pneumococcal carriage in the Netherlands 3-years post-PCV7, to estimate the level of assortativeness in the mixing patterns.
Disease incidence
Infections that progressed into IPD were estimated based on fitting a model to the age-specific incidence of disease attributable to pneumococcal invasive infection as reported by the Netherlands Reference Laboratory for Bacterial Meningitis from 2004–2010, assuming a constant case-carrier ratio over time [40].
Population data
Model outcomes on IPD-cases were extrapolated to national totals using Dutch demographic data for the year 2006 from the Dutch Central Bureau of Statistics (Voorburg, Netherlands).
Sensitivity Analysis
Sensitivity analyses were conducted around the competition and mixing parameters. Specifically, we tested scenarios where cN was equal to 0 or 0.37, representing lower and higher competition, respectively [32]. A higher cN implies a lower replacement of VT by NVT after the introduction of the vaccine. In the base-case the mixing parameter (ε) was estimated at 0.85 using PCV7 data. Subsequently we tested values of ε at 0.50 or 1.00, representing different levels of assortativeness in the mixing patterns (when ε = 1.00, mixing is totally assortative). When a more proportionate mixing pattern (ε = 0.50) was considered, it simulated a situation where additional indirect effects among unvaccinated age groups might occur.
Results
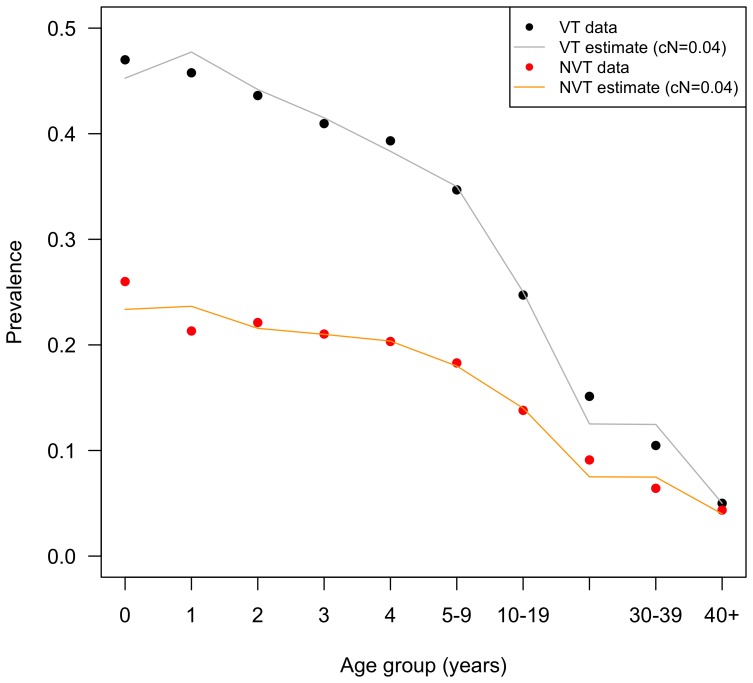
The pre-vaccination model resulted in a good fit to the data with the exception of ages 0 and 1 years. Figure 3 compares observed versus estimated steady-state carriage pre-PCV13 where cN = 0.04.
Figure 3. Carriage pre-PCV13.
Estimated and observed carriage pre-PCV13. Prevalence rates are reported per each age or age group. Note that only the data points in Figure 3 at ages 1 and 2 years, and age group 30–39 years correspond to observed carriage data; other points were interpolated. Abbreviations: cN, competition parameter; NVT, non-vaccine types; PCV13, 13-valent pneumococcal conjugate vaccine; VT, vaccine types.
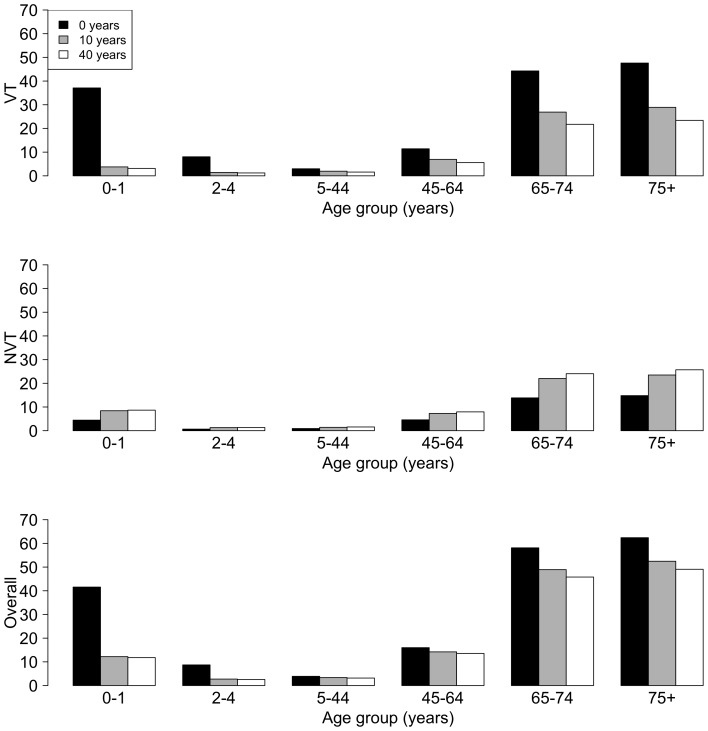
The base-case pre-vaccination, and 10 and 40 years post-PCV13 introduction IPD incidence per 100,000 population for VT, NVT, and overall IPD incidences (VT+NVT diseases) are presented in Figure 4 . The model predicted a rapid decrease of the VT and overall incidence for the age-groups 0–1 years, and also a relevant decrease of the VT and overall incidence for the ≥65 years age groups.
Figure 4. Yearly incidence of IPD.
IPD yearly incidence per 100,000 population pre-vaccination (0 years) and after 10 and 40 years post-PCV13 with the competition parameter cN = 0.04. Abbreviations: cN, competition parameter; IPD, invasive pneumococcal disease; NVT, non-vaccine types; PCV13, 13-valent pneumococcal conjugate vaccine; VT, vaccine types.
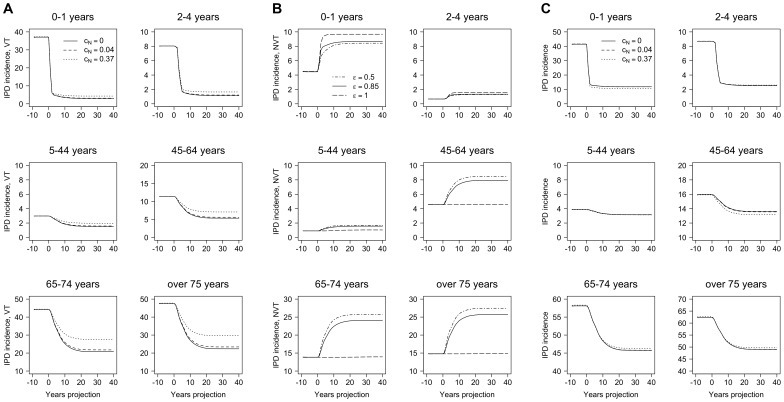
Figure 5 presents VT, NVT, and overall IPD incidence per 100,000 population, over time with ε fixed at 0.85, while varying the competition parameter cN = 0, 0.04, and 0.37. Figures 5A and 5B show that the model was sensitive to cN; a higher cN implies a lower replacement of VT by NVT after the introduction of the vaccine; therefore, the VT incidences are higher and the NVT incidences are lower compared with a lower cN in all age groups. In Figure 5C, the overall incidence is about the same for all three parameters. However, some effects of the different values of cN were apparent once we stratified by VT or NVT incidences. Here, when cN = 0.37, the overall IPD incidence decreased in the age groups 0–1 and 45–64 years, was constant in age groups 2–44 years, and increased for people aged ≥65 years. This is because there was a combination of two opposing effects: increase of VT incidence and decrease of NVT incidence. The model predicted an overall decrease in IPD incidence in the 0–1 years age group. Specifically, the overall IPD incidences (per 100,000 population) 20 years after the introduction of PCV13 in the 0–1 years age group were 118.65 if cN = 0, 118.00 if cN = 0.04, and 105.43 if cN = 0.37. However, the overall IPD incidences (per 100,000 population) 20 years after the introduction of the vaccine in the ≥75 years age group were 491.94 if cN = 0, 492.44 if cN = 0.04, and 498.22 if cN = 0.37.
Figure 5. Yearly incidence of IPD with different competition parameters.
A, IPD incidence, VT; B, IPD incidence, NVT; and C, IPD overall incidence. Yearly incidence per 100,000 population over time when different competition parameters are used: cN = 0, 0.04, and 0.37; and the mixing parameter is ε = 0.85. Each panel corresponds to a different age group. Note: the y-axis scales are different on each graph. Abbreviations: cN, competition parameter; ε, mixing parameter; IPD, invasive pneumococcal disease; NVT, non-vaccine types; VT, vaccine types.
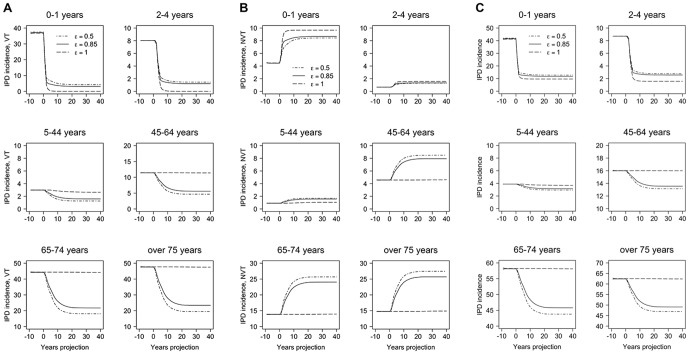
Figure 6 presents VT, NVT, and overall IPD incidence per 100,000 population, over time with cN fixed at 0.04, while varying the mixing parameter ε = 0.50, 0.85, and 1.00. As higher values of ε imply less mixing, IPD incidence, therefore, had a larger decrease over time when the mixing was more proportionate (ε = 0.50) leading to a higher herd protective effect. The opposite happened when mixing was fully assortative (ε = 1.00), and incidence remained nearly unchanged for older unvaccinated age groups.
Figure 6. Yearly incidence of IPD with different mixing parameters.
A, IPD incidence, VT; B, IPD incidence, NVT; and C, IPD overall incidence. Yearly incidence per 100,000 population over time when different mixing parameters are used: ε = 0.50, 0.85, 1.00; and the competition parameter is cN = 0.04. Each panel corresponds to a different age group. Note: the y-axis scales are different on each graph. Abbreviations: cN, competition parameter; ε, mixing paremeter; IPD, invasive pneumococcal disease; NVT, non-vaccine types; VT, vaccine types.
Discussion
Using available Dutch data and a transmission dynamic model, we estimated that 10 years after the introduction of PCV13, the number of IPD cases per year (both VT and NVT) for children <2 years of age would be 45, reduced from 160 cases per year if no vaccination had ever been implemented. At 10 years the equilibrium was not fully reached yet; hence, after 30–40 years, the IPD cases per year for children <2 years of age reached 44. For the elderly ≥75 years of age, IPD cases per year fell from 652 to 573 at equilibrium around 30 years after PCV13 introduction. Overall, IPD cases per year in the Netherlands were predicted to decrease from 2623 cases per year pre-vaccination to 2475, 2289, 2185, 2179, and 2178 cases per year at 5, 10, 20, 30, and 40 years post-vaccination. Therefore, vaccination with PCV13 in the Netherlands was predicted to lower IPD cases annually by up to 445 cases per year in the medium- to long-term.
Rozenbaum et al (2010) [41] recently estimated the cost-effectiveness of PCV13 compared with no vaccination using a static model that included herd protection and serotype replacement in a straightforward linear relationship (termed “net indirect effects”). Using a total vaccination cost of approximately €300 for 4 doses, they estimated cost-effectiveness at approximately €50,000 per quality-adjusted life year, based on a similar reduction in IPD-cases in children aged ≤5 years, as found in this study. However, the linear “net indirect effects” calculus indicated a further reduction of only 181 cases; i.e. a total reduction of 331 cases per year. This is in contrast to our current analysis based on herd protection and serotype replacement within a population dynamic design. Notably, here we estimated a reduction in IPD cases already beyond this number after 10 years, increasing to a reduction of approximately 440 cases per year 20–40 years post-vaccination. Although we acknowledge that our comparison was hampered by the different designs of both models, we do note that both were built on the same dataset for IPD cases. Therefore, a suggestion of the change in cost-effectiveness might have some validity. Inserting the outcomes of the dynamic model developed here into the cost-effectiveness model of Rozenbaum et al (2010) [41] would improve the cost-effectiveness profile of PCV13. Note that this estimate only concerns the reduction in IPD cases; thus, potential further benefits in otitis media and pneumonia might further improve cost-effectiveness.
Our results were quite robust for the sensitivity analyses that we performed on the parameters that regulate herd immunity (ε) and competition (cN). Using PCV7 data, ε was estimated at 0.85, which is quite high, considering that 1.00 reflects full assortativeness. Variation over the range 0.50–1.00 did not influence results dramatically. However, a slight trend could be seen where lower values of ε (more proportionate mixing pattern) facilitated elimination of VT-transmission with correspondingly higher reductions in IPD cases. Furthermore, increasing the value of the competition parameter cN — reflecting lower protection from VT carriage — generally resulted in lower replacement of VT by NVT after introduction of vaccination, and thus resulted in fewer IPD cases, whereas a lower cN showed the opposite effect. However, for the older age groups, the trend may reverse and thus stabilise the overall number of IPD cases, explaining why the results are relatively insensitive to the competition parameter.
As with all models, our model is limited by being a simplification of reality and thus the above considerations should be taken with caution. Our current model consisted of simplified population structures and dynamics involving 100 cohorts of individuals (0, 1, 2, 3…99) each corresponding to one year of age and each of equal size, with a total stable population (i.e. births equal deaths). An additional crucial point of the current work relies on model parameter estimates which, due to the lack of data from the Netherlands, had to be extrapolated from other countries. In particular, the competition parameter between VT and NVT serotypes was based on estimates derived for England and Wales, where post-PCV7 data were available for several years [32]. Vaccine efficacy against VT colonisation and duration of protection were also taken from a study in England and Wales, and were 52% and 5 years, respectively [40]. Although this is clearly limiting the validity of our results, to our knowledge, these represent the best sources of information concerning competition between serotypes, as well as efficacy parameters being derived using pre- and post-vaccination IPD data from the England and Wales surveillance system. Finally, no natural immunity was included in the model because the generation of naturally-acquired immunity to pneumococcal serotypes is too complex and poorly understood to be incorporated in the present model structure. Clearly, all these assumptions can be updated when additional data become available. This also applies to any desired transitions from lower-valent vaccines to PCV13 as suggested by our and potentially other analyses. For example, there are no data yet on the transition in the first year of life from PCV7 to PCV13. Long-term surveillance of IPD, carriage, and non-bacteraemic pneumonia are crucial to understand vaccine effects over the long term.
Another limitation is that this model only captures invasive disease. Efficacy and effectiveness studies of PCV7 and PCV13 have shown a substantial impact on non-invasive disease as well. These disease states are not included in our analysis because serotype-specific data on pneumonia and acute otitis media are too scarce to attempt to predict long-term effects of vaccine pressures on VT and NVT. Again, further surveillance studies on non-IPD will be crucial in evaluating the further impact the vaccine will have on these conditions.
Currently in the Netherlands PCV10 is used having replaced PCV7 in 2011. To date, the impact of PCV10 on carriage remains uncertain [42]–[45], and a herd effect has not yet been demonstrated. For this reason PCV10 was not included in the transmission dynamic analysis. If and when such data become available, the analysis can be updated to include the effects of PCV10.
Miller et al (2011) [46] have shown that the incidence of IPD caused by PCV13-only serotypes halved in children aged <2 years in England and Wales where PCV13 replaced PCV7 in 2010. This is due to protection against serotypes 7F and 19A in particular, which were the main causes of replacement disease. However, Miller et al (2011) [46] claimed that it was too early to see indirect effects in older ages or serotype replacement effects in the vaccinated population. However, in the US, indirect effects have been observed with no evidence yet of serotype replacement in all adult age groups within 1.5 years of implementation of the PCV13 National Immunisation Programme [47], [48].
In conclusion, we have adapted to the Netherlands setting the first application of transmission dynamic modelling for the spread of pneumococci and potential subsequent IPD. We estimated a base-case reduction in IPD due to PCV13 vaccination of up to 445 cases per year, including herd protective and serotype replacement indirect effects. When compared with the previously-published static model [41], which included a linear approximation of indirect effects, we estimated slightly higher reductions in IPD in the Netherlands due to these indirect effects, potentially translating to more favourable cost-effectiveness of PCV13 than previously indicated. Sensitivity analyses showed that results are sensitive to the competition parameter arranging the exchange between VT and NVT, and the type of population mixing. This work is the first attempt to quantify the overall effects of a PCV13 programme in the Dutch population taking into account all the direct and indirect effects on IPD. However, further research is clearly needed in order to evaluate the potential overall effects on non-IPD, which represent a major widespread burden in terms of both morbidity and cost.
Acknowledgments
The authors would like to thank Drs Mark Rozenbaum, Albert van Hoek, Yoon Choi, and Raymond Farkouh for very useful discussions and feedback on the topic.
Funding Statement
This study was funded by Pfizer Inc. The role of Pfizer Inc included study design, decision to publish, and preparation of the manuscript. Pfizer Inc had no role in data collection and analysis.
References
- 1. Hausdorff WP, Feikin DR, Klugman KP (2005) Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 5: 83–93. [DOI] [PubMed] [Google Scholar]
- 2. Fedson DS, Scott JA (1999) The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine 17 Suppl 1 S11–S18. [DOI] [PubMed] [Google Scholar]
- 3. Bingen E, Levy C, Varon E, de la Rocque F, Boucherat M, et al. (2008) Pneumococcal meningitis in the era of pneumococcal conjugate vaccine implementation. Eur J Clin Microbiol Infect Dis 27: 191–199. [DOI] [PubMed] [Google Scholar]
- 4. Dery MA, Hasbun R (2007) Changing epidemiology of bacterial meningitis. Curr Infect Dis Rep 9: 301–307. [DOI] [PubMed] [Google Scholar]
- 5. Macleod CM, Hodges RG, Heidelberger M, Bernhard WG (1945) Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med 82: 445–465. [PMC free article] [PubMed] [Google Scholar]
- 6. Klein JO, Plotkin SA (2007) Robert Austrian: 1917–2007. Clin Infect Dis 45: 2–3. [Google Scholar]
- 7. Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, et al. (2010) Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 201: 32–41. [DOI] [PubMed] [Google Scholar]
- 8. Miller E, Andrews NJ, Waight PA, Slack MP, George RC (2011) Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 11: 760–768. [DOI] [PubMed] [Google Scholar]
- 9. Koshy E, Murray J, Bottle A, Sharland M, Saxena S (2010) Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: national time-trends study, 1997–2008. Thorax 65: 770–774. [DOI] [PubMed] [Google Scholar]
- 10. Roche PW, Krause V, Cooke H, Barralet J, Coleman D, et al. (2008) Invasive pneumococcal disease in Australia, 2006. Commun Dis Intell Q Rep 32: 18–30. [DOI] [PubMed] [Google Scholar]
- 11. van Deursen AM, van Mens SP, Sanders EA, Vlaminckx BJ, de Melker HE, et al. (2012) Invasive pneumococcal disease and 7-valent pneumococcal conjugate vaccine, the Netherlands. Emerg Infect Dis 18: 1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox CM (2012) Early impact of 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease, United States, 2010–11. Presented at the 1st National Immunization Conference Online, March 26–28, 2012 (presentation 30196). https://cdc.confex.com/cdc/nic2012/webprogram/Paper30196.html (accessed 2013 June 24).
- 13.Public Health England. Pneumococcal Disease. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Pneumococcal/ (accessed 2013 June 24).
- 14. Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, et al. (1996) Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate vaccine. J Infect Dis 174: 1271–1278. [DOI] [PubMed] [Google Scholar]
- 15. Obaro SK, Adegbola RA, Banya WA, Greenwood BM (1996) Carriage of pneumococci after pneumococcal vaccination. Lancet 348: 271–272. [DOI] [PubMed] [Google Scholar]
- 16. Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, et al. (1999) Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis 180: 1171–1176. [DOI] [PubMed] [Google Scholar]
- 17. Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. (2003) Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 348: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 18. Givon-Lavi N, Fraser D, Dagan R (2003) Vaccination of day-care center attendees reduces carriage of Streptococcus pneumoniae among their younger siblings. Pediatr Infect Dis J 22: 524–532. [DOI] [PubMed] [Google Scholar]
- 19. Moore MR, Hyde TB, Hennessy TW, Parks DJ, Reasonover AL, et al. (2004) Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J Infect Dis 190: 2031–2038. [DOI] [PubMed] [Google Scholar]
- 20. Talbot TR, Poehling KA, Hartert TV, Arbogast PG, Halasa NB, et al. (2004) Elimination of racial differences in invasive pneumococcal disease in young children after introduction of the conjugate pneumococcal vaccine. Pediatr Infect Dis J 23: 726–731. [DOI] [PubMed] [Google Scholar]
- 21. Black S, Shinefield H, Baxter R, Austrian R, Bracken L, et al. (2004) Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J 23: 485–489. [DOI] [PubMed] [Google Scholar]
- 22. Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, et al. (2005) Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294: 2043–2051. [DOI] [PubMed] [Google Scholar]
- 23. Roche PW, Krause V, Cook H, Barralet J, Coleman D, et al. (2008) Invasive pneumococcal disease in Australia, 2006. Commun Dis Intell Q Rep 32: 18–30. [DOI] [PubMed] [Google Scholar]
- 24. Weinberger DM, Malley R, Lipsitch M (2011) Serotype replacement in disease after pneumococcal vaccination. Lancet 378: 1962–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klugman KP (2001) Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect Dis 1: 85–91. [DOI] [PubMed] [Google Scholar]
- 26. Farkouh RA, Klok RM, Postma MJ, Roberts CS, Strutton DR (2012) Cost-effectiveness models of pneumococcal conjugate vaccines: variability and impact of modeling assumptions. Expert Rev Vaccines 11: 1235–1247. [DOI] [PubMed] [Google Scholar]
- 27. van Hoek AJ, Choi YH, Trotter C, Miller E, Jit M (2012) The cost-effectiveness of a 13-valent pneumococcal conjugate vaccination for infants in England. Vaccine 30: 7205–7013. [DOI] [PubMed] [Google Scholar]
- 28. de Vries R, van Bergen JE, de Jong-van den Berg LT (2008) Postma MJ; PILOT-CT study group (2008) Cost-utility of repeated screening for Chlamydia trachomatis . Value Health 11: 272–274. [DOI] [PubMed] [Google Scholar]
- 29. de Vries R, Kretzschmar M, Schellekens JF, Versteegh FG, Westra TA, et al. (2010) Cost-effectiveness of adolescent pertussis vaccination for the Netherlands: using an individual-based dynamic model. PLoS One 5: e13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rozenbaum MH, De Cao E, Postma MJ (2012) Cost-effectiveness of pertussis booster vaccination in the Netherlands. Vaccine 30: 7327–7331. [DOI] [PubMed] [Google Scholar]
- 31. Melegaro A, Choi YH, George R, Edmunds WJ, Miller E, et al. (2010) Dynamic models of pneumococcal carriage and the impact of the heptavalent pneumococcal conjugate vaccine on invasive pneumococcal disease. BMC Infect Dis 10: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi YH, Jit M, Gay N, Andrews N, Waight PA, et al. (2011) 7-Valent pneumococcal conjugate vaccination in England and Wales: is it still beneficial despite high levels of serotype replacement? PloS One 6: e26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mossong J, Hens N, Jit M, Beutels P, Auranen K, et al. (2008) Social contacts and mixing patterns relevant to the spread of infectious diseases. PloS Med 5: e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rozenbaum MH, Hoek AJ, Hak E, Postma MJ (2010) Huge impact of assumptions on indirect effects on the cost-effectiveness of routine infant vaccination with 7-valent conjugate vaccine (Prevnar). Vaccine 28: 2367–2369. [DOI] [PubMed] [Google Scholar]
- 35. Cauchemez S, Temime L, Valleron AJ, Varon E, Thomas G, et al. (2006) S. pneumoniae transmission according to inclusion in conjugate vaccines: Bayesian analysis of a longitudinal follow-up in schools. BMC Infect Dis 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Melegaro A, Choi Y, Pebody R, Gay N (2007) Pneumococcal carriage in United Kingdom families: estimating serotype-specific transmission parameters from longitudinal data. Am J Epidemiol 166: 228–335. [DOI] [PubMed] [Google Scholar]
- 37.van Lier EA, Oomen PJ, Mulder M, Conyn-van Spaendonck MA, Drijfhout IH, et al. (2013) Vaccinatiegraad Rijksvaccinatieprogramma Nederland: Verslagjaar 2013. RIVM Rapport 150202001. http://www.rivm.nl/dsresource?objectid=rivmp:209251&type=org&disposition=inline&ns_nc=1 (accessed 2013 June 24).
- 38. van Gils EJ, Veenhoven RH, Hak E, Rodenburg GD, Bogaert D, et al. (2009) Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. JAMA 302: 159–167. [DOI] [PubMed] [Google Scholar]
- 39. Spijkerman J, van Gils EJ, Veenhoven RA, Hak E, Yzerman EP, et al. (2011) Carriage of Streptococcus pneumoniae 3 years after start of vaccination program, the Netherlands. Emerg Infect Dis 17: 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi YH, Jit M, Flasche S, Gay N, Miller E (2012) Mathematical modelling long-term effects of replacing Prevnar7 with Prevnar13 on invasive pneumococcal diseases in England and Wales. PloS One 7: e39927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rozenbaum MH, Sanders EA, van Hoek AJ, Jansen AG, van der Ende A, et al. (2010) Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. BMJ 340: c2509. [DOI] [PubMed] [Google Scholar]
- 42. Prymula R, Siegrist CA, Chlibek R, Zemlickova H, Vackova M, et al. (2009) Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet 374: 1339–1350. [DOI] [PubMed] [Google Scholar]
- 43. Prymula R, Hanovcova I, Splino M, Kriz P, Motlova J, et al. (2011) Impact of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) on bacterial nasopharyngeal carriage. Vaccine 29: 1959–1967. [DOI] [PubMed] [Google Scholar]
- 44. Prymula R, Habib A, François N, Borys D, Schuerman L (2013) Immunological memory and nasopharyngeal carriage in 4-year-old children previously primed and boosted with 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) with or without concomitant prophylactic paracetamol. Vaccine 31: 2080–2088. [DOI] [PubMed] [Google Scholar]
- 45.Hammitt LL, Akech DO, Morpeth SC, Kihuha N, Karani A, et al. (2012) Reductions in nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae 6 months after introduction of PCV10 in Kilifi, Kenya. Presented at the 8th International Symposium on Pneumococci and Pneumococcal Diseases, March 11–15, Iguaçu Falls, Brazil (poster 242). http://www2.kenes.com/ISPPD/Scientific/Documents/FinalAbstractbook.pdf (accessed 2013 June 24).
- 46. Miller E, Andrews NJ, Waight PA, Slack MP, George RC (2011) Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine 29: 9127–9131. [DOI] [PubMed] [Google Scholar]
- 47.Moore M, Link-Gelles R, Farley MM, Schaffner W, Thomas A, et al. (2012) Impact of 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease, U.S, 2010-11. Presented at IDWeek™ 2012, October 17–21, San Diego, CA, USA (abstract 1219). https://idsa.confex.com/idsa/2012/webprogram/Paper36569.html (accessed 2013 June 24).
- 48. Link-Gelles R, Taylor T (2013) Moore MR; Active Bacterial Core Surveillance Team (2013) Forecasting invasive pneumococcal disease trends after the introduction of 13-valent pneumococcal conjugate vaccine in the United States, 2010–2020. Vaccine 31: 2572–2577. [DOI] [PubMed] [Google Scholar]