Abstract
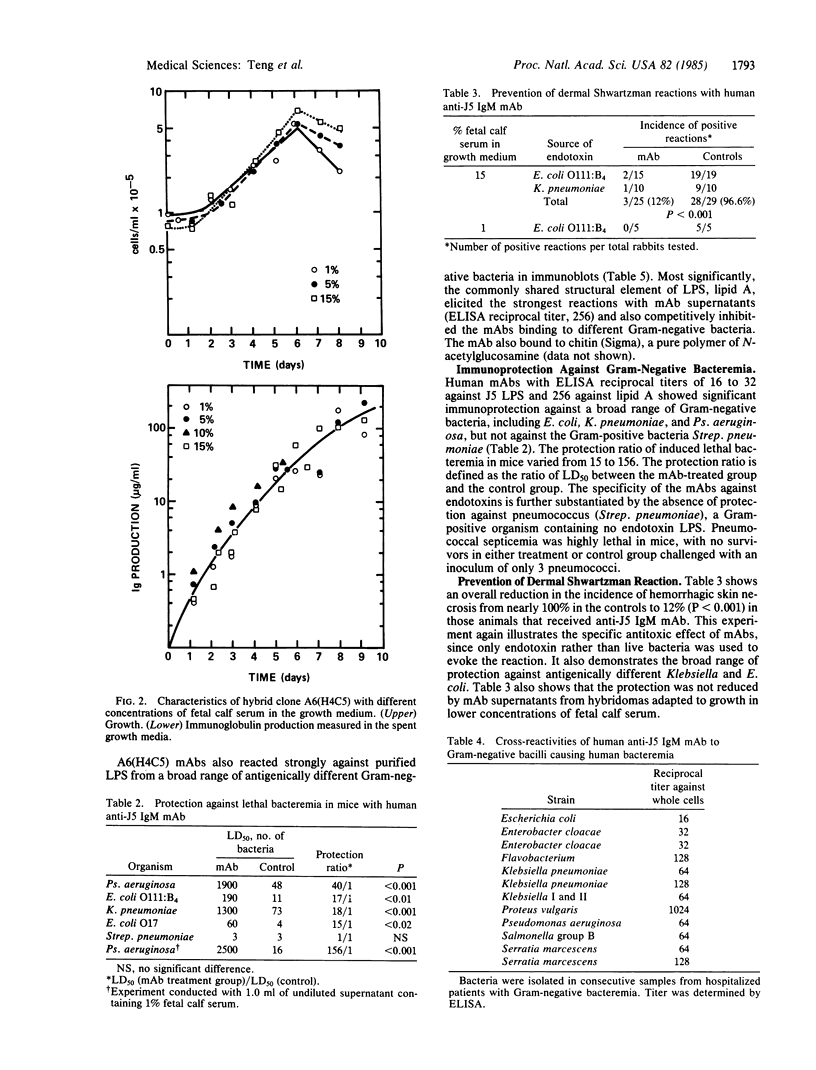
Hybridomas producing human monoclonal IgM antibodies (mAbs) against bacterial lipopolysaccharide (LPS) were generated by fusion of B lymphocytes from sensitized human spleen with heteromyeloma cells. The splenocytes were from patients undergoing splenectomy during staging for Hodgkin disease after vaccination with the J5 mutant of Escherichia coli, which is deficient in O antigenic side chains. This deficiency exposes the core oligosaccharide, common to LPS of all Gram-negative bacteria. The mAbs cross-reacted strongly with endotoxins from a wide range of unrelated species of Gram-negative bacteria. The mAbs also gave strong protection against LPS in the dermal Shwartzman reaction and against lethal Gram-negative bacteremia in mice. These findings indicate that monoclonal IgM against LPS endotoxin can neutralize its toxicity in vivo and might be valuable for treatment of patients with Gram-negative bacteremia. Analysis of one of the hybridoma clones, A6(H4C5), showed that the IgM mAb is directed against the covalently bound lipid A, which represents the most conservative and least variable structural element of LPS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bron D., Feinberg M. B., Teng N. N., Kaplan H. S. Production of human monoclonal IgG antibodies against Rhesus (D) antigen. Proc Natl Acad Sci U S A. 1984 May;81(10):3214–3217. doi: 10.1073/pnas.81.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. A., Miller G. Immunoglobulin expression by human B lymphocytes clonally transformed by Epstein Barr virus. J Immunol. 1982 Jan;128(1):24–29. [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Fischer G. W., Hemming V. G., Wilson S. R., Hartzman R. J., Woody J. N. Antibacterial activity of a human monoclonal antibody to haemophilus influenzae type B capsular polysaccharide. Lancet. 1982 Oct 9;2(8302):798–799. doi: 10.1016/s0140-6736(82)92682-4. [DOI] [PubMed] [Google Scholar]
- Ito J. I., Jr, Wunderlich A. C., Lyons J., Davis C. E., Guiney D. G., Braude A. I. Role of magnesium in the enzyme-linked immunosorbent assay for lipopolysaccharides of rough Escherichia coli strain J5 and Neisseria gonorrhoeae. J Infect Dis. 1980 Oct;142(4):532–537. doi: 10.1093/infdis/142.4.532. [DOI] [PubMed] [Google Scholar]
- Kirkland T. N., Ziegler E. J. An immunoprotective monoclonal antibody to lipopolysaccharide. J Immunol. 1984 May;132(5):2590–2592. [PubMed] [Google Scholar]
- Kreger B. E., Craven D. E., Carling P. C., McCabe W. R. Gram-negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am J Med. 1980 Mar;68(3):332–343. doi: 10.1016/0002-9343(80)90101-1. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETER E., WESTPHAL O., LUDERITZ O., GORZYNSKI E. A., EICHENBERGER E. Studies of enterobacterial lipopolysaccharides; effects of heat and chemicals on erythrocyte-modifying, antigenic, toxic and pyrogenic properties. J Immunol. 1956 May;76(5):377–385. [PubMed] [Google Scholar]
- Nilsson K., Bennich H., Johansson S. G., Pontén J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970 Oct;7(4):477–489. [PMC free article] [PubMed] [Google Scholar]
- Teng N. N., Lam K. S., Calvo Riera F., Kaplan H. S. Construction and testing of mouse--human heteromyelomas for human monoclonal antibody production. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7308–7312. doi: 10.1073/pnas.80.23.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S. M. The treatment of gram-negative bacteremia and shock. N Engl J Med. 1982 Nov 11;307(20):1267–1268. doi: 10.1056/NEJM198211113072010. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]