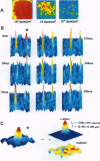
Abstract
In recent years observations at the level of individual atoms and molecules became possible by microscopy and spectroscopy. Imaging of single fluorescence molecules has been achieved but has so far been restricted to molecules in the immobile state. Here we provide methodology for visualization of the motion of individual fluorescent molecules. It is applied to imaging of the diffusional path of single molecules in a phospholipid membrane by using phospholipids carrying one rhodamine dye molecule. For this methodology, fluorescence microscopy was carried to a sensitivity so that single fluorescent molecules illuminated for only 5 ms were resolvable at a signal/noise ratio of 28. Repeated illuminations permitted direct observation of the diffusional motion of individual molecules with a positional accuracy of 30 nm. Such capability has fascinating potentials in bioscience--for example, to correlate biological functions of cell membranes with movements, spatial organization, and stoichiometries of individual components.
Full text
PDF
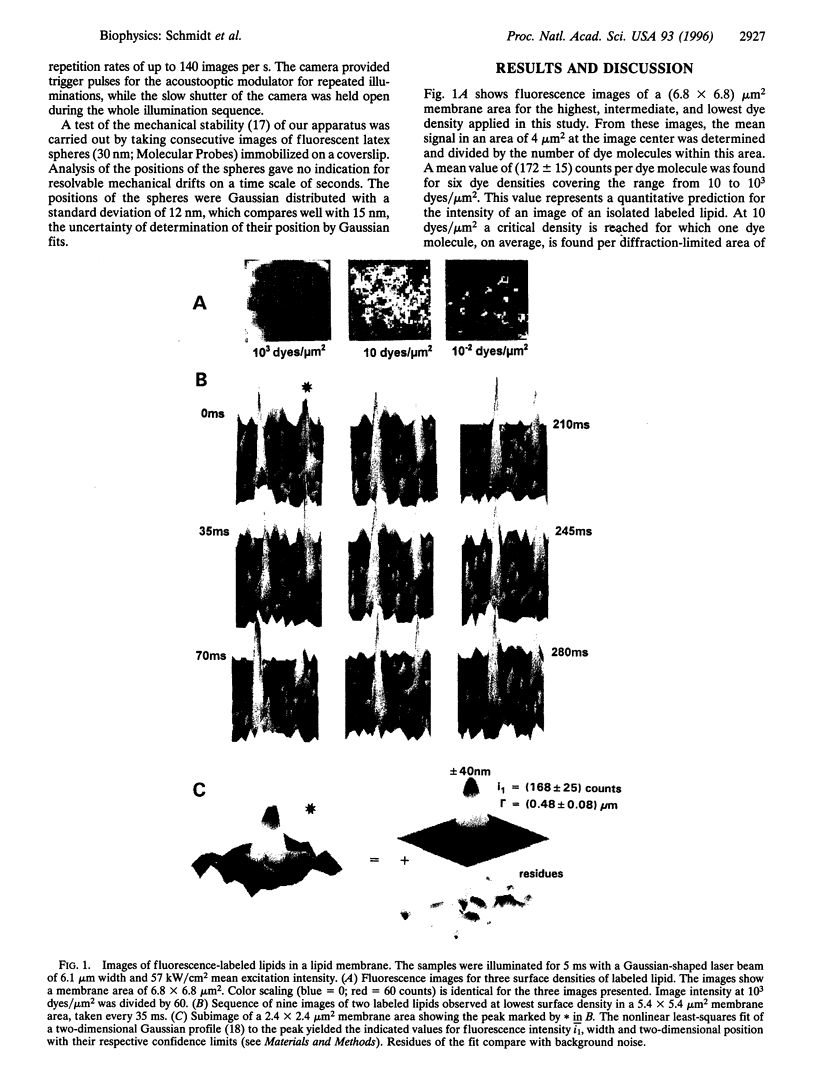
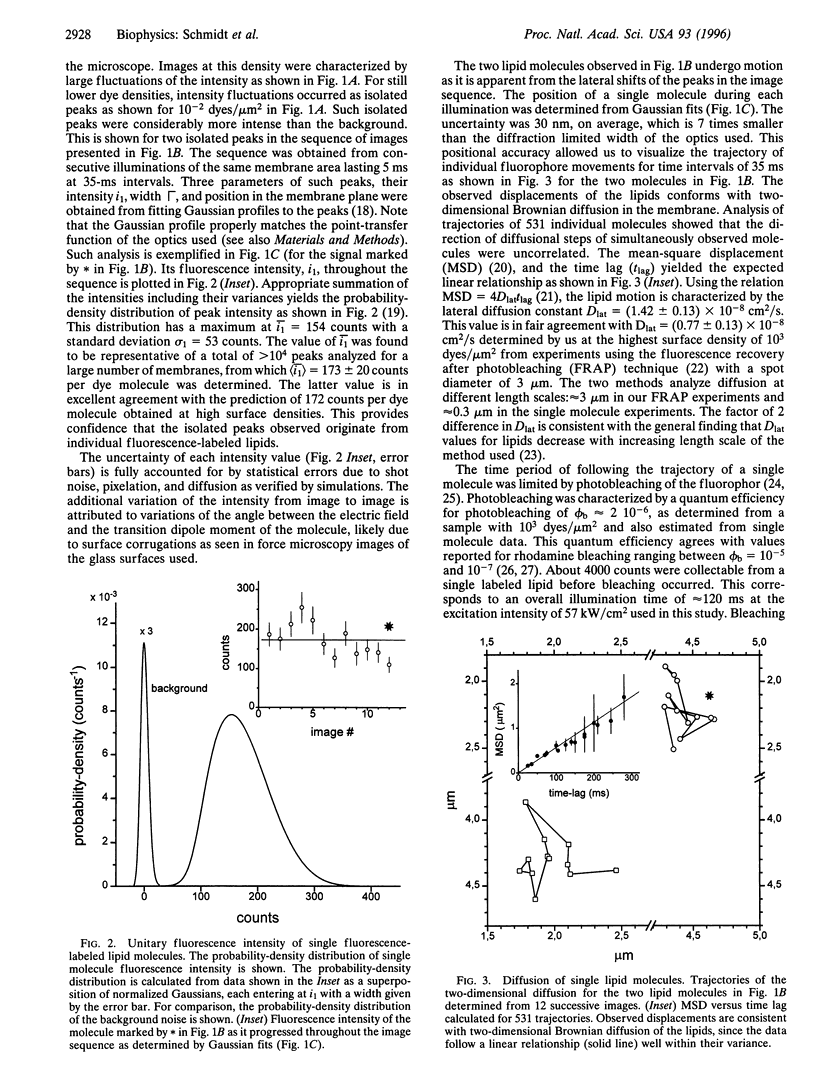

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose WP, Goodwin PM, Martin JC, Keller RA. Single molecule detection and photochemistry on a surface using near-field optical excitation. Phys Rev Lett. 1994 Jan 3;72(1):160–163. doi: 10.1103/PhysRevLett.72.160. [DOI] [PubMed] [Google Scholar]
- Betzig E., Chichester R. J. Single molecules observed by near-field scanning optical microscopy. Science. 1993 Nov 26;262(5138):1422–1425. doi: 10.1126/science.262.5138.1422. [DOI] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Funatsu T., Harada Y., Tokunaga M., Saito K., Yanagida T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature. 1995 Apr 6;374(6522):555–559. doi: 10.1038/374555a0. [DOI] [PubMed] [Google Scholar]
- Gelles J., Schnapp B. J., Sheetz M. P. Tracking kinesin-driven movements with nanometre-scale precision. Nature. 1988 Feb 4;331(6155):450–453. doi: 10.1038/331450a0. [DOI] [PubMed] [Google Scholar]
- Ghosh R. N., Webb W. W. Automated detection and tracking of individual and clustered cell surface low density lipoprotein receptor molecules. Biophys J. 1994 May;66(5):1301–1318. doi: 10.1016/S0006-3495(94)80939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A., Sako Y., Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys J. 1993 Nov;65(5):2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käs J., Strey H., Sackmann E. Direct imaging of reptation for semiflexible actin filaments. Nature. 1994 Mar 17;368(6468):226–229. doi: 10.1038/368226a0. [DOI] [PubMed] [Google Scholar]
- Moerner WE, Kador L. Optical detection and spectroscopy of single molecules in a solid. Phys Rev Lett. 1989 May 22;62(21):2535–2538. doi: 10.1103/PhysRevLett.62.2535. [DOI] [PubMed] [Google Scholar]
- Nie S., Chiu D. T., Zare R. N. Probing individual molecules with confocal fluorescence microscopy. Science. 1994 Nov 11;266(5187):1018–1021. doi: 10.1126/science.7973650. [DOI] [PubMed] [Google Scholar]
- Orrit M, Bernard J. Single pentacene molecules detected by fluorescence excitation in a p-terphenyl crystal. Phys Rev Lett. 1990 Nov 19;65(21):2716–2719. doi: 10.1103/PhysRevLett.65.2716. [DOI] [PubMed] [Google Scholar]
- Peck K., Stryer L., Glazer A. N., Mathies R. A. Single-molecule fluorescence detection: autocorrelation criterion and experimental realization with phycoerythrin. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4087–4091. doi: 10.1073/pnas.86.11.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins T. T., Quake S. R., Smith D. E., Chu S. Relaxation of a single DNA molecule observed by optical microscopy. Science. 1994 May 6;264(5160):822–826. doi: 10.1126/science.8171336. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L. K., McConnell H. M. Supported phospholipid bilayers. Biophys J. 1985 Jan;47(1):105–113. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay T. T., Jacobson K. A. Spatial Fourier analysis of video photobleaching measurements. Principles and optimization. Biophys J. 1991 Aug;60(2):360–368. doi: 10.1016/S0006-3495(91)82061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Almeida P. F. Microscopic versus macroscopic diffusion in one-component fluid phase lipid bilayer membranes. Biophys J. 1991 Dec;60(6):1553–1554. doi: 10.1016/S0006-3495(91)82190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]