Abstract
Background
Autism Spectrum Conditions (ASC) are a group of developmental conditions which affect communication, social interactions and behaviour. Mitochondrial oxidative dysfunction has been suggested as a mechanism of autism based on the results of multiple genetic association and expression studies. SLC25A12 is a gene encoding a calcium-binding carrier protein that localizes to the mitochondria and is involved in the exchange of aspartate for glutamate in the inner membrane of the mitochondria regulating the cytosolic redox state. rs2056202 SNP in this gene has previously been associated with ASC. SNPs rs6716901 and rs3765166 analysed in this study have not been previously explored in association with AS.
Methods
We genotyped three SNPs (rs2056202, rs3765166, and rs6716901) in SLC25A12 in n?=?117 individuals with Asperger syndrome (AS) and n?=?426 controls, all of Caucasian ancestry.
Results
rs6716901 showed significant association with AS (P?=?0.008) after correcting for multiple testing. We did not replicate the previously identified association between rs2056202 and AS in our sample. Similarly, rs3765166 (P?=?0.11) showed no significant association with AS.
Conclusion
The present study, in combination with previous studies, provides evidence for SLC25A12 as involved in the etiology of AS. Further cellular and molecular studies are required to elucidate the role of this gene in ASC.
Keywords: SLC25A12, Asperger syndrome, Association study, Single nucleotide polymorphisms
Background
Autism Spectrum Conditions (ASC) are a group of neurodevelopmental conditions characterised by difficulties in social interaction and communication, alongside unusually narrow interests and repetitive, stereotyped behaviour [1]. Asperger syndrome (AS) is a subset of ASC, where there is no cognitive, developmental or language delay in childhood [2]. ASC has a concordance of 31% in dizygotic twins and 88% in monozygotic twins, suggesting a partly genetic aetiology [3]. Due to the complex and polygenic nature of the condition, the exact cause of ASC is not yet fully understood. Most candidate genes currently implicated in ASC are involved in neurodevelopmental pathways, social-emotional behaviour, or sex hormonal signalling [4].
Several genes mapped also to the region 2q24-q33 have been considered as candidate genes for autism [5-7]. The solute carrier family 25, member 12 gene (SLC25A12) is located at 2q24. It contains 18 exons, spread over 110 kilobases (kb) [8]. SLC25A12 is expressed primarily as 2.9- and 3.2-kb mRNA species, predominantly in skeletal muscle, heart, and brain [6,9]. It encodes a calcium-binding carrier protein, the mitochondrial aspartate-glutamate carrier isoform 1, which localizes to the mitochondria and is involved in the exchange of the aspartate for glutamate in the inner mitochondrial membrane regulating the cytosolic redox state. It enables mitochondrial oxidation of cytosolic nicotinamide adenine dinucleotide (NADH), thought to be important in providing energy for neurons in the central nervous system (CNS) [10,11].
Several studies have identified brain metabolism abnormalities in ASC (increased cytochrome c oxidase activity, increased oxidative stress) which might be a result of mitochondrial oxidative dysfunction in neural cells [9,12,13]. SLC25A12 may play a key role in the pathways that are altered in autism and thus can be considered a candidate gene to test in ASC.
Two SNPs in SLC25A12 (rs2056202, rs2292813) have been associated with ASC [6] and these have been replicated in an Irish sample [14]. Family-based association analyses have provided further support that genetic variants within SLC25A12 contribute to the aetiology of ASC in the Finnish population [15]. The study, however, did not find any association for the small AS-only family subset. rs2056202 and rs2292813 were associated with restricted repetitive behaviour traits in ASC in a small sample [16]. rs2056202 was also associated with levels of routines and rituals in autism and related conditions [17]. Nevertheless, several other studies have been unable to replicate these findings [18,19].
Thus, the role of SLC25A12 in increasing autism risk still remains unclear. Literature provides the evidence that ASC and its subset AS share some genetic factors involved in their aetiology [20]. The aim of our study is to specifically test for association between genetic variants in SLC25A12 and AS, to replicate previously shown results and to better understand the molecular genetics of autism. This is the first study exploring rs6716901 and rs3765166 in association with AS.
Methods
All individuals enrolled in the current study were adults of Caucasian origin from the same geographic region (the United Kingdom). n?=?117 (43 females, 74 males) with a clinical diagnosis of AS. All cases were recruited from our online database and were diagnosed with AS by independent clinicians using either the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV, 1994) or the International Classification of Diseases (ICD-10, 1994) criteria. Participants in the control group were asked to complete an online version of the Autism Spectrum Quotient (AQ) test [21], which is a measure of autistic traits. A score of 32 and above is an excellent predictor of ASC [22], and the mean AQ score in the general population is 16.4 (SD?=?6.3) [21]. Control group includes (n?=?426; 321 females, 195 males) with an AQ score below 24 to ensure a balanced representation of individuals from two ends of the autistic trait continuum. Mean AQ score within the control population was 15.2, with SD 5.2. None of the controls had a clinical diagnosis of any psychiatric conditions. This study was approved by the NHS National Research Ethics Service. Consent was obtained from all participants.
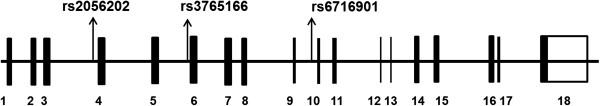
Three SNPs (rs2056202, rs3765166, rs6716901) were selected and analysed in this study. rs2056202 has been reported to be nominally associated with ASC in the Irish and Finnish samples [14,15]. rs3765166 is a part of a common linkage disequilibrium (LD) block with rs2056202 and is a Tag SNP according to HapMap Data Release 27. Even though it belongs to the same LD block as rs2056202, genotyping a Tag SNP would be more informative, as it is in high LD with a greater number of SNPs including rs2056202. rs6716901 is not in LD with either of the two. The SNPs selected were limited by those available in the ABI TaqMan assay that was used for genotyping. All three SNPs are intronic SNPs (see Figure 1).
Figure 1.
Genomic structure of SLC25A12 and the location of tested SNPs. Exons are indicated by boxes; introns are indicated by lines; SNP positions are denoted by arrows (http://www.genome.ucsc.edu).
LD values between SNPs of interest in the HapMap CEPH European samples of the Utah Residents with Northern and Western European Ancestry (CEU) population data were calculated using SNAP (http://www.broadinstitute.org/mpg/snap/). Minor allele frequency (MAF) for the tested SNPs was above 0.05 in the CEPH CEU population as calculated from the dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/). DNA was extracted from buccal swabs and anonymised. SNP genotyping was performed using TaqMan SNP Genotyping Assays (Applied Biosystems Inc., Foster City, CA, USA) using a previously described protocol [4]. Allelic association testing was performed using Plink v1.07 (http://pngu.mgh.harvard.edu/~purcell/plink/) [23]. Bonferroni correction was performed to correct for multiple SNPs. Functional annotation was performed using HaploReg (http://www.broadinstitute.org/mammals/haploreg/haploreg.php) [24], SNPnexus (http://snp-nexus.org/) [25] and F-SNP (http://compbio.cs.queensu.ca/F-SNP/) [26].
Results
In the association analyses, rs2056202 (P?=?0.26) and rs3765166 (P?=?0.11) showed no significant association with AS. rs6716901 showed significant association with AS (P?=?0.008). Results remained significant after correcting for multiple testing. None of the SNPs deviated from the Hardy-Weinberg equilibrium. Genotyping rate was above 98%. Data are summarized in Table 1.
Table 1.
Single SNP association analyses
| dbSNp ID | Alleles a | MAF b | Odds ratio | Confidence interval | F_Ac c | F_U d | Chi-sq e | P -value f | Alpha g |
|---|---|---|---|---|---|---|---|---|---|
| rs6716901 |
G/A |
0.13 |
1.70 |
0.98 to 3.02 |
0.18 |
0.11 |
6.87 |
0.008 |
0.016 |
| rs2056202 |
C/T |
0.13 |
0.76 |
0.39 to 1.46 |
0.10 |
0.13 |
1.29 |
0.26 |
0.016 |
| rs3765166 | G/A | 0.23 | 1.31 | 0.81 to 2.07 | 0.27 | 0.22 | 2.51 | 0.11 | 0.016 |
Significant P-values are written in italics. MAF, minor allele frequency.
acommon allele is listed first.
bcalculated by Plink v1.07 in analysed sample.
cthe frequency of the minor allele in cases.
dthe frequency of the minor allele in controls.
ethe chi-squared statistic for this test (1 df).
fcomputed on the basis of likelihood ratio test.
gdetermined after evaluating the number of completely independent SNPs using SNPSpD.
Discussion
In this study, three SNPs (rs2056202, rs3765166, rs6716901) in SLC25A12 were tested for association with AS. We identified an association between rs6716901 and AS in our sample. rs2056202 has been reported to be nominally associated with ASC in Irish and Finnish samples [14,15]. This association was not replicated in our sample. Some other studies reported discrepant results and found no significant association between SLC25A12 and autism [18,19].
Inconsistency between studies can be explained by several factors. An ASC is a complex condition with considerable variation in the cognitive and behavioural phenotypes. Hence, probands recruited for genetic studies are very heterogeneous. It is likely that different research groups include cases with different clinical symptoms. To the best of our knowledge, this is the first non-family-based association study between SLC25A12 and AS specifically. While this reduces heterogeneity, the dimensional nature of the conditions remains an issue. We demonstrated the association between rs6716901 of SLC25A12 with AS, supporting the role of this gene in the aetiology of ASC, but SLC25A12 might be associated with certain clinical symptoms or behavioural features of autism, rather than the diagnosis of autism itself. In other words, it may be a modifier gene, rather than a causative gene. For example, the A allele of rs2056206 of SLC25A12 was significantly associated with lower levels of routine and ritual behaviour in autism [17].
The functional role of rs6716901 is unclear. It is not in high LD with other common variants that alter transcription or chromatin states when queried on HaploReg. There are no copy number polymorphisms or miRNA binding sites associated with the SNP when queried on SNPnexus. Analyses at a cellular level would need to be carried out to understand how this SNP can contributes to the AS phenotype. Increased activity of the mitochondrial aspartate-glutamate carrier proteins and elevated levels of SLC25A12 have been detected in the superior temporal region of post-mortem brains of people with ASC [27]. Increased expression of SLC25A12 transcript has also been found in the prefrontal cortex of people with ASC [28]. During foetal development, SLC25A12 molecular gradients have been identified in the lateral prefrontal and ventral temporal cortex. These foetal structures show abnormalities in autism [28]. SLC25A12 is also required for the synthesis of myelin lipids in brain neurons [29]. A missense mutation in SLC25A12 leads to changed protein activity, and global hypomyelination in the cerebral hemispheres, suggesting that impaired efflux of aspartate from neuronal mitochondria prevents normal myelin formation [30]. Alternation of SLC25A12 expression in mouse embryonic cortical neurons affects dendrite length and the mobility of dendritic mitochondria [28]. Taken together, variation in SLC25A12 expression may be involved in the pathophysiology of autism, modifying both neuronal structures and metabolism in the CNS.
A limitation of our study is sample size. It has only limited power to reliably detect the role of certain variants in the genetics of the condition. It is worth mentioning that females are over-represented in the control sample compared to the AS group. In the sex-stratified analyses, none of the SNPs are significant after Bonferroni correction, indicating that the over-representation of females in controls is not driving the association (data not shown). Another limitation is the lack of a replication sample. According to our findings, SLC25A12 may contribute to genetic susceptibility of autism in some populations, but further studies with larger sample size are needed to address and clarify the role of this gene in autism. Moreover, a single gene is unlikely to have a major effect in complex conditions like autism, and many other genes are likely to contribute to the phenotype.
Conclusions
Three SNPs (rs2056202, rs3765166, and rs6716901) in SLC25A12 were genotyped in n?=?117 individuals with AS and n?=?426 controls, all of Caucasian ancestry. rs6716901 showed significant association with AS (P?=?0.008) after correcting for multiple testing. The present study, in combination with previous studies, provides evidence for SLC25A12 being involved in the etiology of ASC. Further cellular and molecular studies are required to elucidate the role of this gene in ASC.
Abbreviations
AQ: Autism Spectrum Quotient; AS: Asperger syndrome; ASC: Autism Spectrum Conditions; CEU: European samples of Utah Residents with Northern and Western European Ancestry; CNS: central nervous system; DSM-IV: Diagnostic and statistical manual of mental disorders fourth edition; ICD-10: International classification of diseases; LD: linkage disequilibrium; MAF: minor allele frequency; NADH: nicotinamide adenine dinucleotide; SLC25A12: solute carrier family 25 (aspartate/glutamate carrier); SNP: single nucleotide polymorphism.
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
BC and SBC co-designed the study. SBC obtained funding for the study. VW and JD conducted the analysis. JD wrote the first draft of the paper revised by all authors. All authors read and approved the final manuscript.
Contributor Information
Jaroslava Durdiaková, Email: durdiakova.jaroslava@gmail.com.
Varun Warrier, Email: vw260@medschl.cam.ac.uk.
Simon Baron-Cohen, Email: sb205@cam.ac.uk.
Bhismadev Chakrabarti, Email: b.chakrabarti@reading.ac.uk.
Acknowledgements
This study was funded by grants to SBC by Target Autism Genome (TAG), the Autism Research Trust (ART), the MRC UK, and the Max Planck Institute for Psycholinguistics, Nijmegen, Netherlands. We are grateful to Lindsey Kent, Jonathan Breidbord, Allen Chan, Laura Murphy, Agnese Di Napoli, Simon Fisher, Sally Wheelwright, Carrie Allison, Grant Hill-Cawthorne, Vicky Harris for help with various stages of the project.
References
- Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968;35(4):100–36. [PubMed] [Google Scholar]
- Woodbury-Smith MR, Volkmar FR. Asperger syndrome. Eur Child Adolesc Psychiatry. 2009;18(1):2–11. doi: 10.1007/s00787-008-0701-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med. 2009;163(10):907–14. doi: 10.1001/archpediatrics.2009.98. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Dudbridge F, Kent L, Wheelwright S, Hill-Cawthorne G, Allison C, Banerjee-Basu S, Baron-Cohen S. Genes related to sex steroids, neural growth, and social-emotional behavior are associated with autistic traits, empathy, and Asperger syndrome. Autism Res. 2009;2(3):157–77. doi: 10.1002/aur.80. [DOI] [PubMed] [Google Scholar]
- Bacchelli E, Blasi F, Biondolillo M, Lamb JA, Bonora E, Barnby G, Parr J, Beyer KS, Klauck SM, Poustka A, Bailey AJ, Monaco AP, Maestrini E. Screening of nine candidate genes for autism on chromosome 2q reveals rare nonsynonymous variants in the cAMP-GEFII gene. Mol Psychiatry. 2003;8(11):916–24. doi: 10.1038/sj.mp.4001340. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, Buxbaum JD. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry. 2004;161(4):662–9. doi: 10.1176/appi.ajp.161.4.662. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Cai G, Reichert JG, Silverman JM, Buxbaum JD. An analysis of candidate autism loci on chromosome 2q24-q33: evidence for association to the STK39 gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(7):1152–8. doi: 10.1002/ajmg.b.30739. [DOI] [PubMed] [Google Scholar]
- Saheki T, Kobayashi K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis (NICCD) J Hum Genet. 2002;47(7):333–41. doi: 10.1007/s100380200046. [DOI] [PubMed] [Google Scholar]
- Correia C, Coutinho AM, Diogo L, Grazina M, Marques C, Miguel T, Ataide A, Almeida J, Borges L, Oliveira C, Oliveira G, Vicente AM. Brief report: high frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord. 2006;36(8):1137–40. doi: 10.1007/s10803-006-0138-6. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Pardo B, Lasorsa FM, del Arco A, Kobayashi K, Iijima M, Runswick MJ, Walker JE, Saheki T, Satrustegui J, Palmieri F. Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20(18):5060–9. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolioni V, Persico AM, Porcelli V, Palmieri L. The mitochondrial aspartate/glutamate carrier AGC1 and calcium homeostasis: physiological links and abnormalities in autism. Mol Neurobiol. 2011;44(1):83–92. doi: 10.1007/s12035-011-8192-2. [DOI] [PubMed] [Google Scholar]
- Lombard J. Autism: a mitochondrial disorder? Med Hypotheses. 1998;50(6):497–500. doi: 10.1016/S0306-9877(98)90270-5. [DOI] [PubMed] [Google Scholar]
- Pons R, Andreu AL, Checcarelli N, Vila MR, Engelstad K, Sue CM, Shungu D, Haggerty R, de Vivo DC, DiMauro S. Mitochondrial DNA abnormalities and autistic spectrum disorders. J Pediatr. 2004;144(1):81–5. doi: 10.1016/j.jpeds.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Segurado R, Conroy J, Meally E, Fitzgerald M, Gill M, Gallagher L. Confirmation of association between autism and the mitochondrial aspartate/glutamate carrier SLC25A12 gene on chromosome 2q31. Am J Psychiatry. 2005;162(11):2182–4. doi: 10.1176/appi.ajp.162.11.2182. [DOI] [PubMed] [Google Scholar]
- Turunen JA, Rehnstrom K, Kilpinen H, Kuokkanen M, Kempas E, Ylisaukko-Oja T. Mitochondrial aspartate/glutamate carrier SLC25A12 gene is associated with autism. Autism Res. 2008;1(3):189–92. doi: 10.1002/aur.25. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Silva RM, Flores CG, Jacob S, Guter S, Valcante G, Zaytoun AM, Cook EH, Badner JA. A quantitative association study of SLC25A12 and restricted repetitive behavior traits in autism spectrum disorders. Mol Autism. 2011;2(1):8. doi: 10.1186/2040-2392-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Buxbaum JD, Ramoz N, Schmeidler J, Reichenberg A, Hollander E, Angelo G, Smith CJ, Kryzak LA. Autism-related routines and rituals associated with a mitochondrial aspartate/glutamate carrier SLC25A12 polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2008;147(3):408–10. doi: 10.1002/ajmg.b.30614. [DOI] [PubMed] [Google Scholar]
- Rabionet R, McCauley JL, Jaworski JM, Ashley-Koch AE, Martin ER, Sutcliffe JS, Haines JL, DeLong GR, Abramson RK, Wright HH, Cuccaro ML, Gilbert JR, Pericak-Vance MA. Lack of association between autism and SLC25A12. Am J Psychiatry. 2006;163(5):929–31. doi: 10.1176/appi.ajp.163.5.929. [DOI] [PubMed] [Google Scholar]
- Chien WH, Wu YY, Gau SS, Huang YS, Soong WT, Chiu YN, Chen CH. Association study of the SLC25A12 gene and autism in Han Chinese in Taiwan. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(1):189–92. doi: 10.1016/j.pnpbp.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Persico AM, Napolioni V. Autism genetics. Behav Brain Res. 2013;251:95–112. doi: 10.1016/j.bbr.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31(1):5–17. doi: 10.1023/A:1005653411471. [DOI] [PubMed] [Google Scholar]
- Woodbury-Smith MR, Robinson J, Wheelwright S, Baron-Cohen S. Screening adults for Asperger syndrome using the AQ: a preliminary study of its diagnostic validity in clinical practice. J Autism Dev Disord. 2005;35(3):331–5. doi: 10.1007/s10803-005-3300-7. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–4. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelala C, Khan A, Lemoine NR. SNPnexus: a web database for functional annotation of newly discovered and public domain single nucleotide polymorphisms. Bioinformatics. 2009;25(5):655–61. doi: 10.1093/bioinformatics/btn653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Shatkay H. An integrative scoring system for ranking SNPs by their potential deleterious effects. Bioinformatics. 2009;25(8):1048–55. doi: 10.1093/bioinformatics/btp103. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, Sacco R, Hager J, Rousseau F, Curatolo P, Manzi B, Militerni R, Bravaccio C, Trillo S, Schneider C, Melmed R, Elia M, Lenti C, Saccani M, Pascucci T, Puglisi-Allegra S, Reichelt KL, Persico AM. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry. 2010;15(1):38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- Lepagnol-Bestel AM, Maussion G, Boda B, Cardona A, Iwayama Y, Delezoide AL, Moalic JM, Muller D, Dean B, Yoshikawa T, Gorwood P, Buxbaum JD, Ramoz N, Simonneau M. SLC25A12 expression is associated with neurite outgrowth and is upregulated in the prefrontal cortex of autistic subjects. Mol Psychiatry. 2008;13(4):385–97. doi: 10.1038/sj.mp.4002120. [DOI] [PubMed] [Google Scholar]
- Satrustegui J, Contreras L, Ramos M, Marmol P, del Arco A, Saheki T, Pardo B. Role of aralar, the mitochondrial transporter of aspartate-glutamate, in brain N-acetylaspartate formation and Ca(2+) signaling in neuronal mitochondria. J Neurosci Res. 2007;85(15):3359–66. doi: 10.1002/jnr.21299. [DOI] [PubMed] [Google Scholar]
- Wibom R, Lasorsa FM, Tohonen V, Barbaro M, Sterky FH, Kucinski T, Naess K, Jonsson M, Pierri CL, Palmieri F, Wedell A. AGC1 deficiency associated with global cerebral hypomyelination. N Engl J Med. 2009;361(5):489–95. doi: 10.1056/NEJMoa0900591. [DOI] [PubMed] [Google Scholar]