Abstract
Alcohols and other anesthetic agents dramatically alter neurologic function in a wide range of organisms, yet their molecular sites of action remain poorly characterized. Pentameric ligand-gated ion channels, long implicated in important direct effects of alcohol and anesthetic binding, have recently been illuminated in renewed detail thanks to the determination of atomic-resolution structures of several family members from lower organisms. These structures provide valuable models for understanding and developing anesthetic agents and for allosteric modulation in general. This review surveys progress in this field from function to structure and back again, outlining early evidence for relevant modulation of pentameric ligand-gated ion channels and the development of early structural models for ion channel function and modulation. We highlight insights and challenges provided by recent crystal structures and resulting simulations, as well as opportunities for translation of these newly detailed models back to behavior and therapy.
I. Introduction
Despite their widespread recreational and medical uses throughout human history, alcohol and anesthetic action on the human nervous system remain poorly characterized at a molecular level. Research in the last two decades has supported the direct involvement of protein receptors, culminating recently in the determination of atomic-resolution crystal structures of alcohols and other anesthetics (Nury et al., 2011; Sauguet et al., 2013; Spurny et al., 2013) bound to full-length ion channels that they functionally modulate.
This review focuses on the most thoroughly implicated and characterized of these targets, the family of pentameric ligand-gated ion channels (Campagna et al., 2003). These channels include ionotropic receptors for GABA, glycine, glutamate, acetylcholine, and serotonin. They play a range of inhibitory and excitatory roles in the human nervous system (Stephenson, 2012), as well as in prokaryotic physiology (Tasneem et al., 2005). For reasons yet to be elucidated from an evolutionary standpoint, modulation by alcohols and other anesthetics has also been demonstrated in diverse members of this channel family from both higher (Forman and Miller, 2011) and lower organisms (Weng et al., 2010; Spurny et al., 2013), consistent with one or more conserved sites of action.
Prior to the availability of full-length X-ray crystal structures, the conserved domain topology of this family of receptors was elucidated by sequence analysis, chemical labeling, crystallography of independent domains, and innovative cryoelectron microscopy approaches (Thompson et al., 2010). Each functional channel includes five identical or homologous subunits clustered in parallel around the axis of the ion channel pore. Within each subunit, the ∼200-amino acid N-terminal domain located extracellular to the plasma membrane primarily comprises an immunoglobulin-like β sandwich, and contributes to canonical agonist-binding sites at subunit interfaces (Nys et al., 2013). This extracellular domain in eukaryotic family members features a conserved disulfide bond that contributes to channel assembly (Amin et al., 1994) and to their classification as the “Cys-loop superfamily” (Collingridge et al., 2009)—although the term is less descriptive of their prokaryotic relatives, which lack this feature (Tasneem et al., 2005). Another ∼200 amino acids C-terminal to the extracellular domain comprise the transmembrane domain, which includes four membrane-spanning α-helices (M1–M4) (Bertaccini and Trudell, 2002). Hydrophobic residues from the M1, M3, and M4 helices interface with the lipid bilayer, whereas the M2 helix of each subunit contributes to the channel pore (Unwin, 1993). To simplify comparison of homologous subunits, the M2 helix is often described using prime notation, such that position 2′ occupies the N-terminal intracellular channel vestibule and position 20′ is at the C-terminal extracellular end of the pore (Thompson et al., 2010). The loop between α-helices M3 and M4 is highly variable in both sequence and length, in some cases extending over 250 amino acids residues, and in eukaryotic subtypes forms an independent intracellular domain (Jansen et al., 2008) implicated in channel kinetics, conductance, and cytoplasmic interactions (Baptista-Hon et al., 2013). Although low-resolution cryoelectron microscopy data indicate at least one α-helix in the intracellular loop of nicotinic acetylcholine receptors (Unwin, 2005), the absence of this domain in lower organisms (Tasneem et al., 2005) and its variable, likely flexible, nature in eukaryotes make it an ongoing challenge to structural studies.
In this overview, we place the recent cocrystal structures of pentameric ligand-gated ion channels in historical context, touching on evidence from physiologic, computational, biochemical, and genetic engineering studies. We then highlight critical implications of recent structural data and explore the therapeutic implications of this work, for example, in the understanding and treatment of alcohol use disorders and the development of novel general anesthetics. Note the term “anesthetic” will refer to volatile and intravenous general anesthetic agents; the actions of local anesthetics may, in some cases, overlap but are beyond the scope of this review.
II. Functional Evidence for Modulation
A variety of pentameric ligand-gated ion channels have been implicated in directly mediating physiologic effects of alcohols and anesthetics (Campagna et al., 2003), consistent with the prominence of these receptors in both inhibitory and excitatory neurotransmission related to processes, such as reward, consciousness, nociception, mobility, and learning and memory (Kumar et al., 2009; Davies, 2011; Miwa et al., 2011; Changeux, 2012; Dutertre et al., 2012). Indeed, the diversity of both enhancing and inhibitory responses to allosteric modulators exhibited by distinct ligand-gated ion channel family members has been instrumental in identifying likely alcohol and anesthetic binding site(s) through chimera studies (Mihic et al., 1997; Perkins et al., 2009; Duret et al., 2011). Further optimization of structure/function techniques, including heterologous expression, electrophysiology, mutagenesis, chemical labeling, and spectroscopy (Forman and Miller, 2011) as well as molecular modeling and high-resolution structure techniques described later in this review, has facilitated the identification of multiple candidate sites for direct alcohol and anesthetic modulation and provided critical support for more specific protein theories of anesthesia and alcohol action (Howard et al., 2011b). Notable studies outlined below have elicited provocative suggestions regarding the presence of one or more conserved anesthetic binding sites among these receptors and the evolutionary rationale for their existence and persistence through the family and across species.
A. Eukaryotic Inhibitory Channels
Anion-selective GABAA, glycine, and ligand-gated chloride channels from lower organisms mediate inhibitory neurotransmission in the central and peripheral nervous systems; accordingly, functional enhancement of these channels is associated with nervous system depression (Bowery and Smart, 2006), corresponding to physiologic effects of heavy drinking and anesthesia.
1. GABAA Receptors.
GABAA receptors are molecular targets of sedative hypnotic and anxiolytic agents (Whiting, 1999), and alteration of GABA signaling is implicated in motor, anxiety, and addiction disorders (Kumar et al., 2009; Garcia et al., 2010), as well as mediation of consciousness (Changeux, 2012). Although members of this subfamily are closely related in sequence and general physiology, they arise from at least 20 distinct human gene products (α, β, γ, δ, ε, and ρ subunits), some of which are further diversified by post-translational processing or association with signaling partners (Vithlani et al., 2011). Pentamers consisting of two α, two β, and one γ subunit are the most widely expressed subtypes in brain tissue, but numerous other combinatorial possibilities allow for tissue-specific tuning of channel gating, kinetics, and modulation (Sieghart and Sperk, 2002).
GABAergic signaling mediates alcohol-induced motor impairment in a variety of experimental systems (Kumar et al., 2009). Notably, potentiation of native GABA-induced currents (Nakahiro et al., 1996) was shown to recapitulate the n-alcohol series of potencies for physiologic immobilization (Alifimoff et al., 1989), including a chain length cutoff around 9 carbons. This correspondence was reproduced in heterologously expressed GABAA receptors (Dildy-Mayfield et al., 1996), along with selective potentiation by anesthetics, but not by structurally-related nonimmobilizing compounds (Mihic et al., 1994), further supporting a direct role in modulation. Other receptor subtypes have been associated with even higher sensitivity to alcohols, particularly the α4β3δ and α6β3δ GABAA receptors (Wallner et al., 2006) found extrasynaptically in thalamus and other brain regions involved in consciousness (Brickley and Mody, 2012). Although the finding of high alcohol sensitivity among δ subunit–containing GABAA receptors has not been consistently reproduced (Lovinger and Homanics, 2007), the range of modulatory properties allowed by diverse receptor subtypes is clear. Indeed, the ρ subtype of GABAA receptors is potently inhibited, rather than potentiated, by alcohols and anesthetics, although with a shorter n-alcohol cutoff around 7 carbons (Mihic and Harris, 1996). Originally identified in retina (Cutting et al., 1991), ρ GABAA receptors have since been characterized throughout the central and peripheral nervous systems (Martínez-Delgado et al., 2010), adding further potential complexity to the effects of alcohols and anesthetics on GABAergic signaling.
The search for specific sites of alcohol and anesthetic action on GABAA receptors contributed to the notion of a fundamentally distinct mechanism of binding compared with other drugs. Similar to their effective pharmacological doses, the apparent affinities of these agents for GABAA receptor modulation may be as high as millimolar (Harris et al., 2008); furthermore, their potencies for both clinical immobilization and GABAA receptors tracked more closely with hydrophobicity than any particular pharmacophore, up to a conserved cutoff size (Peoples and Weight, 1999). These properties, along with the small size and paucity of reactive or structurally rigid moieties among anesthetic agents, supported the possibility of multiple drug molecules binding transiently to large, amphiphilic active “sites,” perhaps better considered “cavities.” Notably, the long-documented “pressure reversal” of anesthesia by hyperbaric conditions (Kent et al., 1977) was linked to direct antagonism of GABAA receptors (Davies and Alkana, 1998), supporting a model in which anesthetic binding depends on dynamic motions of flexible cavities (Perkins et al., 2010), in contrast to the precise chemical bonding interactions characteristic of other drugs (Miller and Dill, 1997).
Adding to these complexities, multiple domains of GABAA receptors have been implicated in alcohol and anesthetic action. In the extracellular domain, alcohol effects were antagonized by the benzodiazepine derivative Ro15-4513 [3-ethyl-8-azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]-benzodiazepine-3-carboxylate], consistent with competitive ethanol binding near residue R100 of the extrasynaptic α6 subunit (Wallner et al., 2006). Characterization of the Chinese herbal medicine derivative dihydromyricetin (Shen et al., 2012) has further suggested it counteracts alcohol intoxication and withdrawal via a benzodiazepine-competitive mechanism, supporting the presence of a therapeutic target in this region. Closer to the plasma membrane, chimera studies linked a region of extracellular loop 2 to the putative low-concentration ethanol potentiation of extrasynaptic δ subunit-containing GABAA receptors (Perkins et al., 2009), supporting a role for the extracellular-transmembrane domain interface in channel modulation as well as gating (Bartos et al., 2009).
An increasingly well defined region of the transmembrane domain is arguably the most thoroughly implicated in alcohol and anesthetic modulation of GABAA receptors, particularly at immobilizing concentrations. In mutation studies harnessing the differential profile of ρ GABAA receptors, single amino acid substitutions at ρ1 M2 15′ and a neighboring position in M3 with equivalent residues from the α1/β1 subunits were sufficient to reverse direction and increase cutoff for potentiation (Wick et al., 1998). The volumes of these residues, along with that of a nearby position in M1, were subsequently shown to correlate inversely with sensitivity to a range of volatile anesthetics (Jenkins et al., 2001). Moreover, covalent labeling of engineered cysteines with alcohol analogs at the analogous M2 and M3 positions of α and β GABAA receptor subunits mimicked alcohol potentiation and blocked further modulation by alcohols and anesthetics (Mascia et al., 2000; Jung and Harris, 2006), consistent with involvement of these amino acid residues in a direct binding site. Photolabeling experiments with derivatives of the anesthetics etomidate and propofol on purified GABAA receptors corroborated involvement of the M3 residue and neighboring positions in both M1 and M3 (Li et al., 2006; Chiara et al., 2012, 2013).
Despite the consistency of critical amino acid positions, particularly in the transmembrane domain, it should be noted that different alcohols and anesthetics exhibit differential sensitivity to mutations (Jenkins et al., 2001) and are not universally competitive (Li et al., 2010), suggesting overlapping but nonidentical sites of action for these agents. In the absence of a high-resolution GABAA receptor structure, it also remains to be determined whether these amino acids contribute to one or more binding site(s) for alcohols and anesthetics and where, precisely, they reside—within an individual subunit, between subunits, or elsewhere.
Finally, anesthetic and alcohol inhibition, rather than potentiation, in ρ subtypes (Mihic and Harris, 1996) and engineered GABAA receptor variants (Mihic et al., 1997) suggest the presence of inhibitory, as well as potentiating, sites of action for these agents on at least some subtypes. Indeed, at least one region of the transmembrane domain has been implicated specifically in channel inhibition. Covalent labeling with a small alcohol analog at the pore-facing 6′ residue of α2 GABAA receptors produced persistent channel inhibition and increased the net potentiating effects of alcohols (Johnson et al., 2012), consistent with blockage of an independent inhibitory site. Assuming multiple possible binding sites for small amphiphilic agents on these receptors, with different affinities and efficacies of modulation, it remains to be determined which active sites are particularly critical to alcohol and anesthetic action on GABAA receptors, or whether a dynamic balance of multiple sites underlies their clinical phenotype.
2. Glycine Receptors.
Glycine receptors are the primary mediators of inhibitory neurotransmission in the spinal cord and peripheral nervous system, where they are likely to mediate immobilizing effects of anesthetics (Eger et al., 2008). They have also been shown to play important roles in the central nervous system, including addiction pathways (Dutertre et al., 2012); for instance, the alcohol use disorder therapeutic acamprosate was recently shown to interact with glycine receptors in the nucleus accumbens to reduce dopamine release and ethanol consumption (Chau et al., 2010). Adult glycine receptors are primarily composed of two α subunits (α1, α2, or α3) plus three β subunits (Weltzien et al., 2012). The α1 glycine receptor has been a popular target for structure/function analysis of alcohol and anesthetic binding due, in part, to its highly efficient functional expression as a homopentamer in a variety of systems (Grenningloh et al., 1990). Both physiologic heteromers and heterologously-expressed homomers are potentiated by anesthetic agents, including n-alcohols up to a cutoff (Mascia et al., 1996a) similar to that of GABAA receptors and behavioral assays, and exhibit sensitivity to as little as 20 mM ethanol—slightly above the legal driving limit in most of the United States (Harris et al., 2008).
Consistent with their similar modulation profiles, sites of action for alcohols and anesthetics appear to be conserved between glycine and GABAA receptors. In particular, chimera studies between α1 glycine and ρ1 GABAA receptors identified the importance of the M2 S15′ and M3 A288 residues in ethanol potentiation (Mihic et al., 1997); specifically, molecular volume of the M2 15′ position was inversely correlated with ethanol sensitivity (Ye et al., 1998). Covalent labeling of this position with alcohol analogs enhanced channel function and blocked subsequent potentiation by alcohol. Furthermore, labeling was facilitated by channel opening, consistent with an alcohol-binding cavity whose structure and/or accessibility is coupled to channel gating. Additional transmembrane residues, including M1 I229 (Lobo et al., 2008), M2 Q14′, and M3 M287 (Borghese et al., 2012), and in the extracellular portion of M4 (Lobo et al., 2006),were also shown to influence alcohol and anesthetic modulation, suggesting these positions all contribute to a shared transmembrane binding cavity.
Similarly, residues in extracellular loop 2 have been implicated in alcohol and anesthetic modulation of glycine as well as GABAA receptors. Inspired by a spontaneous disease mutation at residue A52 (Saul et al., 1994), swapping this position in α1 glycine receptors with the less ethanol-sensitive α2 subtype reduced ethanol sensitivity (Mascia et al., 1996b) and removed pressure antagonism of alcohol modulation (Davies et al., 2004), whereas the opposite switch in α2 restored both effects (Perkins et al., 2008). Both charge and geometry of position 52 were subsequently shown to influence ethanol sensitivity (Perkins et al., 2012). The mechanism by which this region influences alcohol modulation may be complex, given that covalent labeling at position 52 enhanced currents but increased, rather than decreased, subsequent ethanol potentiation (Crawford et al., 2007).
Glycine receptors are subject to regulation by cellular signaling factors, suggesting additional mechanisms of ethanol modulation via indirect interactions. For example, basic residues in the intracellular loop domain have been shown to mediate ethanol effects via binding to Gβγ proteins (Yevenes et al., 2010). A peptide designed to mimic the critical loop region disrupted ethanol potentiation without affecting other Gβγ signaling pathways (San Martin et al., 2012) and has been proposed as an intoxication therapeutic. Binding of zinc to residues in the extracellular domain has also been shown to sensitize glycine receptors to both agonist and ethanol, possibly in a synergistic manner (McCracken et al., 2010). Glycine receptors were also weakly potentiated by the antiparasitic agent ivermectin, which was recently proposed as an alcohol therapeutic (Yardley et al., 2012). For both glycine and GABAA receptors, it remains critical to differentiate between several possible mechanisms of alcohol and anesthetic action, and focus on those that are most physiologically and pharmacologically relevant.
3. Glutamate-Gated Chloride Channels.
In addition to GABAA and glycine receptors, other chloride-conducting pentameric ligand-gated ion channels, many of them gated by glutamate, are found in worms, insects, and other lower organisms. The glutamate-gated chloride channel (GluCl) from Caenorhabditis elegans rose to particular significance as the first eukaryotic member of this receptor family whose structure has been determined at atomic resolution (Hibbs and Gouaux, 2011). This receptor may prove a particularly valuable template for homology modeling, given its high degree of sequence conservation with human GABAA and glycine receptors (Bertaccini et al., 2013). In addition to glutamate, this channel is activated by ivermectin (Cully et al., 1994), which also allosterically modulates human homologs, such as GABAA (Sigel and Baur, 1987), glycine (Shan et al., 2001), and nicotinic acetylcholine receptors (Krause et al., 1998). Notably, chronic ivermectin was recently shown to combat ethanol intake and preference in mice (Yardley et al., 2012); these effects were primarily attributed to P2X4 receptors, but may involve pentameric ligand-gated ion channels as well. Ivermectin potency was determined at least in part by the M3 residue equivalent to A288 in α1 glycine receptors (Lynagh and Lynch, 2010), suggesting the agent acts via a function-enhancing site similar to that for alcohols and anesthetics. Further exploration of GluCl promises to reveal structural determinants of allosteric modulation in this channel family at higher resolution than has previously been possible.
B. Eukaryotic Excitatory Channels
Although they play dramatically different roles than GABAA and glycine receptors in neurotransmission, cation-selective pentameric ligand-gated ion channels are also modulated by alcohols and anesthetics in physiologically relevant ways. These channels, including ionotropic receptors for acetylcholine and serotonin, nonselectively import cations (primarily sodium and calcium) down their electrochemical gradients to depolarize membrane potentials and stimulate excitability (Peters et al., 2010). Neurodepressive effects of anesthetics have, in principle, been associated with inhibition of excitatory channels (Campagna et al., 2003), whereas stimulatory effects of ethanol on reward pathways may arise from excitatory channel potentiation (Hurst et al., 2013).
1. Nicotinic Acetylcholine Receptors.
Nicotinic acetylcholine receptors expressed in neurons, which are of primary interest to this review, include up to ten different α subtypes, five of which may coassemble with three different β subunits (Hurst et al., 2013). Of additional pharmacological relevance is the closely related nicotinic receptor expressed in the electric organs used by Torpedo species for predation and defense (Unwin, 1998). The abundance and ease of purification of Torpedo receptors (Cohen et al., 1972) have made them a popular model system for studying ligand-gated ion channel structure, function, and modulation. Recent higher-resolution structures of the ligand-binding domain from mouse α1 (Dellisanti et al., 2007) and transmembrane domains of human nicotinic receptor subunits (Bondarenko et al., 2012), determined by X-ray crystallography and solution-phase NMR, respectively, promise further insights into structure and function in this family.
Nicotinic receptors respond to concentrated synaptic acetylcholine, tonic low extrasynaptic acetylcholine, or exogenous drugs, such as nicotine, to stimulate release of neurotransmitters, such as GABA, glutamate, serotonin, or dopamine (Taly et al., 2009). Accordingly, these receptors are implicated in both acute and chronic effects of nicotine and other drugs, including alcohols and anesthetics (Kamens et al., 2010; Miwa et al., 2011). Long-chain n-alcohols and anesthetics inhibit several subtypes of nicotinic receptors, possibly contributing to their neurodepressive physiologic effects (Flood and Role, 1998). Similar to potentiation of GABAA and glycine receptors, inhibition of nicotinic receptors was shown to increase in potency with chain length up to a cutoff ∼10 carbons (Zuo et al., 2001), consistent with the cutoff for behavioral immobilization (Alifimoff et al., 1989). Conversely, short-chain alcohols, such as ethanol, potentiate at least some nicotinic receptor subtypes (Zuo et al., 2001).
Transmembrane residues throughout the channel pore have been implicated in nicotinic receptor modulation by alcohols and anesthetics. In preparations from Torpedo species, photoaffinity labeling with etomidate analogs implicated a pore-blocking site involving the M2 2′ and 6′ residues of desensitized nicotinic receptors (Ziebell et al., 2004) or the M2 9′ and 13′ residues of closed receptors (Nirthanan et al., 2008). In addition, both octanol (Pratt et al., 2000) and etomidate (Ziebell et al., 2004) analogs photolabeled transmembrane residues even closer to the extracellular mouth of the channel, including M2 20′. Similarly, propofol derivatives photolabeled residues M2 6′, 10′, and 13′, but also an intrasubunit site in the δ subunit involving M1 residues F232 and C236 as well as M2 18′ (Jayakar et al., 2013). In human neuronal nicotinic receptors, mutations at the α2 M2 16′ position blocked ethanol potentiation (Borghese et al., 2003b); furthermore, chemical labeling of an engineered cysteine at this position with alcohol analogs produced persistent channel inhibition and increased net potentiation by ethanol (Borghese et al., 2003a), supporting the presence of an inhibitory binding site in physiologically relevant receptors. In the same work, labeling of the α2 M2 17′ position potentiated channel function and blocked subsequent ethanol potentiation, indicating presence of a potentiating site (Borghese et al., 2003a). Notably, recent saturation transfer NMR data identified amino acid residues involved in halothane binding to isolated nicotinic receptor transmembrane domains (Bondarenko et al., 2013); these included a site at the subunit interface (α4 M1 221 and M3 283, M2 20′), the intrasubunit region photolabeled by propofol (α4 M1 222, β2 M1 212, and M2 22′) (Jayakar et al., 2013), and a second intrasubunit region at the intracellular end of the channel (β2 M1 224 and 231, M2 3′). Thus, amino acid residues implicated in nicotinic receptor modulation have primarily been associated with the channel pore and/or M2 helix interface, although, in the absence of high-resolution structural data for the full-length receptor, their assignments remain uncertain.
2. Serotonin 3 Receptors.
Ionotropic serotonin receptors in brain primarily include homomeric 5-HT3A and heteromeric 5-HT3A/B ion channels, which mediate excitatory neurotransmission in processes such as nausea, anxiety, and seizure; accordingly, 5-HT3 receptor antagonists have been shown to combat these and other symptoms related to alcoholism (Davies, 2011). Similar to their effects on nicotinic receptors, long-chain n-alcohols inhibited 5-HT3 receptors, whereas short-chain alcohols and several volatile anesthetics potentiated channel function (Machu and Harris, 1994; Jenkins et al., 1996). Substitution of a polar residue at the M2 16′ position of 5-HT3A receptors (I294T) resulted in inhibition rather than potentiation by ethanol (Sessoms-Sikes et al., 2003), similar to equivalent mutations in nicotinic receptors (Borghese et al., 2003b). Generally, homomeric 5-HT3A receptors were more sensitive to ethanol potentiation than heteromers with 5-HT3B subunits, suggesting differential alcohol binding to the two subtypes (Stevens et al., 2005). However, evidence for critical physiologic effects relevant to intoxication or anesthesia has been limited, focusing attention instead on inhibitory and nicotinic receptors and on homologs of known structure.
C. Prokaryotic Channels of Known Structure
A variety of pentameric ligand-gated ion channels are found in prokaryotes (Tasneem et al., 2005; Corringer et al., 2010). As in other protein families, bacterial ligand-gated ion channels are generally simplified homologs of human inhibitory and/or excitatory receptors, often lacking the N-terminal helix, extracellular disulfide bond, and long intracellular loop domain of their evolutionary descendants (Tasneem et al., 2005). Nonetheless, multiple bacterial subtypes have been successfully expressed as functional ion channels in heterologous systems (Bocquet et al., 2007; Hilf and Dutzler, 2008; Zimmermann and Dutzler, 2011); moreover, their ease of production in bacterial liquid culture enabled the determination of X-ray crystal structures of two distinct subtypes within the last five years (Hilf and Dutzler, 2008; Bocquet et al., 2009; Hilf and Dutzler, 2009). The availability of high-resolution structures has introduced new opportunities to characterize drug binding at higher resolution, and—assuming conserved mechanisms—extend those principles back to human homologs.
1. Gloeobacter violaceus Ligand-Gated Ion Channel.
Database screening of bacterial genomes (Tasneem et al., 2005) identified the G. violaceus ligand-gated ion channel (GLIC) as a homolog 20% identical to the α7 nicotinic acetylcholine receptor that proved straightforward to express in HEK cells and Xenopus laevis oocytes. Although the biologic role and endogenous ligand (if any) of this receptor are unknown, it is sensitive to activation by protons (low pH), facilitating its pharmacological characterization; GLIC was shown to be cation selective, similar to nicotinic and 5-HT3 receptors, and to exhibit relatively little desensitization at submaximal levels of activation (Bocquet et al., 2007). Notably, like many prokaryotic ligand-gated ion channels (Tasneem et al., 2005), GLIC lacks characteristic structural features of human homologs such as an extended intracellular loop domain, restricting potential determinants of modulation.
GLIC was initially shown to be insensitive to pharmacologically relevant concentrations of ethanol, but was inhibited by volatile and intravenous general anesthetics (Weng et al., 2010) as well as ivermectin (Duret et al., 2011). Anesthetic concentrations of methanol and higher concentrations of ethanol potentiated channel function, whereas long-chain n-alcohols containing three or more carbons inhibited GLIC with increasing potency up to a cutoff around nonanol (Howard et al., 2011a). Thus, this receptor exhibits a similar profile as nicotinic acetylcholine receptors, with a reduced capacity for potentiation.
Localization of critical domains for alcohol and anesthetic action was facilitated by an engineered GLIC chimera containing the transmembrane domain of the human α1 glycine receptor (Duret et al., 2011). As predicted, this construct was activated by low pH, presumably via ionizable residues in the ligand-binding site or other portions of the GLIC extracellular domain. Conversely, the chimera was potentiated by hexanol, propofol, volatile anesthetics, and ivermectin, similar to glycine receptors (Duret et al., 2011). Given that the construct contained the glycine receptor M1–M4 helices but none of the M3–M4 intracellular loop; the allosteric modulators were presumed to act via the transmembrane domain.
Consistent with a critical role for the transmembrane domain in modulation, cysteine scanning mutagenesis of the pore-lining M2 helix of GLIC revealed a limited set of residues that dramatically influenced alcohol and anesthetic effects. In particular, cysteine substitutions at residues L17′ and F14′ increased ethanol potentiation twofold and fivefold, respectively, such that the F14′C mutant was sensitive even to intoxicating concentrations (Howard et al., 2011a). Reducing side chain volume at the 14′ position by substituting alanine further enhanced ethanol potentiation, increased the n-alcohol cutoff for potentiation to hexanol, and resulted in potentiation rather than inhibition by volatile anesthetics (Broemstrup et al., 2013), supporting the presence of an allosteric binding cavity in this region. Indeed, bulky phenylalanine substitutions at neighboring residues I16′ and L17′, or removal of a possible hydrogen-bonding partner at residue N15′, compensated for the enhancing effect of F14′A, reducing or ablating ethanol and anesthetic potentiation (Sauguet et al., 2013). Covalent labeling of engineered cysteines at residues L17′ and V18′ also produced dramatic, persistent current enhancement, whereas alcohol-mimicking substitutions blocked subsequent potentiation, consistent with direct potentiating interactions with alcohols in this region (Howard et al., 2011a).
In contrast to potentiation, identifying amino acid residues responsible for GLIC inhibition by long-chain alcohols and anesthetics has proved complex. Cysteine substitutions in the M2 helix failed to alter long-chain alcohol inhibition, supporting an inhibitory mechanism independent from potentiation (Howard et al., 2011a). Substitutions at M2 V18′ and M3 T255 altered inhibition by volatile and intravenous anesthetics (Nury et al., 2011), and propofol altered the rates of covalent labeling of positions M2 17′ and M3 255 (Ghosh et al., 2013). However, in both studies the structural basis for these effects correlated inconsistently with side chain volume or direction of change. Additional possibilities were raised by tryptophan fluorescence studies, which supported anesthetic interactions with M1 positions distal to the putative potentiating site (W213, W217) along with additional residues in the extracellular domain (Chen et al., 2010). In general, the specific residues or region(s) of GLIC responsible for direct inhibitor binding remain to be clearly elucidated.
2. Erwinia chrysanthemi Ligand-Gated Ion Channel.
Although its atomic-resolution structure (Hilf and Dutzler, 2008) was determined prior to that of GLIC, the E. chrysanthemi ligand-gated ion channel (ELIC) was largely intractable to physiologic study until its agonists were identified. ELIC currents proved to respond to a variety of primary amines, including GABA at high concentrations, and were cation selective, high conductance, and slowly desensitizing (Zimmermann and Dutzler, 2011); they were also modulated by benzodiazepines (Spurny et al., 2012), further supporting their utility as a model system for human channels. ELIC was recently shown to be inhibited by subanesthetic concentrations of chloroform, ethanol, and their brominated derivatives, although it was insensitive to intravenous general anesthetics (Spurny et al., 2013). A summary of the overall effect of ethanol and anesthetics on the ligand-gated ion channels discussed above is shown in Table 1.
TABLE 1.
Effect of alcohols and anesthetics on pentameric ligand-gated ion channels.
Arrows indicate up- or downregulation of ligand-gated ion channel function by ethanol, short-, and long-chain alcohols and anesthetics in various in vitro model systems as described in this review.
| Channel | In Vitro Model | Ethanol/Short-Chain Alcohols | Anesthetics/Long-Chain Alcohols |
|---|---|---|---|
| α/β/γ GABAA | DRG neuronsa Xenopus oocytesb,c | ↑ | ↑ |
| ρ GABAA | Xenopus oocytesd | ↓ | ↓ |
| Glycine | Xenopus oocytese | ↑ | ↑ |
| Nicotinic acetylcholine | Neuronsf,g | ↑ | ↓ |
| HEK cellsg | |||
| 5HT3 | Xenopus oocytesh | ↑ | ↑ (Anesthetics) |
| N1E-115 neuroblastoma cellsi | ↓ (Long-chain alcohols) | ||
| GLIC | Xenopus oocytesj,k | ↑ (Superphysiologic ethanol concentrations) | ↓ |
| ELIC | Xenopus oocytesl | ↓ (Ethanol) | ↓ (Volatile anesthetics) |
DRG, doral root ganglion.
Howard et al., 2011.
III. The Evolution of Accurate Structural Models
In the absence of high-resolution structural data for human pentameric ligand-gated ion channels, computational approaches have facilitated increasingly detailed models of receptor function, including modulation by alcohols and anesthetics. These techniques initially harnessed available data from homologous proteins in lower organisms to elucidate general structure principles, but more recently aim to simulate binding and structural transitions of human receptors in atomic detail.
A. A Brief History of Cys-Loop Receptor Models
A major breakthrough in the field was provided by Unwin (1993) with the first cryoelectron microscopy structure of nicotinic acetylcholine receptors from Torpedo marmorata. However, the mathematical procedures necessary to “flatten” the images made from helical tubes and the need to average many images that were, in fact, heteromeric assemblies of α, β, and γ subunits, resulted in a low resolution of approximately 9 Å—only sufficient to show that the receptors were centrosymmetric pentamers and that α-helical segments lined the ion pore.
A further breakthrough in the structure of nicotinic receptors was provided by the high-resolution X-ray structure of a seemingly unrelated molluscan protein, AChBP (Brejc et al., 2001). When Brejc and coworkers examined this protein, they realized that it not only bound acetylcholine, but also exhibited a homopentameric structure arranged around a central pore. Although AChBP was not a membrane protein and corresponded only to the ligand-binding domain of the nicotinic receptor, its accessibility to high-resolution structure determination allowed alignment of the major ligand-binding residues in nicotinic receptors and suggested a series of loops that could interface with the transmembrane domain.
At this point, the structure of the transmembrane domain was still uncertain, limiting interpretation of the large body of data that was flowing from mutagenesis and photolabeling studies (Blanton and Cohen, 1994; Lee et al., 1994). Most algorithms for predicting secondary structure from primary amino acid sequences were “trained” on water-soluble globular proteins, making them ill-suited to model membrane-bound domains. However, 10 of the best algorithms trained on membrane proteins were applied to a large sample of known sequences of ligand-gated ion channels (Bertaccini and Trudell, 2002). After extensive consensus averaging, the prediction was that each channel subunit would have four transmembrane segments.
This prediction was soon confirmed when Miyazawa et al. (2003) used the AChBP structure to determine the phases for much-improved cryoelectron microscopy images of nicotinic receptors and published a nearly complete model of the ligand-binding and transmembrane domains to ∼4 Å resolution. This structure introduced new possibilities for detailed homology modeling; for example, it was used to build both glycine and GABAA receptor structures (Kash et al., 2003; Trudell and Bertaccini, 2004) in an effort to understand how the binding energy of a ligand was coupled to an ion channel gating motion 40 Å away (Chakrapani and Auerbach, 2005). Recent NMR structures of isolated nicotinic receptor transmembrane domains (Bondarenko et al., 2012) further clarified the topological map and informed its use as a modeling template. Moreover, the determination of homologous X-ray structures from bacteria (Hilf and Dutzler, 2008, 2009; Bocquet et al., 2009) and C. elegans (Hibbs and Gouaux, 2011) to ≤3.3 Å resolution have confirmed the conservation of receptor conformation across species and provided increasingly well-defined templates for homology modeling (Bertaccini et al., 2013).
In the past two years, cocrystal structures of bacterial channels bound to alcohols and anesthetics have allowed these templates to be applied directly to allosteric modulation. For example, modeling of GABAA receptors based on crystal structures of GLIC and GluCl facilitated mapping of residues photolabeled by the anesthetic derivative azietomidate into a coherent allosteric binding site (Chiara et al., 2012). Conversely, alignment of bromoform interaction sites in ELIC with the glycine receptor revealed novel residues involved in anesthetic modulation (Spurny et al., 2013). Modeling of allosteric binding sites in both glycine and nicotinic receptors based on GLIC also recently provided a rationale for differential alcohol sensitivity of multiple channel family members (Sauguet et al., 2013).
B. Elastic Network Calculations
Beyond the static homology-based images described above, a major goal in developing computational models of ion channels is to understand the time course of gating transitions between resting, open, and desensitized states. However, spontaneous gating occurs infrequently, and when it does, the transition takes approximately 20–100 microseconds (Chakrapani and Auerbach, 2005). Because it is difficult to simulate these time scales using molecular dynamics, normal mode analysis has been used to look at long time-scale collective motions (Bertaccini et al., 2005). Briefly, normal mode analysis starts by rendering each amino acid in a protein as a weighted sphere and connecting the spheres with springs of variable force constants (Brooks and Karplus, 1983; Tirion, 1996; Hinsen, 1998; Delarue and Sanejouand, 2002; Lindahl et al., 2006; Bertaccini et al., 2008; Samson and Levitt, 2008). An analysis of all the vibrational modes in this sphere-spring assembly can be accomplished in a matter of hours, after which the results are sorted by frequency and amplitude (Lindahl et al., 2006). Typically, the low-frequency/high-amplitude modes are the most interesting, because they often represent large collective motions of whole helical segments or even subunits. In addition, it is sometimes useful to combine a series of modes to produce a collective motion that best represents an important vibrational motion of a protein (Petrone and Pande, 2006).
Of special interest in the case of ligand-gated ion channels, some large-amplitude modes describe a collective transition of the whole receptor that may be described as a “wringing” motion (Bertaccini et al., 2005, 2007; Taly et al., 2005). That is, the entire pentameric channel behaves like a wet towel that is twisted to dry it out: the ligand-binding domain rotates in one direction and the transmembrane domain in the other. There are two important features of this motion. First, these vibrations follow a low free energy pathway; because they do not tolerate steric clashes, helical segments must all move together to allow a wringing motion without collisions. Second, because the gating transitions of these channels obtain only modest energy from agonist binding and thermal energy to drive this substantial rearrangement of the ion pore, it is important to explore motions that do not create high free energy barriers for the transition. Despite valuable insights from this approach, it is important to note that the sphere and spring model described above does not adequately account for interactions with allosteric ligands, such as alcohols and anesthetics. As a result, a current focus in observing interactions of these modulators has moved to molecular dynamics simulations.
C. Molecular Dynamics
Approximately 15 years ago, it was a significant achievement to run a 1-microsecond molecular dynamics simulation on a small peptide in aqueous solution using a large supercomputer (Duan and Kollman, 1998). More recently, it has been possible to simulate 1 microsecond in a full receptor embedded in a phospholipid bilayer and surrounded by explicit water molecules (Nury et al., 2010). This progress was made possible by more efficient and highly parallel molecular dynamics programs, such as GROMACS (Hess et al., 2008), NAMD (Phillips et al., 2005), and CHARMM (Brooks et al., 1983). Much progress for long time-scale simulations has been made on programs running on Anton computers that are specially configured for molecular dynamics (Shan et al., 2011).
These programs now are beginning to allow simulations of sufficient length to observe the effects of allosteric modulators on ligand-gated ion channel motions. Simulations of nicotinic receptors based on the low-resolution Torpedo structures revealed conformation-dependent binding of the volatile anesthetics halothane and isoflurane that could influence domain interactions or occlude the pore (Vemparala et al., 2006; Brannigan et al., 2010; Liu et al., 2010). More recently, the characterization of both atomic-resolution structure and anesthetic sensitivity in GLIC provided valuable new simulation templates. Multiple binding sites for halothane and isoflurane in GLIC have been computationally identified and predicted to disrupt the channel’s quaternary structure (Chen et al., 2010; Willenbring et al., 2011; Mowrey et al., 2013) or block the pore (Lebard et al., 2012). The discovery of enhanced ethanol and anesthetic potentiation in the F14′A variant of GLIC allowed more direct comparisons: 1-microsecond simulations showed selective intersubunit ethanol binding to ethanol-sensitized (F14′A) receptors, along with expansion of the channel pore (Murail et al., 2012). Building on recent validations of anesthetic docking to GLIC (Liu et al., 2012), we have also used molecular dynamics-based free energy calculations to quantify favorable binding of anesthetics to the intersubunit cavity of the ethanol-sensitized GLIC variant (Broemstrup et al., 2013). Notably, efforts to simulate modulation of human receptors using glycine receptor homology models based on the nicotinic receptor (Cheng et al., 2008) and GLIC (Murail et al., 2011) predicted the preferential binding of ethanol to an intersubunit transmembrane cavity in the channel open state, suggesting that at least some of these modes of modulation are conserved across species.
Of importance for the future, simulations of structural transitions perform best when the beginning and ending states are known, using a directed molecular dynamics approach (Law et al., 2005). There are presently two examples of such double endpoints among ligand-gated ion channels: the open and closed states of the P2X4 receptor (Hattori and Gouaux, 2012), as well as the presumed-open (Bocquet et al., 2009; Hilf and Dutzler, 2009) and “locally closed” states of GLIC (Prevost et al., 2012). Further expansion of the repertoire of ion channel structures and improved computational power promise to reveal new details of receptor gating and modulation in the near future.
IV. Crystallographic Contributions
Despite over a decade of consensus on the broad structural features of pentameric ligand-gated ion channels, technical limitations to membrane protein crystallography (Carpenter et al., 2008) have historically limited structure/function studies of ion channel modulation by alcohols and anesthetics. Even in the presence of moderate-resolution (≥4 Å) structural data (Unwin, 2005), the small size and generic structure of these molecules renders them difficult to distinguish from water or other small solvents (Howard et al., 2011a,b). Consequently, the recent determination of both alcohol- and anesthetic-bound (Nury et al., 2011; Sauguet et al., 2013; Spurny et al., 2013) pentameric ligand-gated ion channel structures at atomic resolution, albeit from model organisms, poses novel opportunities to identify structural hallmarks of anesthetic binding sites, model their effects on ion channel gating, and design novel pharmacological agents.
A. Intersubunit Binding of Potentiators in Gloeobacter violaceus Ligand-Gated Ion Channel
The X-ray structure of wild-type GLIC, determined to 3.1 Å (Hilf and Dutzler, 2009) and 2.9 Å (Bocquet et al., 2009), revealed a transmembrane cavity that includes amino acid residues (F14′ and L17′) associated with alcohol potentiation. This cavity occupies the intersubunit interface, facing the channel pore (Nury et al., 2011). The 2.8-Å structure of the ethanol-sensitizing point mutant F14′A (Sauguet et al., 2013) was superimposable with that of wild-type GLIC, except in the region of the mutation. The loss of the phenylalanine side chain greatly enlarged the intersubunit cavity and filled it with an additional water molecule. Temperature factors in the pore and gating regions of the F14′A mutant were also greater than for the wild-type structure. Given that the GLIC structure has been associated with an open state of the channel, because of its crystallization under activating conditions (pH ≤ 4.6) and the capacity of the pore radius for ion conduction, increased temperature factors may relate to the reduced sensitivity of the F14′A variant to protons and, thus, to destabilization of channel opening.
The ethanol-sensitized GLIC variant was also cocrystallized with potentiating agents, including ethanol to 2.8 Å, and bromoethanol, as well as bromoform, to 3.1 Å each (Sauguet et al., 2013). The anomalous signal provided by bromine derivatization (Choe et al., 2002) of both alcohol and anesthetics confirmed their assignment to the intersubunit cavity enlarged by the F14′A mutation. The structure revealed hydrophobic interactions of ethanol with M2 residues I16′ and L17′ and suggested hydrogen bonds with N15′, E19′, and the backbone carbonyl of M1 N200. Bromoform bound in the same cavity as ethanol, but with exclusively hydrophobic interactions due to its lack of hydrogen bonding partners. Ethanol binding also reduced temperature factors back to levels approximating the wild-type receptor, suggesting stabilization of the putative open state of the structure (Sauguet et al., 2013). Notably, the recently determined crystal structure of GluCl contains the allosteric agonist ivermectin in a position overlapping that of ethanol in GLIC, including a hydrogen bond with the 15′ residue (Hibbs and Gouaux, 2011), consistent with a conserved mechanism of functional enhancement via drug binding in this region.
These structures agree remarkably well with previous functional evidence for modulation of eukaryotic, as well as prokaryotic, receptors described above and provide valuable insights toward further elucidation of ion channel modulation. It is particularly important to connect these snapshots to a mechanistic understanding of how alcohol and anesthetic binding affects channel structure and function, particularly during gating. Recent determination of a locally closed GLIC structure, for example, may facilitate further studies of modulator binding to alternative states of the channel (Prevost et al., 2012).
B. Inhibitory Binding Sites in Gloeobacter violaceus and Erwinia chrysanthemi Ligand-Gated Ion Channels
In addition to the intersubunit cavity occupied by potentiating alcohols and anesthetics, GLIC exhibits an additional transmembrane cavity within each subunit (Nury et al., 2011). Instead of containing water molecules and facing the channel pore, this cavity faces the domain exterior and contains constitutively-bound lipids that persist from the expression system throughout purification and crystallization (Bocquet et al., 2009). In cocrystal structures with wild-type GLIC, both volatile and intravenous general anesthetics occupied this intrasubunit cavity, displacing the bound lipids (Nury et al., 2011). Indeed, in addition to the intersubunit binding cavity described above, the ethanol-sensitized mutant F14′A retained the intrasubunit binding site in cocrystal structures with both bromoethanol and bromoform (Sauguet et al., 2013). This site contains anesthetic-sensitive residues V18′ and T255 (Nury et al., 2011), as well as amino acid positions that display saturation transfer to halothane (Bondarenko et al., 2013) and are photolabeled by propofol (Jayakar et al., 2013) in nicotinic receptors. Thus, the intrasubunit region contains a persistent binding site for alcohols and anesthetics independent of channel potentiation, suggesting it could mediate the inhibitory effects of these modulators.
Complications to this model arose from the apparent open conformation of the GLIC structure. Inhibitors should destabilize the open relative to nonconducting state, yet the inhibitory alcohols and anesthetics appeared to associate tightly with the intrasubunit cavity in the crystallized conformation (Nury et al., 2011). The locally closed GLIC structure showed changes in the size and shape of this cavity, suggesting differences in binding to nonconducting conformations (Prevost et al., 2012). Given mutational evidence inconsistent with direct, conserved binding of anesthetics in this region (Nury et al., 2011; Ghosh et al., 2013), the relevance of this binding mode to channel inhibition remains to be confirmed.
The recently determined structure of ELIC in complex with bromoform (Spurny et al., 2013), which inhibits channel function, implicated additional transmembrane sites of anesthetic action. In this structure, bromoform blocked the channel pore between the M2 L9′ and F16′ residues, consistent with previous nicotinic receptor labeling studies (Borghese et al., 2003a; Nirthanan et al., 2008) and GLIC simulations (Lebard et al., 2012). It is possible that this site of action is also relevant to GLIC inhibition, but has been disfavored under current crystallization conditions because of occupation of the pore by detergent molecules (Bocquet et al., 2009). A second allosteric site for bromoform was identified at the subunit interface, facing the lipid bilayer; a functional role for this site was supported by specific fluorescence quenching of two tryptophan residues by bromoform (Spurny et al., 2013). These residues aligned with tryptophans whose fluorescence was quenched by halothane binding in GLIC (Chen et al., 2010). If conserved, this binding site could have been masked in previous GLIC studies by crystallographic conditions artifactually favoring the open state. Bromoform density was also observed within each subunit of the ELIC extracellular domain (Spurny et al., 2013), although no functional evidence yet supports a role for this site in anesthetic modulation. Generally, the nonconducting state of the known ELIC structure (Hilf and Dutzler, 2008) may favor binding of inhibitors relative to GLIC, improving the likelihood of observing novel modes of interaction.
C. Mechanistic Implications of Crystallographic Binding Sites
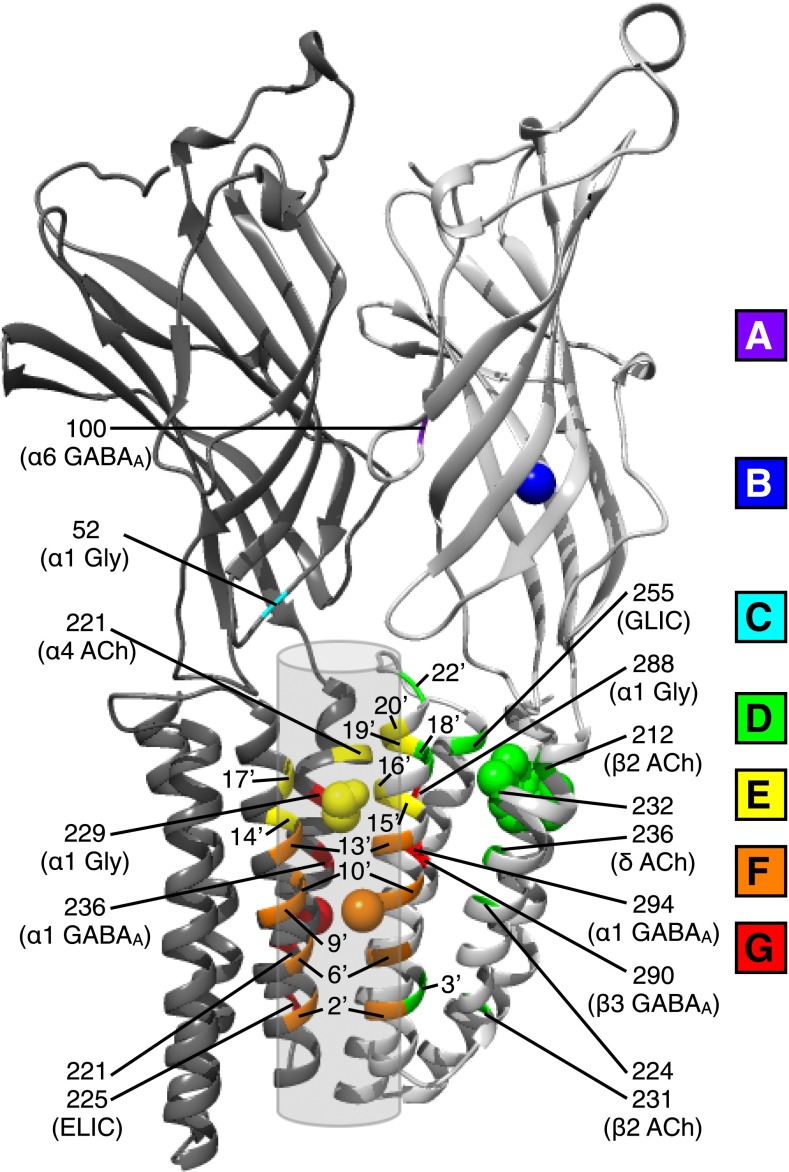
In light of longstanding functional evidence and recent structural studies, a limited set of possible interaction sites for alcohols and anesthetics is emerging in pentameric ligand-gated ion channels (see Fig. 1). Extracellular sites include the intersubunit benzodiazepine cleft in α6 GABAA receptors (Fig. 1A) (Wallner et al., 2006), an intrasubunit crystallographic bromoform site in ELIC (Fig. 1B) (Spurny et al., 2013), and Loop 2 at the extracellular/transmembrane domain interface of GABAA and glycine receptors (Fig. 1C) (Perkins et al., 2012).
Fig. 1.
Spectrum of sites implicated in direct modulation of pentameric ligand-gated ion channels by alcohols and other anesthetic agents. Ribbons depict crystal structure of two neighboring subunits of the prokaryotic GLIC protein, viewed from the channel pore (transparent cylinder). Putative binding sites are indicated by colored ribbons and labeled according to at least one of the GABAA, glycine (Gly), or nicotinic acetylcholine (ACh) receptor subtypes in which they were implicated. Residues in the M2 helix, many of which have been implicated in multiple subtypes, are labeled in prime notation for ease of comparison. (A) Competitive alcohol/benzodiazepine site, defined by the amino acid residue equivalent to position 100 in the α6 GABAA receptor subunit (purple). (B) Bromoform molecule (blue sphere) cocrystallized in the extracellular domains of the right-hand ELIC subunit (Protein Data Bank [PDB] ID 3ZKR) superimposed on the GLIC backbone (blue). (C) Amino acid residue 52 in extracellular loop 2 of the left-hand subunit at which mutations influence alcohol modulation of α1 glycine receptors (cyan). (D) Intrasubunit anesthetic binding site(s), defined by the structure of one propofol molecule (green spheres) cocrystallized in the right-hand GLIC subunit (PDB ID 3P50), along with amino acid positions that influenced anesthetic modulation in GLIC (M2 18′; M3 255) or were photolabeled by propofol derivatives (δ M1 232, 236; M2 18′) or sensitive to saturation transfer from halothane (α4 M1 222; β2 M1 212, 224, 231; M2 3′, 22′) in nicotinic receptors (green). (E) Intersubunit pore-facing potentiation site, defined by the structure of one ethanol molecule (yellow spheres) cocrystallized with an ethanol-sensitive GLIC variant (PDB ID 4HFE), along with amino acid positions that influenced alcohol and anesthetic modulation in multiple receptor subtypes (M2 14′–17′, 19′) or were sensitive to halothane saturation transfer in nicotinic receptors (α4 M1 221; M2 20′) (yellow). (F) Pore-blocking anesthetic binding site(s), defined by the structure of bromoform (orange sphere) cocrystallized with ELIC (PDB ID 3P4W), along with amino acid positions photolabeled by propofol derivatives in nicotinic receptors (M2 2′, 6′, 9′, 10′, 13′) (orange). Additional residues (M2 16′, 17′, 20′) influencing anesthetic modulation of nicotinic receptors are depicted in E (yellow). (G) Intersubunit membrane-facing anesthetic site(s), defined by the structure of bromoform (red sphere) cocrystallized with ELIC (PDB ID 3P4W), along with amino acid positions involved in anesthetic fluorescence quenching in ELIC (M1 221, 225) as well as GLIC, photolabeling of GABAA receptors by anesthetic derivatives (α1 M1 236; M3 294; β3 M3 290), or alcohol and anesthetic modulation of α1 glycine (M1 229; M3 288) and GABAA receptors (red).
In the transmembrane domain, four generalized interaction regions can be distinguished. First, the intrasubunit crystallographic GLIC anesthetic site (Fig. 1D) (Nury et al., 2011) was the first product of cocrystal studies in this field and agrees with recent photolabeling (Jayakar et al., 2013) and NMR studies (Bondarenko et al., 2013). However, the physiologic impact, if any, of binding in this region remains unclear (Nury et al., 2011; Ghosh et al., 2013); that is, ligand binding does not necessarily imply efficacy. Additional transmembrane domain residues facing more deeply buried portions of the subunit interior have also been implicated by NMR and could represent either long-range extensions of the same binding mode or a novel intrasubunit site (Bondarenko et al., 2013). Second, in contrast to the intrasubunit site, the intersubunit crystallographic GLIC potentiating site (Fig. 1E) (Sauguet et al., 2013) agrees remarkably well with previous literature on GABAA, glycine (Mihic et al., 1997), nicotinic (Borghese et al., 2003a), and 5-HT3 receptors (Sessoms-Sikes et al., 2003), as well as ivermectin binding to GluCl (Hibbs and Gouaux, 2011). Third, pore-facing residues throughout M2 (Fig. 1F) are implicated in anesthetic inhibition of nicotinic (Ziebell et al., 2004; Nirthanan et al., 2008) and GABAA receptors (Johnson et al., 2012), as well as ELIC (Spurny et al., 2013). Some of these residues also overlap portions of the intersubunit cavity, suggesting these binding regions could be contiguous. Finally, residues at the “outer” intersubunit interface (Fig. 1G), facing the lipid bilayer rather than the channel pore, influence modulation of GABAA receptors (Chiara et al., 2013), glycine receptors (Mihic et al., 1997; Lobo et al., 2008), and ELIC (Spurny et al., 2013). Given the large size of ivermectin, its binding site in GluCl also overlaps this region (Hibbs and Gouaux, 2011), along with the crystallographic inner intersubunit site. These sites may or may not be truly distinct, but mapping them onto the known structure of GLIC provides a starting point for testing new principles of binding and modulation.
A few general themes continue to emerge from structural data on anesthetic binding. Although the importance of certain sites, such as the intersubunit transmembrane cavity, seems corroborated now by multiple methods, it remains likely that small amphiphilic agents are capable of binding multiple locations on ligand-gated ion channels, perhaps mimicking endogenous lipid modulators. It may be a combination of binding events with either additive or competitive efficacies that results in the net modulation observed in functional assays. In addition, it is interesting to note the preponderance of implicated sites closely associated with the channel pore. Given the long distance over which agonist binding must propagate its pharmacological signal to open the ion channel, it is plausible that alcohols and anesthetics bind the receptor much closer to its “active site” (pore) than do neurotransmitter ligands; indeed, the “agonist” may turn out to be more of an “allosteric” agent than the modulators themselves (Taly et al., 2009).
V. From Structure Back to Function
The primary motivation for structural studies is to gain functional insight into the biologic importance of these proteins. In this section, we will discuss the application of structural data to translational research, connecting molecular models back to function and behavior and exploring promising avenues for therapeutic development.
A. Behavioral Pharmacology in Rodent Models
A central question in neuropharmacology is the behavioral consequence of drug action on a single protein. It is usually not possible to predict the effects of activation or inhibition of a single protein on specific behaviors, and the finding that most drugs act on multiple targets further complicates this problem. One approach has been genetic deletion (null mutant or knockout) of the protein. Although this tactic prevents drug action on a specific receptor, it also eliminates all receptor function, which greatly complicates interpretation of drug actions in null mutant animals (Crabbe et al., 2006). A much more powerful approach is to replace the normal gene with one that produces a mutated protein that is resistant to drug modulation but otherwise is normal in function (knock-in). Of course, this can only be successful if there is substantial information about the site of drug action as well as the structural mechanisms of normal receptor function. This approach has been applied to several pentameric ligand-gated ion channels and has been quite successful in linking specific receptor subunits with drug actions. One of the earliest and most elegant examples is the construction of knock-in mice with specific GABAA receptor subunits lacking actions of benzodiazepines or general anesthetics (Rudolph and Möhler, 2004; Morris et al., 2006; Tan et al., 2011). For example, these studies showed that benzodiazepine modulation of the α2 subunit of the GABAA receptor is critical for the antianxiety actions of these drugs and the general anesthetic action of propofol is due to action on GABAA receptors containing the β3 subunit (Jurd et al., 2003).
Ethanol provides an unusual challenge because it has the potential to alter many brain proteins, including multiple ligand-gated ion channels (Harris et al., 2008). Remarkably, the knock-in approach has nonetheless linked specific GABAA and glycine receptors to behavioral actions of alcohol (Boehm et al., 2006; Crabbe et al., 2006; Blednov et al., 2012). In particular, action of ethanol on the α2 subunit of the GABAA receptor appears important for the stimulating and aversive effects of ethanol, but not for other alcohol-related behaviors (Blednov et al., 2011). An important contribution of the approach is the ability to rule out potential targets. For example, the α1 subunit of the glycine receptor was considered one of the most likely mediators of spinal immobility produced by inhaled anesthetics (Eger et al., 2008), but this was shown not to be the case through use of knock-in mice (Borghese et al., 2012). A different approach was taken for nicotinic receptors, where gain of function was engineered into specific receptor subunits in mice (Drenan and Lester, 2012). These animals have proven useful in defining the role of specific nicotinic receptor subunits in electrophysiologic and behavioral phenotypes; for example, high-sensitivity α4 and α6 nicotinic receptors were recently shown to enhance alcohol-related reward behaviors (Liu et al., 2013; Powers et al., 2013). As evidenced by the identification of a novel anesthetic modulation site in glycine receptors based on crystallographic data from the ELIC homolog (Spurny et al., 2013), the advent of high-resolution structural data for alcohol and anesthetic binding to receptors in this family, along with improved simulation techniques, promises to enable higher certainty prediction of mutant phenotypes. These advances may allow engineering of mutations with much more specific and potent effects on drug sensitivity than has previously been possible.
B. Therapeutic Potential of Existing Agents
The discovery of amphiphilic cavities in pentameric ligand-gated ion channels raises the possibility of covert modulation of these receptors by pharmacologic agents that are only known to affect other proteins. One example is the finding that the analgesic action of cannabinoids is attributable, at least in part, to their actions on the α3 subunits of glycine receptors (Xiong et al., 2011, 2012). Cannabinoids were earlier thought to act only on the CB1/CB2 G protein–coupled receptors, demonstrating the potential for unexpected action of a long-studied class of compounds (Oz, 2006). Ivermectin, mentioned earlier, is another potential therapeutic based on its limitation of alcohol intake and preference in mice, presumably by acting at P2X4, GABAA, glycine, or nicotinic receptors rather than the GluCl channels it targets in worms. Understanding the role of specific receptor sites and subunits in discrete behaviors will allow new therapeutic approaches by repurposing of known compounds.
C. Opportunities for Novel Drug Design for Under-Targeted Ligand-Gated Ion Channels
The advent of high-resolution structural data for pentameric ligand-gated ion channel family members poses new opportunities to develop novel modulators to combat intoxication or improve induction of anesthesia. Docking to known receptor structures has recently been shown to recapitulate crystallographic binding sites (Liu et al., 2012; Broemstrup et al., 2013), validating the use of computational techniques in screening these novel targets. However, the utility of these methods in identifying new drugs remains to be demonstrated, particularly given that the receptors of greatest therapeutic interest (GABAA and glycine receptors) exhibit ion selectivity and modulation properties distinct from structurally-characterized receptors (GLIC and ELIC). The recently determined structure of GluCl (Hibbs and Gouaux, 2011) may prove a more useful template given its selectivity for anions and excellent alignment with other eukaryotic receptors (Bertaccini et al., 2013); however, the structural relevance of the bulky ivermectin molecule cocrystallized at the transmembrane subunit interface in this structure remains to be determined. Notably, docking of etomidate to GABAA receptor homology models based on both GLIC and GluCl corroborated functional evidence for binding (Chiara et al., 2012), supporting these structures as templates for future drug screening. Molecular dynamics may prove particularly valuable to this goal, as microsecond simulations in both GLIC (Murail et al., 2012) and a homology model of the glycine receptor (Murail et al., 2011) predicted the subsequently determined crystallographic binding site for ethanol (Sauguet et al., 2013).
Among human ligand-gated ion channels, GABAA receptors have been the most heavily targeted for drug development, yielding positive allosteric modulators, such as benzodiazepines, that are widely used as antianxiety and sedative drugs (Saari et al., 2011). In addition, many surgical procedures involve drugs such as midazolam and propofol, which are employed to enhance GABAA function (Sneyd and Rigby-Jones, 2010). In particular, the ρ subtypes of GABAA receptors (previously referred to as GABAC receptors) may prove particularly important targets for future therapeutic applications. For one thing, ρ GABAA receptors may be better modeled than other subtypes by prokaryotic homologs, which generally exhibit inhibition rather than potentiation by anesthetics (Weng et al., 2010; Spurny et al., 2013). For another, several lines of evidence link ρ GABAA receptors to physiologically relevant actions of ethanol. In addition to retina, ρ GABAA subunits have been found in many brain regions, and specific antagonists to ρ GABAA receptors enhance anxiety in the elevated plus maze, as well as learning and memory in the Morris water maze (Chebib et al., 2009; Flores-Gracia et al., 2010). Furthermore, ρ1 GABAA receptor expression in the nucleus accumbens is correlated with ethanol consumption and motor activation in mice (r = 0.77, ethanol preference in two-bottle choice test, 10% ethanol; r = −0.48, ethanol-induced motor response, distance traveled, 0- to 5-minute time interval; from genenetwork.org). In humans, family-based association analyses demonstrated that single nucleotide polymorphisms in both ρ1 (GABRR1) and ρ2 (GABRR2) genes are significantly associated with alcohol dependence, particularly with early onset (Xuei et al., 2010). Thus, emerging evidence supports a role for ρ GABAA receptors in alcohol-related processes, including anxiety, learning, memory, and dependence, and implicates this receptor as a new therapeutic target.
In addition, glycine receptors offer increasingly promising targets for pharmacologic development. Recent interest in this field has shifted focus to supraspinal glycine receptors found in reward-related pathways (Söderpalm and Ericson, 2013). Glycine receptors in the brain may contain α1, α2, or α3 subunits and may be homomeric (α only), as well as heteromeric (α + β) (Muller et al., 2008; Aroeira et al., 2011), making it more straightforward to model physiologically relevant receptors on homomeric prokaryotic templates. These receptors are synaptic, as well as extrasynaptic, and provide both phasic and tonic inhibition (Martin and Siggins, 2002; Zhang and Thio, 2007). In addition to their modulation properties in recombinant systems (Mascia et al., 1996a), the importance of glycine receptors in alcohol actions was recently highlighted in an examination of alcohol inhibition of lateral orbitofrontal cortex neurons. Surprisingly, GABAA receptors made no contribution to the inhibitory effects of ethanol and there was only a minor role for glutamate receptors; instead, most of the inhibition was attributable to enhancement of glycine receptor function (Badanich et al., 2013). In humans, genetic analysis of alcohol dependence in African Americans found the strongest association with markers near GLRA3, the gene encoding the α3 glycine receptor subunit (Han et al., 2013). Thus, both ρ GABAA and glycine receptors may mediate important actions of alcohol, including dependence. Moreover, recent validation of molecular dynamics approaches to characterizing ethanol binding sites in glycine receptor homology models suggests these channels may prove tractable to future drug design. Emerging structural information at the atomic level, including alterations of binding cavities during activation of these receptors, will allow for improved in silico screening and design of new ligands, providing a level of subunit and receptor specificity that has been difficult to achieve prior to structural data.
Abbreviations
- ELIC
Erwinia chrysanthemi ligand-gated ion channel
- GLIC
Gloeobacter violaceus ligand-gated ion channel
- GluCl
glutamate-gated chloride channel
- M1–M4
transmembrane helices 1–4
- PDB
Protein Data Bank
- Ro15-4513
3-ethyl-8-azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]-benzodiazepine-3-carboxylate
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Howard, Trudell, Harris.
Footnotes
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants R01-AA06399 (to R.A.H.) and R01-AA013378 (to J.R.T.)]; and startup funds from Skidmore College.
References
- Alifimoff JK, Firestone LL, Miller KW. (1989) Anaesthetic potencies of primary alkanols: implications for the molecular dimensions of the anaesthetic site. Br J Pharmacol 96:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J, Dickerson IM, Weiss DS. (1994) The agonist binding site of the gamma-aminobutyric acid type A channel is not formed by the extracellular cysteine loop. Mol Pharmacol 45:317–323 [PubMed] [Google Scholar]
- Aroeira RI, Ribeiro JA, Sebastião AM, Valente CA. (2011) Age-related changes of glycine receptor at the rat hippocampus: from the embryo to the adult. J Neurochem 118:339–353 [DOI] [PubMed] [Google Scholar]
- Badanich KA, Mulholland PJ, Beckley JT, Trantham-Davidson H, Woodward JJ. (2013) Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism. Neuropsychopharmacology 38:1176–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista-Hon DT, Deeb TZ, Lambert JJ, Peters JA, Hales TG. (2013) The minimum M3–M4 loop length of neurotransmitter-activated pentameric receptors is critical for the structural integrity of cytoplasmic portals. J Biol Chem 288:21558–21568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Corradi J, Bouzat C. (2009) Structural basis of activation of cys-loop receptors: the extracellular-transmembrane interface as a coupling region. Mol Neurobiol 40:236–252 [DOI] [PubMed] [Google Scholar]
- Bertaccini E, Trudell JR. (2002) Predicting the transmembrane secondary structure of ligand-gated ion channels. Protein Eng 15:443–454 [DOI] [PubMed] [Google Scholar]
- Bertaccini EJ, Lindahl E, Sixma T, Trudell JR. (2008) Effect of cobratoxin binding on the normal mode vibration within acetylcholine binding protein. J Chem Inf Model 48:855–860 [DOI] [PubMed] [Google Scholar]
- Bertaccini EJ, Trudell JR, Lindahl E. (2005) Normal mode analysis reveals the channel gating motion within a ligand gated ion channel model. Int Congr Ser 1283:160–163 [Google Scholar]
- Bertaccini EJ, Trudell JR, Lindahl E. (2007) Normal-mode analysis of the glycine α1 receptor by three separate methods. J Chem Inf Model 47:1572–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaccini EJ, Yoluk O, Lindahl ER, Trudell JR. (2013) Assessment of homology templates and an anesthetic binding site within the γ-aminobutyric acid receptor. Anesthesiology 119:1087–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton MP, Cohen JB. (1994) Identifying the lipid-protein interface of the Torpedo nicotinic acetylcholine receptor: secondary structure implications. Biochemistry 33:2859–2872 [DOI] [PubMed] [Google Scholar]
- Blednov YA, Borghese CM, McCracken ML, Benavidez JM, Geil CR, Osterndorff-Kahanek E, Werner DF, Iyer S, Swihart A, Harrison NL, et al. (2011) Loss of ethanol conditioned taste aversion and motor stimulation in knockin mice with ethanol-insensitive α2-containing GABA(A) receptors. J Pharmacol Exp Ther 336:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Mayfield RD, Belknap J, Harris RA. (2012) Behavioral actions of alcohol: phenotypic relations from multivariate analysis of mutant mouse data. Genes Brain Behav 11:424–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux J-P, Delarue M, Corringer P-J. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457:111–114 [DOI] [PubMed] [Google Scholar]
- Bocquet N, Prado de Carvalho L, Cartaud J, Neyton J, Le Poupon C, Taly A, Grutter T, Changeux J-P, Corringer P-J. (2007) A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature 445:116–119 [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Ponomarev I, Blednov YA, Harris RA. (2006) From gene to behavior and back again: new perspectives on GABAA receptor subunit selectivity of alcohol actions. Adv Pharmacol 54:171–203 [DOI] [PubMed] [Google Scholar]
- Bondarenko V, Mowrey D, Liu LT, Xu Y, Tang P. (2013) NMR resolved multiple anesthetic binding sites in the TM domains of the α4β2 nAChR. Biochim Biophys Acta 1828:398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko V, Mowrey D, Tillman T, Cui T, Liu LT, Xu Y, Tang P. (2012) NMR structures of the transmembrane domains of the α4β2 nAChR. Biochim Biophys Acta 1818:1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Blednov YA, Quan Y, Iyer SV, Xiong W, Mihic SJ, Zhang L, Lovinger DM, Trudell JR, Homanics GE, et al. (2012) Characterization of two mutations, M287L and Q266I, in the α1 glycine receptor subunit that modify sensitivity to alcohols. J Pharmacol Exp Ther 340:304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Henderson LA, Bleck V, Trudell JR, Harris RA. (2003a) Sites of excitatory and inhibitory actions of alcohols on neuronal α2β4 nicotinic acetylcholine receptors. J Pharmacol Exp Ther 307:42–52 [DOI] [PubMed] [Google Scholar]
- Borghese CM, Wang L, Bleck V, Harris RA. (2003b) Mutation in neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes blocks ethanol action. Addict Biol 8:313–318 [DOI] [PubMed] [Google Scholar]
- Bowery NG, Smart TG. (2006) GABA and glycine as neurotransmitters: a brief history. Br J Pharmacol 147 (Suppl 1):S109–S119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannigan G, LeBard DN, Hénin J, Eckenhoff RG, Klein ML. (2010) Multiple binding sites for the general anesthetic isoflurane identified in the nicotinic acetylcholine receptor transmembrane domain. Proc Natl Acad Sci USA 107:14122–14127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411:269–276 [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. (2012) Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 73:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broemstrup T, Howard RJ, Trudell JR, Harris RA, Lindahl E. (2013) Inhibition versus potentiation of ligand-gated ion channels can be altered by a single mutation that moves ligands between intra- and inter-subunit sites. Structure 21: 1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B, Karplus M. (1983) Harmonic dynamics of proteins: normal modes and fluctuations in bovine pancreatic trypsin inhibitor. Proc Natl Acad Sci USA 80:6571–6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. (1983) CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J Comput Chem 4:187–217 [Google Scholar]
- Campagna JA, Miller KW, Forman SA. (2003) Mechanisms of actions of inhaled anesthetics. N Engl J Med 348:2110–2124 [DOI] [PubMed] [Google Scholar]
- Carpenter EP, Beis K, Cameron AD, Iwata S. (2008) Overcoming the challenges of membrane protein crystallography. Curr Opin Struct Biol 18:581–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani S, Auerbach A. (2005) A speed limit for conformational change of an allosteric membrane protein. Proc Natl Acad Sci USA 102:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J-P. (2012) Conscious processing: implications for general anesthesia. Curr Opin Anaesthesiol 25:397–404 [DOI] [PubMed] [Google Scholar]
- Chau P, Höifödt-Lidö H, Löf E, Söderpalm B, Ericson M. (2010) Glycine receptors in the nucleus accumbens involved in the ethanol intake-reducing effect of acamprosate. Alcohol Clin Exp Res 34:39–45 [DOI] [PubMed] [Google Scholar]
- Chebib M, Hinton T, Schmid KL, Brinkworth D, Qian H, Matos S, Kim H-L, Abdel-Halim H, Kumar RJ, Johnston GAR, et al. (2009) Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J Pharmacol Exp Ther 328:448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Cheng MH, Xu Y, Tang P. (2010) Anesthetic binding in a pentameric ligand-gated ion channel: GLIC. Biophys J 99:1801–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MH, Coalson RD, Cascio M. (2008) Molecular dynamics simulations of ethanol binding to the transmembrane domain of the glycine receptor: implications for the channel potentiation mechanism. Proteins 71:972–981 [DOI] [PubMed] [Google Scholar]
- Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. (2012) Mapping general anesthetic binding site(s) in human α1β3 γ-aminobutyric acid type A receptors with [³H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry 51:836–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. (2013) Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 γ-aminobutyric acid type A (GABAA) receptor. J Biol Chem 288:19343–19357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J, Suresh S, Wisedchaisri G, Kennedy KJ, Gelb MH, Hol WGJ. (2002) Anomalous differences of light elements in determining precise binding modes of ligands to glycerol-3-phosphate dehydrogenase. Chem Biol 9:1189–1197 [DOI] [PubMed] [Google Scholar]
- Cohen JB, Weber M, Huchet M, Changeux J-P. (1972) Purification from Torpedo marmorata electric tissue of membrane fragments particularly rich in cholinergic receptor protein. FEBS Lett 26:43–47 [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. (2009) A nomenclature for ligand-gated ion channels. Neuropharmacology 56:2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer P-J, Baaden M, Bocquet N, Delarue M, Dufresne V, Nury H, Prevost M, Van Renterghem C. (2010) Atomic structure and dynamics of pentameric ligand-gated ion channels: new insight from bacterial homologues. J Physiol 588:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. (2006) Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol 11:195–269 [DOI] [PubMed] [Google Scholar]
- Crawford DK, Trudell JR, Bertaccini EJ, Li K, Davies DL, Alkana RL. (2007) Evidence that ethanol acts on a target in Loop 2 of the extracellular domain of α1 glycine receptors. J Neurochem 102:2097–2109 [DOI] [PubMed] [Google Scholar]
- Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. (1994) Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371:707–711 [DOI] [PubMed] [Google Scholar]
- Cutting GR, Lu L, O’Hara BF, Kasch LM, Montrose-Rafizadeh C, Donovan DM, Shimada S, Antonarakis SE, Guggino WB, Uhl GR, et al. (1991) Cloning of the gamma-aminobutyric acid (GABA) ρ 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci USA 88:2673–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Alkana RL. (1998) Direct antagonism of ethanol’s effects on GABA(A) receptors by increased atmospheric pressure. Alcohol Clin Exp Res 22:1689–1697 [PubMed] [Google Scholar]
- Davies DL, Crawford DK, Trudell JR, Mihic SJ, Alkana RL. (2004) Multiple sites of ethanol action in α1 and α2 glycine receptors suggested by sensitivity to pressure antagonism. J Neurochem 89:1175–1185 [DOI] [PubMed] [Google Scholar]
- Davies PA. (2011) Allosteric modulation of the 5-HT(3) receptor. Curr Opin Pharmacol 11:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue M, Sanejouand Y-H. (2002) Simplified normal mode analysis of conformational transitions in DNA-dependent polymerases: the elastic network model. J Mol Biol 320:1011–1024 [DOI] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang Z-Z, Chen L. (2007) Crystal structure of the extracellular domain of nAChR α1 bound to α-bungarotoxin at 1.94 A resolution. Nat Neurosci 10:953–962 [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Mihic SJ, Liu Y, Deitrich RA, Harris RA. (1996) Actions of long chain alcohols on GABAA and glutamate receptors: relation to in vivo effects. Br J Pharmacol 118:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Lester HA. (2012) Insights into the neurobiology of the nicotinic cholinergic system and nicotine addiction from mice expressing nicotinic receptors harboring gain-of-function mutations. Pharmacol Rev 64:869–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Kollman PA. (1998) Pathways to a protein folding intermediate observed in a 1-microsecond simulation in aqueous solution. Science 282:740–744 [DOI] [PubMed] [Google Scholar]
- Duret G, Van Renterghem C, Weng Y, Prevost M, Moraga-Cid G, Huon C, Sonner JM, Corringer P-J. (2011) Functional prokaryotic-eukaryotic chimera from the pentameric ligand-gated ion channel family. Proc Natl Acad Sci USA 108:12143–12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre S, Becker C-M, Betz H. (2012) Inhibitory glycine receptors: an update. J Biol Chem 287:40216–40223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger EI, 2nd, Raines DE, Shafer SL, Hemmings HC, Jr, Sonner JM. (2008) Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg 107:832–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood P, Role LW. (1998) Neuronal nicotinic acetylcholine receptor modulation by general anesthetics. Toxicol Lett 100-101:149–153 [DOI] [PubMed] [Google Scholar]
- Flores-Gracia C, Nuche-Bricaire A, Crespo-Ramírez M, Miledi R, Fuxe K, Pérez de la Mora M. (2010) GABA(A) ρ receptor mechanisms in the rat amygdala and its role in the modulation of fear and anxiety. Psychopharmacology (Berl) 212:475–484 [DOI] [PubMed] [Google Scholar]
- Forman SA, Miller KW. (2011) Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels. Can J Anaesth 58:191–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia PS, Kolesky SE, Jenkins A. (2010) General anesthetic actions on GABA(A) receptors. Curr Neuropharmacol 8:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B, Satyshur KA, Czajkowski C. (2013) Propofol binding to the resting state of the gloeobacter violaceus ligand-gated ion channel (GLIC) induces structural changes in the inter- and intrasubunit transmembrane domain (TMD) cavities. J Biol Chem 288:17420–17431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G, Schmieden V, Schofield PR, Seeburg PH, Siddique T, Mohandas TK, Becker CM, Betz H. (1990) α subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J 9:771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Gelernter J, Kranzler HR, Yang B-Z. (2013) Ordered subset linkage analysis based on admixture proportion identifies new linkage evidence for alcohol dependence in African-Americans. Hum Genet 132:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Trudell JR, Mihic SJ. (2008) Ethanol’s molecular targets. Sci Signal 1:re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Gouaux E. (2012) Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 485:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B, Kutzner C, van der Spoel D, Lindahl E. (2008) Gromacs 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447 [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf RJC, Dutzler R. (2008) X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452:375–379 [DOI] [PubMed] [Google Scholar]
- Hilf RJC, Dutzler R. (2009) Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457:115–118 [DOI] [PubMed] [Google Scholar]
- Hinsen K. (1998) Analysis of domain motions by approximate normal mode calculations. Proteins 33:417–429 [DOI] [PubMed] [Google Scholar]
- Howard RJ, Murail S, Ondricek KE, Corringer P-J, Lindahl E, Trudell JR, Harris RA. (2011a) Structural basis for alcohol modulation of a pentameric ligand-gated ion channel. Proc Natl Acad Sci USA 108:12149–12154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, Slesinger PA, Davies DL, Das J, Trudell JR, Harris RA. (2011b) Alcohol-binding sites in distinct brain proteins: the quest for atomic level resolution. Alcohol Clin Exp Res 35:1561–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst R, Rollema H, Bertrand D. (2013) Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther 137:22–54 [DOI] [PubMed] [Google Scholar]
- Jansen M, Bali M, Akabas MH. (2008) Modular design of Cys-loop ligand-gated ion channels: functional 5-HT3 and GABA ρ1 receptors lacking the large cytoplasmic M3M4 loop. J Gen Physiol 131:137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakar SS, Dailey WP, Eckenhoff RG, Cohen JB. (2013) Identification of propofol binding sites in a nicotinic acetylcholine receptor with a photoreactive propofol analog. J Biol Chem 288:6178–6189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A, Franks NP, Lieb WR. (1996) Actions of general anaesthetics on 5-HT3 receptors in N1E-115 neuroblastoma cells. Br J Pharmacol 117:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A, Greenblatt EP, Faulkner HJ, Bertaccini E, Light A, Lin A, Andreasen A, Viner A, Trudell JR, Harrison NL. (2001) Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J Neurosci 21:RC136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WD, 2nd, Howard RJ, Trudell JR, Harris RA. (2012) The TM2 6′ position of GABA(A) receptors mediates alcohol inhibition. J Pharmacol Exp Ther 340:445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Harris RA. (2006) Sites in TM2 and 3 are critical for alcohol-induced conformational changes in GABA receptors. J Neurochem 96:885–892 [DOI] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, et al. (2003) General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor β3 subunit. FASEB J 17:250–252 [DOI] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. (2010) Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 208:613–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Jenkins A, Kelley JC, Trudell JR, Harrison NL. (2003) Coupling of agonist binding to channel gating in the GABA(A) receptor. Nature 421:272–275 [DOI] [PubMed] [Google Scholar]
- Kent DW, Halsey MJ, Eger EI, 2nd, Kent B. (1977) Isoflurane anesthesia and pressure antagonism in mice. Anesth Analg 56:97–101 [DOI] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer P-J, Galzi JL, Changeux J-P, Bertrand D. (1998) Ivermectin: a positive allosteric effector of the α7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol 53:283–294 [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. (2009) The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 205:529–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RJ, Henchman RH, McCammon JA. (2005) A gating mechanism proposed from a simulation of a human α7 nicotinic acetylcholine receptor. Proc Natl Acad Sci USA 102:6813–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBard DN, Hénin J, Eckenhoff RG, Klein ML, Brannigan G. (2012) General anesthetics predicted to block the GLIC pore with micromolar affinity. PLOS Comput Biol 8:e1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Li L, Lasalde J, Rojas L, McNamee M, Ortiz-Miranda SI, Pappone P. (1994) Mutations in the M4 domain of Torpedo californica acetylcholine receptor dramatically alter ion channel function. Biophys J 66:646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G-D, Chiara DC, Cohen JB, Olsen RW. (2010) Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J Biol Chem 285:8615–8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G-D, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. (2006) Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci 26:11599–11605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl E, Azuara C, Koehl P, Delarue M. (2006) NOMAD-Ref: visualization, deformation and refinement of macromolecular structures based on all-atom normal mode analysis. Nucleic Acids Res 34 (Web Server issue):W52–W56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hendrickson LM, Guildford MJ, Zhao-Shea R, Gardner PD, Tapper AR. (2013) Nicotinic acetylcholine receptors containing the α4 subunit modulate alcohol reward. Biol Psychiatry 73:738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LT, Haddadian EJ, Willenbring D, Xu Y, Tang P. (2010) Higher susceptibility to halothane modulation in open- than in closed-channel α4β2 nAChR revealed by molecular dynamics simulations. J Phys Chem B 114:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Perez-Aguilar JM, Liang D, Saven JG. (2012) Binding site and affinity prediction of general anesthetics to protein targets using docking. Anesth Analg 114:947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo IA, Harris RA, Trudell JR. (2008) Cross-linking of sites involved with alcohol action between transmembrane segments 1 and 3 of the glycine receptor following activation. J Neurochem 104:1649–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo IA, Trudell JR, Harris RA. (2006) Accessibility to residues in transmembrane segment four of the glycine receptor. Neuropharmacology 50:174–181 [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Homanics GE. (2007) Tonic for what ails us? high-affinity GABAA receptors and alcohol. Alcohol 41:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynagh T, Lynch JW. (2010) A glycine residue essential for high ivermectin sensitivity in Cys-loop ion channel receptors. Int J Parasitol 40:1477–1481 [DOI] [PubMed] [Google Scholar]
- Machu TK, Harris RA. (1994) Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther 271:898–905 [PubMed] [Google Scholar]
- Martin G, Siggins GR. (2002) Electrophysiological evidence for expression of glycine receptors in freshly isolated neurons from nucleus accumbens. J Pharmacol Exp Ther 302:1135–1145 [DOI] [PubMed] [Google Scholar]
- Martínez-Delgado G, Estrada-Mondragón A, Miledi R, Martínez-Torres A. (2010) An update on GABAρ receptors. Curr Neuropharmacol 8:422–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Machu TK, Harris RA. (1996a) Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br J Pharmacol 119:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. (1996b) A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol 50:402–406 [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. (2000) Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA 97:9305–9310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Trudell JR, Goldstein BE, Harris RA, Mihic SJ. (2010) Zinc enhances ethanol modulation of the α1 glycine receptor. Neuropharmacology 58:676–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Harris RA. (1996) Inhibition of ρ1 receptor GABAergic currents by alcohols and volatile anesthetics. J Pharmacol Exp Ther 277:411–416 [PubMed] [Google Scholar]
- Mihic SJ, McQuilkin SJ, Eger EI, 2nd, Ionescu P, Harris RA. (1994) Potentiation of γ-aminobutyric acid type A receptor-mediated chloride currents by novel halogenated compounds correlates with their abilities to induce general anesthesia. Mol Pharmacol 46:851–857 [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. (1997) Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389:385–389 [DOI] [PubMed] [Google Scholar]
- Miller DW, Dill KA. (1997) Ligand binding to proteins: the binding landscape model. Protein Sci 6:2166–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester HA. (2011) Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron 70:20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423:949–955 [DOI] [PubMed] [Google Scholar]
- Morris HV, Dawson GR, Reynolds DS, Atack JR, Stephens DN. (2006) Both α2 and α3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. Eur J Neurosci 23:2495–2504 [DOI] [PubMed] [Google Scholar]
- Mowrey D, Cheng MH, Liu LT, Willenbring D, Lu X, Wymore T, Xu Y, Tang P. (2013) Asymmetric ligand binding facilitates conformational transitions in pentameric ligand-gated ion channels. J Am Chem Soc 135:2172–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E, Le-Corronc H, Legendre P. (2008) Extrasynaptic and postsynaptic receptors in glycinergic and GABAergic neurotransmission: a division of labor? Front Mol Neurosci 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murail S, Howard RJ, Broemstrup T, Bertaccini EJ, Harris RA, Trudell JR, Lindahl E. (2012) Molecular mechanism for the dual alcohol modulation of Cys-loop receptors. PLOS Comput Biol 8:e1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murail S, Wallner B, Trudell JR, Bertaccini E, Lindahl E. (2011) Microsecond simulations indicate that ethanol binds between subunits and could stabilize an open-state model of a glycine receptor. Biophys J 100:1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahiro M, Arakawa O, Nishimura T, Narahashi T. (1996) Potentiation of GABA-induced Cl- current by a series of n-alcohols disappears at a cutoff point of a longer-chain n-alcohol in rat dorsal root ganglion neurons. Neurosci Lett 205:127–130 [DOI] [PubMed] [Google Scholar]
- Nirthanan S, Garcia G, 3rd, Chiara DC, Husain SS, Cohen JB. (2008) Identification of binding sites in the nicotinic acetylcholine receptor for TDBzl-etomidate, a photoreactive positive allosteric effector. J Biol Chem 283:22051–22062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nury H, Poitevin F, Van Renterghem C, Changeux J-P, Corringer P-J, Delarue M, Baaden M. (2010) One-microsecond molecular dynamics simulation of channel gating in a nicotinic receptor homologue. Proc Natl Acad Sci USA 107:6275–6280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, Changeux J-P, Sonner JM, Delarue M, Corringer P-J. (2011) X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469:428–431 [DOI] [PubMed] [Google Scholar]
- Nys M, Kesters D, Ulens C. (2013) Structural insights into Cys-loop receptor function and ligand recognition. Biochem Pharmacol 86:1042–1053 [DOI] [PubMed] [Google Scholar]
- Oz M. (2006) Receptor-independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmacol Ther 111:114–144 [DOI] [PubMed] [Google Scholar]
- Peoples RW, Weight FF. (1999) Differential alcohol modulation of GABA(A) and NMDA receptors. Neuroreport 10:97–101 [DOI] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Asatryan L, Davies DL, Alkana RL. (2012) Charge and geometry of residues in the loop 2 β hairpin differentially affect agonist and ethanol sensitivity in glycine receptors. J Pharmacol Exp Ther 341:543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. (2008) Targets for ethanol action and antagonism in loop 2 of the extracellular domain of glycine receptors. J Neurochem 106:1337–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. (2010) Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol Ther 127:53–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Asatryan L, Alkana RL, Davies DL. (2009) Loop 2 structure in glycine and GABA(A) receptors plays a key role in determining ethanol sensitivity. J Biol Chem 284:27304–27314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JA, Cooper MA, Carland JE, Livesey MR, Hales TG, Lambert JJ. (2010) Novel structural determinants of single channel conductance and ion selectivity in 5-hydroxytryptamine type 3 and nicotinic acetylcholine receptors. J Physiol 588:587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone P, Pande VS. (2006) Can conformational change be described by only a few normal modes? Biophys J 90:1583–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K. (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MS, Broderick HJ, Drenan RM, Chester JA. (2013) Nicotinic acetylcholine receptors containing α6 subunits contribute to alcohol reward-related behaviours. Genes Brain Behav 12:543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt MB, Husain SS, Miller KW, Cohen JB. (2000) Identification of sites of incorporation in the nicotinic acetylcholine receptor of a photoactivatible general anesthetic. J Biol Chem 275:29441–29451 [DOI] [PubMed] [Google Scholar]
- Prevost MS, Sauguet L, Nury H, Van Renterghem C, Huon C, Poitevin F, Baaden M, Delarue M, Corringer P-J. (2012) A locally closed conformation of a bacterial pentameric proton-gated ion channel. Nat Struct Mol Biol 19:642–649 [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. (2004) Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol 44:475–498 [DOI] [PubMed] [Google Scholar]
- Saari TI, Uusi-Oukari M, Ahonen J, Olkkola KT. (2011) Enhancement of GABAergic activity: neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacol Rev 63:243–267 [DOI] [PubMed] [Google Scholar]
- Samson AO, Levitt M. (2008) Inhibition mechanism of the acetylcholine receptor by α-neurotoxins as revealed by normal-mode dynamics. Biochemistry 47:4065–4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martin L, Cerda F, Jimenez V, Fuentealba J, Muñoz B, Aguayo LG, Guzman L. (2012) Inhibition of the ethanol-induced potentiation of α1 glycine receptor by a small peptide that interferes with Gβγ binding. J Biol Chem 287:40713–40721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Howard RJ, Malherbe L, Lee US, Corringer P-J, Harris RA, Delarue M. (2013) Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun 4:1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul B, Schmieden V, Kling C, Mülhardt C, Gass P, Kuhse J, Becker CM. (1994) Point mutation of glycine receptor α 1 subunit in the spasmodic mouse affects agonist responses. FEBS Lett 350:71–76 [DOI] [PubMed] [Google Scholar]
- Sessoms-Sikes JS, Hamilton ME, Liu L-X, Lovinger DM, Machu TK. (2003) A mutation in transmembrane domain II of the 5-hydroxytryptamine(3A) receptor stabilizes channel opening and alters alcohol modulatory actions. J Pharmacol Exp Ther 306:595–604 [DOI] [PubMed] [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. (2001) Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem 276:12556–12564 [DOI] [PubMed] [Google Scholar]
- Shan Y, Kim ET, Eastwood MP, Dror RO, Seeliger MA, Shaw DE. (2011) How does a drug molecule find its target binding site? J Am Chem Soc 133:9181–9183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Lindemeyer AK, Gonzalez C, Shao XM, Spigelman I, Olsen RW, Liang J. (2012) Dihydromyricetin as a novel anti-alcohol intoxication medication. J Neurosci 32:390–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. (2002) Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem 2:795–816 [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R. (1987) Effect of avermectin B1a on chick neuronal gamma-aminobutyrate receptor channels expressed in Xenopus oocytes. Mol Pharmacol 32:749–752 [PubMed] [Google Scholar]
- Sneyd JR, Rigby-Jones AE. (2010) New drugs and technologies, intravenous anaesthesia is on the move (again). Br J Anaesth 105:246–254 [DOI] [PubMed] [Google Scholar]
- Söderpalm B, Ericson M. (2013) Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr Top Behav Neurosci 13:127–161 [DOI] [PubMed] [Google Scholar]
- Spurny R, Billen B, Howard RJ, Brams M, Debaveye S, Price KL, Weston DA, Strelkov SV, Tytgat J, Bertrand S, et al. (2013) Multisite binding of a general anesthetic to the prokaryotic pentameric Erwinia chrysanthemi ligand-gated ion channel (ELIC). J Biol Chem 288:8355–8364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurny R, Ramerstorfer J, Price K, Brams M, Ernst M, Nury H, Verheij M, Legrand P, Bertrand D, Bertrand S, et al. (2012) Pentameric ligand-gated ion channel ELIC is activated by GABA and modulated by benzodiazepines. Proc Natl Acad Sci USA 109:E3028–E3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson FA. (2012) Introduction to thematic minireview series on celebrating the discovery of the cysteine loop ligand-gated ion channel superfamily. J Biol Chem 287:40205–40206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R, Rüsch D, Solt K, Raines DE, Davies PA. (2005) Modulation of human 5-hydroxytryptamine type 3AB receptors by volatile anesthetics and n-alcohols. J Pharmacol Exp Ther 314:338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Corringer P-J, Guedin D, Lestage P, Changeux J-P. (2009) Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov 8:733–750 [DOI] [PubMed] [Google Scholar]
- Taly A, Delarue M, Grutter T, Nilges M, Le Novère N, Corringer P-J, Changeux J-P. (2005) Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophys J 88:3954–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Rudolph U, Lüscher C. (2011) Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci 34:188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasneem A, Iyer LM, Jakobsson E, Aravind L. (2005) Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol 6:R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lester HA, Lummis SCR. (2010) The structural basis of function in Cys-loop receptors. Q Rev Biophys 43:449–499 [DOI] [PubMed] [Google Scholar]
- Tirion MM. (1996) Large amplitude elastic motions in proteins from a single-parameter, atomic analysis. Phys Rev Lett 77:1905–1908 [DOI] [PubMed] [Google Scholar]
- Trudell JR, Bertaccini E. (2004) Comparative modeling of a GABAA α1 receptor using three crystal structures as templates. J Mol Graph Model 23:39–49 [DOI] [PubMed] [Google Scholar]
- Unwin N. (1993) Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol 229:1101–1124 [DOI] [PubMed] [Google Scholar]
- Unwin N. (1998) The nicotinic acetylcholine receptor of the Torpedo electric ray. J Struct Biol 121:181–190 [DOI] [PubMed] [Google Scholar]
- Unwin N. (2005) Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol 346:967–989 [DOI] [PubMed] [Google Scholar]
- Vemparala S, Saiz L, Eckenhoff RG, Klein ML. (2006) Partitioning of anesthetics into a lipid bilayer and their interaction with membrane-bound peptide bundles. Biophys J 91:2815–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithlani M, Terunuma M, Moss SJ. (2011) The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev 91:1009–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. (2006) Low dose acute alcohol effects on GABA A receptor subtypes. Pharmacol Ther 112:513–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzien F, Puller C, O’Sullivan GA, Paarmann I, Betz H. (2012) Distribution of the glycine receptor β-subunit in the mouse CNS as revealed by a novel monoclonal antibody. J Comp Neurol 520:3962–3981 [DOI] [PubMed] [Google Scholar]
- Weng Y, Yang L, Corringer P-J, Sonner JM. (2010) Anesthetic sensitivity of the Gloeobacter violaceus proton-gated ion channel. Anesth Analg 110:59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ. (1999) The GABA-A receptor gene family: new targets for therapeutic intervention. Neurochem Int 34:387–390 [DOI] [PubMed] [Google Scholar]
- Wick MJ, Mihic SJ, Ueno S, Mascia MP, Trudell JR, Brozowski SJ, Ye Q, Harrison NL, Harris RA. (1998) Mutations of gamma-aminobutyric acid and glycine receptors change alcohol cutoff: evidence for an alcohol receptor? Proc Natl Acad Sci USA 95:6504–6509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbring D, Liu LT, Mowrey D, Xu Y, Tang P. (2011) Isoflurane alters the structure and dynamics of GLIC. Biophys J 101:1905–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Cheng K, Cui T, Godlewski G, Rice KC, Xu Y, Zhang L. (2011) Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat Chem Biol 7:296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Wu X, Li F, Cheng K, Rice KC, Lovinger DM, Zhang L. (2012) A common molecular basis for exogenous and endogenous cannabinoid potentiation of glycine receptors. J Neurosci 32:5200–5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuei X, Flury-Wetherill L, Dick D, Goate A, Tischfield J, Nurnberger J, Jr, Schuckit M, Kramer J, Kuperman S, Hesselbrock V, et al. (2010) GABRR1 and GABRR2, encoding the GABA-A receptor subunits ρ1 and ρ2, are associated with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet 153B:418–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley MM, Wyatt L, Khoja S, Asatryan L, Ramaker MJ, Finn DA, Alkana RL, Huynh N, Louie SG, Petasis NA, et al. (2012) Ivermectin reduces alcohol intake and preference in mice. Neuropharmacology 63:190–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, Harris RA. (1998) Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position α267. J Biol Chem 273:3314–3319 [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Avila A, Guzmán L, Figueroa M, Peoples RW, Aguayo LG. (2010) Molecular requirements for ethanol differential allosteric modulation of glycine receptors based on selective Gbetagamma modulation. J Biol Chem 285:30203–30213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HX, Thio LL. (2007) Zinc enhances the inhibitory effects of strychnine-sensitive glycine receptors in mouse hippocampal neurons. J Neurophysiol 98:3666–3676 [DOI] [PubMed] [Google Scholar]
- Ziebell MR, Nirthanan S, Husain SS, Miller KW, Cohen JB. (2004) Identification of binding sites in the nicotinic acetylcholine receptor for [3H]azietomidate, a photoactivatable general anesthetic. J Biol Chem 279:17640–17649 [DOI] [PubMed] [Google Scholar]
- Zimmermann I, Dutzler R. (2011) Ligand activation of the prokaryotic pentameric ligand-gated ion channel ELIC. PLoS Biol 9:e1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Aistrup GL, Marszalec W, Gillespie A, Chavez-Noriega LE, Yeh JZ, Narahashi T. (2001) Dual action of n-alcohols on neuronal nicotinic acetylcholine receptors. Mol Pharmacol 60:700–711 [PubMed] [Google Scholar]