Abstract
It has become increasingly clear that the accumulation of proteins in specific regions of the plasma membrane can facilitate cellular communication. These regions, termed signaling microdomains, are found throughout the blood vessel wall where cellular communication, both within and between cell types, must be tightly regulated to maintain proper vascular function. We will define a cellular signaling microdomain and apply this definition to the plethora of means by which cellular communication has been hypothesized to occur in the blood vessel wall. To that end, we make a case for three broad areas of cellular communication where signaling microdomains could play an important role: 1) paracrine release of free radicals and gaseous molecules such as nitric oxide and reactive oxygen species; 2) role of ion channels including gap junctions and potassium channels, especially those associated with the endothelium-derived hyperpolarization mediated signaling, and lastly, 3) mechanism of exocytosis that has considerable oversight by signaling microdomains, especially those associated with the release of von Willebrand factor. When summed, we believe that it is clear that the organization and regulation of signaling microdomains is an essential component to vessel wall function.
I. Introduction
It has become clear that proteins do not randomly accumulate at cellular foci but are instead organized at particular regions of the cell to exert their function in a more efficient manner. The vast majority of proteins does not act alone but are highly coordinated by a network of associated molecules that can modify, activate, or inhibit the protein’s function. Concordantly, the numerous signaling molecules involved in intracellular signaling pathways often have a short half-life; thus their target must frequently be spatially localized to their site of production. For example, the half-life of inositol 1,4,5-trisphosphate (IP3) produced by phospholipase C (PLC) is of the order of 30 ms with a diffusion coefficient of approximately 300 µm2/s because of its rapid degradation by localized 5-phosphatases (Wang et al., 1995). Therefore, having IP3 receptors (IP3R) in close proximity to the region where the IP3 is produced maximizes the effect of the messenger (Berridge, 2006). This has been shown to be the case with PLC, because the enzyme has been found to reside in close proximity to IP3R on the endoplasmic reticulum (ER) (e.g., Nomura et al., 2007; Weerth et al., 2007). Overall, there are few proteins that can diffuse to notable distances within the cell without being modified, activated, or inhibited in some way. Thus, it is important to have associated proteins within close proximity to efficiently maintain their function.
However, the question arises as to how the proteins associated with a particular function congregate to a precise location within the cell. It is now recognized that this can be accomplished by multiple factors, including but not exclusive to 1) differing lipid composition of the membrane, 2) unique addressing sequences within proteins directing them to the apical, basal, or lateral regions of the cells, 3) sequestration of proteins transcribed in local regions of the cell, and/or 4) associated protein-protein interaction into macromolecular structures (Lippincott-Schwartz et al., 2000). This last example forms the basis of signaling microdomains, where a group of proteins form a macromolecular complex that in turn can regulate cell-to-cell (paracrine) or cell-to-self (autocrine) signaling processes. There is currently no specific definition for a signaling microdomain, and so we have put forth a set of guidelines to define these nexuses (Table 1).
TABLE 1.
Rubrics defining a signaling microdomain
In this review, at least two rules listed below are required to consider a group of proteins as part of a signaling microdomain that can regulate cellular communication. Examples of signaling microdomain applicable to each guideline are indicated in the right column.
| Guideline | Example |
|---|---|
| 1. Proteins are concentrated to a region of the cell (i.e., apical membrane, myoendothelial junction) and altogether participate in a specific cellular function. | Exocytosis at the apical membrane (section IV) |
| Endothelium-dependent hyperpolarization-mediated response (section III.C) | |
| 2. There is an accumulation of two or more proteins contained within a membranous phospholipid region (i.e., lipid raft, caveolae), and the loss of this structure alters cell-cell communication. | eNOS localized in caveolae (section II.A) |
| 3. There is a direct protein-protein interaction, and disruption of this interaction alters cell-cell communication. | eNOS and caveolin 1 (section II.A.1) |
| eNOS and Hbα (section II.A.4) | |
| Cx43 and ZO-1 (section III.A.4) | |
| 4. There is evidence for close localization of proteins, with a loss of one of the proteins (function or expression) altering the way in which cell-cell communication occurs. | Endothelium-dependent hyperpolarization-mediated response (section III.A) |
A. Definition of a Signaling Microdomain
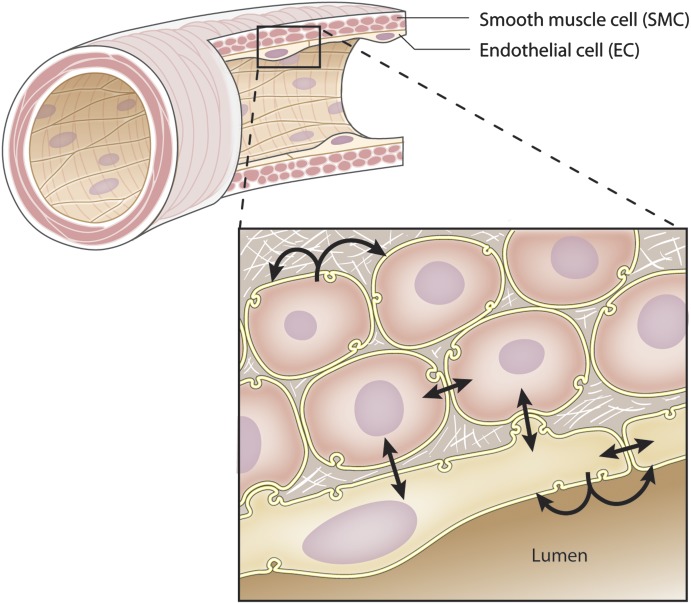
The first characteristic of a signaling microdomain is that proteins are concentrated to a specific region within the cell (Table 1). As mentioned above, it would be difficult for proteins at opposite ends of a cell to have rapid, nonrandom associations, because they are not located in the same cellular location. The closer the protein association is, the more the effect could be deemed nonrandom and deliberate. This is especially true in specialized cellular structures such as the myoendothelial junction where hemoglobin α (Hbα) has been shown to accumulate and regulate nitric oxide diffusion to surrounding smooth muscle cells (Straub et al., 2012).
The next characteristic of a signaling microdomain is that the proteins are within specific regions of the plasma membrane (Table 1). The plasma membrane is composed of a variety of lipids, and it is now well understood that specialized lipid regions, especially those enriched with cholesterol, can harbor proteins together to create a signaling platform at the plasma membrane. Perhaps the most well-known of these specialized plasma membrane regions are lipid rafts and caveolin 1 (Cav1)-enriched caveolae, which are known to concentrate membrane receptors, transporters, and other signaling proteins (for review, see Popescu et al., 2006). The association of endothelial nitric-oxide synthase (eNOS) with Cav1 can be spatially enriched in the vicinity of a number of cofactors and substrates (Mineo and Shaul, 2012). The membrane compartmentalization then can serve an important role in keeping proteins organized that together can regulate a particular function.
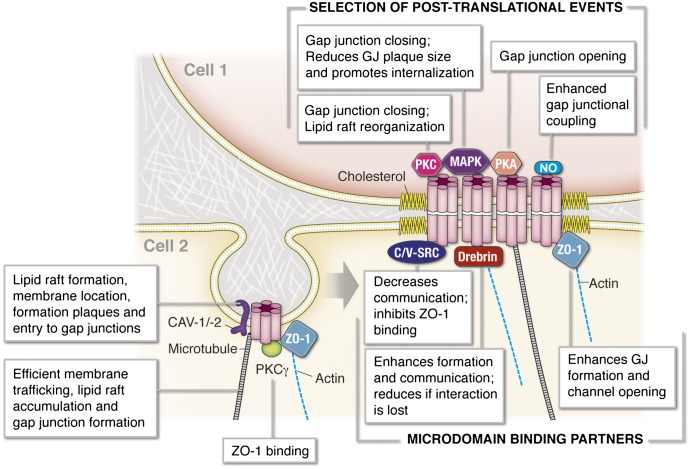
The third rule is that two or more proteins directly interact with each other (Table 1). There are numerous instances where two proteins actually bind together, and their tertiary states can affect one another’s activation or inhibition (e.g., Jones, 2012). This would indicate that the proteins require direct association. An example of this can be found with connexin 43 (Cx43) and zonula occludens-1 (ZO-1) (see section III.A). There is a unique binding sequence for ZO-1 on Cx43 via a PDZ domain, and this interaction dictates both Cx43 trafficking to the plasma membrane and actual function of the fully formed gap junction (see section III.A). Thus the direct interaction of these two proteins serves as a model of how the discreet direct interaction of two proteins plays an important part in cellular communication.
Lastly, proteins may regionally associate without direct protein-protein interaction (Table 1). In this instance, the proteins may not be directly associated, but may be part of a larger macromolecular complex or spatially localized to a similar region of the cell where they work in concert. In this case, the functional association of several proteins is usually revealed using pharmacological tools and often involves an intracellular messenger with a short half-life (e.g., nitric oxide, superoxide anion, IP3, calcium ion).
With a definition in hand, the focus of this review is centered on the organization of signaling microdomains and how they are functionally used by cells of the blood vessel wall, specifically the endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), for cellular communication (Fig. 1). The areas of cellular communication this review will focus on include 1) paracrine release of molecules such as nitric oxide or superoxide anion, 2) channel communication via gap junctions and other ion channels, and 3) exocytosis. There are other examples of released molecules, channels, transporter, or other proteins that play a role in cellular communication, but because they have yet to be fully recognized as being part of a signaling microdomain we have not discussed them in detail because they do not fit into the focus of this review (e.g., pannexin channels). There are other aspects of cellular communication including the role of integrins and chemotaxis that are not discussed here but can be found in multiple reviews (Herbert and Stainier, 2011; Hoffman et al., 2011).
Fig. 1.
Schematic representation of intercellular communication in the arterial wall. The SMCs and ECs composing the vascular wall can communicate with each other either by releasing molecules to neighboring cells (paracrine communication) or directly via gap junction channels that link the cytoplasm of two adjacent cells. The different types of intercellular communications are represented by the arrows.
B. On the Importance of Calcium Compartmentalization
Calcium is a ubiquitous second messenger that controls numerous cellular functions. How calcium is compartmentalized in cells and the consequences on vascular function must be discussed because such compartmentalization directly relates to both the coordination of intercellular communication between cells and their corresponding signaling microdomains. In addition, the calcium signaling microdomains at the origin of such compartmentalization are an excellent example of how cells accumulate proteins together to efficiently regulate cellular functions. The concept of calcium compartmentalization described in this section is by no means exhaustive and will not cover in detail the dynamics of calcium homeostasis; we invite the reader to refer to excellent reviews on this topic (Berridge, 2006; Bolton, 2006; Parekh, 2008; Putney and Bird, 2008; Hill-Eubanks et al., 2011; van Breemen et al., 2013).
Cells need to maintain a low intracellular calcium concentration ([Ca2+]i) to avoid inappropriate and random activation of signaling pathways. The cytosolic calcium concentration is maintained at a low level ([Ca2+]i ∼100 nM) through a multitude of pumps and calcium transporters at the plasma membrane and at the ER membrane, respectively, extruding calcium outside of the cell or storing calcium in the ER [or in the sarcoplasmic reticulum (SR), a specialized ER in muscle cells] (ZhuGe et al., 1999). Because the equivalent of the total free cytosolic calcium enters the cells every half second, these pumps and transporters have to be constitutively active to maintain a low [Ca2+]i (Lee et al., 2002). The extracellular calcium concentration and the calcium concentration within the ER ([Ca2+]ER) are higher (approximately 2 mM and 200 µM, respectively). Additionally, it is now accepted that the mitochondria constitutes another buffer organelle to regulate cytoplasmic calcium concentration (for review, see McCarron et al., 2012).
Because calcium is a central molecule for cellular functions, the dynamics of calcium are complex to temporally and spatially control specific signaling pathways. Thus, it has become evident that cells do not regulate their [Ca2+]i as a whole but more in discrete regions to activate specific, localized signaling pathways (i.e., the calcium signaling is compartmentalized). The activity level of the multiple calcium pumps, channels, and transporters are thus responsible for high calcium concentrations that can develop close to the plasma membrane as well as close to the ER membrane (Cheng et al., 1993; Nelson et al., 1995; Perez et al., 2001; Berridge et al., 2003; Navedo et al., 2005; Berridge, 2006; Niggli and Shirokova, 2007; Feletou, 2011b). To help with this, the different intracellular calcium compartments are close to one another and/or to the plasma membrane to form a restricted space, where the calcium can be confined. The best known examples of such intracellular membrane junctions are found in cardiac myocytes where the T-tubules of the sarcolemma and the terminal cisternae of the SR come into contact, a contact that is central in the excitation-contraction coupling of the cardiac muscle (Fabiato and Fabiato, 1972; McNutt, 1975).
Some of the best examples of compartmentalized calcium are in SMCs because they are the best described to date. Compartmentalization of calcium allows for the activation of specific cellular pathways, mainly through the activation of calcium-dependent enzymes located close to the sources of calcium, near the ER or the plasma membrane (Berridge, 2006). In contrast, other calcium-dependent enzymes located further from the sources of calcium (i.e., further from the ER or the plasma membrane) are not activated because of the rate of calcium diffusion (Berridge, 2006). A striking example of calcium compartmentalization is the observation that, although increases in whole [Ca2+]i cause contraction of SMCs, a local subplasmalemmal increase in calcium facilitates relaxation (Nelson et al., 1995); this example will be discussed in detail in this section (see section I.A.1.a). Calcium homeostasis has particularly been investigated in vascular SMCs (VSMCs) because of the central role of calcium in the contractile process (Nelson et al., 1990; Fleischmann et al., 1994). Heterogeneous and high local calcium concentrations have been observed in VSMCs in multiple reports (e.g., Deth and van Breemen, 1977; Van Breemen, 1977; van Breemen et al., 1986; Laskey et al., 1992; Kargacin, 1994; Nelson et al., 1995; Rembold et al., 1995), and computer modeling of calcium signaling within the VSMCs showed that high calcium concentrations could occur in restricted spaces and persist for 100–200 ms (Kargacin, 1994, 2003; Naraghi and Neher, 1997). Based in part on these reports, calcium compartmentalization was conceptualized where localized [Ca2+]i could activate the contractile apparatus without altering other calcium-dependent pathways (Karaki, 1989).
1. Spatial Organization of Intracellular Organelles Is Crucial For Efficient Calcium Compartmentalization.
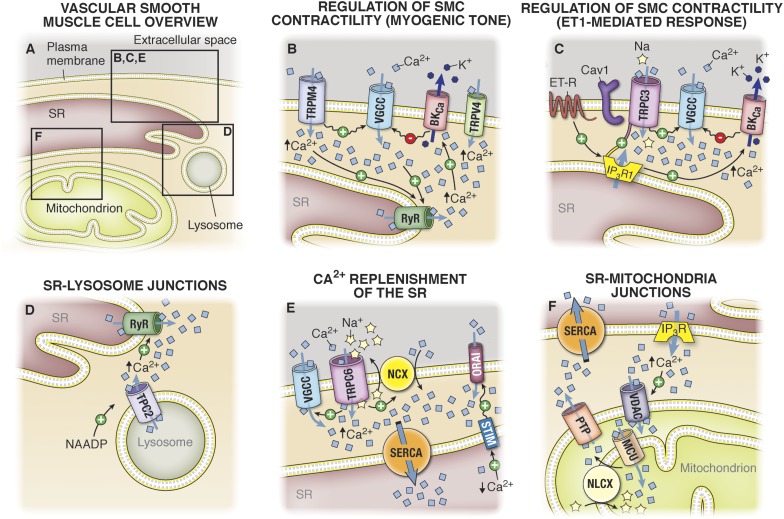
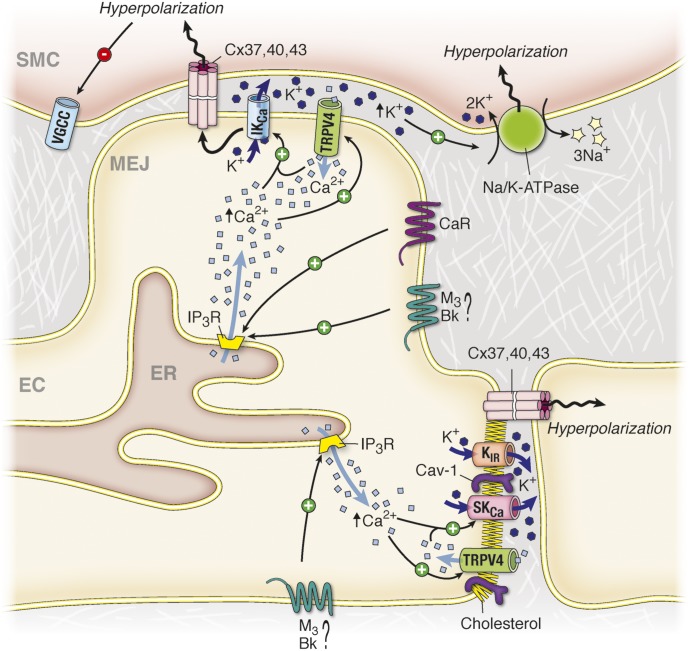
There is evidence that spatial localization of organelles can contribute to calcium compartmentalization, including (but not limited to) proximity of the SR and plasma membrane and proximity of the SR and mitochondria (Fig. 2A). Thus, the location of calcium entry from the outside of the cell or the location of the release of calcium from the SR into the cytoplasm is not only important in regard to the signaling proteins that are surrounding the calcium channel but also in regard to the intracellular organization of organelles (Poburko et al., 2004). Indeed, if calcium influxes occur at a location where intracellular organelles come into close contact with the plasma membrane, the latter will prevent free diffusion of calcium, making localized calcium concentration persist for longer periods of time. Conversely, if calcium influxes occur in a region of the plasma membrane where there are no intracellular organelles, the calcium will diffuse freely and dilute in the cytoplasm, and its effect on the surrounding signaling proteins will be lower (Kargacin, 1994). One example of importance in cell-cell communication is the localization of ER at the myoendothelial junctions (MEJ), a cellular structure linking ECs and SMCs that is embedded in extracellular matrix (for review, see Heberlein et al., 2009). The local release of calcium in this compartment, presumably from ER, has been observed in numerous instances (Ledoux et al., 2008; Bagher et al., 2012), which could act to regulate eNOS or other localized channels such as IKCa channels (see sections II.A and III.B).
Fig. 2.
Schematic representation of calcium compartmentalization. (A) In VSMCs, calcium (Ca2+) can be stored in both the SR and in the mitochondria. Calcium release from both organelles is tightly coordinated to calcium influx at the PM, and this coordination is facilitated by the close proximity between the organelles and the PM. (B) In cerebral VSMCs, after increased intravascular pressure, there is a coordinated action of TRPV4, TRPM4, VGCC, and large conductance potassium channels (BKCa) at the plasma membrane and ryanodine receptors RyR at the SR membrane. In this configuration, increased pressure activates calcium influx in VSMCs via TRPV4, which stimulates calcium release from the SR through RyR, whereas opening of TRPM4 results in calcium influx through VGCC also activating calcium release from the SR through RyR. Calcium release from RyR (also termed calcium “sparks”) further activates potassium (K+) efflux via BKCa channels. The hyperpolarization resulting from potassium efflux reduces the activity of VGCC, making BKCa key in the autoregulation of calcium homeostasis in VSMCs. (C)Upon cerebral VSMC stimulation with ET-1, activation of IP3-R1 at the SR membrane activates calcium influx through TRPC3 independently of calcium release via IP3R but via a direct protein interaction between IP3-R and TRPC3. This IP3R/TRPC3 interaction is facilitated by the presence of Cav1. Calcium release via IP3R upon ET-1 stimulation further activates BKCa channels at the plasma membrane in a similar manner as RyR activates BKCa channels in (B). Activation of BKCa induces hyperpolarization of the plasma membrane, thus attenuating the activation of VGCC by cation influx through TRPC3. (D) After stimulation with ET-1, nicotinic acid adenine dinucleotide phosphate (NAADP) activates the release of calcium from intracellular lysosomes via the two pore calcium channel (TCP2). The calcium released from the lysosome further activates calcium release from the SR via RyRs. (E) The compartmentalization of VGCC, TRP channels, the NCX, and the SERCA are part of a signaling microdomain controlling calcium replenishment of the PM-SR junction. In this configuration, calcium and Na+ influxes via TRPC6 activate the adjacent VGCC and the NCX in reverse mode. Calcium influx via the VGCC and the NCX provide sources of calcium for ER/SR replenishment via the SERCA pump. Additionally, the STIM present at the SR membrane is capable of sensing decreased levels of calcium in the SR and activates calcium influx via Orai at the plasma membrane, again providing calcium for SR replenishment via the SERCA pumps. (F) Mitochondria also play a major role as a buffer and as a source of calcium for the SR. After stimulation of VSMCs, mitochondria take up the calcium released from IP3R via the VDAC on the outer mitochondrial membrane and the mitochondrial calcium uniporter (MCU) on the inner mitochondrial membrane. The buffering role of mitochondria is essential to prevent the formation of high local calcium concentrations surrounding the IP3-R, which would inhibit the IP3-R activity. The release of calcium from the mitochondria via the mitochondrial sodium/calcium exchanger (NLCX) present on the inner mitochondrial membrane and the permeability transition pore (PTP) on the outer mitochondrial membrane provides a source of calcium for SR replenishment by the SERCA pumps. Straight arrows with positive and negative signs indicate activation and inhibition by Ca2+, respectively. Wavy arrows with a positive or negative sign indicate activation by depolarization or an inhibition by hyperpolarization respectively.
Observations of plasma membrane-SR junctions (PM-SR junctions) were reported nearly 50 years ago (Fawcett, 1961; Rosenbluth, 1962; Franzini-Armstrong, 1964; McNutt, 1975). In these articles, the authors observed the close proximity of two biological membranes separated by a cytoplasmic space of 10–30 nm wide over a few hundred nanometers in both skeletal and cardiac muscle cells. These junctions were functionally associated with the calcium-induced calcium release (CICR) present in cardiac and skeletal muscles (Fawcett, 1961; Rosenbluth, 1962; Franzini-Armstrong, 1964; McNutt, 1975). In the VSMCs, the close apposition of the SR to the plasma membrane has been suggested by several investigators (Somlyo et al., 1971, 1979; Somlyo, 1985; Benham and Bolton, 1986; Hermsmeyer and Sturek, 1986), and the presence of a CICR and very high local calcium concentrations in the cytoplasmic space between the two membranes were both evidenced in this cell type (Van Breemen, 1977; van Breemen et al., 1986). Since the presence of PM-SR junctions were identified, van Breemen et al. (2013) proposed that the SR in VSMCs is capable of forming at least eleven types of junctions with other intracellular organelles and with itself. However, the junctions formed by apposition of the plasma membrane and the SR membrane are the most abundant SR junctions in the VSMCs (van Breemen et al., 2013).
Structurally, various reports have identified a series of proteins that play an important role in the architectural organization of PM-SR junctions. Junctophilins (Takeshima et al., 2000; Komazaki et al., 2002) and junctate (Treves et al., 2010; Srikanth et al., 2012) are two transmembrane proteins expressed at the SR membrane that constitute protein bridges that keep the SR and plasma membranes close and may prevent their fusion (Carrasco and Meyer, 2011). For example, PM-SR junctions are absent in cardiac myocytes from mice deficient in the cardiac isoform of junctophilins gene (Takeshima et al., 2000). Further studies on these proteins could help reveal another key component to localization of calcium-related organelles.
2. Examples of Calcium Compartmentalization Involved in Vascular Smooth Muscle Cells Contractile State.
When VSMCs are stimulated, the coordination between all of the molecular players responsible for calcium entry into the cell and calcium release from the intracellular organelles is crucial for a homogeneous and regulated contraction. In this section, we describe three examples where compartmentalization of calcium signaling plays an important role in the regulation of VSMC contraction.
a. Regulation of myogenic tone in cerebral arteries.
Calcium release from the SR and coordination with channels and other transporters at the plasma membrane of cerebral VSMCs was first described in 1995 by Nelson et al. (1995). In the seminal article, the group defined calcium sparks as a temporal and spatial release of intracellular calcium from the SR via the ryanodine receptors (RyR), which further activate calcium-dependent large conductance potassium channels (BKCa) at the plasma membrane (Nelson et al., 1995) (Fig. 2B). Activation of BKCa channels induced hyperpolarization of the plasma membrane, making the coordination between RyR and BKCa central to the regulation of voltage gated calcium channel (VGCC) expressed at the plasma membrane (Fig. 2B) (Nelson et al., 1995; Jaggar et al., 1998b). Conversely, calcium influx from channels at the plasma membrane can also activate RyR at the SR membrane. Calcium influx through channels from the transient receptor potential (TRP) family, namely TRPV4 channels, is expressed at the plasma membrane of cerebral VSMCs and activates calcium release from the SR via RyR channels, making TRPV4 and RyR part of a CICR mechanism (Earley et al., 2005) (Fig. 2B). Of note, high calcium concentrations between RyR and BKCa were demonstrated in stomach SMCs where the calcium concentration between both channels could reach 10 µM in an area of 1 μm2 during a calcium spark (ZhuGe et al., 1999; Zhuge et al., 2002). At the arterial level, the coordination between RyR, BKCa, and TRPV4 is crucial in the regulation of smooth muscle contraction as shown specifically in pressure-induced constriction, where the hyperpolarization induced by BKCa channels negatively feeds back on the depolarization occurring during increases in intravascular pressure (Jaggar et al., 1998a,b; Knot and Nelson, 1998; Knot et al., 1998; Jaggar, 2001; Wellman et al., 2002; Ledoux et al., 2006). Accordingly, cerebral arteries isolated from mice deficient in the β subunit of BKCa channels are significantly more constricted at a given intraluminal pressure compared with control mice (Brenner et al., 2000). This negative feedback is key in the autoregulation of cerebral blood flow, a process that is impaired during subarachnoid hemorrhage, thus resulting in a decreased activation of the BKCa and a higher constriction of cerebral arteries (Koide et al., 2011).
During pressure-induced contraction of cerebral arteries, other calcium channels expressed at the plasma membrane of cerebral VSMCs are activated by calcium release from the SR, specifically TRPM4 channels (Earley et al., 2005). However, as opposed to BKCa channels that are activated by calcium sparks released via RyR channels, TRPM4 channels are activated by calcium release via IP3R present at the SR membrane (Fig. 2B) (Gonzales et al., 2010a). In cerebral VSMCs, TRPM4 channels at the plasma membrane are less than 50 nm from the SR membrane but are not physically coupled to the IP3R, as shown by immunofluorescence overlap and immuno-fluorescence resonance energy transfer (Zhao et al., 2010; Gonzales and Earley, 2012). It is noteworthy that translocation of the TRPM4 channels at the plasma membrane via a PKC-dependent pathway is key for the channel activation by calcium release through IP3R (Crnich et al., 2010; Garcia et al., 2011). Because activation of TRPM4 by a PKC-dependent pathway is involved in the myogenic response to increased intravascular pressure (Earley et al., 2004, 2007; Gonzales et al., 2010b), it has been hypothesized that the functional complex formed by IP3R, TRPM4, and PKC could play a role in the depolarization of VSMCs observed upon increase in intravascular pressure (Earley, 2013). However, neither the origin of IP3R activation by increased levels of IP3 (Narayanan et al., 1994) nor the origin of PKC activation upon increase intravascular pressure has been elucidated (Earley, 2013). Mechanical activation of Gq receptors by increased intravascular pressure has been suggested (Mederos y Schnitzler et al., 2008; Brayden et al., 2013) and could reconcile the ideas that both PKC and IP3R are activated during increased intravascular pressure, which would, respectively, result in relocation of the TRPM4 at the plasma membrane and in its activation. Further investigation is needed, because activation of Gq receptors upon increased intravascular pressure is controversial (Anfinogenova et al., 2011; Earley, 2013).
b. Agonist-induced constriction in cerebral arteries.
As opposed to the functional but indirect interactions between channels at the plasma membrane and at the SR membranes described above, TRPC3 channels expressed at the plasma membrane of VSMCs have been shown to be physically coupled to IP3R1 on the SR. This direct interaction was demonstrated using coimmunoprecipitation in intact rat cerebral arteries and by immuno-fluorescence resonance energy transfer (Adebiyi et al., 2011). Functionally, TRPC3:IP3R1 coupling is important in the endothelin-1 (ET-1)-mediated response where activation of IP3R1 directly activates a cation influx via TRPC3 channels at the plasma membrane, producing a sustained constriction (Xi et al., 2008; Adebiyi et al., 2010, 2011). It is noteworthy that activation of TRPC3 by IP3R1 occurs independently of calcium release from the SR via the IP3R1, because exogenous IP3 or ET-1 applied to isolated cerebral myocytes induces a cation influx via TRPC3, even when the SR was depleted of calcium (Xi et al., 2008). The TRPC3:IP3R1 interaction occurs via a calmodulin and IP3R binding domain (CIRB) that is present on the TRPC3 channels and can be disrupted using a peptide corresponding to the N-terminal sequence of the IP3R1 known to interact with CIRB domain (Adebiyi et al., 2010). Conversely, the functional effect of TRPC3:IP3R1 interaction can be mimicked by a peptide corresponding to the CIRB domain of TRPC3, which simulates IP3R1 interaction to TRPC3 and results in the activation of TRPC3 at the plasma membrane (Adebiyi et al., 2010).
The presence of Cav1 is key in the assembling of IP3R and TRPC3 complex as shown by the decrease in IP3-induced cation influx via TRPC3 when VSMCs were treated with methyl β cyclodextrin (MβCD) or with shRNA targeting Cav1 (Adebiyi et al., 2011). The same group also demonstrated that IP3-induced cation influx via TRPC3 was inhibited by a peptide that competes with endogenous Cav1 for interaction with protein partners (Adebiyi et al., 2011). Concordantly, MβCD, shRNA targeting Cav1, and the competing peptide all abolished IP3-induced constriction of cerebral arteries (Adebiyi et al., 2011). In parallel, it was also shown that local calcium release via IP3R1 could activate BKCa channels, similarly to BKCa activation by calcium sparks (see above) (Zhao et al., 2010).
These observations clearly demonstrate the impact of compartmentalized calcium signaling, especially as it relates to VSMCs. These areas of compartmentalized calcium signaling have also been demonstrated in the systemic circulation, where IP3R1, TRPC3, and Cav1 also interact together (Adebiyi et al., 2012). Additionally, another TRP channel can activate BKCa at the plasma membrane of systemic VSMCs, namely TRPC1, which coimmunoprecipitate and colocalize with BKCa channels in freshly isolated aortic SMCs (Kwan et al., 2009). Functionally, the authors demonstrated that TRPC1 channels are involved in the responses to several contractile agonists including ET-1, but also phenylephrine and U-46619. Agonist-induced activation of TRPC1 further activates a potassium efflux via BKCa at the plasma membrane, thus controlling the contractile state of VSMCs (Kwan et al., 2009). It is noteworthy that the IP3R:TRPC3 coupling is increased in spontaneously hypertensive rats, along with an increase in TRPC3 expression and ET-1-induced vasoconstriction (Adebiyi et al., 2012).
c. Lysosome-sarcoplasmic reticulum junctions.
The membrane appositions between the lysosomes and the SR have important implications in processes such as autophagy and cholesterol metabolism (van Breemen et al., 2013). Recent studies reported a role of these junctions in the regulation of CICR from the SR induced by the second messenger nicotinic acid adenine dinucleotide phosphate. This second messenger, which can be produced in response to agonists such as ET-1, stimulates the release of calcium from the lysosomes via the two pore segment channel subtype 2 (TPC2; Fig. 2D) (Calcraft et al., 2009). The released calcium further activates release of calcium from the SR via RyR3 found at SR-lysosomes nanojunctions in a CICR manner (Kinnear et al., 2004, 2008). After activation of RyR3, the RyR2 isoform is activated in a CICR manner, and the calcium released from the SR is propagated as a wave in the cytoplasm to activate contraction of SMCs (Kinnear et al., 2004, 2008; Clark et al., 2010). By use of a lysosome marker and labeled ryanodine, Kinnear et al. (2008) demonstrated a close proximity between the lysosomes and ryanodine receptors. These studies strongly point to a calcium compartmentalization between the SR and the lysosome that may play important roles in the regulation of calcium homeostasis.
3. Calcium Signaling Microdomains Involved in the Regulation of Calcium Concentration in the Sarcoplasmic Reticulum.
The SR is able to autoregulate its own calcium content and maintain a constant calcium concentration. The capacity of SR to store calcium is attributed to the presence of high-capacity, low-affinity calcium-binding proteins in its lumen such as calsequestrin and calreticulin (Michalak et al., 1992; Milner et al., 1992; Raeymaekers et al., 1993). After stimulation of a cell, the SR is able to replenish its content by pumping calcium via the sarco/endoplasmic reticulum calcium ATPase (SERCA) pumps localized strategically in close apposition to the plasma membrane and the mitochondria (Fig. 2, E and F) (Putney, 1986; Floyd and Wray, 2007; Satoh et al., 2011). Thus, the SR is able to refill its calcium stock from both the extracellular space and the mitochondria. To do this, the cells have established different mechanisms to transfer calcium from the extracellular space or the mitochondrial matrix to the SR in a highly coordinated manner to avoid diffusion into the cytosol and/or unwanted activation of calcium-dependent signaling pathways.
a. Signaling microdomains at the plasma membrane-sarcoplasmic reticulum junctions.
Upon VSMC stimulation, the opening of TRPC6 channels at the plasma membrane allows for the entry of sodium along with calcium, which reverses the sodium and calcium exchanger (NCX) by increasing subplasmalemmal concentration of sodium and activating calcium entry via VGCC, respectively (Lee et al., 2001; Lemos et al., 2007; Poburko et al., 2007; Fameli et al., 2009) (Fig. 2E). Activation of VGCCs can be induced solely by cation influx from TRPC6 and independently of the NCX; however, reversal of the NCX offers an additional source of calcium specifically for calcium refilling (Poburko et al., 2008). Disruption of the PM-SR junctions using the cytoskeleton-disrupting agent calyculin A prevents calcium refilling of the SR, inhibits calcium influx, but increases sodium entry presumably due to a disruption of the refilling mechanism involving NCX, TRPC6, and SERCA presented in Fig. 2E (Dai et al., 2005a; Lemos et al., 2007). At the mitochondrial level, a similar mechanism involving the mitochondrial NCX has been demonstrated (see section I.B.3.b).
In parallel, the SR can also replenish its calcium content by stromal interaction molecule (STIM) and Orai expressed, respectively, at the SR and plasma membrane (Williams et al., 2001; Liou et al., 2005; Roos et al., 2005; Zhang et al., 2005; Feske et al., 2006; Prakriya et al., 2006). In this system, STIM serves as a [Ca2+]SR sensor due to an EF-hand located in the SR lumen (Zhang et al., 2005) and relocate to SR regions that are close to the plasma membrane (approximately 10–25 nm) when [Ca2+]SR decreases (Luik et al., 2006; Wu et al., 2006; Calloway et al., 2009). After STIM relocation at the PM-SR junctions, STIM interact physically with the Orai channels at the plasma membrane and activate Ca2+ entry (Fig. 2E) (Liou et al., 2005; Zhang et al., 2005; Prakriya et al., 2006; Ong et al., 2007; Muik et al., 2008; Navarro-Borelly et al., 2008; Park et al., 2009a; Zheng et al., 2013a). Recently, the store operated channel entry associated regulatory factor was found to associate with STIM at the plasma membrane so as to regulate calcium influx via Orai to avoid excessive refill of the SR (Palty et al., 2012).
b. Calcium signaling microdomains at the sarcoplasmic reticulum-mitochondria junctions.
Fifty years ago, mitochondria were shown to accumulate calcium (Deluca and Engstrom, 1961; Vasington and Murphy, 1962; Lehninger et al., 1963), but the physiologic relevance of the process was initially dismissed because of the discordance between the mitochondria’s low affinity for the ion (in the millimolar range) and the measured physiologic cytosolic values of calcium (<1 μM) (Patron et al., 2013). The role of mitochondria in calcium homeostasis re-emerged in the early 1990s with the development of calcium probes targeted to the mitochondria (Rizzuto et al., 1992). Since then, the discrepancy between the low affinity of the mitochondria for calcium and the low cytosolic calcium concentration has been explained by the close proximity between the organelle and channels that release calcium both at the SR and at the plasma membrane (Mannella et al., 1998; Rizzuto et al., 1998; Csordas et al., 1999).
In VSMCs, the functional role of mitochondria in calcium homeostasis was demonstrated using mitochondria protonophores, which cause the mitochondrial membrane potential to collapse or pharmacologically block the mitochondrial calcium uniporter (MCU) known to drive calcium influx into the mitochondria (Drummond and Fay, 1996; McCarron and Muir, 1999). Because the mitochondrial calcium uptake relies on the large proton electrochemical driving force, these mitochondrial inhibitors were shown to increase cytosolic calcium concentration upon depolarizing stimulation (Drummond and Fay, 1996; McCarron and Muir, 1999; Kamishima and Quayle, 2002; Cheranov and Jaggar, 2004). Blockers of the mitochondrial ATP synthase, however, did not affect mitochondrial calcium uptake, suggesting that the role of mitochondria in calcium homeostasis was not due to a depletion of cellular ATP and subsequent inactivation of the calcium pumps present at the SR (e.g., SERCA) or at the plasma membrane (e.g., PMCA) (Drummond and Fay, 1996; McCarron and Muir, 1999). It recently became more clear that the mitochondria acts as a buffer of the calcium released from the SR, because an increase in mitochondrial calcium concentration ([Ca2+]mit) occurs after release of the ion from the SR (Drummond and Fay, 1996). Indeed, application of caffeine or a GqPCR agonist, which stimulate the RyRs and the IP3R, respectively, or flash photolysis of caged IP3 induced increases in [Ca2+]mit (Drummond and Fay, 1996; Drummond and Tuft, 1999; McCarron and Muir, 1999; Gurney et al., 2000; Kamishima and Quayle, 2002; Chalmers and McCarron, 2008). This increase in [Ca2+]mit was sensitive to mitochondrial protonophores (Drummond and Tuft, 1999). Given the buffering role of mitochondria, their contribution to the return of [Ca2+]i to baseline levels after stimulation has also been demonstrated (Drummond and Tuft, 1999; McCarron and Muir, 1999; Kamishima and Quayle, 2002; Chalmers and McCarron, 2008 ). For example, mitochondrial protonophores increased the time of recovery of [Ca2+]i after caffeine application in rat PASMCs (Drummond and Tuft, 1999).
The temporal buffering of calcium by the mitochondria was also demonstrated using ATP in rat PASMCs, where application of the purine induced oscillations in [Ca2+]i synchronized with oscillations of [Ca2+]mit (Drummond and Tuft, 1999). More direct evidence has been observed in HeLa cells where [Ca2+]mit, [Ca2+]SR, and [Ca2+]i have been measured simultaneously (Arnaudeau et al., 2001). These studies indicated a larger ER depletion when calcium uptake by the mitochondria was blocked by inhibitors of the mitochondrial respiratory chain (Arnaudeau et al., 2001). The same study also demonstrated a larger ER depletion when calcium efflux from the mitochondria by the mitochondrial Na+-Ca2+ exchanger was pharmacologically blocked, indicating that mitochondria calcium stores aid in the refilling of the ER by locally extruding calcium proximal to the SERCA pump on the ER (Arnaudeau et al., 2001). Interestingly, ER regions that are close to mitochondria refilled and released more calcium than ER regions that are more distant from mitochondria (Arnaudeau et al., 2001).
Because calcium has been shown to accumulate in the mitochondrial matrix, the ion has to traverse both the external mitochondrial membrane and the inner mitochondrial membrane. It is presumed that calcium ions, driven by the negative charge of the mitochondrial membrane potential established by the respiratory chain, cross the external mitochondrial membrane via the voltage-gated anion channel. Calcium residing in the intermembrane space is then imported into the mitochondrial matrix via the MCU (Madesh and Hajnoczky, 2001; Rapizzi et al., 2002; Kirichok et al., 2004). Calcium is slowly released from the mitochondria via the Na+-Ca2+ exchanger and the mitochondrial permeability transition pore and is used to refill the SR via the SERCA pump (Landolfi et al., 1998; Szado et al., 2003; Ishii et al., 2006; Chalmers and McCarron, 2009; Poburko et al., 2009; Giacomello et al., 2010) (Fig. 2F). Several reports demonstrated that calcium buffering by the mitochondria is especially important in the spatial area surrounding the IP3R to prevent its inhibition by cytosolic calcium accumulation (Hajnoczky et al., 1999; Olson et al., 2010).
4. Calcium Signaling Microdomains Involved in Protein Expression and Cell Proliferation.
During cellular proliferation, the transcription factor nuclear factor of activated T-cells can be activated by calcium-bound calcineurin, which induces the translocation to the nucleus (Hogan et al., 2003; Aubart et al., 2009). Several SR and plasma membrane calcium channels have been involved in the calcium-induced translocation of nuclear factor of activated T-cells in the nuclei, and it appears that the STIM/Orai complex described above is central in this process (Aubart et al., 2009; Baryshnikov et al., 2009; Zhang et al., 2011). Interestingly, in contractile quiescent VSMCs, STIM and Orai are expressed at very low levels; however, when VSMCs dedifferentiate and transition to a proliferative phenotype, the expression of these two proteins is significantly increased (Aubart et al., 2009; Potier et al., 2009; Bisaillon et al., 2010). For example, several reports have demonstrated an increased expression of the STIM1 and Orai1 isoforms in VSMCs after carotid balloon injury, where VSMCs adopt a highly proliferative phenotype (Aubart et al., 2009; Guo et al., 2009; Bisaillon et al., 2010; Zhang et al., 2011). In these studies, the genetic knock down of STIM1 and Orai1 in vivo resulted in significant inhibition of neointimal growth (Aubart et al., 2009; Guo et al., 2009; Bisaillon et al., 2010; Zhang et al., 2011).
Calcium influx secondary to calcium release from the SR (i.e., calcium capacitive influx) plays a key role in the increased PASMC proliferation observed during pulmonary hypertension (Sylvester et al., 2012; Firth et al., 2013). Both TRPC1 and the STIM/Orai molecular complex are key in this capacitive calcium influx (Ng et al., 2009, 2010a,b) and have been shown to play a critical role in the calcium response of PASMC under hypoxic conditions (Lu et al., 2008, 2009; Ng et al., 2012). Although multiple lines of in vitro data demonstrate the importance of the STIM/Orai/TRPC1 complex in PASMCs, the physiologic relevance of the complex in vivo remains unclear. However, because it is well accepted that the capacitive calcium entry in PASMCs in vivo is involved in the development of pulmonary arterial hypertension, the STIM/Orai/TRPC1 complex could be an important molecular component in this pathology. Indeed, a recent investigation reported that platelet-derived growth factor, a growth factor known to be elevated in patients with pulmonary arterial hypertension, enhances the expression of STIM and Orai in human PASMCs, along with an increase in capacitive calcium entry and proliferation of the cells (Ogawa et al., 2012).
The main conclusion that can be drawn from the work described above is that calcium is highly regulated and compartmentalized by the cell. To do so, cells harbor a complex organization of intracellular organelles but also assemble calcium pumps, calcium channels, and calcium-binding proteins in calcium signaling microdomains. The result of such highly organized microdomains is a very efficient regulation of calcium homeostasis. Therefore, calcium signaling microdomains provide a valuable example to both understand and provide a basis for signaling microdomains that regulate intercellular communication work.
II. Gaseous Molecule Cellular Communication by Signaling Microdomains
The biologic prevalence of physiologic and pathologic signaling cascades utilizing diffusible gaseous molecules in the blood vessel wall has been extensively documented. Of particular note, a number of reactive nitrogen and oxygen species have been implicated in a vast array of cellular signaling pathways in the vascular wall. Among these gaseous molecules, nitric oxide (NO) and its oxidized derivatives, nitrate, nitrite, and peroxinitrite, have been studied the most extensively, with a number of other free radical species [notably superoxide anion (O2•−) and hydrogen peroxide (H2O2)] having come into focus over the last decade with conclusive work demonstrating an equally important role in cellular communication (reviewed by Ronson et al., 1999; Wolin, 2000; Ignarro, 2002; Ardanaz and Pagano, 2006; Bian et al., 2008; Touyz et al., 2011; Sparacino-Watkins et al., 2012; Bueno et al., 2013). How these molecules are regulated by signaling microdomains is discussed below, as well as potential contributions from carbon monoxide (CO) and hydrogen sulfide (H2S).
A. A Case for Endothelial Nitric-Oxide Synthase and Nitric Oxide
The initial discovery by Murad in 1977 that exogenous NO can act as a bioactive messenger to activate soluble guanylate cyclase (sGC) in SMCs (Katsuki et al., 1977), along with the work by Furchgott and Zawadzki (1980) identifying the presence of an endothelium derived relaxing factor that has since been identified as NO (Ignarro et al., 1987; Palmer et al., 1987), fueled the concept that NO is an essential player in regulating blood vessel physiology. Since then, a number of intra- and intercellular targets for bioactive NO have been identified with the biological effects ranging from enzyme activation or inhibition, posttranslational modifications altering protein function, including S-nitrosylation and tyrosine nitrosation, and the generation of complex reactive nitrogen and oxygen species through the rapid spontaneous reaction of NO with other gaseous molecules in the cell (Ignarro, 1991; Davidge et al., 1995; Xu et al., 1998; Handy and Loscalzo, 2006; Yoshida et al., 2006; Kang-Decker et al., 2007; Selemidis et al., 2007; Zuckerbraun et al., 2007; Illi et al., 2008; Briones et al., 2009; Fernhoff et al., 2009; Lima et al., 2010; Thibeault et al., 2010; Bess et al., 2011; Choi et al., 2011; Straub et al., 2011; Marin et al., 2012; Haldar and Stamler, 2013; Korkmaz et al., 2013).
It has now become evident that the spatial and temporal regulation of reactive nitrogen and oxygen species generation can dictate the functional impact of these signaling molecules on the homeostatic maintenance of vascular function, where dysregulation can lead to complications including, but not limited to, endothelial dysfunction, inflammation, and atherosclerosis (reviewed by Giles, 2006; Pacher et al., 2007; Muller and Morawietz, 2009). The biologic half-life of NO is extremely short (<5 seconds), because of the rapid diffusion to surrounding cells, chemical reactions with other cellular oxidants, and scavenging by heme-containing proteins, most notably hemoglobin (Nathan, 1992; Archer, 1993; Hakim et al., 1996). These observations suggest that the generation of NO may be spatially confined to microdomains within the cell where induction of NO-dependent signaling cascades can be poised in close proximity to downstream targets.
Although the potent effects of NO as a vasodilator and anti-inflammatory mediator were first recognized nearly 30 years ago, the enzymes responsible for its synthesis were not identified until the early 1990s. Three nitric-oxide synthase (NOS) enzymes have since been cloned and characterized with differential tissue distributions and regulatory elements. These NOS isoforms were subsequently termed nNOS (neuronal NOS; NOS1) (Bredt et al., 1990; Bredt and Snyder, 1990), inducible NOS (NOS2) (Charles et al., 1993; Sherman et al., 1993; Maier et al., 1994), and eNOS (endothelial NOS; NOS3) (Busse and Mulsch, 1990; Lamas et al., 1992; Marsden et al., 1992). With respect to the cells comprising the blood vessel wall, eNOS is the most abundant isoform with robust expression in the ECs lining the vascular intima under physiologic conditions. The other two NOS isoforms have also been identified in the vessel wall, with nNOS expression being detectable at low levels in the VSMCs in certain vascular beds (Boulanger et al., 1998; Brophy et al., 2000) and inducible NOS expression increasing in both ECs and VSMCs in response to vascular damage or cellular activation by proinflammatory cytokines (Hansson et al., 1994; Kanno et al., 1994; Ikeda et al., 1997; Hecker et al., 1999). In addition to the vascular cells comprising the blood vessel wall, sympathetic perivascular nerves innervate the resistance arteries express nNOS, providing another source of NO for the regulation of vascular function (Sosunov et al., 1995; Faraci, 2002). Based on the current myriad of literature implicating eNOS in vascular NO generation and the complex regulatory networks dictating compartmentalized eNOS signaling, this section of the review will focus on the signaling microdomains important for control of eNOS activity and signal transduction cascades in the vascular endothelium and how these domains impart discrete control over NO production in this tissue.
1. Structural Organization and Functional Regulation of Endothelial Nitric-Oxide Synthase.
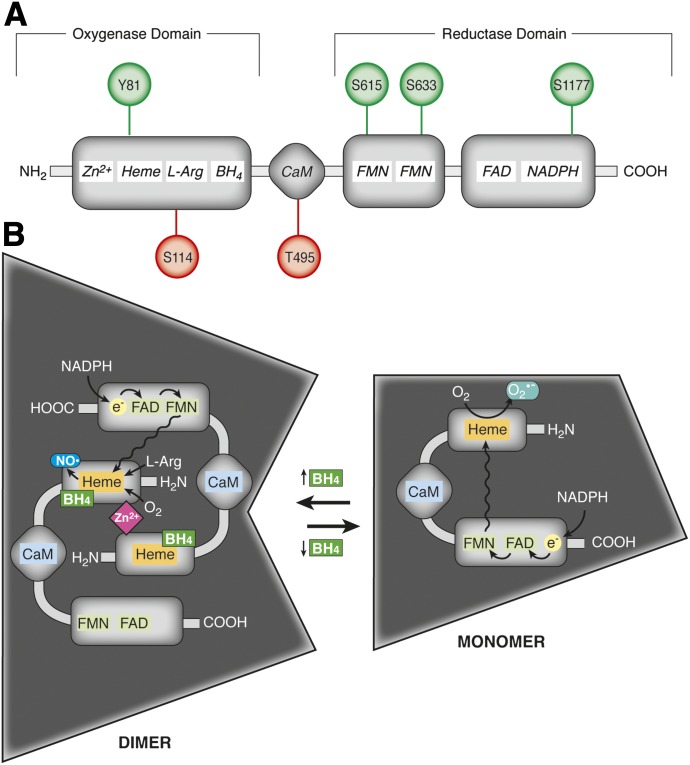
Endothelial NOS is a highly regulated enzyme in the vasculature, with a multifaceted control of its enzymatic activity conferred by numerous factors including local substrate and cofactor availability, regulatory protein binding partners, and dynamic posttranslational modifications, predominantly by phosphorylation of specific serine, tyrosine, and threonine residues. The eNOS enzyme has a constitutive low level of activity for NO generation that is tightly modulated by each of the aforementioned factors (Palmer et al., 1988; Bredt and Snyder, 1990; Busse and Mulsch, 1990; Lamas et al., 1992; Garcia-Cardena et al., 1996; Michel et al., 1997; Presta et al., 1997). The enzyme contains an N-terminal oxygenase domain harboring a heme prosthetic group and a C-terminal reductase domain with the latter containing binding sites for flavin mononucleotide, flavin adenine dinucleotide, and NADPH (Sessa et al., 1992; Fleming and Busse, 1999) (Fig. 3A). The oxygenase and reductase domains are separated by a linker region containing a calmodulin binding domain (Sessa et al., 1992; Fleming and Busse, 1999) (Fig. 3A). Translated eNOS forms a homodimer where the N-terminal oxygenase domain from one monomer participates in oxidation-reduction reactions with the C-terminal reductase domain of the second monomer (Fig. 3B). As a dimer, eNOS uses NADPH, l-arginine, and O2 to synthesize NO and the byproduct l-citrulline through a reaction driven by electron transport from the enzyme’s reductase domain to the heme moiety located in the oxygenase domain of the second monomer (Fig. 3B). This reaction by the heme iron promotes binding and subsequent reduction of O2 and incorporation into l-arginine, ultimately terminating with the production of bioactive NO (Palmer et al., 1988; Presta et al., 1997; Fleming and Busse, 1999). This process is dependent on a number of cofactors including 1) tetrahydrobiopterin (BH4), which binds to the oxygenase domain and is required for eNOS dimerization; 2) CaM, which binds to the linker region of dimeric eNOS conferring an activating conformational change; and 3) heat shock protein 90. Transient increases in intracellular Ca2+ recruit CaM to its regulatory binding site in the eNOS linker, promoting NADPH-dependent electron flux between the reductase domain of one eNOS monomer to the oxygenase domain of the second monomer (Abu-Soud and Stuehr, 1993; Chen et al., 1997; List et al., 1997; Presta et al., 1997). In addition, BH4 is required for coordinating the electron transport from the reductase to oxygenase domains and a decrease in the bioavailability of this essential cofactor leads to eNOS uncoupling and the production of superoxide anion (O2•−) instead of NO, imparting oxidative stress on the cell (Wever et al., 1997; Stuehr et al., 2001) (Fig. 3B).
Fig. 3.
eNOS: protein domains, phosphorylation sites, and higher order organization. (A) eNOS is composed of an N-terminal oxygenase domain containing binding sites for tetrahydrobiopterin (BH4), Zn2+, heme, and l-arginine and a C-terminal reductase domain containing NADPH, flavin adenine dinucleotide, and flavin mononucleotide binding sites. The oxygenase and reductase domains are separated by a linker region that harbors a regulatory CaM binding domain. Binding domains are indicated in italics. eNOS also harbors several serine, threonine, and tyrosine residues that are targeted for phosphorylation. The most extensively characterized phosphorylated residues are depicted with those that promote enzyme activation in green (Tyr81, Ser615, Ser633, and Ser1177; human sequence) and sites imparting inhibition in red (Ser114 and Thr495). (B) eNOS forms a homodimer coordinated by BH4 and Zn2+ binding in the N-terminal oxygenase domains of each monomer. Dimeric eNOS synthesizes NO from l-arginine and O2 through NADPH-dependent electron flux from the C-terminal reductase domain of one monomer to the heme moiety located on the oxygenase domain. Depletion of BH4 promotes eNOS uncoupling, leaving the enzyme in a monomeric form, resulting in the production of superoxide rather than NO.
In addition to the array of regulatory cofactors that control eNOS activity, the enzyme is dynamically modulated by phosphorylation, with modification of multiple serine, threonine, and tyrosine residues influencing NO synthesis (Fig. 3A). Although there are several potential phosphorylation sites on eNOS, Tyr81, Ser615, Ser633, and Ser1177 have been identified as target residues for enzyme activation by phosphorylation, and Ser114 and Thr495 for enzyme inhibition (Dimmeler et al., 1999; Fleming et al., 2001; Scotland et al., 2002; Chen et al., 2003; Fulton et al., 2005; Li et al., 2007; Fisslthaler et al., 2008; Loot et al., 2009; Watts and Motley, 2009). Phosphorylation of Ser1177 in the C-terminal reductase domain has been the most extensively characterized, and this activating modification increases electron flux and NO synthesis by eNOS (Dimmeler et al., 1999; Scotland et al., 2002). Ser1177 is modified by a number of kinases in a context-dependent manner. For instance, shear-stress induces the activation of the kinases Akt and protein kinase A (PKA), which phosphorylate eNOS at Ser1177 and promote NO-dependent arterial relaxation (Dimmeler et al., 1999; Gallis et al., 1999; Boo et al., 2002b). Akt-mediated phosphorylation of eNOS has also been observed in response to VEGF and estrogen stimulation, whereas the vasodilation observed in response to bradykinin is controlled by phosphorylation of eNOS Ser1177 by CaM kinase II (Papapetropoulos et al., 1997; Bernier et al., 2000; Yang et al., 2000; Fleming et al., 2001; Chambliss and Shaul, 2002; Chen et al., 2006; Gentile et al., 2013). Site-directed mutagenesis studies have found that mutating this residue to an alanine prevents Akt-dependent NO synthesis, whereas a phosphomimicking mutation of Ser1177 to aspartate renders eNOS constitutively active (Dimmeler et al., 1999; Fulton et al., 1999). In addition to Ser1177, Ser633 can be phosphorylated in response to shear stress and adiponectin stimulation in a PKA and 5′-AMP-activated protein kinase-dependent manner, respectively, leading to eNOS activation and NO synthesis in arterial endothelial cells (Boo et al., 2002a; Chen et al., 2003; Osuka et al., 2012). More recently, phosphorylation of Tyr81 in the N-terminal oxygenase domain has been reported to increase eNOS activity in a c-Src-dependent manner (Fulton et al., 2005). Phosphorylation of Ser615 has also been observed in response to bradykinin stimulation and has been associated with increased eNOS activity (Michell et al., 2002). In contrast to these activating modifications, targeted phosphorylation of Ser114 or Thr495 exerts inhibitory effects on eNOS. Modification of Ser114 renders eNOS less active in response to VEGF stimulation, and mutagenesis studies have concurrently revealed that phosphorylation of this residue promotes eNOS interaction with its scaffolding regulatory protein Cav1 (Li et al., 2007). Of the most studied inhibitory residues in eNOS, Thr495 located in the CaM binding site plays a dynamic role in regulating eNOS activity. In resting ECs, Thr495 is constitutively phosphorylated by 5′-AMP-activated protein kinase and PKC, antagonizing CaM binding in response to Ca2+-mobilizing agonists and functionally inhibiting NO synthesis by the enzyme (Fleming et al., 2001; Watts and Motley, 2009). The myriad of evidence for eNOS regulation by phosphorylation has made it clear that the dynamic balance between phosphorylation of activating and inhibiting residues of eNOS imparts strict control over the enzyme’s ability to produce NO and propagate NO-dependent signaling cascades. Based on the growing literature for phosphorylation in regulating interactions of eNOS with its cofactors, these posttranslational modifications may prove to play an important role in the regulation of eNOS in distinct signaling microdomains by controlling the localization and binding interactions between other known signaling partners that are discussed in the following sections. Characterization of the catalytic activity of eNOS and the requirement of indispensable cofactors and substrates have prompted numerous investigations into the key factors conferring specificity to localization of the enzyme to specific regions in the cell where these substrates are concentrated and interactions between eNOS and other protein binding partners that can regulate its activity. At the axis of eNOS regulation in the blood vessel wall, evidence has emerged suggesting distinct signaling microdomains containing the enzyme at the level of the Golgi, plasma membrane caveolae, and the MEJ.
2. Compartmentalized Endothelial Nitric-Oxide Synthase Regulation in the Endothelial Cells Golgi Apparatus.
eNOS resides in several distinct locations within ECs, notably the Golgi apparatus, cholesterol-enriched microdomains at the plasma membrane (including lipid rafts and caveolae), and the MEJ where heterocellular communication can occur through direct cell-to-cell coupling. Proper trafficking of eNOS to these domains requires the coordinated cotranslational modification of eNOS by N-myristoylation at amino acid residue Gly2 and posttranslational modification by S-palmitoylation at residues Cys15 and Cys26 (Sessa et al., 1993; Liu and Sessa, 1994; Liu et al., 1995; Robinson and Michel, 1995; Shaul et al., 1996; Fernandez-Hernando et al., 2006). N-Myristoylation is an absolute requirement for eNOS trafficking through the Golgi because a deficiency in the covalent attachment of a myristoyl group to Gly2 results in cytoplasmic sequestration of the enzyme and decreased catalytic activity for NO generation. Retention of eNOS in the cytoplasmic compartment of the cell probably results in decreased NO generation because of suboptimal exposure of the enzyme to its required cofactors and substrates for NO synthesis. Following N-myristoylation, eNOS is targeted to the trans-Golgi, where a class of acetyltransferases of the Asp-His-His-Cys motif (DHHC) palmitoyltransferase family palmitoylate eNOS at Cys15 and Cys26 (Fernandez-Hernando et al., 2006).
The eNOS enzyme has been shown to polarize to the Golgi along with five members of the DHHC family of palmitoyl transferases including DHHC 2, 3, 7, 8, and 21. In particular, regional colocalization of eNOS and the DHHC 21 isoform has been observed by coimmunoprecipitation and immunofluorescence overlap studies, suggesting that these two enzymes may form a functional complex required for eNOS palmitoylation and activity (Fernandez-Hernando et al., 2006). Modification of eNOS by S-palmitoylation confers proper trafficking and subcellular localization of the enzyme to cellular membranes where eNOS activity has been shown to be maximal, notably at plasma membrane caveolae and lipid rafts (Michel, 1999; Sessa, 2004). Dysregulated acylation of eNOS by DHHC 21 results in diminished NO production both basally and in response to vasodilatory agonists such as ATP, which may ultimately perturb communication between the endothelium and smooth muscle in the blood vessel wall (Fernandez-Hernando et al., 2006). This is particularly evident because eNOS constructs with mutations at the myristoylation and/or palmitoylation sites are less active than wild-type eNOS, and genetic knockdown of DDHC 21 in ECs impairs trafficking of eNOS to plasma membrane compartments and reduces NO generation in response to the calcium ionophore ionomycin and adenosine triphosphate (ATP) (Fernandez-Hernando et al., 2006). Taken together, these studies suggest that a signaling microdomain poised in the EC Golgi is important for eNOS processing and subsequent trafficking to peripheral membranes in the cell where its biologic activity is optimal. Improper lipid modification of eNOS can therefore have detrimental effects on signaling events in the vasculature, which can lead to pathologies including hypertension, impairments in angiogenesis and atherosclerosis.
3. Signaling Microdomains Involving Endothelial Nitric-Oxide Synthase Regulation at the Plasma Membrane.
a. Endothelial nitric-oxide synthase and caveolin 1 interactions in the endothelium.
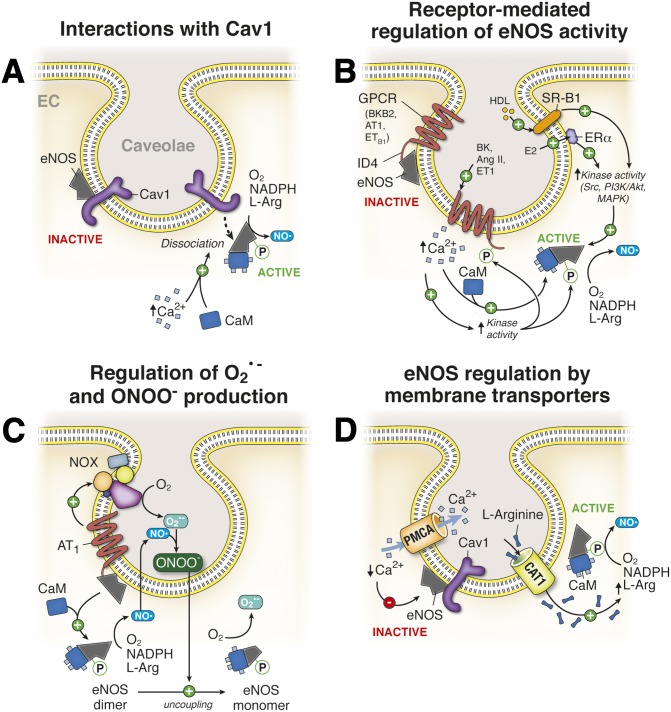
The major fraction of eNOS in ECs is localized to specialized regions of the plasma membrane, including cholesterol-rich lipid rafts and caveolae where its activity has been shown to be optimal (Zhang et al., 2006b). In ECs, eNOS colocalizes with the caveolin 1 (Cav1) isoform in caveolae, anchoring the enzyme at the cytoplasmic face of the invagination. A direct protein-protein binding interaction between eNOS and Cav1 has been observed both in vitro and in vivo, and characterization of this interaction identified the ability of Cav1 to impart an inhibitory clamp on the catalytic activity of eNOS, acting as an allosteric inhibitor controlling NO production in the endothelium (Garcia-Cardena et al., 1997; Ju et al., 1997; Bucci et al., 2000). Furthermore, studies aimed at mapping the interacting domains of eNOS and Cav1 identified this protein-protein interaction to occur between the N-terminal oxygenase domain of eNOS and the intracellular N-terminal scaffolding domain of Cav1 (Ju et al., 1997; Bucci et al., 2000; Bernatchez et al., 2005). In addition, a synthetic peptide corresponding to residues 82–101 of Cav1 called cavtratin was capable of binding to and inhibiting eNOS activity (Ju et al., 1997; Bucci et al., 2000).
Functionally, Cav1 binding to eNOS antagonizes the interaction of the enzyme with its activating cofactor calmodulin under resting conditions. Further investigation of the Cav1/eNOS interaction has identified three residues (Thr90, Thr91, and Phe92) in the Cav1 scaffolding domain that impart the negative regulation of Cav1 on eNOS activity (Bernatchez et al., 2005). The inhibitory clamp can be relieved by stimulating endothelial cells with agonists that mobilize intracellular Ca2+, including fluid shear stress on the endothelium and a number of vasodilator agonists. This process leads to dissociation of eNOS from Cav1, binding of CaM to its allosteric site on eNOS, and activation of the enzyme to facilitate NO production (Fig. 4A). Disruption of the eNOS/Cav1 interaction by genetic deletion of Cav1 from ECs increases NO production by eNOS and facilitates SMC relaxation. To this end, Cav1−/− mice develop systemic hypotension due to increased activity of eNOS (Murata et al., 2007). On the basis of these observations, it has been proposed that Cav1 binding to eNOS in caveolae serves to impart allosteric inhibition of the enzyme under basal conditions to prevent excessive NO production and nitrosative stress. In addition to the systemic vascular effects of Cav1 depletion, mice lacking Cav1 acquire pulmonary hypertension attributed to dysregulated eNOS activity (Zhao et al., 2009). The mechanism responsible for this pathology is proposed to be mediated through increased protein kinase G tyrosine-nitration due to nitrosative stress, which inhibits protein kinase G, thus preventing smooth muscle relaxation and increasing vascular resistance in the pulmonary vasculature. This pulmonary hypertension has been shown to be ablated in mice lacking both Cav1 and eNOS or by eNOS inhibition in Cav1−/− mice with l-NAME treatment (Zhao et al., 2009). In addition to the effects of Cav1 deficiency on the vascular tone, ECs isolated from Cav1−/− mice have an impaired barrier function due in part to increased nitrosative stress and nitration of regulatory proteins involved in adherens junction assembly and maintenance (Siddiqui et al., 2011).
Fig. 4.
Nitric oxide regulation at plasma membrane caveolae. (A) eNOS localizes to plasma membrane caveolae, where it directly binds to Cav1. This interaction inhibits basal eNOS activity and NO synthesis. Increases in Ca2+ facilitate activation of CaM, which is recruited to eNOS and promotes dissociation of the enzyme from Cav1. Binding of CaM to free eNOS increases its enzymatic activity, resulting in NO production from the substrates l-arginine, NADPH, and O2. (B) eNOS colocalizes with a number of membrane receptors in endothelial cell caveolae, including the angiotensin II type 1 receptor (AT1), the bradykinin B2 receptor (BKB2), the endothelin-1 type B receptor, the estrogen receptor (ERα), and the scavenger receptor (SR-B1). The GPCRs bind eNOS directly through an interaction with their fourth intracellular domain (ID4) and inhibit basal eNOS activity. Binding of GPCR ligands to their complement receptors promotes eNOS dissociation from these receptors, relieving the inhibitory clamp that is mediated through increases in intracellular Ca2+ and phosphorylation of eNOS and the GPCR, leading to NO production by eNOS. Activation of SR-B1 by high-density lipoprotein (HDL) or ERα by estradiol promotes activation of protein kinases, including Src, PI3K/Akt, and MAPKs, which promote eNOS phosphorylation and enzyme activation. (C) Activation of the AT1 receptor in caveolae signals eNOS activation as described in (B) as well as the recruitment and activation of NOX to caveolae. NOX activation produces superoxide anion (O2•−), which uncouples dimeric eNOS to its monomeric form, resulting in O2•− production. NO and O2•− generated at EC caveolae rapidly react to form the free radical peroxinitrite (ONOO−). See Fig. 6 for NOX regulation. (D) eNOS enriched at EC caveolae colocalizes with and is regulated by the activity of the cationic amino acid transporter 1 (CAT1) and the plasma membrane Ca2+ ATPase (PMCA). In caveolae, PMCA functions to extrude Ca2+ from the local cytosolic compartment and the subsequent reduction in free intracellular Ca2+ prevents CaM recruitment and Cav1 dissociation from eNOS, inhibiting NO production. Conversely, CAT1 facilitates the cellular uptake of the eNOS substrate l-arginine in spatial proximity to the synthase, providing local enrichment in the precursor for NO synthesis.
After the characterization of this signaling complex in caveolae, the Sessa laboratory developed a noninhibitory analog of cavtratin that contains alanine substitutions at Thr90, Thr91, and Phe92 called cavnoxin (Bernatchez et al., 2011). Cavnoxin competes with Cav1 for binding the oxygenase domain of eNOS but, unlike cavtratin, does not inhibit eNOS activity. Instead, cavnoxin increases basal NO production in eNOS-expressing cells, decreases vascular resistance in isolated arterioles, and reduces blood pressure in mice (Bernatchez et al., 2011).
b. Endothelial nitric-oxide synthase interactions with membrane receptors localized to caveolae in endothelial cells.
Multiple plasma membrane receptors have been implicated in signal transduction pathways that regulate the activity of eNOS in caveolae including the bradykinin receptor B2, the angiotensin receptor, the endothelin 1 receptor, estrogen receptor α (ERα), and the scavenger receptor class B type 1 (SR-B1) (Fig. 4B) (Ju et al., 1998; Chen et al., 1999; Bernier et al., 2000; Chambliss et al., 2000; Golser et al., 2000; Haynes et al., 2000; Waid et al., 2000; Hisamoto et al., 2001; Yuhanna et al., 2001; Suzuki et al., 2006). The G-protein coupled receptors (GPCRs) have been shown to bind to eNOS in caveolae through a C-terminal intracellular domain (ID4) and inhibit the enzyme under basal conditions (Ju et al., 1998). Activation of these GPCRs by their complement ligands relieves the inhibitory clamp on eNOS by inducing an increase in intracellular Ca2+ (see above) and recruitment of CaM to eNOS or it can stimulate phosphorylation cascades, ultimately targeting activating residues in eNOS or the receptor itself causing dissociation of the receptor from eNOS (McDuffie et al., 1999; Bernier et al., 2000; Golser et al., 2000; Waid et al., 2000; Suzuki et al., 2006). Both mechanisms result in eNOS activation and NO production in ECs. Incubation of purified ID4 domains with purified eNOS decreases the enzyme’s catalytic activity and NO bioavailability, providing direct evidence for a GPCR:eNOS signaling complex (Ju et al., 1998; Golser et al., 2000).
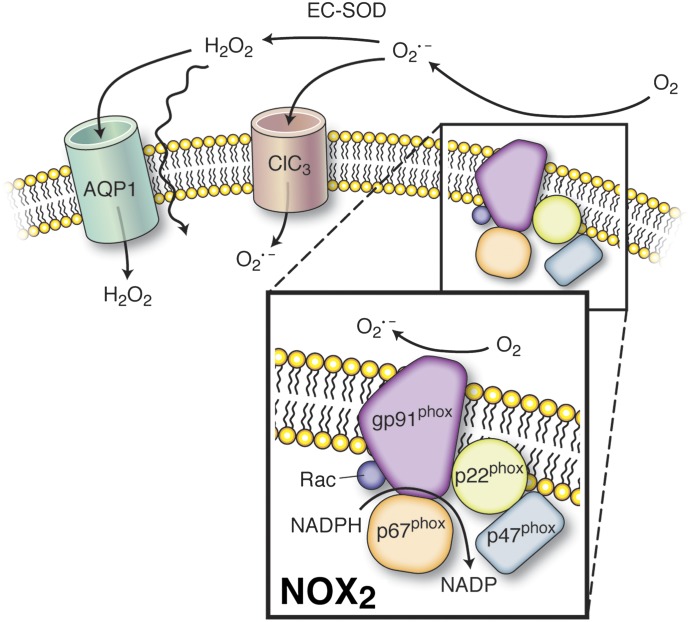
Of particular note, eNOS regulation at caveolae in ECs by the AT1 receptor provides complex control over the production of NO and its oxidized derivative peroxinitrite (ONOO−) (Fig. 4C). Activation of the caveolae angiotensin receptor by angiotensin II (Ang II) promotes eNOS activation through the mechanisms described above but may also promote recruitment and activation of NADPH oxidase (NOX) to this microdomain (Pueyo et al., 1998; Lobysheva et al., 2011). This process results in the generation of O2•−, which can readily react with eNOS-derived NO to form ONOO−. Upon ONOO− accumulation, eNOS dimers uncouple to their monomeric form in the caveolae resulting in the synthesis of O2•− rather than NO. In addition, ONOO− may diffuse within the cell or to surrounding cells in the vascular wall, imparting oxidative stress (Huie and Padmaja, 1993; Hogg et al., 1994).
Additional evidence has supported a role for high-density lipoprotein (HDL) mediated eNOS activation by binding to and activating SR-B1, conferring atheroprotective NO production (Yuhanna et al., 2001). Binding of HDL to SR-B1 localized to EC caveolae leads to activation of several protein kinases including Src, MAPK, and PI3K/Akt, which function to phosphorylate and activate eNOS. Likewise, the estrogen receptor ERα colocalizes with eNOS in plasma membrane caveolae, and binding of estradiol to this receptor activates eNOS and promotes NO generation in ECs through the coordinated action of protein kinase-mediated eNOS phosphorylation (Chambliss et al., 2000; Haynes et al., 2000; Hisamoto et al., 2001). This signaling mechanism has been suggested to be an important determinant for atheroprotection in females that can be mimicked in the male population by estrogen supplementation.
c. Regulation of endothelial nitric-oxide synthase by membrane transporters and channels in caveolae.
The sequestration of eNOS to caveolae in ECs poises the enzyme in a spatial region of the cell where specific membrane channels and transporters have been shown to colocalize and regulate eNOS activity by tightly controlling the abundance of essential cofactors and substrates. Notably, the cationic amino acid transporter 1 (CAT1) colocalizes with eNOS and Cav1 in endothelial cell caveolae, where its function has been proposed to regulate the cellular import of l-arginine, the prerequisite substrate for NO synthesis by eNOS, in close proximity to the enzyme (Fig. 4D). Studies have shown that the CAT1 coimmunoprecipitates with eNOS and Cav1 from isolated endothelial cell membranes, suggesting a regionalized interaction between these proteins in caveolae (McDonald et al., 1997). Therefore, the regulated activity of CAT1 in vascular endothelial cell caveolae may impart functional effects on NO synthesis through localized modulation of substrate bioavailability.
In addition to CAT1, the plasma membrane calcium ATPase (PMCA) has also been found to localize to plasma membrane caveolae. Notably, PMCA is concentrated 18- to 25-fold higher in caveolae compared with noncaveolae plasma membrane fractions (Fujimoto, 1993; Schnitzer et al., 1995). PMCA is a P-type ATPase that plays a crucial role in the regulation of cell calcium homeostasis (Di Leva et al., 2008). As its name indicates, it utilizes energy from ATP hydrolysis to extrude calcium from the cytosol to the extracellular space (Di Leva et al., 2008). In human umbilical vein endothelial cells (HUVECs), treatment with methyl β cyclodextrin (MβCD), which disrupts caveolae by sequestering cholesterol from the compartment, significantly reduces Ca2+ efflux mediated by PMCA (Zhang et al., 2009). Conversely, replenishment of cholesterol to MβCD-treated cells restored PMCA-mediated calcium efflux from ECs (Zhang et al., 2009). Because eNOS activity is highly dependent on calcium, PMCA was shown to negatively regulate eNOS activity by extruding Ca2+ from the cytosol, decreasing the local concentration of Ca2+ in spatial proximity to eNOS in caveolae (Holton et al., 2010). This active shuttling of Ca2+ to the extracellular compartment decreases CaM recruitment to Cav1 bound eNOS, preventing dissociation of the two proteins and ultimately preventing NO synthesis (Fig. 4A). In addition to the control of local Ca2+ availability at the caveolae membrane, recent work has identified a novel binding interaction between endothelial cell PMCA and eNOS that may directly antagonize eNOS activity and NO production partly through effects on eNOS phosphorylation status (Holton et al., 2010). Taken together, these observations provide evidence for a regulated signaling microdomain between CAT1, PMCA, and eNOS isoforms in EC caveolae.
4. Compartmentalized Nitric Oxide Signaling at the Myoendothelial Junction.
The MEJ is a distinct anatomic structure in the blood vessel wall where (predominantly) ECs send cellular projections through small holes in the internal elastic lamina separating the intima and media that allow for direct cell-to-cell contact with the overlying smooth muscle cell layer (Moore and Ruska, 1957; for review, see Heberlein et al., 2009). In small resistance arteries, MEJs are numerous compared with larger conduit arteries and coordinate signal transduction between the smooth muscle and endothelium by facilitating intercellular transport of small molecules and ions as well as harboring polarized proteins involved in vascular cell crosstalk (Sandow and Hill, 2000; Dora et al., 2003a; Isakson and Duling, 2005; Isakson et al., 2007; Isakson, 2008; Straub et al., 2011).
a. Nitric oxide regulation of gap junction permeability at the myoendothelial junction.
The MEJ has emerged as an important signaling microdomain in the vasculature with a number of signaling proteins localized to the junction that influence vascular homeostasis, most notably gap junctions comprised of connexins.
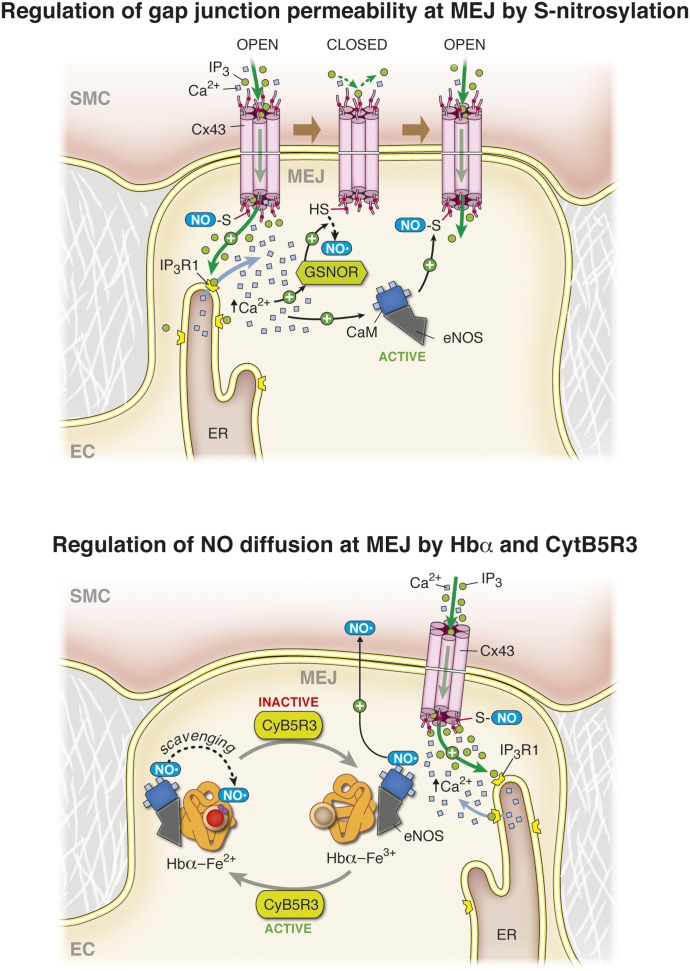
The presence of gap junctions at the MEJ influences smooth muscle-endothelial cell coupling, and the permeability of connexin 43 (Cx43)-based gap junctions is tightly regulated by nitric oxide (Straub et al., 2011). Use of a vascular cell coculture model has revealed an enrichment of Cx43 and eNOS localized to the MEJ in vitro, and characterization of this enrichment in vivo by immunolabeling coupled to transmission electron microscopy has confirmed this observation in the intact arterial wall (Straub et al., 2011). Concurrent with a localized enrichment of these two proteins at the MEJ, a novel signaling microdomain involving eNOS and Cx43 has been identified in which the posttranslational modification of Cx43 at the MEJ by S-nitrosylation of Cys271 regulates the permeability of these intercellular channels. S-Nitrosylation of Cx43 promotes an open-channel conformation allowing exchange of cytosolic constituents between smooth muscle and endothelium. Cx43 is constitutively S-nitrosylated at the MEJ due to colocalization of eNOS, which harbors a low level of basal activity (Straub et al., 2011). In small resistance arteries, stimulation of smooth muscle α1-adrenergic receptors promotes induction of Gq-dependent signaling cascades, leading to the activation of phospholipase C that cleaves the phospholipid phosphatidylinositol 4,5-bisphosphate to IP3 and diacylglycerol, increasing the cytosolic inositol triphosphate (IP3) concentration. The generated IP3 induces Ca2+ release from the smooth muscle cell SR (see above) but can also directly traverse gap junctions at the MEJ to activate the IP3 receptor type 1 (IP3R1) in ECs (Isakson et al., 2007; Isakson, 2008). This has been proven in vivo in mesenteric and cremasteric arteries, where IP3 diffusion from SMCs can elicit a local increase in [Ca2+]i in ECs specifically at the MEJ where a subset of the IP3R1 isoform is poised at extensions of the ER within the MEJ (Isakson, 2008; Ledoux et al., 2008). The initial rise in endothelial calcium activates another enzyme polarized and enriched at the MEJ, the S-nitrosoglutatione reductase (GSNOR), whose activation facilitates denitrosylation of Cx43 and channel closure (Straub et al., 2011) (Fig. 5, top). After activation of GSNOR and denitrosylation of Cx43, the rise in endothelial cell [Ca2+]i promotes eNOS activation and subsequent generation of NO at the MEJ, which diffuses to and relaxes the SMCs and ultimately renitrosylates Cx43 at the MEJ, presumably providing rapid spatial and temporal control of heterocellular communication between the vascular cells (Straub et al., 2011). In addition to the role of smooth muscle derived IP3 in regulating endothelial cell [Ca2+]i signaling at the MEJ, smooth muscle Ca2+ released from the SR downstream of IP3 mobilization may also influence heterocellular communication by traversing the gap junctions at the MEJ to control eNOS and GSNOR activity (Isakson et al., 2007). Regardless of the second messenger to elicit the increase in [Ca2+]i, the NO-related dynamics on the EC side of the MEJ likely remain the same. These studies have shown a novel interaction between Cx43, IP3R1, eNOS, and GSNOR localized to the MEJ, providing evidence for a signaling microdomain that can regulate cellular communication between the EC and SMC in the blood vessel wall.
Fig. 5.
Nitric oxide regulation at the MEJ. Top, Nitric oxide posttranslationally modifies the gap junction protein connexin 43 (Cx43) at the MEJ through S-nitrosylation. Under basal conditions, Cx43 is constitutively S-nitrosylated, which renders the channel in an open, permeable state. IP3 and Ca2+ from the smooth muscle cell layer diffuse through these open gap junctions and bind to IP3 receptor type 1 (IP3R1) on the EC endoplasmic reticulum that is poised at the MEJ, resulting in Ca2+ release from the internal store. This local increase in Ca2+ promotes the activation of the denitrosylase enzyme S-nitrosoglutathione reductase (GSNOR) whose activity leads to denitrosylation of Cx43. Reduction of the S-nitrosothiols on Cx43 closes the channel, preventing additional ion and metabolite diffusion into the ECs. After this event, the local rise in Ca2+ activates the eNOS localized at the MEJ, resulting in increased NO production and renitrosylation of Cx43 gap junctions, restoring gap junctional communication between ECs and SMCs in the arterial wall. The black dashed arrow indicates reduction of the nitrosothiol, and the red dots on Cx43 correspond to the cysteine residue on the carboxyl tail of Cx43 that is S-nitrosylated. Bottom, Hemoglobin α (Hbα) is synthesized by vascular ECs and is enriched at the MEJ, where it forms a complex with eNOS and the reductase CytB5R3. NO generated by eNOS at the MEJ is able to diffuse to the overlying smooth muscle cell layer when Hbα resides in the Fe3+ state (methemoglobin, maroon). Reduction of Hbα to the Fe2+ state (oxyhemoglobin, red) by the activity of CytB5R3 promotes NO scavenging by Hbα and prevents NO diffusion.
b. Nitric oxide signaling regulation at the myoendothelial junction by hemoglobin α and cytochrome B5 reductase 3.
Polarization and enrichment of eNOS at the EC side of the MEJ poises the enzyme in close proximity to the overlying SMC layer, allowing for rapid NO diffusion upon eNOS activation. Recent evidence has shown that the alpha chain of hemoglobin (Hbα) is actively synthesized by arterial ECs and polarizes to the MEJ in small resistance arteries (Straub et al., 2012). In addition, the Hbα subunit has been shown to be expressed in small mesenteric arteries (Burgoyne et al., 2012). In red blood cells, hemoglobin is known to scavenge NO through reactive chemistry involving its heme center (Cassoly and Gibson, 1975). Recent work has also found a similar function of Hbα at the MEJ, where monomeric Hbα acts to regulate NO diffusion to SMCs and functionally impacts arterial reactivity (Straub et al., 2012).
NO scavenging by hemoglobin in the red blood cell can be regulated by the reducing activity of the enzyme cytochrome B5 reductase 3 (CytB5R3), where the enzyme acts to reduce the heme iron in Hb from the Fe3+ state to the Fe2+ state (Hultquist and Passon, 1971). Importantly, the reaction kinetics of NO with heme iron in the Fe2+ state is extremely rapid (2.4 × 107 M−1⋅s−1) (Cassoly and Gibson, 1975), whereas the control of NO diffusion by heme iron in the Fe3+ state occurs more slowly (3.3×103 M−1⋅s−1) (Sharma et al., 1987). As a result, Hbα residing at the MEJ in the Fe2+ state may scavenge NO rapidly, preventing diffusion to SMCs and maintaining a more constricted state. Conversely, Hbα in the Fe3+ state may allow for greater NO diffusion to SMCs, enhancing vasorelaxation. Concurrently, NO scavenging by heme iron in the Fe2+ state can limit the amount of NO diffusing to the overlying SMC layer, whereas heme iron in the Fe3+ binds NO at a slower rate. It is noteworthy that CytB5R3 was also present at the MEJ and that coimmunoprecipitation studies using in vitro MEJ fractions, intact arteries, and purified proteins have indicated that eNOS, Hbα, and CytB5R3 form a protein complex enriched at this signaling nexus (Straub et al., 2012).
In isolated resistance arteries, siRNA knockdown of Hbα in EC potently increased NO diffusion through the arterial wall, indicating the functional importance of Hbα in scavenging endothelium-derived NO (Straub et al., 2012). Furthermore, pressure myography studies on these arteries have provided functional evidence for NO scavenging by EC Hbα in response to phenylephrine and acetylcholine stimulation, two agonists that induce NO synthesis by the endothelium (Straub et al., 2012). Further analysis revealed that CytB5R3 actively reduces Hbα to the Fe2+ state at the MEJ, imparting strict control over the ability of Hbα to scavenge diffusible NO at the interface of endothelium and smooth muscle (Straub et al., 2012). These observations have identified a novel signaling microdomain at the MEJ in small resistance arteries where Hbα, eNOS, and CytB5R3 work in concert to regulate arterial tone (Fig. 5, bottom).
The identification of multiprotein signaling microdomains at the MEJ has shed light on the regulation of heterocellular communication in the resistance vasculature and provides insight into the complex control of arterial tone. At one axis, it is now clear that gap junctions at the MEJ can be direct targets of bioactive NO that is synthesized in close proximity to the junction by eNOS. Furthermore, the eNOS:Hbα:CytB5R3 ternary complex that resides at the MEJ plays a role in regulating the diffusion of NO to the junction and surrounding SMCs to influence arterial tone. These new insights into signal propagation between ECs and SMCs may prove to be intimately associated where Hbα may not only function to control the amount of NO diffusing to the SMC to activate cGMP-dependent relaxation, but also regulate the extent of Cx43 S-nitrosylation and ultimately gap-junction-mediated heterocellular communication. In this respect, decreased NO scavenging by Hbα at the MEJ may favor S-nitrosylation of Cx43 poising the junctions in an open conformation and allow diffusion of IP3 and calcium from the SMC to EC, promoting vasodilation (Fig. 5).
In conclusion, eNOS activation and NO bioavailability results from a complex regulating process involving direct protein interaction with multiple players (Cav1, GPCR, Hbα, CytB5R3, PMCA, etc.) that accumulate within a specialized phospholipid region, caveolae, and, in some cases, are polarized to a specific region of the cells, the MEJ. With regard to our definition in Table 1 and the work described above, eNOS and its protein partners are an important signaling microdomain for intercellular communication in the blood vessel wall.
B. Reactive Oxygen Species Are Signaling Molecules Involved in Intercellular Communication
Oxygen is a necessary molecule for cellular function by fueling the respiratory chain in the mitochondria. Paradoxically, oxygen is also the main source of reactive oxygen species (ROS) that cause a multitude of cellular damages within the cell. The superoxide anion (O2•−) is at the origin of the formation of other ROS such as hydrogen peroxide (H2O2) or the hydroxyl radical (⋅OH). The superoxide anion can also react with NO and form peroxynitrite (ONOO⋅), which can further generate reactive nitrogen species that also have deleterious cellular effects (for review, see Martinez and Andriantsitohaina, 2009). When ROS are produced in large amount, due to upregulation of the proteins orchestrating their synthesis and/or decreased ROS degradation or scavenging, it results in cellular oxidative stress. Oxidative stress occurs in multiple pathologic conditions, including hypertension, atherosclerosis, and myocardial infarction where ROS are involved in VSMC proliferation and migration, monocyte infiltration, endothelial dysfunction, and remodeling of the extracellular matrix (for review, see Touyz et al., 2011; Montezano and Touyz, 2012; Sedeek et al., 2012). The plasma level of the ROS H2O2 is increased in patients with hypertension compared with healthy patients, suggesting its importance as a paracrine molecule mediating oxidative stress (Varma and Devamanoharan, 1991; Lacy et al., 1998; Halliwell et al., 2000).
The short half-life of ROS and their high reactivity has led to the hypothesis that these oxidant molecules may be produced and exert their effects in a restricted space (Davidson and Duchen, 2006). Although the concept of a “ROS microdomain” has never been clearly established, O2•− production was observed in discrete regions at the plasma membrane and in the area surrounding the mitochondria (Zorov et al., 2000; Aon et al., 2003; Brady et al., 2004; Davidson and Duchen, 2006; Yi et al., 2006; Garcia-Perez et al., 2012; Siddall et al., 2013). Superoxide anion can be produced via several pathways, both intracellularly and extracellularly. The main intracellular source of O2•− is the mitochondria, which “leaks” electrons from the respiratory chain that react with molecular oxygen. At the plasma membrane, NADPH oxidase is the principal source of O2•− and produces O2•− extracellularly. Given the extremely short half-life of the superoxide anion and the presence of an extracellular form of superoxide dismutase (EC-SOD), it has been hypothesized that O2•− is rapidly dismutated to H2O2 in the extracellular space. Because H2O2 is a lipophilic molecule, it can readily diffuse through the plasma membrane and exert its oxidative effects intracellularly. Conversely, due to the lipophilic nature and relative stability of H2O2 compared with O2•−, it has been suggested that H2O2 is the central molecule imparting oxidative stress. However, it is becoming increasingly clear that O2•− produced extracellularly by Nox has an effect intracellularly, which has subsequently led several groups to investigate candidate proteins for the transport of the O2•− across the plasma membrane.
1. NADPH Oxidase as a Signaling Microdomain.
The NADPH oxidase (Nox) was first characterized in phagocytic cells where it plays an important role in the antimicrobial defense via the production of superoxide anion (Parkos et al., 1987; Rotrosen et al., 1992). Nox is a multimeric protein complex that could be considered in itself a signaling microdomain because it is composed of two distinct enzymatic subunits that interact with multiple small regulating proteins. The intrinsic transmembrane component of the Nox is formed by a heterodimeric flavocytochrome, which comprises two subunits, gp91phox and p22phox (Fig. 6) (Parkos et al., 1987; Rotrosen et al., 1992). This heterodimeric flavocytochrome is also called cytochrome b558, based on its spectroscopic properties. These subunits were named after their molecular mass on gel electrophoresis whereas the letters indicate a protein (p) or glycoprotein (gp) of the phagocyte oxidase (phox) (Dinauer et al., 1987; Parkos et al., 1987, 1988; Rotrosen et al., 1992). Several homologs of gp91phox have been identified and are termed Nox1, Nox3, Nox4, Nox5, Duox1, and Duox2, with the isoform gp91phox subsequently renamed Nox2 (for review, see Bedard and Krause, 2007; Montezano et al., 2011). These isoforms have different modes of regulation, expression, and activity but all harness the capacity to produce O2•−, with the exception of Nox4, which has been shown to mainly produce H2O2 (Wu et al., 2010). The Nox1, Nox2, Nox4, and Nox5 isoforms have all been identified in the vasculature, with differential expression in both ECs and VSMCs (Touyz et al., 2011). Although each Nox isoform (with the exception of Nox5) requires the presence of the enzymatic subunit p22phox, their activity is differentially regulated: Nox1 is regulated by NOX1A, NOX1B, and Rac1, whereas Nox2 is regulated by p47phox, p67phox, and the small GTPase (Rac) (Nunoi et al., 1988; Volpp et al., 1988; Abo et al., 1991; Bedard and Krause, 2007). Although the regulators of Nox1 and 2 have been well characterized, the mechanisms controlling Nox4 and Nox5 activity are not as well understood; however, it appears that Nox4 is constitutively active and Nox5 does not require the subunit p22phox (Montezano et al., 2011).
Fig. 6.
ROS microdomain at the plasma membrane. NADPH oxidase is the main source of O2•− at the plasma membrane where it produces O2•− in the extracellular space. The NADPH oxidase protein complex represented here corresponds to the Nox2 isoform (previously termed gp91phox). Upon stimulation, the regulators of gp91phox, including gp22phox, gp67phox, gp47phox, and Rac assemble with the gp91phox. The O2•− produced extracellularly can either be dismuted by the extracellular superoxide dismutase (EC-SOD) or traverse the chloride channel ClC3 at the plasma membrane. In the extracellular space, the dismutation of O2•− results in the production of H2O2, which can either cross the plasma membrane directly because of its lipophilic property or via the aquaporin channels (AQP1).
In resting conditions, the different elements composing the Nox enzymatic complex are colocalized at the plasma membrane, but they only interact with each other upon stimulation, which brings each subunit and regulating protein in close apposition (Sumimoto et al., 1996; Han et al., 1998; Groemping et al., 2003). Once activated, two heme groups present in the transmembrane portion of the gp91phox subunit allow for the transfer of electrons from a cytosolic NADPH to a molecular oxygen on the extracellular side of the plasma membrane, thus producing O2•− in the extracellular milieu (Bedard and Krause, 2007). In the vasculature, production of O2•− can be activated by a variety of stimuli such as Ang II, inflammatory mediators, and shear stress (Fisher, 2009). The activation of Nox, specifically Nox2, by Ang II has been at the center of a multitude of investigations, and it is now well described that Ang II induces the phosphorylation of the regulatory subunits p47phox and p67phox, causing them to relocate to the plasma membrane with the heterodimeric flavocytochrome b558 (Griendling et al., 1994). In these conditions, p38 MAPK is activated by the Nox2-derived ROS and results in VSMC hypertrophy (Ushio-Fukai et al., 1998).
The superoxide anion produced extracellularly by the Nox can exert its effect via several pathways. The most well described pathway involves dismutation of O2•− to H2O2 in the extracellular space by the EC-SOD. Thus, H2O2 has been considered a second messenger that is involved in intercellular communication as well as in intracellular signalization (Bedard and Krause, 2007). The role of H2O2 as a paracrine molecule has been demonstrated in the vascular wall, where H2O2 is key in the communication between the vascular adventitia and VSMCs and is involved in VSMC hypertrophy (Liu et al., 2004). In parallel, studies indicate that H2O2 derived from the adventitia plays a critical role in the relaxation of rat carotid artery in response to acetylcholine by activating p38 MAPK and inhibiting the protein tyrosine phosphatase SHP-2 in VSMCs (Cascino et al., 2011). In addition to the damaging effect of O2⋅−-dependent H2O2 production, it has become evident that despite its short half-life, O2•− may itself play an active role in oxidative cellular damage. Studies incorporating exogenous SOD to deplete O2•− have aided in the investigation of a primary role for the free radical during oxidative stress. For example, mice deficient in EC-SOD exhibit higher systemic blood pressure upon Ang II infusion compared with control mice (Welch et al., 2006). Likewise, a recombinant heparin-binding form of SOD acutely decreased blood pressure in spontaneously hypertensive rats (Chu et al., 2003). These observations have raised questions regarding the exact mechanism responsible for intracellular oxidative damages caused by the extracellular production of O2•−, and several investigators suggested different mechanisms that may facilitate the transport of O2•− across the plasma membrane, including the voltage-gated chloride channel ClC-3 and aquaporin (Fig. 6).
2. Candidates for Transport of Reactive Oxygen Species across the Plasma Membrane.
a. ClC-3 channels.
ClC-3 is a member of the family of voltage-gated chloride channels that is abundantly expressed in the VSMCs and ECs where it can be activated by increases in cell volume or cellular stretch (Duan, 2011). Additionally, ClC-3 channels have been shown to open in conditions where the cells are stimulated by Ang II, ET-1, CaM kinase II, and ROS (for review, see Duan, 2011). Evidence for a flux of O2•− across the plasma membrane has been demonstrated in pulmonary microvascular ECs using the superoxide-sensitive dye hydroethidine (Hawkins et al., 2007). When O2•− was added to the extracellular milieu, the fluorescence of EC loaded with hydroethidine increased significantly, and this was abolished when cells were pretreated with the anion channel blocker (4,4′-diisothiocyanato-2,2′-stilbenedisulfonic acid disodium salt) or with siRNA targeting ClC-3 (Hawkins et al., 2007). The authors observed an increase in [Ca2+]i when cells were treated with exogenous superoxide anion (Hawkins et al., 2007). The calcium appeared to be originating from the ER, and in turn, calcium altered the mitochondrial membrane potential, inducing more superoxide anion production from the mitochondria (Madesh et al., 2005; Hawkins et al., 2007).
In pulmonary hypertension, increased ROS production has been correlated with increased Nox expression and/or increased activity of Nox (for review, see Freund-Michel et al., 2013). Additionally, the increased ROS production has been shown to be involved in VSMC proliferation and endothelial dysfunction (Freund-Michel et al., 2013). Interestingly, in a model of monocrotalin-induced pulmonary hypertension, the expression of the gene coding the ClC-3 channels was upregulated in rat pulmonary arteries and cultured canine PASMCs (Dai et al., 2005b). Although no direct link has been established between the overexpression of Nox4 and ClC-3, the in vitro data reported by Hawkins et al. (2007) could lead to the hypothesis that the channel overexpression contributes to the influx of extracellular O2•− produced by the enzyme.
b. Aquaporin.
As discussed above, H2O2 has been assumed to be the best candidate for intracellular damage given its longer half-life and lipophilic properties. Because of these characteristics, it was assumed that H2O2 exerts its intracellular effects by diffusing across the plasma membrane. This notion was recently challenged by Miller et al. (2010), who reported that aquaporin can mediate H2O2 uptake in mammalian cells. In this study, human embryonic kidney-293 and HeLa cells overexpressing different isoforms of aquaporin showed increased uptake of the chemoselective H2O2 indicator Py1-ME (Miller et al., 2010).
In the vasculature, Pagano’s laboratory was the first to describe the transport of H2O2 through aquaporin-1 channels at the plasma membrane of VSMCs (Al Ghouleh et al., 2013). In their model, H2O2 applied exogenously on rat aortic SMCs penetrated into the cells via aquaporin-1 and activated superoxide anion production by Nox1 (Al Ghouleh et al., 2013). This signaling cascade leads to the phosphorylation of the protein Ask1, which results in hypertrophy of SMCs.
The presence of multiple regulators directly interacting with the different Nox isoforms makes the NADPH oxidase complex a signaling microdomain by itself. Additionally, because the structural characteristic of the enzyme results in extracellular production of O2•− and the presence of 1) channels that allow the transfer of the ROS into the cell and 2) EC-SOD that degrades the O2•− into H2O2, it could be argued that a ROS signaling microdomain is an important component of vascular cell communication.
C. Signaling Microdomains in the Release of Hydrogen Sulfide and Carbon Monoxide?
In addition to the well-defined nitrogen-related gaseous species described above, carbon monoxide (CO) and hydrogen sulfide (H2S) have also been shown to have potent effects in the blood vessel wall as specific cellular signaling molecules that can alter local vasoconstriction/dilation and blood pressure [e.g., CO (Suematsu et al., 1994; Johnson et al., 1997; Caudill et al., 1998; Leffler et al., 1999); H2S (Zhao et al., 2001; Yang et al., 2008; Leffler et al., 2011a; Liang et al., 2011)]. Both gases are produced by ubiquitously expressed enzymes. Although their production and activation have yet to be identified as being regulated by signaling microdomains, emerging evidence may indicate a possible role in CO production.
Heme oxygenase [HO; found in two isoforms, HO-1 (inducible) and HO-2 (constitutive)] catalyzes the O2-dependent degradation of heme into free iron, biliverdin IXa, and CO. NADPH-cytochrome P450 reductase provides the electrons for this catalysis (for review, see Kim et al., 2006; Leffler et al., 2011b). Although both HO-1 and HO-2 are primarily found in the endoplasmic reticulum (e.g., Tenhunen et al., 1968), there is now sufficient evidence that both enzymes can associate with Cav1 at the plasma membrane of mesengial (Jung et al., 2003) and endothelial cells (Kim et al., 2004). Further reports indicated that Cav1 is a competitive inhibitor for HO-1, identifying a minimum sequence required for binding (Taira et al., 2011) and possibly regulating activation of BKCa channels in endothelium (Riddle and Walker, 2012) [although this Cav1 interaction has yet to be detected in smooth muscle cells where the link between BK and CO was originally identified and well-described (Wang et al., 1997; Jaggar et al., 2002, 2005; Xi et al., 2004)]. One report indicated that cytochrome P450 reductase is also associated with Cav1 (Jung et al., 2003), potentially forming a compartmentalized signaling microdomain for CO production. Further work on this concept is required to elucidate the role for sequestration of these proteins into microdomains and the importance in cellular signaling. In addition, because CO is potently scavenged by hemoglobin, it will be interesting to determine how the Hbα expressed in the endothelium of small arteries (Burgoyne et al., 2012; Straub et al., 2012) may interact and modulate CO function.
The production of H2S is by a series of steps starting with serine and homocysteine that proceeds through cystathionine beta synthase (with H2S as a substrate) producing cystathionine, which feeds into cystathionine γ-lyase (with H2S as a substrate) producing cysteine; this is the trans-sulfuration pathway (Hildebrandt and Grieshaber, 2008). The cysteine produced from this pathway or other endogenous sources can then feed into the cysteine catabolism pathway, which converts cysteine into mercaptopyruvate via cysteine aminotransferase (Hildebrandt and Grieshaber, 2008). Lastly, mercaptopyruvate sulfurtransferase converts the meraptopyruvate into pyruvate and H2S (although the exact acceptors remain unidentified) (Hildebrandt and Grieshaber, 2008; Kabil and Banerjee, 2010). There remains much to understand in regard to H2S, including the following. 1) How does H2S penetrate the plasma membrane? Under physiologic pH, H2S does not readily move through plasma membranes and there are currently no known transports for this molecule (Mathai et al., 2009), 2) How can H2S specifically be inhibited? The current pharmacological inhibitors have been called into question (Kabil and Banerjee, 2010). 3) What is the physiologic concentration of H2S? The amount of H2S needed to produce biologic effects is an order of magnitude above physiologically relevant levels (Furne et al., 2008; Whitfield et al., 2008; Olson, 2009). The answers to these questions may lead to a novel understanding of how such a fundamental gas could function in the vasculature. Currently there is no known localization of the H2S enzymes together in a particular region of the cell that may constitute a signaling microdomain.
III. Channel-Based Cellular Communication by Signaling Microdomains
In section II, we described intercellular communication via free diffusion across the plasma membranes or extracellular production of signaling molecules by cells within the vascular wall. In this section, we will first discuss intercellular communication through the direct cytoplasmic transfer of molecules from one cell to another via gap junctions. We will further describe the multiple ion channels structured in a signaling microdomain, especially those involved in the endothelium-derived hyperpolarization (EDH) mediated response.
A. Gap Junction Channels.
One of the first indications of a physiologic role for gap junctions in the vasculature came from studies by Segal and Duling (1986) that identified a “conducted vasodilation” in arterioles upstream of a vasodilator stimulus, suggesting that some form of direct communication was involved. Gap junction channels coordinate responses through the passage of small molecules between cells and are important in normal vascular physiology but are also highly implicated in vascular pathophysiology such as hypertension and arteriogenesis. The gap junctions as membrane proteins contain highly flexible intracellular regions that make them ideal for forming protein-protein interactions to regulate their function, and decades of studies have established their roles in signaling microdomains.
The gap junctions as a cellular structure were described nearly 50 years ago (Dewey and Barr, 1964). These membrane channels permit direct cell-to-cell (intercellular) transfer of ions, metabolites, and small molecules between two different cell cytoplasms and are essential for the maintenance of normal vascular functions. The small molecules include (but are not limited to) ATP, IP3, nicotinamide adenine dinucleotide, and other metabolites up to 1 kDa in size (see Table 2). Gap junctions are ubiquitously expressed in almost all cells of the body, existing transiently at the cell surface and at points of cell-to-cell contact (Laird, 2006; Johnstone et al., 2009a). The protein subunit comprising gap junctions is the connexin (Cx), which assembles in hexameric channels at the plasma membrane. In this state, the hexameric channel is referred to as a connexin hemichannel or connexon, which is transported to the plasma membrane. Two connexin hemichannels residing on apposed cells further dock to each other, forming a gap junction channels. In total, 21 human connexin isoforms have been identified with a primary role of direct intercellular communication. The classification of connexin isoforms is based on their molecular weights, with further classification based on isoform interaction (Eastman et al., 2006). Within the cells of the blood vessel wall, four connexins have been well characterized: Cx37, Cx40, Cx43, and Cx45. In addition, it was recently shown that Cx32 is expressed within vascular cells and may contribute to direct cell-to-cell communication through gap junctions (Okamoto et al., 2009, 2011; Fowler et al., 2013). Typically, ECs predominantly express Cx37, Cx40, and Cx43, whereas VSMCs have been shown to express Cx37, Cx43, and Cx45 (Johnstone et al., 2009a,b; Okamoto et al., 2009). Additionally, within the resistance vasculature at sites of endothelial to smooth muscle contact (i.e., MEJ), Cx37, Cx40, and Cx43 have all been shown to be expressed (Haddock et al., 2006; Sandow et al., 2006; Isakson et al., 2008; Straub et al., 2010, 2011). However, connexin expression across the vascular wall varies widely depending on the animal species and the vascular bed examined. A number of factors may influence this, including sheer stress or changes in vessel wall structure (as can be found in vascular disease states such as atherosclerosis and hypertension) (Kwak et al., 2002; Johnson and Nerem, 2007; Vorderwulbecke et al., 2012). A more detailed examination of vascular specific differences in connexin expression profiles in EC and VSMC in vitro and in vivo can be found in Table 3.
TABLE 2.
Substrates and ions permeable to gap junctions
Table summarizes molecules reported to traverse gap junction channels. Key publication(s) showing evidence for transfer of a molecule via gap junctions is indicated in the right column
TABLE 3.
Connexin expression in vascular cells
Key references indicating the expression of Cx32, 37, 40, 43, and 45 reported in ECs, SMCs, and at the MEJ in cell culture and in vivo. [Apologies to authors of any relevant studies missing from this table.]
| System | Cell Type/ Location | Cx32 | Cx37 | Cx40 | Cx43 | Cx45 |
|---|---|---|---|---|---|---|
| Cell culture (static/ with flow) | EC | HUVEC [Im, mRNA, WB (Okamoto et al., 2009, 2011)] | HUVEC [Im (Kameritsch et al., 2005)] | Mouse cremaster EC [Im, WB (Isakson and Duling, 2005)] | Mouse EC [Im, WB (Isakson and Duling, 2005; Straub et al., 2010)] | ND (Isakson and Duling, 2005)e |
| HAEC, HMVEC, PAEC [mRNA (Okamoto et al., 2009)] | bEnd [Im (Pfenniger et al., 2012)] | HUVEC [Im, mRNA, WB (Okamoto et al., 2009, 2011; Straub et al., 2011)] | Bovine aortic EC [mRNA (Hirschi et al., 2003)] | |||
| Mouse EC [Im (Isakson and Duling, 2005)] | HAEC, HMVEC, PAEC [mRNA (Okamoto et al., 2009)] | |||||
| bEnd, increased with flow [Im, mRNA, WB (Pfenniger et al., 2012)] | Decreased expression in sheer stress models [WB (Feaver et al., 2008)] | |||||
| Sheer stress and stretch increases Cx43 in bEnd cells (Kwak et al., 2005) | ||||||
| MEJa | ND | Mouse cremaster VCCC, EC side only [Im (Isakson and Duling, 2005)] | Mouse cremaster VCCC, EC side only [Im (Isakson and Duling, 2005; Isakson, 2008)] | EC and VSMC [Im, WB (Isakson and Duling, 2005; Straub et al., 2010)] | ND | |
| HUVEC/HUVSMC VCCC [WB (Straub et al., 2011)] | ||||||
| SMC | ND | Mouse aortic [Im, WB (Isakson and Duling, 2005; Johnstone et al., 2012b)] | NP mouse cremaster VSMC [Im (Isakson and Duling, 2005; Isakson, 2008)] | HUVEC [WB (Straub et al., 2011)] | Mouse aortic (Johnstone et al., 2012b) | |
| Mouse and rat aortic [Im, WB (Johnstone et al., 2009b, 2012b; Straub et al., 2010)] | ||||||
| Resistance/ muscular arteriesb | EC | ND | Mouse/rat cremaster [Im (Sandow et al., 2006; Isakson et al., 2008)] | Mouse/ Rat cremaster [Im (Mather et al., 2005; Sandow et al., 2006; Isakson et al., 2008)] | Mouse cremaster [Im (Isakson et al., 2008; Straub et al., 2010)] | Mouse cremaster [Im (Isakson et al., 2008)] |
| Mouse renal [Im (Braunstein et al., 2009)] | Mouse renal [Im (Braunstein et al., 2009)] | Mouse renal [Im (Braunstein et al., 2009)] | NP in transgenic mouse GFP reporter (Schmidt et al., 2012) | |||
| Rat basilar artery [Im (Haddock et al., 2006)] | Mouse afferent arteriole [Im (Zhang et al., 2006a; Sorensen et al., 2012)] | Rat basilar artery [Im (Haddock et al., 2006)] | Mouse lung [Im (Wang et al., 2012)] | |||
| Mouse afferent arteriole [Im (Zhang et al., 2006a; Sorensen et al., 2012)] | Rat Basilar artery [Im (Haddock et al., 2006)] | Mouse efferent arteriole ECs, lost in transgenic eNOS-null diabetic mice [Im (Zhang et al., 2006a)] | ||||
| MEJf | ND | Mouse/rat cremaster [Im (Sandow et al., 2006; Isakson et al., 2008)] | Mouse/rat cremaster [Im (Mather et al., 2005; Sandow et al., 2006; Isakson et al., 2008)] | Mouse/rat cremaster, also present as Cx43-pS368 [Im (Isakson et al., 2008; Straub et al., 2010)] | NP, Mouse/ rat cremaster [Im (Isakson et al., 2008)] | |
| Rat Basilar artery [Im (Haddock et al., 2006)] | Rat basilar artery [Im (Haddock et al., 2006)] | NP Rat basilar artery [Im (Haddock et al., 2006)] | ||||
| SMC | ND | Mouse cremaster [Im (Isakson et al., 2008)]. | NP-mouse cremaster [Im (Isakson et al., 2008)] | Mouse cremaster [Im (Isakson et al., 2008; Straub et al., 2010)] | Mouse cremaster [Im (Isakson et al., 2008), present in transgenic mouse GFP reporter (Schmidt et al., 2012)] | |
| Rat basilar artery [Im (Haddock et al., 2006)] | NP-rat basilar artery [Im (Haddock et al., 2006)] | Rat basilar artery [Im (Haddock et al., 2006)] | ||||
| Mouse afferent arteriole [Im (Zhang et al., 2006a)] | Mouse afferent arteriole [Im (Zhang et al., 2006a)] | |||||
| Conduit arteriesc | EC | NP-mouse aorta [Im (Lopez et al., 2009)] | Mouse carotid, decreased in mice at regions of turbulent flow [Im (Pfenniger et al., 2012)] | Mouse aorta and Carotid [Im (Kwak et al., 2002; Johnstone et al., 2009b; Koval et al., 2011)] | Mouse carotid [Im (Johnstone et al., 2009b)] | Mouse aorta and carotid [Im (Johnstone et al., 2009b; Koval et al., 2011)] |
| Human femoral [Im (Bol et al., 2013)] | Human femoral [Im (Bol et al., 2013)] | Cx43 is increased in aorta of hypertensive rat carotid [Im (Haefliger et al., 2004)] | ||||
| Decreased in ApoE−/− mice [Im (Johnstone et al., 2009b)]. | Human femoral artery [Im (Bol et al., 2013)] | |||||
| SMC | NP, mouse aorta [Im (Lopez et al., 2009)] | NP, mouse carotid [Im (Kwak et al., 2002; Johnstone et al., 2009b)] | NP, mouse carotid [Im (Johnstone et al., 2009b)] | Mouse carotid [Im (Johnstone et al., 2009b)] | Mouse carotid [Im (Johnstone et al., 2009b)] | |
| Low levels in human femoral artery [Im (Bol et al., 2013)] | Human femoral artery [Im (Bol et al., 2013)] | Human femoral artery [Im (Bol et al., 2013)] | ||||
| Increased in ApoE−/− mouse carotid and aorta [Im (Johnstone et al., 2009b)] | Increased. | |||||
| Expression in ApoE−/− mouse aorta/carotid [Im (Johnstone et al., 2009b, 2012b)] | ||||||
| Conduit veins | EC | Mouse inferior vena cava [Im (Okamoto et al., 2009)] | Rat cranial/ caudal vena cava [Im (Inai and Shibata, 2009)] | Rat cranial/caudal vena cava [Im (Inai and Shibata, 2009)] | Rat cranial/caudal vena cava [Im (Inai and Shibata, 2009)] | Rat cranial/caudal vena cava [Im (Inai and Shibata, 2009)] |
| Human saphenous vein [Im (Bol et al., 2013)] | Human saphenous vein [Im (Bol et al., 2013)] | Low levels in human saphenous vein [Im, WB (Deglise et al., 2005; Bol et al., 2013)] | human saphenous vein [Im, WB (Deglise et al., 2005)]. Expression lost in 14 week ApoE−/− mice aorta/ carotid [Im (Kwak et al., 2002)] | |||
| Expression lost in 14 week ApoE−/− mice aorta/ carotid [Im (Kwak et al., 2002)] | ||||||
| SMC | Mouse inferior vena cava [Im (Okamoto et al., 2009)] | Low levels in human saphenous vein [Im, mRNA (Deglise et al., 2005; Bol et al., 2013)] | Human saphenous vein [Im, mRNA, WB (Deglise et al., 2005; Bol et al., 2013)] | Rat cranial/caudal vena cava [Im (Inai and Shibata, 2009)]. | Low levels in human saphenous vein ]mRNA (Deglise et al., 2005)] | |
| Significant increase in human saphenous vein [Im, mRNA, WB (Deglise et al., 2005; Inai and Shibata, 2009; Bol et al., 2013)] |
Im, immunodetection, e.g., immunofluorescence, immunohistochemistry, immunolabeling coupled to transmission-electron microscopy, ND, not determined; NP, not present; VCCC, vascular cell coculture (in vitro); WB, Western blot.
Refers to the in vitro MEJ as described (Heberlein et al., 2010).
Resistance/muscular arteries refers to vessels where EC and VSMC make contacts, i.e., MEJ. These include cremasteric, mesenteric, pulmonary and thoracodorsal.
Conduit vessels, e.g., aorta, carotid, coronary artery
Typically only found in sheer stress regions of the vascular.
Several reports suggest poor antigenicity and specificity of Cx45 antibody (Theis et al., 2004; Isakson et al., 2006b).
For table of MEJ identified in vivo, see review (Sandow et al., 2012).
1. Intrinsic Characteristics of Connexins Allowing for Protein Interactions.
Connexin proteins possess an intracellular N terminus, four transmembrane-spanning domains, two extracellular loops, a single intracellular loop, and a highly flexible intracellular C-terminal region containing multiple sites for potential protein interactions and posttranslational modifications (Evans and Martin, 2002; Kovacs et al., 2007). Within the connexin isoforms expressed in the vasculature, C-terminal regions are essentially unstructured, allowing for rapid changes as a result of protein modifications such as phosphorylation and nitrosylation events (Sorgen et al., 2004a; Bouvier et al., 2009; Straub et al., 2012). These random coil or disordered domains are the main sites of connexin protein-protein interactions, and a diverse array of proteins have now been demonstrated to regulate connexin protein assembly, trafficking, and gating, ultimately affecting cellular communication involved in cell proliferation and migration (Wei et al., 2004; Johnstone et al., 2012b). Eliminating the C-terminal domain of Cx43, the most ubiquitously expressed isoform, significantly reduces the formation of large gap junction plaques but does not alter the proper trafficking and insertion into the plasma membrane, suggesting that interactions between the C-terminal domain and other proteins are required (but not essential) for efficient gap junction plaque formation (Duffy et al., 2004; Simek et al., 2009).
A further unique feature of connexin-protein interactions that can be crucial for incorporation into signaling microdomains is the apparent requirement for dimerization of two connexin proteins to allow for interactions such as interactions between the C terminus and the intracellular loop within the same Cx protein (Ponsaerts et al., 2010), interactions between Cx and the cell cycle regulating protein cyclin E (Johnstone et al., 2012b), and interactions with other connexins (Sorgen et al., 2004b; Zhou et al., 2007b). An alteration in the C-terminal structural conformation resulting from phosphorylation may play a central role in connexin C-terminal dimerization and interactions with other proteins, although the exact nature of this dimerization has not been determined (Kopanic and Sorgen, 2013; Grosely et al., 2013).
Oligomerization of the connexins primarily occurs within the ER for Cx37, Cx40, and Cx45 or later in the trans-Golgi network (TGN) for Cx32 and Cx43 (Das Sarma et al., 2001; Vanslyke et al., 2009; Smith et al., 2012). Composition of a connexin hemichannel can either be formed of six of the same connexin isoform (homomeric) or a mixture of multiple isoforms (heteromeric). Within the connexin isoforms expressed in the vasculature, heteromeric channels have been identified for Cx37/Cx40 (Ayad et al., 2006; Laird, 2006; Smith et al., 2012), Cx37/Cx43 (Brink et al., 1997; Larson et al., 2000; Wang et al., 2005; Beyer et al., 2013), Cx40/43 (He et al., 1999; Stergiopoulos et al., 1999; Bouvier et al., 2009), Cx40/Cx45 (Martinez et al., 2002); Cx43/Cx45 (Moreno et al., 1991; Koval et al., 1995), and Cx43:Cx32 (Lagree et al., 2003; Vanslyke et al., 2009). Typically, compared with homomeric hemichannels, these heteromeric associations produce channels with altered gating sensitivities to voltage, pH, or Ca2+; altered channel opening; different ion selectivity; and permeability properties, but their membrane targeting is not altered (Beyer et al., 2000, 2001; Cottrell and Burt, 2001; Wei et al., 2004). The specific characterization of the functional properties of these heteromeric channels has been extensively reviewed by others (e.g., Moreno, 2004).
2. Microdomains Regulating the Intracellular Trafficking of Connexins.
Once formed, vascular connexin hemichannels are actively transported to the plasma membrane through direct binding of their C-terminal and intracellular loop regions with proteins such as tubulin (α and β) (Giepmans et al., 2001b; Thomas et al., 2005; Kang et al., 2009; Saidi Brikci-Nigassa et al., 2012). Associations between Cx43 and tubulins (α and β) have been shown through coimmunoprecipitation, immunofluorescence overlap, analytical size exclusion chromatography, nuclear magnetic resonance, pull-down assays, and site-directed mutagenesis in a variety of studies (e.g., Giepmans et al., 2001b; Saidi Brikci-Nigassa et al., 2012). The interaction between Cx43 and tubulins occurs in a 26 amino acid region (in the region of aa 228–262) in the Cx43 C terminus, which results in the formation of helical regions at the point of interaction between the two proteins. This process was recently demonstrated to be regulated through v-src phosphorylation at the Tyr247 of the Cx43 C terminus (Giepmans et al., 2001b; Saidi Brikci-Nigassa et al., 2012). The interaction between connexins and tubulins regulates membrane targeting, localization within the plasma membrane, gap junction plaque formation, and TGF-β signaling (Shaw et al., 2007; Saidi Brikci-Nigassa et al., 2012). Despite the presence of this binding site, microtubule blockers do not block trafficking or gap junctions assembly, which can be explained by redundancies that allow for alternative trafficking pathways to the cell membrane (Jordan et al., 1999; Majoul et al., 2009; del Castillo et al., 2010). Additionally, removal of the microtubule-binding domain or complete removal of the Cx43 C terminus does not stop plasma membrane trafficking or gap junction assembly (although gap junction plaques are smaller) (Jordan et al., 1999; Omori and Yamasaki, 1999; Rhee et al., 2009). However, under these conditions, there is a reduction in available hemichannels at the plasma membrane and subsequent transition to gap junctions (Johnson et al., 2002; Maass et al., 2007). Although the connexins can bypass the tubulin pathway to target the membrane, it may be a critical domain for normal development. In transgenic mice, expression of a mutated form of Cx43 where there is a truncated Cx43 C terminus (δ258, within the tubulin binding domain) produces a lethal phenotype in neonatal mice (Maass et al., 2004, 2007). Many deletion and mutagenesis studies have shown that the C terminus is at least in part dispensable for membrane trafficking of Cx43. However, a recent study demonstrated that deletion mutations within the tubulin binding domain i.e., aa 235–242 (unlike previous mutations that retain this domain) inhibit gap junctions and gap junction intercellular communication (Wayakanon et al., 2012). These studies clearly define that interactions between connexins and the microtubule network result in efficient signaling microdomain formation but demonstrate that the C-terminal binding site is not essential.
The trafficking of Cx43 in monomer form to the TGN is facilitated through interactions with the GTPase protein rab20 (Das Sarma et al., 2008). By use of GST pull-down or yeast-2-hybrid assays, connexins have also been found to specifically interact with consortin within the TGN. Consortin further interacts with clathrin adapter proteins to regulate vesicular trafficking of proteins (del Castillo et al., 2010). Reduced interaction with consortin alters the membrane transport of Cx43, Cx45, and Cx32 (del Castillo et al., 2010). The ability of connexin hemichannels to reach the membrane significantly dictates their ability to form functional gap junction channels with the ultimate outcome being reduced gap junctional activity through reduced membrane targeting. Altogether, consortin and tubulins are key protein partners to connexin proteins and ensure the trafficking of connexin hemichannels to the plasma membrane.
3. The “Functional” Connexin Hemichannel Versus Pannexin Channel.
Connexin hemichannels are inserted into the membrane in an arbitrary fashion in that that they are not directed to specific cellular regions prior to membrane insertion (Lauf et al., 2002; Simek et al., 2009). Once at the membrane, connexin hemichannels are then lateralized to areas of cell-to-cell contact where they aggregate in clusters based on plasma membrane lipid composition, thus forming direct interactions with opposing connexin hemichannels to form gap junctions (Wang et al., 2013). Before their insertion into a gap junction cluster, connexin hemichannels are in a closed conformation under physiologic conditions.
Connexin hemichannels at the cell surface have also been extensively studied for their singular functional role (besides that of a gap junction). Many studies have suggested that connexin hemichannels can act by releasing a number of small ionic molecules such as ATP, IP3, or NAD+ and by uptake of a number of fluorescent molecules. However, the ability for connexin hemichannels to function in a physiologic context remains contentious (Spray et al., 2006; Bosco et al., 2011; Fasciani et al., 2013). The primary concerns center around whether these gap junction intermediaries are really signaling hemichannels, with only a few studies directly indicating that certain connexin hemichannels open under physiologic conditions (Bukauskas et al., 2002, 2006; Contreras et al., 2003; Bukauskas and Verselis, 2004), with calls to clearly demonstrate the signaling potentials of these channels (Spray et al., 2006; Sosinsky et al., 2011). Every experiment involving connexin hemichannels require that no extracellular calcium be present for the connexin hemichannels to be opened (Spray et al., 2006). In addition, alterations in intercellular ionic concentrations of K+ and variation in pH have been showed to regulate the opening and closing of connexin hemichannels (Retamal et al., 2007). Specific pathways for the regulation of channel opening include intracellular loop and C-terminal regions interactions (Ponsaerts et al., 2010) and the specific posttranslational modifications of connexin C-terminal amino acids (Bao et al., 2004a,b; Retamal et al., 2006; De Vuyst et al., 2007) as recently well reviewed (Saez et al., 2010; Ek-Vitorin and Burt, 2013; Fasciani et al., 2013; Herve and Derangeon, 2013; Verselis and Srinivas, 2013; Wang et al., 2013).
The role of connexin hemichannels in a physiologic setting, particularly within the vasculature, has been further polarized by the fact that connexin-mimetic peptides as well as a large number of chemical agents that have been used to demonstrate connexin hemichannels specific activity also block pannexin channel activity (Wang et al., 2013) (see Table 4). The pannexin class of proteins was only recently discovered in the early 2000s (Panchin et al., 2000). The pannexins have similar membrane topology to connexins, with four membrane-spanning domains and intracellular N terminus, loop, and C terminus (MacVicar and Thompson, 2010). Similar to connexin hemichannels, six pannexin proteins assemble to form hexameric membrane channels that have been shown to be expressed in virtually all cell types, including vascular cells (Billaud et al., 2011; Sosinsky et al., 2011; Lohman et al., 2012b). Significantly, pannexin channels are not currently known to form intercellular channels (as opposed to connexin hemichannels that form gap junctions) but act as a paracrine channel for the generation of directional signaling across the surface of the cell (Dahl and Locovei, 2006; Sosinsky et al., 2011; Lohman et al., 2012a). To date, their role as a purine-releasing channel as well as nonspecific anion channel has been clearly demonstrated (Chekeni et al., 2010; Lohman et al., 2012c; Wang et al., 2013). In the vascular wall, the three pannexin isoforms are expressed with variation depending on the type of circulation (systemic, pulmonary, coronary, hepatic) (Lohman et al., 2012b). To date, only a few studies have investigated the role of pannexin channels in vascular functions, and it is now clear that they participate in the adrenergic signaling pathway in SMCs as well as in the thrombin signaling pathway in ECs (Billaud et al., 2011; Godecke et al., 2012). Lastly, although pannexins have not been identified in any clear microdomains and are not localized within caveoli, as demonstrated in a rat mammary tumor cell line (Gehi et al., 2011), they have been associated with a number of receptors that can initiate their opening, including the α1D-adrenergic receptor, the NMDA receptor, and PAR-1 (Billaud et al., 2011; Godecke et al., 2012; Weilinger et al., 2012). Ongoing and future studies of these channels could provide interesting clues into Panx1 integration in signaling microdomains in the blood vessel wall.
TABLE 4.
Pharmacology of connexins and pannexins
Table summarizing the pharmacological inhibitors of connexin and pannexin channels. For each drug, the range of concentration tested as well as their potency on pannexin channels, and connexin hemichannels and gap junctions is indicated.
| Drug | Characteristics | Concentration | Magnitude of inhibition | References | |
|---|---|---|---|---|---|
| Panx | Cx | ||||
| Mefloquine | Prevention and treatment of malaria (many side effects reported). Interacts with a number of ion channels and proteins with nonspecific effects and targets, e.g., neuronal calcium homeostasis, the endoplasmic reticulum, calcium pump, acetylcholinesterase, P-glycoproteins in the blood-brain barrier, connexins pannexins, A2A receptors, potassium, and anion channels. | 1–10 μM | ++(S) | +(R) | Dow et al., 2005; Iglesias et al., 2008, 2009a; Li et al., 2010; Nevin, 2011 |
| Probenecid (Pro) | Used in the treatment of gout and hyperuricemia, works by interfering with the kidneys organ anion transporter (OAT). Blocks channel based release of cAMP, cGMP, and ATP from various cell types. Reportedly specific to Panx1 by Silverman et al. (2008). | 150 μM–1 mM | ++ | −/+ (Cx46/Cx50) | Hsyu et al., 1988; Paul et al., 1991; Beahm and Hall, 2002; Silverman et al., 2008, 2009; Ma et al., 2009; Li et al., 2010 |
| 10Panx1 | Used experimentally, acts to specifically inhibit Panx1 channels, targeted to the extracellular loop region of Panx1. Some effects demonstrated for Cx46 channel inhibition. | 100 μM | ++ | −/+ (Cx46) | Pelegrin and Surprenant, 2006; Wang et al., 2007; Bargiotas et al., 2011; Billaud et al., 2011 |
| 18-α/β-Glycyrrhetinic acid | Derived from licorice herb. Inhibits several channels, e.g., IKCa/SKCa, voltage-dependent channels, pannexin, and connexin channels. | 25 μM | + | ++/+++ | Bruzzone et al., 2005; Ozkan and Uma, 2009; Johnstone et al., 2010; Boedtkjer et al., 2013 |
| Carbenoxolone | Glycyrrhetinic acid derivative. Used in treatment of oral ulcerations and lesions, nonspecific ion channel inhibitor, suppresses hyperpolarization. | 50–100 μM | +++ | ++ | Sagar and Larson, 2006; Thompson et al., 2006; Iglesias et al., 2008, 2009a; Chekeni et al., 2010; Behringer et al., 2012; Gairhe et al., 2012; Boedtkjer et al., 2013; Zhang et al., 2013 |
| 40Gap27, 37,43Gap27 | Used experimentally to inhibit connexin (Cx37, Cx43) and has been showed to affect pannexin channels. Inhibition of Ca2+ oscillations, depolarization, and contraction of smooth muscle cells | 300 μM | + | + | Dora et al., 1999; Haddock et al., 2006; Pelegrin and Surprenant, 2006; Wang et al., 2007; Islam et al., 2012 |
| Flufenamic acid | Fenamate, nonsteroidal anti-inflammatory drugs. Nonspecific ion channel inhibitor, including: nonselective cation channels, voltage-gated NA+/K+/ Ca2+ channels, connexins (e.g., Cx46), pannexins (Panx1), and calcium activated channels. | 100–300 μM | −/+ | ++ (reversible) | Harks et al., 2001; Bruzzone et al., 2005; Iglesias et al., 2008 |
| Arachidonic acid and eicosatetraynoic acid (ETYA) | Nonspecific effects on voltage-gated potassium channels, blocker of connexins (e.g., Cx43) and pannexin channels. Arachidonic acid promotes endothelium-induced vasorelaxation, ETYA inhibits endothelium-dependent vasodilation. | 100 μM | +++ | +++ | De Mey et al., 1982; Martinez and Saez, 1999; Kehl, 2001; Samuels et al., 2013 |
| Ouabain | Cardiotonic steroid, identified in human blood. Widely used experimentally as a Na+/K+-ATPase inhibitor and inhibitor of connexins (not known for pannexins). | 100 μM–1 mM | n/a | −/++ | Weingart, 1977; Martin et al., 2004; Turner et al., 2004; Aperia, 2007; Boedtkjer et al., 2013 |
| Heptanol | Aliphatic alcohol, gap junction uncoupler/inhibitor | 200 μM–3 mM | −/+ | ++ | Takens-Kwak et al., 1992; Garcia-Dorado et al., 1997; Pelegrin and Surprenant, 2006; Nishida et al., 2008; Li et al., 2010; Li et al., 2012b |
+, Partial inhibition; −/+, conflicting reports; ++, greater than 50% inhibition; −, no effect; +++, complete inhibition; n/a, no information available
R and S represent the two stereoisomers of mefloquine (Iglesias et al., 2009b)
Evidence that heptanol effect is not specific to gap junctions in smooth muscle cells (Chaytor et al., 1997).
4. Gap Junctional Plaque as a Signaling Microdomain.
Gap junctions form through accretion and docking of opposing connexin hemichannels between adjacent cells and have been identified between EC, VSMC, and at the MEJ in a large number of vascular beds (Brisset et al., 2009). Although gap junctional communication has been clearly identified between ECs in numerous vascular beds, the presence of gap junction between VSMCs and at the MEJ seems to differ according to the vascular beds and the species (Johnstone et al., 2009a; Billaud et al., 2011). Within the blood vessel wall, gap junctions play an integral role in key physiologic responses, including vascular resistance, vascular growth, and cell differentiation, and are highly adapted during diseases, implicating them as key members in modulating different aspects of disease responses (Kwak et al., 2002; Liao et al., 2007; Chadjichristos et al., 2008) (see Table 5). The formation of gap junctions and gap junction channel properties are highly regulated through intracellular environment but also through direct protein interactions in signaling microdomains.
TABLE 5.
Examples of physiologic and pathophysiological role of gap junction in the vasculature
There is a plethora of physiologic functions for gap junctions in the vasculature having been described, with a sample of the more recent referenced below.
Evidence that gap junction independent pathways are involved in this regulation (Johnstone et al., 2012b).
Evidence that although connexin expression and/or gap junction communication levels correlated to disease they do not appear to be directly linked to phenotypic modulation (Matsushita et al., 2007; Behringer et al., 2012).
Evidence that gap junctions are not involved in EC wound repair migration or proliferation (Bearden et al., 2010).
a. Caveolin.
At the plasma membrane, connexin hemichannels are held within discrete cholesterol- and sphingolipid-rich membrane regions known as lipid rafts (Schubert et al., 2002; Locke et al., 2005; Locke and Harris, 2009; Defamie and Mesnil, 2012). Connexins are differentially expressed in a number of different lipid rafts of variable composition characterized by their sensitivity to detergents such as Triton X-100, Nonidet P-40, or Brijj and by the presence of Cav1 (Locke et al., 2005). Multiple interaction studies (e.g., immunofluorescence overlap, coimmunoprecipitation, Far Western) have now identified that vascular connexins, including Cx32, Cx37, Cx43, and Cx40, can interact with both Cav1 and Cav2 (Schubert et al., 2002; Locke et al., 2005; Langlois et al., 2008; Saliez et al., 2008). Protein truncation studies have revealed that Cav1 and Cx43 interact at residues 244–256 on Cx43 C terminus (Langlois et al., 2008). Although caveolin interactions are primarily considered at the plasma membrane in caveolae, newly synthesized Cx43 can also interact with Cav1 and Cav2 in the Golgi, indicating that both isoforms of caveolin may also be involved in the transport of connexins to the plasma membrane (Langlois et al., 2008). Connexins within caveolae at the plasma membrane are presumably connexin hemichannels not gap junctions but are an integral step in gap junction channel assembly at the plasma membrane (Locke et al., 2005; Langlois et al., 2008). In experimental models, the expression levels of Cx37, Cx40, and Cx43 and gap junction formation are significantly reduced in Cav1−/− mouse arteries (Saliez et al., 2008). Additionally, interactions between Cx43 and Cav1 and Cav2 are regulated via PKCγ in a model, suggesting that phosphorylation of Cx43 by PKCγ may lead to a redistribution of Cx43 hemichannels in the lipid rafts at the cell membrane, reducing their accumulation into gap junctional plaques (Lin et al., 2003). Therefore, it is likely that Cav1 and Cav2 interact with connexins in a manner that promote their incorporation into a signaling microdomain to regulate their function.
b. Zonula occludens-1.
At the point of insertion to the plasma membrane, connexin hemichannels become directly associated with the tight junction proteins ZO-1/2/3 (Toyofuku et al., 1998, 2001; Singh et al., 2005), as recently reviewed in Palatinus et al. (2012). Direct interactions between ZO-1 and Cx43 and Cx45 have been demonstrated at the plasma membrane (Kausalya et al., 2001; Laing et al., 2001, 2005a,b). These interactions have been extensively studied and shown by numerous biochemical techniques such as coimmunoprecipitation, immunofluorescence, proximity ligation assay, nuclear magnetic resonance, peptide competition, and pull-down assays (Toyofuku et al., 1998, 2001; Kausalya et al., 2001; Sorgen et al., 2004a; Laing et al., 2005a,b; Bouvier et al., 2008; Chen et al., 2008; O’Quinn et al., 2011; Tence et al., 2012). The interaction between Cx43 and ZO-1 is not required for proper gap junction channel functionality per se but instead appears to negatively regulate accumulation of gap junction channels at the plasma membrane, limiting plaque size and stability (Giepmans, 2004, 2006; Hunter et al., 2005; Rhett et al., 2011; Arora et al., 2012). Plaque size is regulated by this interaction in two main ways: 1) by limiting incorporation of connexins into gap junction plaques and 2) by promoting endocytosis of connexins from gap junction plaques (Palatinus et al., 2012). Accordingly, it has been proposed that ZO-1 acts as a “connexon switch,” regulating the transition from nonjunctional to junctional form of Cx43 hemichannels (Rhett et al., 2011). Newly formed connexons aggregate at the cell surface in noncoupled plaques called “perinexus” (Rhett and Gourdie, 2012). Interaction of Cx43 with ZO-1 facilitates movement between the perinexus and gap junction plaque in this way, regulating the size of the plaque. Indeed, when Cx43/ZO-1 interactions are inhibited (e.g., through siRNA approaches, inclusion of a Cx43-CT-GFP, or site directed mutation of the PDZ domain of ZO-1 or of the C-terminal region of Cx43), the ability of these two proteins to interact is abolished, resulting in decreased size of gap junction plaques (Hunter et al., 2003, 2005; Palatinus et al., 2011a, 2012).
The second function for Cx43/ZO-1 interactions is to increase gap junction disassembly and endocytosis. Phosphorylation of Cx43 at the Ser368 residue is known to decrease gap junction communication and is associated to internalization of the protein; in other words, it is associated with reduced plaque stability. This phosphorylation is reportedly dependent on Cx43 interaction with ZO-1, because in the absence of ZO-1, Cx43 can interact with but cannot be phosphorylated by PKCε and efficiency of disassembly of gap junctions is reduced (Akoyev and Takemoto, 2007). These results suggest that ZO-1 interactions cause structural changes in the Cx43-CT that allow for PKC phosphorylation (Akoyev and Takemoto, 2007; O’Quinn et al., 2011). Thus, although ZO-1 interaction with Cx43 may not be critical in the formation of gap junction channels, ZO-1 may act to regulate their function through membrane targeting and molecular inhibition by limiting gap junction formation and promoting endocytosis from the gap junction plaque.
As mentioned above, ZO-1 plays a role in maintaining gap junction stability not only by binding to Cx43 directly but also through binding to actin (Giepmans et al., 2001c; Kostin, 2007). In addition, drebrin, another actin-binding protein originally identified as neuronal cell-specific, has since been detected in ECs and VSMCs where it is involved in gap junction function (Peitsch et al., 1999, 2005; Yamada et al., 2005). Recent studies using coimmunoprecipitation, immunofluorescence, and immunolabeling coupled to transmission electron microscopy have identified that Cx43 and drebrin directly interact, and drebrin acts to stabilize actin cytoskeleton association that is required for the maintenance of stable and functional gap junctions (Butkevich et al., 2004; Majoul et al., 2007; Park et al., 2009b). Taken together, studies of connexins reveal an intricate signaling microdomain involving tubulins, ZO proteins, and binding partners, including drebrin and actin, that maintain the ability of connexins to traffic to the plasma membrane and form functional gap junctions.
c. Calmodulin.
It has been well documented that connexin hemichannels and gap junctions are sensitive to calcium environments, although the exact mechanisms of the interaction are not fully defined (Harris, 2001; Lurtz and Louis, 2007; Zhou et al., 2007b; Herve et al., 2012; Herve and Derangeon, 2013). The first observation made in the early 1980s using freeze fracture studies indicated that connexins twist the gap junction channel shut in response to supraphysiological levels of Ca2+ (Peracchia, 1978; Unwin and Ennis, 1983; Bruzzone et al., 1996). Several studies now suggest that this may be regulated through binding of the calcium sensing subunit calmodulin. Calmodulin interacts with both Cx32 and Cx43 to maintain a closed gap junction channel, potentially by altering channel conformation or through physical interactions with calmodulin or calcium itself (Van Eldik et al., 1985; Torok et al., 1997; Sotkis et al., 2001; De Vuyst et al., 2006; Stauch et al., 2012; Xu et al., 2012). This interaction is not homologous between connexin isoforms: gap junction channels formed of Cx40 isoforms are insensitive to high levels of calcium, potentially because of a lack of the putative binding sites for calmodulin (Lurtz and Louis, 2007; Xu et al., 2012).
d. Posttranslational modifications.
It has become increasingly evident that the C-terminal region of Cx43 acts as a binding domain for many molecules that are involved in its trafficking, membrane stability, and gap junctional communications (Niger et al., 2010) (see section III.A.1). The C terminus is an unstructured, highly dynamic region that contains multiple sites for posttranslational modifications, including phosphorylation, nitrosylation, palmitoylation, sumoylation, and ubiquitination (Palatinus et al., 2011b; Straub et al., 2011; Johnstone et al., 2012a; Chen et al., 2013). As described in section III.A.1, the connexin C terminus is primarily unfolded and rich in serine, threonine, and tyrosine residues (Ser/Thr/Tyr) that are targeted for posttranslational modifications (Solan et al., 2003, 2009; Chen et al., 2013). Additionally, through a number of techniques including coimmunoprecipitation, immunofluorescence overlap, and pull-down assays, direct interactions have been identified within the Cx43 C terminus, with the suggestion that this can reduce the signaling properties of the gap junctional pore (Niger et al., 2010). Kinases and sites within Cx43 have been well characterized, including phosphorylation by kinases from the src family of Tyr265, and phosphorylated by MAPK of Ser255/(Ser262)/Ser279/Ser282 residues (Solan and Lampe, 2009; Johnstone et al., 2012a; Chen et al., 2013). Presumptively, these posttranslational modifications induce structural changes within the connexin C-terminal region that allow for further protein interactions with this region (Saidi Brikci-Nigassa et al., 2012). However, given the lack of data on the effect of posttranslational modifications on the structure of connexin C terminus, the exact consequences of these modifications remain unclear.
Further studies have shown that connexins form aggregate called “formation plaques” prior to accretion as gap junction plaques in a process that appears highly regulated by PKC phosphorylation of the C-terminal domain of Cx43 (Johnson et al., 2012). As with previously mentioned studies, removal of the C-terminal region after the tubulin-binding domain demonstrates that these sites are not essential but promote efficient gap junction assembly (see section III.A.1). In the heart, phosphorylation of the Ser368 residue (the main PKC-associated site in Cx43 C terminus) allows for the interaction with the ZO-1 proteins, which facilitates Cx43 aggregation in gap junctions but may also be a key factor in Cx43 cellular distribution. For example, phosphorylation of Cx43 Ser368 in cardiac myocytes induces lateralization of Cx43 hemichannels by removal from the intercalated disc where Cx43 is known to interact with ZO-1, desmin, and interleukins (Giepmans, 2004; Severs, 2007). These proteins form a stable signaling microdomain that facilitate Cx43 cellular localization and gap junctional signaling in the myocardium.
The src kinase family has been demonstrated to interact with and modulate Cx43 gap junctions through tyrosine phosphorylation of its C-terminal region (Warn-Cramer and Lau, 2004). The Cx43 C terminus contains two binding sites for the SH2 and SH3 domains of v-src and c-src (Kanemitsu et al., 1997; Loo et al., 1999; Giepmans et al., 2001a; Lin et al., 2006). Interaction with these kinases and subsequent phosphorylation of Cx43 decreases gap junctional communication (Duffy et al., 2004; Duffy, 2012; Geletu et al., 2012). One potential mechanism for this is through disruption of the interaction between ZO-1 and Cx43 (Toyofuku et al., 2001). Although Cx40 does not contain the consensus sequence for binding to src kinases, it has been proposed that src interacts with proline-rich domains that are found in Cx40 (Bouvier et al., 2008). As with previous reports on the C terminus, interactions of Cx43 with diverse kinases seem to be involved in the overall regulation of gap junction plaque formation and function but do not present as an absolute requirement given that gap junction plaque can form in the absence of phosphorylation.
Taken together, it is clear that vascular gap junction proteins are regulated through specific protein interactions in specialized signaling microdomains (Fig. 7). The studies mentioned in this section report that connexins 1) can interact with numerous protein partners, 2) are polarized to specific region of the cells (e.g., at the MEJ), and 3) can accumulate in specialized areas of the plasma membrane as they have been detected in caveoli. However, although the “gap junction microdomain” can be defined in terms of the protein partners, the consequences of the protein-protein interactions still need to be clarified. This is hindered by the lack of specificity of pharmacological agents currently available to inhibit gap junctions (see Table 4). Indeed, a number of inhibitors of gap junctions are widely used but are generally considered to be nonspecific, and their mechanism of inhibition have not been clearly defined (Evans and Boitano, 2001; Evans et al., 2012). For example, studies of the functional role of connexins in arterioles have extensively used inhibitors such as glycyrrhetinic acid and carbenoxolone, but recent studies have identified nonspecific effects of these blockers, including inhibition of calcium-activated potassium channels (Behringer et al., 2012). The development of transgenic animals was first thought to be a solution to cope with the lack of specific pharmacological inhibitors, but it is now clear that a number of compensations and/or redistribution of other connexin isoforms occur in Cx KO animals, which could further hinder interpretation of connexins on physiologic function (e.g., Kruger et al., 2002; Simon and McWhorter, 2003; Isakson et al., 2006a). It appears more specific molecular (e.g., small molecule inhibitors) or genetic (inducible cell line-specific knockouts) studies are required to more clearly delineate connexin function in the vasculature.
Fig. 7.
Connexins and gap junction signaling microdomains. The formation of gap junctional structures is regulated through organization of connexin hemichannels in caveolae that further assemble to a gap junction plaque through association with multiple protein partners. The C-terminal domain of connexins interacts with microtubule, PKC, and ZO-1, and these interactions promote integration of connexin hemichannels to the plasma membrane at lipid-enriched caveolae. Assembling of hemichannels in the lipid raft structures surrounding a gap junction plaque is enhanced through interactions with ZO-1/2 and drebrin. The different steps leading to the formation of gap junction assembly as well as gap junctional permeability are modulated through a dynamic process of posttranslational modifications at the C-terminal regions of connexins including phosphorylations by PKC and MAPK, which both reduce gap junction communication, and by PKA, which increases gap junctional signaling.
B. Potassium and Calcium Channels: A Case for the Endothelium-Derived Hyperpolarization-Mediated Response
In the late 1970s, the Kuriyama group reported that acetylcholine was capable of inducing a hyperpolarization of the VSMC plasma membrane in guinea pig coronary and mesenteric artery as well as in rabbit mesenteric artery (Kuriyama and Suzuki, 1978; Karashima and Kuriyama, 1981; Takata and Kuriyama, 1980). In their preparation, VSMC hyperpolarization was paradoxically simultaneous to arterial constriction, which was the mechanical response commonly observed in in vitro arterial preparations at the time (Kitamura and Kuriyama, 1979; Karashima and Kuriyama, 1981). Over the same period of time, the identification of an endothelium-derived relaxant factor (EDRF, see section II.A) led scientists to realize that the contractile effect of acetylcholine on in vitro preparation was caused by damage to the endothelial layer during arterial isolation (Furchgott and Zawadzki, 1980; Furchgott, 1999). After this groundbreaking work, care was taken to work on in vitro preparation with intact endothelium, and in 1984, Bolton et al. (1984) demonstrated that the VSMC hyperpolarization induced by acetylcholine also depended on the integrity of the endothelium. In the late 1980s, a clear distinction between EDRF [then identified as NO (Ignarro et al., 1987; Khan and Furchgott, 1987; Palmer et al., 1987)] and the endothelium-dependent hyperpolarization (EDH) of VSMCs was drawn. In their seminal article, Chen et al. (1988) reported that hemoglobin and methylene blue (which, respectively, scavenges NO and blocks the guanylate cyclase) reduced acetylcholine-induced relaxation and abolished cGMP production but had no effect on the VSMC hyperpolarization or on the 86Rb efflux (a marker for potassium) in rat aorta and pulmonary artery (Chen et al., 1988). With the discovery of l-arginine analogs as specific inhibitors of NO production (Hibbs et al., 1987a,b; Marletta et al., 1988; Knowles et al., 1989; Stuehr et al., 1989), it became clear that the EDH was resistant to prostacyclin blockers and NO-synthase blockers, alone or combined (Ishii et al., 1990; Nagao and Vanhoutte, 1992; Rand and Garland, 1992; Cowan et al., 1993; Waldron and Garland, 1994a). These observations pointed to the fact that NO and prostacyclin derived from ECs could not be responsible for the endothelium-dependent hyperpolarization of VSMC observed in acetylcholine dilation, which resulted in a surge of interest in this NO- and prostacyclin-independent vasodilation.
The concept of an endothelium-derived hyperpolarizing factor (EDHF) emerged in the 1980s (Taylor and Weston, 1988). Since then, numerous laboratories have tried to identify the “hyperpolarizing factor” itself and have worked on characterizing the hyperpolarization response induced by acetylcholine and other endothelial agonists. It was generally accepted that the endothelium-dependent hyperpolarization involved potassium ions (K+), because 1) the amplitude of hyperpolarization was inversely correlated to the extracellular concentration of K+ (Chen et al., 1989; Chen and Suzuki, 1989; Nagao and Vanhoutte, 1992), 2) K+ efflux was observed in cells preloaded with a radioactive form of K+ (42K) or a marker for K+ ions (86Rb) upon stimulation with acetylcholine (Chen et al., 1988; Taylor et al., 1988), and 3) the EDH was abolished by potassium channels blockers (Chen et al., 1991; Nagao and Vanhoutte, 1992; Van de Voorde et al., 1992). The role of extracellular calcium as well as calcium from the intracellular stores in EDH-mediated relaxation was also demonstrated by a number of laboratories (e.g., Chen and Suzuki, 1990). The role of extracellular calcium was also confirmed by application of the calcium ionophore A23187, which also resulted in an EDH that was insensitive to eNOS blockers and to inhibitors of the prostacyclin pathway (Chen and Suzuki, 1990; Parsons et al., 1994; Plane et al., 1995; Zygmunt and Hogestatt, 1996).
1. Role of Potassium Channels.
The role of K+ in acetylcholine response was first proven by the Kuriyama group’s work on guinea pig coronary arteries that showed that the amplitude of acetylcholine-induced hyperpolarization was reduced in low K+ solutions, whereas removal of other ions (namely Na+ and Cl−) did not affect the hyperpolarization (Kitamura and Kuriyama, 1979). When the concept of EDHF emerged 25 years later, the identification that K+ efflux is a fundamental step in the EDH-mediated response became a major milestone in our understanding of the pathway (Chen et al., 1988; Taylor and Weston, 1988). Later, treatment with potassium channel blockers such as glibenclamide, an inhibitor of ATP-sensitive potassium channel (Chen et al., 1991; Garland and McPherson, 1992; Van de Voorde et al., 1992; Plane and Garland, 1993, 1994; Plane et al., 1995; Corriu et al., 1996), or 4-aminopyridine, an inhibitor of voltage-dependent potassium channels (Zygmunt and Hogestatt, 1996; Hashitani and Suzuki, 1997), failed to consistently inhibit EDH in several vascular beds. Conversely, nonselective KCa blockers such as tetraethylammonium or tetrabutylammonium were shown to inhibit EDH of VSMCs (Chen et al., 1991; Van de Voorde et al., 1992; Cowan et al., 1993; Zygmunt and Hogestatt, 1996). Several laboratories further observed that the EDH was attenuated by drugs such as apamin, a blocker of small conductance KCa channels (SKCa,), and charybdotoxin, a nonspecific blocker of large conductance KCa (BKCa), and intermediate conductance KCa (IKCa) (Adeagbo and Triggle, 1993; Cowan et al., 1993; Holzmann et al., 1994). However, in 1994, the EDH-mediated response for the first time was abolished using a combination of apamin and charybdotoxin (Waldron and Garland, 1994b). This observation was followed by the demonstration that iberiotoxin, a blocker of BKCa, had no effect on the EDH-mediated response (Zygmunt and Hogestatt, 1996). Because specific blockers of IKCa were not available at the time, it was then concluded that both IKCa and SKCa were involved in the EDH-mediated response. This was proven to be true with the further development of the IKCa inhibitors TRAM39 and TRAM34 (Wulff et al., 2000, 2001), which clarified a role of these channels in the EDH-mediated relaxation (Crane et al., 2003; Hinton and Langton, 2003). In parallel, IKCa channel openers such as 1-ethyl-2-benzimidazolinone (1-EBIO) or SKCa openers (riluzole) were able to reproduce the EDH induced by acetylcholine (Edwards et al., 1999a,b; Walker et al., 2001; Crane and Garland, 2004). These pharmacological studies led to the conclusion that the hyperpolarization observed in the EDH pathway reflects the activation of two potassium channels, IKCa and SKCa, which was a key step in our understanding of the EDH-mediated response.
In 1998, the exact role, location, and temporal activation of these channels became more clear as a result of an investigation published in Nature by Edwards et al. (1998). In this study, the authors measured the membrane potential of ECs and demonstrated that the K+ efflux observed in EDH-mediated response was due to the activation of SKCa and IKCa channels at the plasma membrane of ECs and not of SMCs as it was assumed at this time (Garland et al., 2011). Edwards et al. (1998) further showed that this K+ efflux creates an accumulation of K+ in the extracellular space between ECs and VSMCs, which in turn activates the Na/K/ATPase as well as the KIR channels at the plasma membrane of the VSMC, thus hyperpolarizing and relaxing the VSMCs (Edwards et al., 1998). This K+ accumulation was demonstrated using potassium-selective electrodes, allowing for the measurement of local K+ concentration in the blood vessel wall measuring a concentration of approximately 10 mM in the space between ECs and VSMCs in intact rat hepatic arteries stimulated with acetylcholine (Edwards et al., 1998). In parallel, another study confirmed the role of IKCa and SKCa located at the plasma membrane of EC in rat mesenteric arteries (Doughty et al., 1999).
It became clear that although both IKCa and SKCa were involved in the EDH-mediated response, their location, mode of activation, and their role were different. Several studies from the early 2000s showed that the role of KCa channels appears to differ depending on the contractile state of the smooth muscle: when no depolarizing preconstrictor is present (i.e., when the contractile state of the smooth muscle is due to basal tone), acetylcholine produces a “true” hyperpolarization, which is due to activation of SKCa, whereas in the case of agonist-induced SMC depolarization (in presence of phenylephrine, for example), EDH-mediated relaxation in response to acetylcholine can be separated in two components reflecting SKCa and IKCa activities (Dora et al., 2000; Dora and Garland, 2001; Crane et al., 2003; Takano et al., 2004).
Because both IKCa and SKCa are activated by the calcium-calmodulin sensor (Xia et al., 1998) with similar sensitivity to calcium (Carignani et al., 2002), their functional difference has been suggested to be the result of a differential intracellular location in the ECs. The development of isoform-specific antibodies as well as knockout mouse models helped to increase our knowledge regarding the contribution of each isoform of KCa channels. Several reports observed a spatial separation of IKCa and SKCa in ECs with SKCa predominantly expressed at EC-EC junctions, whereas IKCa channels are mainly present at the points of contacts between ECs and VSMCs and in close proximity to the ER (Sandow et al., 2006; Dora et al., 2008; Ledoux et al., 2008). It is not clear how this channel polarization occurs, but it is possible that it may be due to local plasma membrane composition because IKCa is not located in caveolae, whereas SKCa is located in caveolae in ECs as demonstrated by coimmunoprecipitation and experiments using sucrose gradient (Absi et al., 2007). The SKCa portion of the EDH-mediated relaxation can be inhibited in presence of the caveolae disrupting agent MβCD, an inhibition that was reversed by addition of cholesterol (Graziani et al., 2004; Absi et al., 2007).
With the development of transgenic mouse models, the importance of the EDH-mediated response at the whole animal level has become more clear. Carotid and resistance arteries isolated from the global KCa3.1 (= IKCa) knockout (KO) mice exert decreased hyperpolarization of ECs and VSMCs in response to acetylcholine, as well as decreased associated vasodilation (Si et al., 2006; Wolfle et al., 2009; Milkau et al., 2010). Interestingly, KCa3.1 KO mice are hypertensive [approximately 10 to 15 mm Hg higher compared with wild-type mice), highlighting a crucial role for this potassium channel in the control of vascular tone and blood pressure (Si et al., 2006)]. The double KO of both KCa2.3 (= SKCa) and KCa3.1 exhibited an impaired acetylcholine-induced EDH and dilation in conduit and resistance arteries measured in vivo (Brahler et al., 2009). In a mouse model in which the expression level of SKCa can be manipulated with dietary doxycycline, the amount of SKCa expression in the EC was inversely correlated with the blood pressure (Taylor et al., 2003). It is noteworthy that several studies in small and large animals demonstrated that activation of IKCa and SKCa channels using the drug SKA-31 could decrease blood pressure, making these channels a potential therapeutic target for treatment of hypertension (Sankaranarayanan et al., 2009; Hasenau et al., 2011; Damkjaer et al., 2012).
2. Importance of Local Calcium Release: Role of Calcium Channels.
In addition to being an important second messenger in the regulation of the contractile state of the VSMCs during the EDH-mediated response, calcium has been identified as a key element in the induction of the endothelial hyperpolarization itself in the late 1990s. Suzuki’s group used submucosal arterioles loaded with Fura-2 for 1 hour or for 3 hours to investigate the calcium response from ECs and VSMCs, respectively, and demonstrated for the first time that acetylcholine elevates fluorescence in ECs, whereas it has the ability to reduce Ba2+-induced increase in [Ca2+]i in VSMCs (Fukuta et al., 1999). More importantly, their experiments showed that the decrease in [Ca2+]i in VSMCs was not blocked by a combination of inhibitors of the EDRF/NO and the prostaglandin pathways (Fukuta et al., 1999). Lastly, although charybdotoxin had no effect on the acetylcholine-induced increase in [Ca2+]i in ECs, it reduced the acetylcholine-induced decrease in [Ca2+]i in VSMCs, suggesting that acetylcholine may modulate [Ca2+]i in VSMCs indirectly via activation of potassium channels in the ECs (Fukuta et al., 1999).
Once the KCa channels involved in the EDH-mediated response were identified in the ECs (see above), the source of calcium activating the endothelial KCa channels has been at the center of numerous investigations. In mouse mesenteric arteries, IKCa are directly activated by calcium released from IP3R upon stimulation with acetylcholine (Ledoux et al., 2008). The role of extracellular calcium has also been suggested by several investigations, initially showing that a capacitive entry of calcium is involved in the EDH-mediated relaxation (e.g., in Taylor et al., 2001). These initial observations have since been confirmed by other groups, identifying channels from the TRP family as the molecular protein responsible for extracellular calcium influx. Namely, the isoforms TRPV1, TRPV3, TRPV4, TRPC3, and TRPC1 have all been reported in the EDH-mediated response (Kohler and Hoyer, 2007; Loot et al., 2008; Earley et al., 2009; Schmidt et al., 2010; Senadheera et al., 2012; Ma et al., 2013).
Among the vanilloid family of TRP channels, TRPV1, TRPV3, and TRPV4 all appear in some way to be involved in the EDH-mediated response, with TRPV4 being the most studied. The isoform TRPV4 is of particular interest because 1) TRPV4 KO mice exhibit an impaired EDH-mediated relaxation and 2) the TRPV4 specific opener 4αPDD induced an endothelium-dependent hyperpolarization (Kohler and Hoyer, 2007). A recent study in rats showed that TRPV4 and SKCa coimmunoprecipitate and colocalize in isolated ECs, suggesting a functional interaction between the two channels (Ma et al., 2013). Interestingly, Fleming’s group established a correlation between the EDH-mediated relaxation and the translocation of TRPV4 channel from a perinuclear localization to the cell membrane (Loot et al., 2008). At the whole animal level, activation of TRPV4 channels using 4αPDD resulted in an increase in local blood flow in the mesenteric vascular bed as well as in a decreased blood pressure (Ma et al., 2013). Lastly, when TRPV4 KO mice were treated with an inhibitor of eNOS, the increased in blood pressure was greater compared with control mice, showing a prominent role of these channels in the regulation of blood pressure (Earley et al., 2009). Regarding other TRPV channels, stimulation of TRPV3 appears to induce an endothelium-dependent hyperpolarization of adjacent VSMCs as well as a decreased [Ca2+]i (Earley et al., 2010). The EDH-mediated response observed upon stimulation of TRPV3 was further reduced by blockers of SKCa and IKCa (Earley et al., 2010). Although a lot of important information has been gained in regard to TRPV channels with EDH, the more recent experiments have relied on knockout mice. A future critical point in these experiments would be to demonstrate the lack of other TRPV channels when one is deleted, because developmental compensation has been clearly demonstrated in a very large variety of germline knockout animals.
In addition to channels from the TRPV family, TRP channels from the canonical family such as TRPC1 and TRPC3 have been studied in the EDH-mediated response. The role of TRPC3 was recently demonstrated using Pyr3, which inhibited hyperpolarization generation (Senadheera et al., 2012). In this study, the authors concluded that TRPC3 activity is involved in the triggering of SKCa and IKCa at the EC plasma membrane, making TRPC3 a possibly important component to the EDH-mediated response. Conversely, TRPC1 channels appeared to have an inhibitory effect on the EDH-mediated response, because the TRPC1 KO mice exhibited increased EDH-mediated relaxation and a greater hyperpolarization of ECs (Schmidt et al., 2010). In this study, the authors also showed that TRPC1 KO mice exhibited a reduced arteriolar tone as well as reduced blood pressure.
In addition to being an activator of the hyperpolarization on the EC side, calcium is also central in the relaxation process in the EDH-mediated response. As mentioned above, investigations of calcium dynamics simultaneously in the ECs and in the SMCs showed that the EDH-mediated component of acetylcholine response is characterized by an increase in calcium in the ECs, whereas the calcium in VSMCs decreases (Bolz et al., 1999; Fukuta et al., 1999). The KCa blockers tetrabutylammonium and charybdotoxin abolished calcium decrease and hyperpolarization of VSMCs but did not affect calcium increase and hyperpolarization in ECs (Bolz et al., 1999; Fukuta et al., 1999). These observations suggest that the decrease of calcium in VSMCs is due to the activity of KCa channels in the ECs, which presumably close voltage-gated calcium channels in the VSMCs by reducing the membrane potential of the VSMCs (Nelson et al., 1990; Bolz et al., 1999).
Another potentially important component in the EDH-mediated response is the calcium-sensitive receptor (CaR or CaSR). CaR is a G protein-coupled receptor activated by millimolar concentrations of calcium, which results in the release of calcium from intracellular stores via IP3R (for review, see Ward et al., 2012). The role of CaR in the EDH-mediated response was demonstrated in rat mesenteric arteries where activation of CaR in ECs induced a hyperpolarization of the VSMCs, which was abolished by the IKCa inhibitor TRAM34 or by denudation of the endothelium (Weston et al., 2005). The same group also demonstrated that CaR and IKCa are both located in noncaveolae fractions. Conversely, CaR does not colocalize with SKCa channels (Weston et al., 2005). The colocalization of CaR and IKCa might be involved in the differential activation of IKCa and SKCa in the EDH-mediated response (Dora et al., 2008).
3. Gap Junction Channels.
The central role of gap junction channels in the EDH-mediated response has been observed for several decades with the use of nonspecific blockers such as carbenoxolone, 18α-glycyrrhetinic acid, octanol, d-mannitol, sucrose, or heptanol, which reduced the NO-independent relaxation in response to vasodilators in numerous vascular beds (Kuhberger et al., 1994; Yamamoto et al., 1998, 1999; Brandes et al., 2000; Sandow and Hill, 2000; Dora et al., 2003a). With the development of gap junction mimetic peptides such as 37-43Gap 27, 40Gap27, 37-40Gap26, and 43Gap26, the role of specific connexin isoforms was initially thought to be further clarified. Several reports demonstrated an inhibitory effect of these connexin-mimetic peptides on indomethacin- and l-NAME-resistant relaxation to acetylcholine (Chaytor et al., 1998, 2001, 2003, 2005; Dora et al., 1999; Sandow et al., 2002; Ellis et al., 2009). It is noteworthy that the 37-43Gap 27, 40Gap27, and 37-40Gap26 were shown to inhibit VSMCs hyperpolarization upon acetylcholine stimulation, whereas they had no effect on the hyperpolarization of ECs (Sandow et al., 2002; Chaytor et al., 2003, 2005; Ellis et al., 2009). In contrast, the use of 43Gap 26, 40Gap 27, and 37,43Gap 27 in a different study failed to inhibit the EDH-mediated response, which is in line with other studies that have now called into question the specificity of the connexin mimetics [e.g., Table 4 (Mather et al., 2005; Dahl, 2007; Wang et al., 2007)]. However, loading ECs of intact pressurized mesenteric arteries with an antibody blocking Cx40 resulted in an impaired EDH-mediated dilation, whereas loading of EC with antibodies against Cx37 or Cx43 had no effect on EDH-mediated response (Mather et al., 2005). Lastly, the use of Cx40 KO mice also provided possible evidence that in particular vascular beds, the Cx40 isoform may be important (Figueroa et al., 2003; Milkau et al., 2010).
Structurally, the presence of gap junction communication in the vessel wall has been reported by numerous investigators (see above). Transmission electron microscopy as well as immunolabeling for different connexin isoforms (mostly Cx37, Cx40, and Cx43) considerably enhanced our knowledge on the location and the nature of gap junctions within the vascular wall of arteries presenting EDH-mediated responses. By use of transmission electron microscopy, the presence of the typical gap junction pentalaminar structure has been observed between endothelial and smooth muscle layers as well as between ECs in several vascular beds (Sandow and Hill, 2000; Sandow et al., 2002, 2003b; Straub et al., 2011; Billaud et al., 2012). Although the presence of functional gap junctions between VSMCs has been observed consistently in large vessels, their occurrence in smaller arteries presenting EDH-mediated responses is more debated (Little et al., 1995; Welsh and Segal, 1998; Yamamoto et al., 2001; Sandow et al., 2002; Looft-Wilson et al., 2004a,b; Fanchaouy et al., 2005; Hakim et al., 2008). There are reports of Cx37 and Cx43 immunostaining in VSMCs, with variable expression of both isoforms depending on the vascular bed studied (Yamamoto et al., 2001; Chaytor et al., 2003, 2005; Sandow et al., 2003b). Consequently, the role of gap junctional communication between VSMCs in the transfer of hyperpolarization in the EDH-mediated response is unclear. In contrast, the presence and the role of gap junction communication between ECs in the EDH-mediated response is well accepted, with Cx40 being key in the conduction of the hyperpolarization along the endothelial layer (Welsh and Segal, 1998; Emerson and Segal, 2000; Yamamoto et al., 2001; Chaytor et al., 2003, 2005). Indeed, several reports using Cx40 KO mice observed a decreased EDH-mediated response as well as a decreased conduction of the hyperpolarization and relaxation along the endothelial layer (de Wit et al., 2000; Figueroa and Duling, 2008; Milkau et al., 2010). In addition, the presence and the contribution of Cx37 and Cx43 at the EC junctions in the EDH-mediated response have been reported, but their exact role is not clear (Haddock et al., 2006; Sandow et al., 2006).
The presence of gap junctional communication at the junctions between ECs and SMCs was also demonstrated by measuring membrane potentials of both cells types in vascular beds with and without EDH-mediated responses (Yamamoto et al., 1998, 1999; Emerson and Segal, 2000; Sandow et al., 2002; Haddock et al., 2006). In these studies, there was a strong EDH-mediated response upon acetylcholine stimulation, and the resting membrane potentials of ECs and SMCs were similar. In contrast, the rat femoral artery does not present EDH-mediated response when stimulated with acetylcholine, and the resting membrane potentials of ECs and VSMCs are significantly different, strongly suggesting an absence of gap junctional coupling between both cell types (Sandow et al., 2002). At the electron microscopy level, most of the gap junctions between endothelial and smooth muscle layers are organized in one single plaque that is smaller in size compared with the plaques observed between ECs (Little et al., 1995; Sandow and Hill, 2000; Dora et al., 2003a; Sandow et al., 2003b).
With regard to all of these observations, the role of gap junctions in the EDH-mediated response is clear. It is noteworthy that a recent study highlighted the influence of the methodology in the investigation of gap junctional coupling in the EDH-mediated response by comparing the EDH-mediated response in arteries in vivo and in arteries mounted in a wire myograph or in a pressure myograph using global Cx40 KO mice as well as mice deficient in Cx40 specifically in ECs (Boettcher and de Wit, 2011). The EDH-mediated response was completely dependent on Cx40 in arteries mounted in a wire myograph (isometric conditions), whereas the EDH-mediated responses studied in a vessel mounted in a pressure myograph (isobaric conditions) or in vivo were not affected by the deletion of Cx40 (Boettcher and de Wit, 2011). Although the role of Cx40 is clear in the EDH-mediated response (Mather et al., 2005; Boettcher and de Wit, 2011), it appears that the EDH-mediated response is a highly sensitive mechanism [e.g., changes in posttranslational modification of connexins after artery manipulation (Straub et al., 2010)] that requires careful interpretation according to the methodology used.
4. Other Important Players in the Endothelium-Dependent Hyperpolarization-Mediated Response.
Since the first demonstration of an EDRF/NO− and prostaglandin-independent component of endothelial relaxation in the late 1980s, it was assumed that this component was due to the release of a hyperpolarizing factor from ECs (Taylor and Weston, 1988). Since then, it is debated whether the endothelium-induced hyperpolarization of VSMCs is induced by a diffusible factor released by ECs and targeting VSMCs and/or is attributable to direct transfer of hyperpolarization from the endothelium via myoendothelial gap junctions. However, several molecules such as arachidonic acid metabolites and hydrogen peroxide (H2O2) have also been suggested to constitute the endothelium-derived hyperpolarizing factor, EDHF.
a. Metabolites of arachidonic acids.
Metabolites of arachidonic acid such as epoxyeicosatrienoic acids have been shown to contribute to EDH-mediated response in the late 1990s (Campbell and Harder, 1999; Fisslthaler et al., 1999). Several authors were able to reproduce an endothelium-dependent hyperpolarization of the VSMC and/or arterial vasodilation by applying exogenous arachidonic acid itself or its metabolites (Pinto et al., 1987; Rosolowsky and Campbell, 1993; Oltman et al., 1998; Campbell and Harder, 1999; Fisslthaler et al., 1999). In parallel, inhibitors of the enzymes involved in the arachidonic acid metabolism [phospholipase A2, cytochrome P450 (P450)] are able to significantly decrease EDH-mediated relaxation in several vascular beds (Pinto et al., 1987; Rubanyi and Vanhoutte, 1987; Rosolowsky and Campbell, 1993). Given that the role of TRPV4 channels in the EDH-mediated response had been discovered earlier (see section III.B.2) but their gating was still unknown, the discovery that arachidonic acid activates TRPV4 channels in ECs later helped understanding the role of these lipid compounds in the EDH-mediated response (Watanabe et al., 2003). Later work found that arachidonic acid metabolites such as epoxyeicosatrienoic acids produced via cytochrome P450 could be an endogenous activator of TRPV4, and thus an important player in the EDH-mediated response in many vascular beds (e.g., Earley et al., 2005). It is now accepted that arachidonic acid metabolites activate TRPV4 and calcium influx in ECs, leading to the activation of KCa channels and thus to hyperpolarization (Earley et al., 2005; Vriens et al., 2005; Marrelli et al., 2007). However, the exact isoform of KCa channels activated in this case remains under debate and is likely vascular bed-dependent (Campbell and Fleming, 2010; Dora, 2010; Feletou, 2011a). Activation of arachidonic acid metabolism is thought to happen after activation of the phospholipase A2 by diacylglycerol produced by phospholipase C when GPCR (such as muscarinic receptors) are stimulated (Saliez et al., 2008; Ella et al., 2010). More recently, a study demonstrated that TRPV4 is phosphorylated by PKA in EC, a phosphorylation that seems to be required for arachidonic activation of the channel in human coronary arteries (Zheng et al., 2013b).
b. Hydrogen peroxide.
Although it is not clear whether H2O2 is a “true” EDHF, this reactive oxygen species has also been suggested to be produced and may be further released by the endothelium to contribute to the EDH-mediated response. Supporting this hypothesis, the addition of exogenous H2O2 or the combination of xanthine and xanthine oxidase induces a typical EDH-mediated dilation that is dependent on IKCa and/or SKCa channels and that is characterized by VSMCs hyperpolarization (Sobey et al., 1997; Pomposiello et al., 1999; Matoba et al., 2000, 2002, 2003; Chaytor et al., 2003; Matoba and Shimokawa, 2003; Miura et al., 2003; Rabelo et al., 2003). In parallel, numerous studies using H2O2-sensitive probes have demonstrated that H2O2 is produced in arteries stimulated with agonists such as acetylcholine, the calcium ionophore A23187, or bradykinin (Matoba et al., 2000, 2003; Chaytor et al., 2003; Miura et al., 2003). Functional experiments using catalase that degrades H2O2 also resulted in a decreased EDH-mediated dilation (Sobey et al., 1997; Pomposiello et al., 1999; Matoba et al., 2000, 2002; Edwards et al., 2008). In contrast, the evidence against H2O2 as an EDHF is that, although exogenous addition of H2O2 is capable of hyperpolarizing VSMCs (Beny and von der Weid, 1991; Chaytor et al., 2003), catalase often failed to reduce the smooth muscle hyperpolarization (Beny and von der Weid, 1991; Matoba et al., 2002; Chaytor et al., 2003; Gluais et al., 2005). However, research on this work is ongoing.
The exact contribution of H2O2 in the EDH-mediated response is still unclear and appears variable depending on the vascular bed investigated. Recently, experiments in culture ECs supported that H2O2 potentiates calcium release from EC stores, probably via redox modification of the IP3R (Edwards et al., 2008). The calcium release is suggested to further activate KCa channels and induce the EDH-mediated response (Sobey et al., 1997). The source of H2O2 production is also variable according to the literature, because NADPH oxidase, uncoupled eNOS, and P450 have all been involved in H2O2 production in the EDH-mediated response (Shimokawa, 2010). It has been suggested that NADPH oxidase, uncoupled eNOS, and P450 generate H2O2 indirectly by first producing superoxide anion, which is further transformed in H2O2 via superoxide anion dismutase, mostly the soluble isoform Cu,Zn SOD (Morikawa et al., 2003).
The identification of a single solitary factor mediating the EDH response has been at the center of multiple investigations in the past decades, and its identity has been debated because it seems to be highly dependent on the vascular bed and the species in question, the preconstrictor used, or the methodology. In addition, the necessity of the term “factor” is highly debated “because it masks the real identity of the signal(s) involved” (Feletou and Vanhoutte, 2013).
5. Altogether at the Myoendothelial Junction.
The importance of MEJ in the EDH-mediated response has been reported by a number of investigators in the past 20 years, and it is now well accepted that the EDH-mediated signaling pathway is likely located at the MEJ (Mulvany and Aalkjaer, 1990; Hwa et al., 1994; Shimokawa et al., 1996; Sandow and Hill, 2000; Berman et al., 2002). Whether the EDH-mediated response requires an EDH factor or simple transfer of hyperpolarization from the ECs to the VSMCs, the presence of these close contacts between ECs and VSMCs seems to be essential in the EDH-mediated response since a lot of the molecular players described above are present at the MEJ.
The potassium channel IKCa and to a lesser extent the SKCa; the plasma membrane calcium channels TRPV4 and TRPC3; the α2/α3 subunits of Na/K/ATPase; and the gap junction proteins Cx37 and Cx40 have all been detected at the MEJ in arteries (Isakson et al., 2006a; Sandow et al., 2006, 2012; Dora et al., 2008; Ledoux et al., 2008; Chadha et al., 2011; Bagher et al., 2012; Senadheera et al., 2012; Sonkusare et al., 2012; Kirby et al., 2013). Functionally, the presence of gap junctions at the MEJ is thought to be key in the transfer of the hyperpolarization from ECs to VSMCs (Edwards et al., 2010). In parallel, the close apposition of the two cell types at the MEJ is believed to be essential for the accumulation of potassium released from EC via the SKCa and the IKCa channels and the further activation of KIR and Na/K/ATPase in the VSMC, inducing their hyperpolarization (Edwards et al., 2010). With regard to calcium, its dynamics at the MEJ are crucial in the EDH-mediated response, because calcium concentration is specifically increased at the MEJ (Dora et al., 2008; Ledoux et al., 2008; Bagher et al., 2012; Sonkusare et al., 2012). The presence of a source of calcium (ER) at the MEJ, as well as the close localization of the calcium release channels IP3R and the calcium-dependent IKCa channels at the MEJ is also of importance in the EDH-mediated response. Indeed, this aggregation at the MEJ is crucial for the activation of IKCa by calcium release from the ER via IP3R in presence of acetylcholine (Isakson, 2008; Ellis et al., 2009; Sonkusare et al., 2012; Kirby et al., 2013). Lastly, given the functional role of TRP channels in the EDH-mediated response, it has been suggested that TRPC3 and TRPV4 channels present at the MEJ could either participate in the ER replenishment or in the activation of the KCa channels (Bagher et al., 2012; Senadheera et al., 2012; Sonkusare et al., 2012)
Although the MEJ is a key region for EDH-mediated response, the points of contact between ECs are equally important in this response. Namely, the spread of the hyperpolarization throughout the endothelium is central to ensure a coordinated response along the vascular bed (de Wit, 2010; Bagher and Segal, 2011). Accordingly, SKCa, Cx37, Cx40, and Cx43 have been evidenced at the points of contact between ECs (Emerson and Segal, 2000; Isakson et al., 2006a; Sandow et al., 2006, 2012; Dora et al., 2008; de Wit, 2010).
Structurally, it appears that caveolae play an important role in maintaining a functional EDH signaling microdomain at both EC contacts and the MEJ, because the EDH-mediated response is virtually abolished in mesenteric arteries from Cav1 KO mice (Saliez et al., 2008). In this study, the authors also observed an impaired calcium homeostasis in ECs, possibly resulting from a decreased activity of TRPV4, because the function of TRPV4 channels in HUVECs was impaired when cells were treated with Cav1 siRNA (Saliez et al., 2008). In parallel, Cav1 KO mice also exhibit decreased Cx37, 40, and 43 expressions at the MEJ (Saliez et al., 2008). Lastly, TRPV4, Cx37, Cx40, Cx43, and SKCa are localized in caveolae and also all colocalize and/or coimmunoprecipitate with Cav1 (Graziani et al., 2004; Absi et al., 2007; Saliez et al., 2008).
The importance of the MEJ in the EDH-mediated response has also been highlighted in pathologic conditions where their number increases along with the degree of EDH-mediated relaxation (Sandow et al., 2003a; Chadha et al., 2011). Additionally, IKCa channels and connexin expression at the MEJ is upregulated in arteries of obese rats (Sandow et al., 2003a; Haddock et al., 2011). In these studies, endothelium-dependent relaxation tends to shift from a NO component to an EDH component (Sandow et al., 2003a; Haddock et al., 2011). Thus, it is tempting to speculate that the increased number of MEJ in disease states compensate for other dysfunction in the arterial wall (Sandow et al., 2003a; Heberlein et al., 2009).
Several reports observed that the calcium released by the ER in EC and the calcium entering via TRP channels seem to be selectively involved in the hyperpolarization of ECs and appear not to induce NO generation from eNOS (e.g., Sonkusare et al., 2012). This fits well with the demonstration that eNOS and Hbα are closely localized at specific regions in the EC [i.e., the MEJ (see above)] where they regulate the amount of NO generated and released (Straub et al., 2012). With Hbα being expressed only in MEJs from resistance arteries and not conduit arteries, it is tempting to suggest that the Hbα “allows” for EDH to occur by binding any NO produced by eNOS activation after localized calcium release. Further studies will be required to tease this potentially important mechanism apart.
The studies described above involve a number of ion channels that are located in close proximity at the MEJ working in tandem to communicate a specific response in a spatially and temporally restricted manner (Fig. 8). Because the EDH-mediated response involves proteins that are 1) polarized to the MEJ, 2) accumulate in caveolae, and 3) are closely located to each other as demonstrated by coimmunoprecipitation or double immunolabeling, the EDH-mediated response is likely mediated by a signaling microdomain so as to regulate cellular communication in the blood vessel wall.
Fig. 8.
The endothelium-dependent hyperpolarization microdomain. The EDH response starts with the activation of IP3R at the ER in EC by a GqPCR [for example, muscarinic (M3), BK, or the calcium sensing receptor (CaR)]. The calcium released from the ER activates the calcium-sensitive potassium channels SKCa at the EC-EC junctions and IKCa at the MEJ, which induces hyperpolarization of the EC. In parallel, the calcium release from the ER activates the capacitive entry of calcium at both the EC-EC junctions and the MEJ via TRPV4 channels, which sustains the opening of the IKCa and SKCa channels. The activation of SKCa and IKCa at the plasma membrane of ECs activates the efflux of potassium and its accumulation in the extracellular space between the VSMCs and the ECs. This potassium accumulation further activates the sodium/potassium ATPase (Na/K/ATPase) at the plasma membrane of VSMC, producing hyperpolarization and relaxation of the VSMC by closing the voltage-gated calcium channels (VGCC). The hyperpolarization of EC can also be transferred to the adjacent ECs and VSMCs via gap junctions channels located at the MEJ and at the EC-EC junctions. Wavy arrows indicate hyperpolarization.
IV. Vesicular Communication: The Exocytosis Microdomain
Thus far, we have discussed communication involving diffusion of signals across the membrane or through plasma membrane channels. We now turn to the process of exocytosis, which allows for the storage and movement of signaling molecules in specialized vesicles. In the vascular wall, exocytosis provides ECs with a mechanism of rapid response to changes in the microenvironment to potentiate thrombosis, hemostasis, or an inflammatory response. The secretory vesicles of the endothelium, known as Weibel-Palade bodies (WPB), were originally discovered as the storage depot of von Willebrand factor (vWF) (Weibel and Palade, 1964). Since their initial discovery via electron microscopy, WPBs have also been found to store and release P-selectin (Bonfanti et al., 1989; McEver et al., 1989), interleukin-8 (Utgaard et al., 1998; Wolff et al., 1998), endothelin-1 (Ozaka et al., 1997; Russell et al., 1998), angiopoietin-2 (Fiedler et al., 2004), tissue-type plasminogen activator (Huber et al., 2002), and other proteins that play important roles in the signaling pathways of inflammation, hemostasis, and tissue repair (for review, see Rondaij et al., 2006b; Valentijn et al., 2011).
A. Components of Exocytosis Signaling Microdomains
Exocytosis of the WPBs takes place in a series of discrete steps. The process starts with the synthesis of the WPBs, which is mostly driven by the presence of vWF. Once the vesicle has been formed, interactions with the cytoskeleton either keep the WPBs in the perinuclear space or move them to a more peripheral position. To have its contents released into the extracellular space, the vesicle is primed and then fused to the plasma membrane. Upon secretion of its contents, the WPB dissociates from the membrane (Fig. 9). These steps require the coordinated movements of several key proteins organized into signaling microdomains.
Fig. 9.
The exocytosis microdomain. (A) Epinephrine induces increased cAMP via Gs protein-coupled receptors, resulting in the activation of RalA by RalGDS. The exocytosis of the Weibel-Palade bodies present at the PM is activated, whereas the progression of the WPBs located in the perinuclear region is inhibited by the presence of a more prominent peripheral actin rim. This process is accompanied by an increase in barrier function. (B) Upon activation of GqPCR in ECs by agonists such as histamine or thrombin, there is an increase in [Ca2+]i, which associates with CaM. The Ca2+-CaM further binds to the amino terminus of Ral GDS, which leads to the dissociation from the inhibitory beta-arrestin and to activation of RalA. This whole process allows WPB migration to the surface and concurrently decreases the strength of the barrier function between ECs. (C) RalA promotes fusion of the membrane by increasing PLD activity. Rab27a helps to determine when exocytosis will occur via its ratio of fractional occupancy by MyRIP and Slp4a. (D) The V-SNARE VAMP3 and the t-SNAREs syntaxin4 and SNAP23 interact to pull the two membranes in close proximity for fusion to occur. Munc18 acts to inhibit the SNAREs from binding prematurely. (E) vWF is released into the lumen, where it can bind to and attract platelets in addition to exerting effects on neighboring ECs. (F) NSF/α-SNAP bind to SNAREs to facilitate their disassembly.
1. von Willebrand Factor.
von Willebrand factor is a 2050-amino acid-long protein synthesized as a prepro–vWF precursor protein in the ER and transported to the Golgi, where pro-vWF assembles in ultra-large multimers (Sadler, 1998). The vWF multimers are stored in WPBs, which act to transport and release vWF into the lumen of blood vessels. Once released, vWF multimers anchor themselves to the ECs where they are proteolyzed by ADAMTS13 (Dong et al., 2002). The vWF allows platelets to adhere to ECs and can travel via blood to sites of blood vessel injury and attach to exposed collagen fibers. These functions ascribe vWF an important role in the responses of ECs, which cause coagulation, form thrombi, and repair damaged tissue (for a detailed review on vWF, see Lenting et al., 2012; De Ceunynck et al., 2013).
2. Synthesis of Weibel-Palade Bodies.
The presence of vWF drives the synthesis of WPBs, as shown by the reduced number of WPBs in animal models lacking vWF and in humans with von Willebrand disease, a condition where the protein is mutated or not produced (Denis et al., 2001; Haberichter et al., 2005). In parallel, a study in dogs with von Willebrand disease showed that formation of WPBs was restored upon administration of vWF (Haberichter et al., 2005). Although the WPB is an EC-specific structure in vivo, the presence of vWF is enough to induce the formation of cigar-shaped organelles similar to WPBs in other cell types in vitro, such as monkey kidney CV-1 cells (Wagner et al., 1991; Voorberg et al., 1993).
The WPBs originate from clathrin and clathrin adaptor-protein 1-coated vesicles, filled with vWF multimers, that bud from the trans-Golgi network [TGN (Lui-Roberts et al., 2005; Zenner et al., 2007)]. The WPBs lose these clathrin/AP-1 protein coats as they mature (Lui-Roberts et al., 2005; Zenner et al., 2007), and the vWF contained within them becomes highly multimeric so it is able to efficiently anchor platelets once secreted into the lumen (Wagner and Marder, 1984).
The physiologic importance of proper WPB synthesis can be observed in the various disease states associated with release of immature, altered, or higher levels of vWF. In humans, several genetic mutations have been shown to affect the biosynthesis of vWF by altering its multimerization in the Golgi and its tubular packing in the WPB. In addition, the recruitment of other proteins stored within the vesicles is altered in these genetic disorders (for review, see Valentijn et al., 2011).
3. Direct Protein-Protein Interactions of the Weibel-Palade Bodies.
After the WPB leaves the TGN, it moves to the outer regions of the cell. As the WPBs mature, they recruit proteins important in the exocytosis process to the membranes. This includes small monomeric G proteins of the Rab family (members of the Ras superfamily of proteins) and vesicle soluble N-ethylmaleimide sensitive factor (NSF) protein receptor proteins (v-SNAREs). These components in conjunction with additional proteins help the vesicles attach to the membrane.
The Rabs are a family of over 60 members of small GTPases that help to control membrane identity and actions of intracellular vesicles; each secretory vesicle of the cell hosts a unique set of Rab family members (for review, see Stenmark, 2009). Rab proteins found in association with the WPBs are Rab3b (Bierings et al., 2012) Rab3d (Knop et al., 2004), Rab27a (Hannah et al., 2003), Rab3a, Rab15, Rab33a, and Rab37 (Zografou et al., 2012). Rab27a can either inhibit the secretion of WPBs by associating with its effector MyRIP (myosin VIIA and Rab interacting protein), thus anchoring WPBs to actin filaments and keeping them from attaching to the plasma membrane (Nightingale et al., 2009) or activate the secretion of WPBs by associating with an alternate effector, Slp4-a (synaptotagmin-like protein 4), which links the secretory granules to the plasma membrane (Gomi et al., 2005). Rab27a is central to the process of vesicle secretion, because the ratio of fractional occupancy of Rab27a by Slp4-a and MyRIP plays a role in determining the balance for or against exocytosis (Bierings et al., 2012). Furthermore, Rab27a may work in conjunction with Rab15 to help control exocytosis; Zografou et al. (2012) recently showed that simultaneous knockdown of the two Rabs using siRNA in HUVECs leads to a greater reduction in vWF secretion compared with knockdown of either Rab individually. Their experiments further showed that Munc13-4, an already known effector of Rab27a, colocalized with Rab15 at WPBs. Taken together, this information suggests that the three proteins Rab27a, Rab15, and Munc13-4 likely work in tandem to help regulate exocytosis of vWF (Zografou et al., 2012).
RalA, a small GTP-binding protein, is another central actor in the process of exocytosis that has been shown to cosediment with WPBs in density gradients (de Leeuw et al., 1999). RalA helps to promote exocytosis in two ways: 1) it promotes fusion of the membranes by increasing phospholipase D1 activity (Vitale et al., 2005) and 2) it interacts with components of the exocyst (Moskalenko et al., 2002). The exocyst is an octameric protein complex consisting of Sec and Exo protein subunits (Hsu et al., 1996; TerBush et al., 1996) identified as having a role in vesicle tethering and fusion in nonendothelial cells (Grindstaff et al., 1998; Bao et al., 2008; Grote et al., 2000). However, to our knowledge, this complex has not been well characterized in ECs. In neutrophil, RalA is activated by its exchange factor, Ral guanine-nucleotide dissociation stimulator (RalGDS), which acts to promote the loss of the bound GDP and the uptake of GTP. This small protein is kept inactive by its attachment to β-arrestin, which uncouples from RalGDS upon stimulation of a GPCR, leaving the exchange factor in an active state (Bhattacharya et al., 2002) (Fig. 9). Downregulation of RalGDS expression using siRNA in HUVECs leads to a significant increase in the number of WPBs remaining in the cell after thrombin and noradrenaline stimulation, showing the importance of the exchange factor in the exocytosis process (Rondaij et al., 2008). Therefore, the RalGDS/β-arrestin complex is an important component of the signaling microdomain because of its role in linking GPCR activation with WPB exocytosis.
SNAREs are a family of transmembrane proteins that fuse to both vesicle (v-SNARE) and target (t-SNARE) membranes; they are defined by an extended coiled-coil stretch known as the “SNARE motif” (Jahn and Fasshauer, 2012). The three SNAREs that are known to target WPBs to the endothelial cell membrane are syntaxin 4 (t-SNARE) (Matsushita et al., 2003; Fu et al., 2005), SNAP-23 (t-SNARE), and VAMP3 (v-SNARE) (Pulido et al., 2011). When the vesicle is shuttled to the target membrane with the help of myosin and actin, it is aligned in such a way that the SNAREs from both membranes assemble in a α-helix to the forming bundle, and these helices “zipper” pull the membranes together (Fig. 9) (Sutton et al., 1998; Stein et al., 2009). This zippering model has been more fully characterized at neuronal synapses, but the players are analogous to those in the endothelium. Other proteins within the signaling microdomain potentially help to hold the SNAREs in a stabilized state before the zippering interaction, which facilitates exocytosis at a rate much faster than kinetics of the zippering would allow from a nonprimed state (Pobbati et al., 2006). Although this stabilized complex has not been clearly shown in ECs, complexes of SNAP23, syntaxin4, and VAMP3 have been coimmunoprecipitated, showing that at least these SNAREs interact to promote exocytosis (Pulido et al., 2011).
Munc18 is one such factor thought to interact with SNARE proteins to render the zippering model more efficient and decrease the time required for fusion. An inhibitory role has been ascribed to Munc18, because its binding to syntaxin isoforms keeps the SNARE in a closed conformation (Dulubova et al., 1999; Misura et al., 2000), but its function remains somewhat elusive. It has been suggested that a modification of Munc18, such as phosphorylation by PKC, is necessary before WBP exocytosis can occur (Fu et al., 2005). This suggests that Munc18 may play a role beyond pure inhibition and instead contribute to the stability of the SNARE complex before exocytosis.
Disassembly of the SNARE complex is required before the vesicle can be released from its membrane attachment. N-Ethylmaleimide sensitive factor (NSF) is an ATPase that binds to SNARE complexes to facilitate the disassembly of the “zippered” bundles (Zhao et al., 2012). Because NSF lacks a direct binding domain for members of the SNARE family, it connects to the complex via an effector, the α-soluble NSF attachment protein (α-SNAP) (Clary et al., 1990). Six NSF assemble together at the plasma membrane, and each NSF hexamer requires three α-SNAPs to mediate binding to the SNAREs (Wimmer et al., 2001). Once the NSF/αSNARE complex is formed, the NSF hydrolyzes ATP, providing the energy necessary for the disassembly of the SNARE complex (Whiteheart et al., 1994). Interestingly, α-SNAP may do more than just mediate the disassembly of SNAREs, because Gα proteins were recently shown to work in conjunction with α-SNAP to aid in the exocytosis process. Indeed, Rusu et al. (2013) provided strong evidence through the use of HUVECs and knockout animal models that Gα12 subunits interact with α-SNAP to promote the docking and fusion of WPBs. Gα12 binding sites for α-SNAP were mutated and evaluated for promotion of exocytosis by testing for a direct interaction between the two (Rusu et al., 2013). They further showed that Gα12 and Gαq subunits may regulate actin polymerization via activation of RhoA (a Ras-related GTPase), which leads to WPB docking, providing a direct link between GPCR activation and SNARE complex formation (Rusu et al., 2013).
4. Importance of Cellular Structure.
Cytoskeletal elements are important to the exocytotic process because they facilitate the transport of WPBs from the TGN to more peripheral locations and eventually to the plasma membrane (Manneville et al., 2003). The cytoskeleton is also integral to the idea of the exocytotic machinery being organized into a signaling microdomain, because it serves as the scaffolding, which keeps the exocytosis complex confined to a specific area of the cell. Microtubules are polarized within the ECs, with the “minus” ends oriented perinuclearly and the “plus” ends extending toward the cell periphery; these microtubules help to move the WPBs over long distances (Nightingale et al., 2009). Motor proteins of the kinesin family help facilitate movement in the plus-end direction, and dynein motors facilitate movement in the minus-end direction (Rondaij et al., 2006a). Actin combines with cross-linking proteins and with myosin II motors to form stress fibers (Burridge, 1981). Stress fiber components rearrange to either cause cell retraction or a strengthening of the cortical actin rim in response to different mediating pathways of exocytosis, providing structural changes that affect WPB movement and interaction with the membrane (Vischer et al., 2000).
The role of actin in exocytosis of WPBs has been given much attention. Many studies concluded that the actin filaments function as a physical barrier to prevent secretion (Doreian et al., 2008; Berberian et al., 2009; Bittins et al., 2009; Deng et al., 2009), whereas others proposed a different, although not mutually exclusive, idea that the stress fibers interact directly with the WPBs to anchor them in the cell until their complete maturity is reached (Desnos et al., 2003; Waselle et al., 2003; Hume et al., 2007; Nightingale et al., 2009). Recently, Nightingale et al. (2011) proposed a new function for actin in WPB exocytosis: after fusion of the WPB with the membrane, actin filaments and myosin II are recruited to form a ring around the base of the WPB. They believe that this ring acts to exert force at the base of the open granule, pushing vWF out into the lumen (Nightingale et al., 2011). The same group further demonstrated that myosin Va (MyoVa), an actin dependent motor, is capable of binding MyRIP, which, as described in section IV.A.3, works in unison with Rab27a to anchor WPBs in the periphery until they are fully matured (Fukuda and Kuroda, 2002; Desnos et al., 2003; Nightingale et al., 2009). In their study, Rojo Pulido et al. (2011) showed that knockdown of MyoVa in HUVECs using siRNA leads to a significant increase in the amount of immature vWF released after histamine stimulation (Rojo Pulido et al., 2011). They further demonstrated that Rab27a, MyRIP, and MyoVa are colocalized and coprecipitate in HUVECs (Rojo Pulido et al., 2011). This indicates that MyoVa is responsible for holding WPBs at the membrane until they are fully matured and ready for exocytosis (Rojo Pulido et al., 2011), at which point the Rab27a switches from MyRIP to the exocytosis activator Slp4-a, as detailed in section IV.A.3.
The lipid composition of the plasma membrane appears to be crucial in the exocytotic process, as illustrated by the specific changes in the phospholipid composition of the plasma membrane that occur before exocytosis takes place in ECs. For example, there is an increase in phosphatidic acid (PA) in HUVECs upon histamine-induced exocytosis of WPBs (Disse et al., 2009). The increased in PA observed was caused by recruitment to the membrane and activation of phospholipase D1 (PLD1), an enzyme that hydrolyzes phosphatidylcholine to produce PA (Disse et al., 2009). The central role of this enzyme in WPB exocytosis was further evidence using shRNA to reduce the expression of PLD1, which resulted in a reduced secretion of vWF upon histamine stimulation (Disse et al., 2009). PLD1 is commonly thought as a general promoter of membrane fusion because of its role in producing fusogenic cone-shaped lipids such as PA (Roth, 2008). Depending on cell type and activation pathway, PLD1 requires activation by one or more factors, including small GTPases such as those of the ADP-ribosylation factor, Rho families as well as RalA, RalGDS, or protein kinase C (for review, see Jenkins and Frohman, 2005). Thus, RalA and RalGDS not only play a role in the exocytosis process itself, as discussed in section IV.A.4, but are additionally associated with WPBs and help to increase PLD1 activity (Rondaij et al., 2008). Taken together, this information suggests that RalA could serve as an upstream activator of PLD1, causing its movement to the membrane and the subsequent generation of PA-enriched domains, which could aid in the membrane fusion process.
In addition to PA, annexin-A2 has been found to promote the formation of microdomains with increased cholesterol content, which correlates with sites of exocytosis in chromaffin cells (Chasserot-Golaz et al., 2005). Likewise, expression of annexin-A2 has been shown via immunofluorescence in the cytoplasm and at the plasma membrane in HUVECs (Knop et al., 2004). Functionally, when HUVECs were depleted of the annexin-A2 along with its main protein partner S100A10, secretion of vWF was decreased (Knop et al., 2004). This suggests that annexin-A2 is necessary for EC exocytosis, possibly because of its role in formation of cholesterol-rich microdomains as demonstrated in other cell types.
B. Activation Pathways for Exocytosis
Exocytosis of vWF from ECs can be triggered by many events such as hypoxia, ischemia, or acute injury. Exocytosis in response to a biologic stimulus is often induced via activation of GPCR through the binding of ligands such as histamine (Hamilton and Sims, 1987), thrombin (Levine et al., 1982), serotonin (Schluter and Bohnensack, 1999), epinephrine (Vischer and Wollheim, 1997), and vasopressin (Kaufmann et al., 2000). The components of the GPCRs are of particular importance, because they bind to downstream signaling effectors such as heterotrimeric G protein complexes, kinases, and arrestins (Rasmussen et al., 2011; Katritch et al., 2012). Depending on the nature of the Gα protein coupled to a GPCR, exocytosis of WPBs can be activated via a calcium-dependent pathway (if the stimulated GPCR is coupled to a Gq protein) or via a cAMP-dependent pathway (if the stimulated GPCR is coupled to a Gs protein).
1. Calcium-Dependent Activation of Exocytosis.
Calcium’s ability to stimulate exocytosis has been demonstrated experimentally using Ca2+ ionophores, which were sufficient to promote the release of vWF (Loesberg et al., 1983; van den Eijnden-Schrauwen et al., 1997). In parallel, experiments in HUVECs using the caged Ca2+ chelator DM-nitrophen showed that the cell capacitance increases upon elevation of intracellular calcium via photolysis, which is indicative of exocytosis (Zupancic et al., 2002). Calmodulin inhibitors and intracellular calcium chelators are also able to decrease thrombin-induced exocytosis of vWF in HUVECs (Birch et al., 1992; van den Eijnden-Schrauwen et al., 1997). The blocking effect of calmodulin inhibitors was explained recently using a peptide that mimics the calmodulin-binding domain present in the N terminus of RalGDS, showing that RalGDS association to calmodulin is crucial for RalA activation (Rondaij et al., 2008). When HUVECs are treated with the mimetic peptide, RalA activation is inhibited along with WPB exocytosis initiated by thrombin (Rondaij et al., 2008). Thus, it appears that calcium indirectly activates RalGDS by binding to calmodulin, further activating RalA and WPB exocytosis.
Thrombin and histamine stimulation, angiotensin II (Ge et al., 2007), sphingosine-1-phosphate (S1P) (Matsushita et al., 2004), and ATP/ADP (Vischer and Wollheim, 1998) all cause increases in [Ca2+]i via GPCR activation, which is a prime potentiator of exocytosis (Tse et al., 1997), but there is also evidence for extracellular calcium involvement. For example, influx of extracellular calcium via the T-type calcium channels Cav3.1 occurs upon stimulation of pulmonary microvascular ECs with thrombin, resulting in a procoagulant endothelial phenotype (Wu et al., 2003). In a more recent work, the same group linked exocytosis to external calcium influx via Cav3.1 using the Cav3.1 channels blocker mifbefradil and by direct targeting with shRNA, which both inhibit thrombin-induced vWF secretion in pulmonary microvascular ECs (Zhou et al., 2007a). In this study, the authors also showed that there is differential regulation of exocytosis between microvessels and conduit arteries in the pulmonary circulation, with exocytosis being entirely calcium dependent in smaller vessels, whereas exocytosis is regulated by two distinct pathways involving calcium or cAMP in larger vessels (Zhou et al., 2007a). This flexibility is thought to be a result of different populations of WPBs and might allow for a faster or slower response to inflammation (Zhou et al., 2007a). Further evidence for the relevance of T-type calcium channels in mediating exocytosis is that the mRNA of Cav3.1 in EC is increased upon Ang II stimulation (Wang et al., 2006).
The sphingolipid S1P is similar to thrombin and histamine in that it can potentiate inflammation in the vasculature via exocytosis of vWF and angiopoietin 2 from WPBs (Matsushita et al., 2004; Jang et al., 2009). Matsushita et al. (2004) showed that S1P-mediated exocytosis of WPBs occurs in part via PLCγ but also involves extracellular calcium, because both PLC inhibitors and calcium-free media blocked S1P-induced secretion of vWF from human aortic ECs. Given that the receptor was pertussis toxin sensitive, the authors concluded that the S1P must act via a Gi-linked receptor (Matsushita et al., 2004).
2. Cyclic AMP Activation of Exocytosis.
The regulation of vWF secretion by cAMP was first evidenced in HUVECs in the late 1990s by two different groups (Vischer and Wollheim, 1997; Hegeman et al., 1998). In both studies, the authors were able to measure an increase in cAMP concomitant with vWF secretion after stimulation with epinephrine and the adenylate cyclase activator forskolin (Vischer and Wollheim, 1997; Hegeman et al., 1998). In parallel, pharmacological blockers of the cAMP pathway completely abolished vWF secretion (Vischer and Wollheim, 1997; Hegeman et al., 1998). Other agonists are now known to activate WPBs exocytosis in a cAMP-dependent manner, including serotonin (Schluter and Bohnensack, 1999) and vasopressin (Kaufmann et al., 2000), all of which induce vWF secretion independently of a rise in [Ca2+]i. The exocytosis of WPBs caused by an increase in cAMP is partly due to a PKA-dependent signaling pathway, which leads to the activation of RalA (de Leeuw et al., 1999; Rondaij et al., 2004, 2008). Exocytosis can also be activated independently of PKA activity through a cAMP-induced activation of Rap1 (Ras-related protein 1) mediated by the guanine nucleotide exchange factor Epac (van Hooren et al., 2012). It is noteworthy that activation of both Rap1 and RalA pathways may be coordinated, allowing both pathways to contribute to the exocytotic process in a synchronous fashion (van Hooren et al., 2012).
3. Differential Activation of Weibel-Palade Bodies Exocytosis by Calcium and cAMP.
Although both calcium- and cAMP-mediated pathways lead to release of vWF from WPBs in ECs, there are subtle differences that may have important consequences. The two pathways lead to different rearrangements of cytoskeletal elements as demonstrated by Vischer et al. (2000) in HUVECs, where stress fibers are rearranged differently upon activation of cAMP or calcium pathways. Namely, histamine and thrombin, which both activate calcium-mediated exocytosis, induced the formation of more prominent stress fibers that aligned in a parallel, longitudinal fashion, whereas the myosin II was redistributed to underlying stress fibers (Vischer et al., 2000). However, when cAMP was increased via forskolin or IBMX, there was a rapid disappearance of stress fibers, and the peripheral actin rim became more prominent at the basis of WPBs (Vischer et al., 2000). The secretion of vWF in response to Ca2+-elevating agonists was inhibited in HUVECs treated with the microtubule disrupters colchicine or nocodazole but was potentiated when treated with the actin disrupter cytochalasin E (Vischer et al., 2000). However, the secretion of vWF in response to cAMP-elevating agonists is not affected by either treatment (Vischer et al., 2000; Manneville et al., 2003; Nightingale et al., 2009;).
Interestingly, calcium- and cAMP-dependent pathways lead to differential WPB exocytosis. Specifically, vWF depletion of almost all WPBs is observed when an increase in Ca2+ occurs, whereas an increase in cAMP leads to a clustering of WPB in the perinuclear space with only the most peripherally located vesicles releasing their contents (Cleator et al., 2006). This perinuclear clustering of WPBs seen with increased cAMP is due to movements facilitated by the dynein-dynactin complex (Rondaij et al., 2006a).
These different responses also lead to opposite consequences on the barrier function of endothelial cells: increased Ca2+ leads to the disassembly of tight and adherens junctions via RhoA activation (van Nieuw Amerongen et al., 2000; Wojciak-Stothard et al., 2001), whereas activation of Rap1 through a cAMP-dependent pathway leads to improved barrier function due to promotion of cell-cell contacts mediated by VE-cadherin (Cullere et al., 2005; Fukuhara et al., 2005; Kooistra et al., 2005). The differing responses in cytoskeletal rearrangement and barrier function could play a role in determining the effects of the exocytotic process in response to different stimuli. Although the machinery involved in getting the content of WPBs to the lumen is similar, cAMP-mediated WPB release is slower (>10 minutes), whereas calcium-dependent WPB release is rapid (<5 minutes) (Vischer et al., 2000; Rondaij et al., 2004). Taken together, these differences provide mechanisms by which ECs can use the same process of exocytosis to bring about proper physiologic change in response to varying triggers.
Although there are many molecular players and different modes of activation in this complex choreography, and although the details of the entire pathway are still elusive, it is known that the components work closely together to form a macromolecular complex to ensure proper coordination of the exocytosis pathway. From the activation of a GPCR to the fusing of the vesicle at the membrane, this process is tightly regulated by a dependence on several protein-protein interactions and polarization of the individual components to a specific cellular location, making the multiple proteins involved in the exocytotic process part of an important signaling microdomain involved with cellular communication.
V. Conclusion
In the vascular wall, cells have to act in a highly coordinated manner to ensure proper function. To do this, cellular communication must be present and highly regulated. Behind this tight regulation lies a highly coordinated intracellular machinery organized as signaling microdomains where the second messenger calcium is of utter importance. In this review, we described and defined examples of intracellular proteins that act in an orchestrated manner to ensure proper intercellular communication.
The signaling microdomains described in this review are the results of considerable work using pharmacological and molecular tools coupled, in a majority of cases, to high-resolution microscopy. However, in some instances, it was difficult for us to evaluate whether two or more proteins are part of the same signaling microdomain. The main reason for this was the lack of correlation between observed functional data coupled to detection of the proximity of the different proteins involved. Recently, several technical advances have been made in the field, allowing scientists to observe the function of a single protein. For example, the scanning ion conductance microscopy (Lab et al., 2013), measurements of elementary calcium events (Sonkusare et al., 2012), or evaluation of the proximity of proteins directly in tissues (Gullberg et al., 2003) considerably enhanced our understanding of signaling microdomains. The expansion of these, as well as several other unique molecular techniques, will certainly open even more possibilities in the future to understand how cellular interactions regulate their ability to efficiently communicate.
Acknowledgments
The authors sincerely apologize to those who were not cited in this review. Please be assured that this was not attributed to any other reason than the need to condense the vastness of the field or the adherence to the rubrics laid out in Table 1.
Abbreviations
- 4αPDD
4-α-phorbol-12,13-didecanoate
- ADAMTS13
A Disintegrin And Metalloproteinase with Thrombospondin type1 Motifs, member 13
- Ang II
angiotensin II
- α-SNAP
α-soluble NSF attachment protein
- bEnd
immortalized mouse brain endothelial cell line
- BH4
tetrahydrobiopterin
- BKCa
calcium-dependent large conductance potassium channels
- Ca2+
calcium
- [Ca2+]I
intracellular calcium concentration
- [Ca2+]ER
calcium concentration in the endoplasmic reticulum
- [Ca2+]mi
mitochondrial calcium concentration
- CaM
calmodulin
- CaR
calcium-sensitive receptor
- CAT1
cationic amino acid transporter 1
- Cav1
caveolin-1
- CICR
calcium-induced calcium release
- CIRB
calmodulin and inositol 1,4,5-phosphate receptor binding domain
- Cl−
chloride ion
- CO
carbon monoxide
- Cx
connexin
- CytB5R3
cytochrome B5 reductase 3
- DHHC
aspartate-histidine-histidine-cystine motif
- EC
endothelial cells
- EDH
endothelium-dependent hyperpolarization
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxant factor
- eNOS
endothelial nitric-oxide synthase
- ER
endoplasmic reticulum
- ERα
estrogen receptor α
- GPCR
G protein-coupled receptor
- GSNOR
S-nitrosoglutathione reductase
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- Hbα
hemoglobin α
- HDL
high-density lipoprotein
- HUVEC
human umbilical vein endothelial cells
- ID4
intracellular domain 4
- IKCa
calcium-dependent intermediate conductance potassium channels
- IP3
inositol 1,4,5-phosphate
- IP3R
IP3 receptors
- K+
potassium
- KO
knockout
- l-NAME
Nω-nitro-l-arginine methyl ester
- MAPK
mitogen-activated protein kinase
- MβCD
methyl β-cyclodextrin
- MCU
mitochondrial calcium uniporter
- MEJ
myoendothelial junction
- MyRIP
myosin VIIA and Rab-interacting protein
- NCX
sodium/calcium exchanger
- nNOS
neuronal nitric-oxide synthase
- NO
nitric oxide
- Nox
NADPH oxidase
- NSF
N-ethylmaleimide sensitive factor
- O2•−
superoxide anion
- ONOO
peroxinitrite
- P450
cytochrome P450
- PA
phosphatidic acid
- Panx
pannexin
- PASMC
pulmonary arterial smooth muscle cells
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PLD1
phospholipase D1
- PM
plasma membrane
- PMCA
plasma membrane calcium ATPase
- Rab
Ras-related protein
- RalGDS
Ral guanine-nucleotide dissociation stimulator
- RyR
ryanodine receptor
- S1P
sphingosine-1-phosphate
- SERCA
sarco/endoplasmic reticulum calcium ATPase
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- SKCa
calcium-dependent small conductance potassium channels
- Slp4-a
synaptotagmin-like protein 4
- SMC
smooth muscle cell
- SNARE
soluble NSF attachment protein receptor
- SOD
superoxide dismutase
- STIM
stromal interaction molecule
- SR
sarcoplasmic reticulum
- SR-B1
scavenger receptor type B1
- TGN
trans-Golgi network
- TRAM 34
1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
- TRAM 39
2-(2-chlorophenyl)-2,2-diphenylacetonitrile
- TRP
transient receptor potential channels
- U-46619
(5Z)-7-[(1R,4S,5S,6R)-6-[(1E,3S)-3-hydroxy-1-octenyl]-2-oxabicyclo[2.2.1]hept-5-yl]-5-heptenoic acid
- VGCC
voltage-gated calcium channels
- VSMC
vascular smooth muscle cells
- vWF
von Willebrand factor
- WPB
Weibel-Palade bodies
- ZO-1
zonula occludens-1
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Billaud, Lohman, Johnstone, Biwer, Mutchler, Isakson.
Footnotes
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL088554 (to B.E.I.) and HL107963 (to B.E.I.)]; and an American Heart Association predoctoral fellowship (to A.W.L.).
References
- Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. (1991) Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353:668–670 [DOI] [PubMed] [Google Scholar]
- Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. (2007) Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SK(Ca) channels and caveolin-rich domains. Br J Pharmacol 151:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Soud HM, Stuehr DJ. (1993) Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci USA 90:10769–10772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeagbo AS, Triggle CR. (1993) Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J Cardiovasc Pharmacol 21:423–429 [PubMed] [Google Scholar]
- Adebiyi A, Narayanan D, Jaggar JH. (2011) Caveolin-1 assembles type 1 inositol 1,4,5-trisphosphate receptors and canonical transient receptor potential 3 channels into a functional signaling complex in arterial smooth muscle cells. J Biol Chem 286:4341–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebiyi A, Thomas-Gatewood CM, Leo MD, Kidd MW, Neeb ZP, Jaggar JH. (2012) An elevation in physical coupling of type 1 inositol 1,4,5-trisphosphate (IP3) receptors to transient receptor potential 3 (TRPC3) channels constricts mesenteric arteries in genetic hypertension. Hypertension 60:1213–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebiyi A, Zhao G, Narayanan D, Thomas-Gatewood CM, Bannister JP, Jaggar JH. (2010) Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ Res 106:1603–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoyev V, Takemoto DJ. (2007) ZO-1 is required for protein kinase C gamma-driven disassembly of connexin 43. Cell Signal 19:958–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Ghouleh I, Frazziano G, Rodriguez AI, Csányi G, Maniar S, St Croix CM, Kelley EE, Egaña LA, Song GJ, Bisello A, et al. (2013) Aquaporin 1, Nox1, and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc Res 97:134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfinogenova Y, Brett SE, Walsh MP, Harraz OF, Welsh DG. (2011) Do TRPC-like currents and G protein-coupled receptors interact to facilitate myogenic tone development? Am J Physiol Heart Circ Physiol 301:H1378–H1388 [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Marbán E, O’Rourke B. (2003) Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278:44735–44744 [DOI] [PubMed] [Google Scholar]
- Aperia A. (2007) New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J Intern Med 261:44–52 [DOI] [PubMed] [Google Scholar]
- Archer S. (1993) Measurement of nitric oxide in biological models. FASEB J 7:349–360 [DOI] [PubMed] [Google Scholar]
- Ardanaz N, Pagano PJ. (2006) Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 231:237–251 [DOI] [PubMed] [Google Scholar]
- Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. (2001) Mitochondria recycle Ca(2+) to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem 276:29430–29439 [DOI] [PubMed] [Google Scholar]
- Arora AI, Johar K, Gajjar DU, Ganatra DA, Kayastha FB, Pal AK, Patel AR, Rajkumar S, Vasavada AR. (2012) Cx43, ZO-1, alpha-catenin and beta-catenin in cataractous lens epithelial cells. J Biosci 37:979–987 [DOI] [PubMed] [Google Scholar]
- Aubart FC, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P, Lechat P, Lompre AM, Hulot JS. (2009) RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther 17:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad WA, Locke D, Koreen IV, Harris AL. (2006) Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. J Biol Chem 281:16727–16739 [DOI] [PubMed] [Google Scholar]
- Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. (2012) Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA 109:18174–18179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagher P, Segal SS. (2011) Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 202:271–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Altenberg GA, Reuss L. (2004a) Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am J Physiol Cell Physiol 286:C647–C654 [DOI] [PubMed] [Google Scholar]
- Bao X, Reuss L, Altenberg GA. (2004b) Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of serine 368. J Biol Chem 279:20058–20066 [DOI] [PubMed] [Google Scholar]
- Bao Y, Lopez JA, James DE, Hunziker W. (2008) Snapin interacts with the Exo70 subunit of the exocyst and modulates GLUT4 trafficking. J Biol Chem 283:324–331 [DOI] [PubMed] [Google Scholar]
- Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H, Schwaninger M. (2011) Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci USA 108:20772–20777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA. (2009) Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol 297:C1103–C1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahm DL, Hall JE. (2002) Hemichannel and junctional properties of connexin 50. Biophys J 82:2016–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden SE, Beard RS, Jr, Pfau JC. (2010) Extracellular transsulfuration generates hydrogen sulfide from homocysteine and protects endothelium from redox stress. Am J Physiol Heart Circ Physiol 299:H1568–H1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beblo DA, Veenstra RD. (1997) Monovalent cation permeation through the connexin40 gap junction channel. Cs, Rb, K, Na, Li, TEA, TMA, TBA, and effects of anions Br, Cl, F, acetate, aspartate, glutamate, and NO3. J Gen Physiol 109:509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 [DOI] [PubMed] [Google Scholar]
- Bedner P, Niessen H, Odermatt B, Kretz M, Willecke K, Harz H. (2006) Selective permeability of different connexin channels to the second messenger cyclic AMP. J Biol Chem 281:6673–6681 [DOI] [PubMed] [Google Scholar]
- Behringer EJ, Socha MJ, Polo-Parada L, Segal SS. (2012) Electrical conduction along endothelial cell tubes from mouse feed arteries: confounding actions of glycyrrhetinic acid derivatives. Br J Pharmacol 166:774–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Bolton TB. (1986) Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol 381:385–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bény JL, Schaad O. (2000) An evaluation of potassium ions as endothelium-derived hyperpolarizing factor in porcine coronary arteries. Br J Pharmacol 131:965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bény JL, von der Weid PY. (1991) Hydrogen peroxide: an endogenous smooth muscle cell hyperpolarizing factor. Biochem Biophys Res Commun 176:378–384 [DOI] [PubMed] [Google Scholar]
- Berberian K, Torres AJ, Fang Q, Kisler K, Lindau M. (2009) F-actin and myosin II accelerate catecholamine release from chromaffin granules. J Neurosci 29:863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RS, Martin PE, Evans WH, Griffith TM. (2002) Relative contributions of NO and gap junctional communication to endothelium-dependent relaxations of rabbit resistance arteries vary with vessel size. Microvasc Res 63:115–128 [DOI] [PubMed] [Google Scholar]
- Bernatchez P, Sharma A, Bauer PM, Marin E, Sessa WC. (2011) A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. J Clin Invest 121:3747–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatchez PN, Bauer PM, Yu J, Prendergast JS, He P, Sessa WC. (2005) Dissecting the molecular control of endothelial NO synthase by caveolin-1 using cell-permeable peptides. Proc Natl Acad Sci USA 102:761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier SG, Haldar S, Michel T. (2000) Bradykinin-regulated interactions of the mitogen-activated protein kinase pathway with the endothelial nitric-oxide synthase. J Biol Chem 275:30707–30715 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. (2006) Calcium microdomains: organization and function. Cell Calcium 40:405–412 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4:517–529 [DOI] [PubMed] [Google Scholar]
- Bess E, Fisslthaler B, Frömel T, Fleming I. (2011) Nitric oxide-induced activation of the AMP-activated protein kinase α2 subunit attenuates IκB kinase activity and inflammatory responses in endothelial cells. PLoS ONE 6:e20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevans CG, Kordel M, Rhee SK, Harris AL. (1998) Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem 273:2808–2816 [DOI] [PubMed] [Google Scholar]
- Beyer EC, Gemel J, Martínez A, Berthoud VM, Valiunas V, Moreno AP, Brink PR. (2001) Heteromeric mixing of connexins: compatibility of partners and functional consequences. Cell Commun Adhes 8:199–204 [DOI] [PubMed] [Google Scholar]
- Beyer EC, Gemel J, Seul KH, Larson DM, Banach K, Brink PR. (2000) Modulation of intercellular communication by differential regulation and heteromeric mixing of co-expressed connexins. Braz J Med Biol Res 33:391–397 [DOI] [PubMed] [Google Scholar]
- Beyer EC, Lin X, Veenstra RD. (2013) Interfering amino terminal peptides and functional implications for heteromeric gap junction formation. Front Pharmacol 4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Anborgh PH, Babwah AV, Dale LB, Dobransky T, Benovic JL, Feldman RD, Verdi JM, Rylett RJ, Ferguson SS. (2002) Beta-arrestins regulate a Ral-GDS Ral effector pathway that mediates cytoskeletal reorganization. Nat Cell Biol 4:547–555 [DOI] [PubMed] [Google Scholar]
- Bian K, Doursout MF, Murad F. (2008) Vascular system: role of nitric oxide in cardiovascular diseases. J Clin Hypertens (Greenwich) 10:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierings R, Hellen N, Kiskin N, Knipe L, Fonseca AV, Patel B, Meli A, Rose M, Hannah MJ, Carter T. (2012) The interplay between the Rab27A effectors Slp4-a and MyRIP controls hormone-evoked Weibel-Palade body exocytosis. Blood 120:2757–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, et al. (2011) Pannexin1 regulates α1-adrenergic receptor-mediated vasoconstriction. Circ Res 109:80–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billaud M, Lohman AW, Straub AC, Parpaite T, Johnstone SR, Isakson BE. (2012) Characterization of the thoracodorsal artery: morphology and reactivity. Microcirculation 19:360–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch KA, Pober JS, Zavoico GB, Means AR, Ewenstein BM. (1992) Calcium/calmodulin transduces thrombin-stimulated secretion: studies in intact and minimally permeabilized human umbilical vein endothelial cells. J Cell Biol 118:1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd’heuil D, Trebak M. (2010) Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol 298:C993–C1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittins CM, Eichler TW, Gerdes HH. (2009) Expression of the dominant-negative tail of myosin Va enhances exocytosis of large dense core vesicles in neurons. Cell Mol Neurobiol 29:597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtkjer E, Kim S, Aalkjaer C. (2013) Endothelial alkalinisation inhibits gap junction communication and endothelium-derived hyperpolarisations in mouse mesenteric arteries. J Physiol 591:1447–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher M, de Wit C. (2011) Distinct endothelium-derived hyperpolarizing factors emerge in vitro and in vivo and are mediated in part via connexin 40-dependent myoendothelial coupling. Hypertension 57:802–808 [DOI] [PubMed] [Google Scholar]
- Bol M, Van Geyt C, Baert S, Decrock E, Wang N, De Bock M, Gadicherla AK, Randon C, Evans WH, Beele H, Cornelissen R, Leybaert L. (2013) Inhibiting connexin channels protects against cryopreservation-induced cell death in human blood vessels. Eur J Vasc Endovasc Surg 45:382–390 [DOI] [PubMed] [Google Scholar]
- Bolton TB. (2006) Calcium events in smooth muscles and their interstitial cells; physiological roles of sparks. J Physiol 570:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton TB, Lang RJ, Takewaki T. (1984) Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol 351:549–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz SS, de Wit C, Pohl U. (1999) Endothelium-derived hyperpolarizing factor but not NO reduces smooth muscle Ca2+ during acetylcholine-induced dilation of microvessels. Br J Pharmacol 128:124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti R, Furie BC, Furie B, Wagner DD. (1989) PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood 73:1109–1112 [PubMed] [Google Scholar]
- Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. (2002a) Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol 283:H1819–H1828 [DOI] [PubMed] [Google Scholar]
- Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. (2002b) Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277:3388–3396 [DOI] [PubMed] [Google Scholar]
- Bosco D, Haefliger JA, Meda P. (2011) Connexins: key mediators of endocrine function. Physiol Rev 91:1393–1445 [DOI] [PubMed] [Google Scholar]
- Boulanger CM, Heymes C, Benessiano J, Geske RS, Lévy BI, Vanhoutte PM. (1998) Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells: activation by angiotensin II in hypertension. Circ Res 83:1271–1278 [DOI] [PubMed] [Google Scholar]
- Bouvier D, Kieken F, Kellezi A, Sorgen PL. (2008) Structural changes in the carboxyl terminus of the gap junction protein connexin 40 caused by the interaction with c-Src and zonula occludens-1. Cell Commun Adhes 15:107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier D, Spagnol G, Chenavas S, Kieken F, Vitrac H, Brownell S, Kellezi A, Forge V, Sorgen PL. (2009) Characterization of the structure and intermolecular interactions between the connexin40 and connexin43 carboxyl-terminal and cytoplasmic loop domains. J Biol Chem 284:34257–34271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady NR, Elmore SP, van Beek JJ, Krab K, Courtoy PJ, Hue L, Westerhoff HV. (2004) Coordinated behavior of mitochondria in both space and time: a reactive oxygen species-activated wave of mitochondrial depolarization. Biophys J 87:2022–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brähler S, Kaistha A, Schmidt VJ, Wölfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, et al. (2009) Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 119:2323–2332 [DOI] [PubMed] [Google Scholar]
- Brandes RP, Schmitz-Winnenthal FH, Félétou M, Gödecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. (2000) An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci USA 97:9747–9752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein TH, Sorensen CM, Holstein-Rathlou NH. (2009) Connexin abundance in resistance vessels from the renal microcirculation in normo- and hypertensive rats. APMIS 117:268–276 [DOI] [PubMed] [Google Scholar]
- Brayden JE, Li Y, Tavares MJ. (2013) Purinergic receptors regulate myogenic tone in cerebral parenchymal arterioles. J Cereb Blood Flow Metab 33:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Snyder SH. (1990) Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347:768–770 [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. (1990) Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 87:682–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. (2000) Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407:870–876 [DOI] [PubMed] [Google Scholar]
- Brink PR, Cronin K, Banach K, Peterson E, Westphale EM, Seul KH, Ramanan SV, Beyer EC. (1997) Evidence for heteromeric gap junction channels formed from rat connexin43 and human connexin37. Am J Physiol 273:C1386–C1396 [DOI] [PubMed] [Google Scholar]
- Brink PR, Robinson RB, Rosen MR, Cohen IS. (2010) In vivo cellular delivery of siRNA. IDrugs 13:383–387 [PubMed] [Google Scholar]
- Briones AM, Padilha AS, Cogolludo AL, Alonso MJ, Vassallo DV, Pérez-Vizcaino F, Salaices M. (2009) Activation of BKCa channels by nitric oxide prevents coronary artery endothelial dysfunction in ouabain-induced hypertensive rats. J Hypertens 27:83–91 [DOI] [PubMed] [Google Scholar]
- Brisset AC, Isakson BE, Kwak BR. (2009) Connexins in vascular physiology and pathology. Antioxid Redox Signal 11:267–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy CM, Knoepp L, Xin J, Pollock JS. (2000) Functional expression of NOS 1 in vascular smooth muscle. Am J Physiol Heart Circ Physiol 278:H991–H997 [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. (2005) Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92:1033–1043 [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. (1996) Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem 238:1–27 [DOI] [PubMed] [Google Scholar]
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. (2000) In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6:1362–1367 [DOI] [PubMed] [Google Scholar]
- Bueno M, Wang J, Mora AL, Gladwin MT. (2013) Nitrite signaling in pulmonary hypertension: mechanisms of bioactivation, signaling, and therapeutics. Antioxid Redox Signal 18:1797–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Bukauskiene A, Verselis VK. (2002) Conductance and permeability of the residual state of connexin43 gap junction channels. J Gen Physiol 119:171–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Kreuzberg MM, Rackauskas M, Bukauskiene A, Bennett MV, Verselis VK, Willecke K. (2006) Properties of mouse connexin 30.2 and human connexin 31.9 hemichannels: implications for atrioventricular conduction in the heart. Proc Natl Acad Sci USA 103:9726–9731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Verselis VK. (2004) Gap junction channel gating. Biochim Biophys Acta 1662:42–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne JR, Prysyazhna O, Rudyk O, Eaton P. (2012) cGMP-dependent activation of protein kinase G precludes disulfide activation: implications for blood pressure control. Hypertension 60:1301–1308 [DOI] [PubMed] [Google Scholar]
- Burridge K. (1981) Are stress fibres contractile? Nature 294:691–692 [DOI] [PubMed] [Google Scholar]
- Busse R, Mülsch A. (1990) Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett 265:133–136 [DOI] [PubMed] [Google Scholar]
- Butkevich E, Hulsmann S, Wenzel D, Shirao T, Duden R, Majoul I. (2004) Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr Biol 14:650–658 [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459:596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloway N, Vig M, Kinet JP, Holowka D, Baird B. (2009) Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol Biol Cell 20:389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Fleming I. (2010) Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch 459:881–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Harder DR. (1999) Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res 84:484–488 [DOI] [PubMed] [Google Scholar]
- Carignani C, Roncarati R, Rimini R, Terstappen GC. (2002) Pharmacological and molecular characterisation of SK3 channels in the TE671 human medulloblastoma cell line. Brain Res 939:11–18 [DOI] [PubMed] [Google Scholar]
- Carrasco S, Meyer T. (2011) STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu Rev Biochem 80:973–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascino T, Csanyi G, Al Ghouleh I, Montezano AC, Touyz RM, Haurani MJ, Pagano PJ. (2011) Adventitia-derived hydrogen peroxide impairs relaxation of the rat carotid artery via smooth muscle cell p38 mitogen-activated protein kinase. Antioxid Redox Signal 15:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassoly R, Gibson Q. (1975) Conformation, co-operativity and ligand binding in human hemoglobin. J Mol Biol 91:301–313 [DOI] [PubMed] [Google Scholar]
- Caudill TK, Resta TC, Kanagy NL, Walker BR. (1998) Role of endothelial carbon monoxide in attenuated vasoreactivity following chronic hypoxia. Am J Physiol 275:R1025–R1030 [DOI] [PubMed] [Google Scholar]
- Chadha PS, Liu L, Rikard-Bell M, Senadheera S, Howitt L, Bertrand RL, Grayson TH, Murphy TV, Sandow SL. (2011) Endothelium-dependent vasodilation in human mesenteric artery is primarily mediated by myoendothelial gap junctions intermediate conductance calcium-activated K+ channel and nitric oxide. J Pharmacol Exp Ther 336:701–708 [DOI] [PubMed] [Google Scholar]
- Chadjichristos CE, Matter CM, Roth I, Sutter E, Pelli G, Lüscher TF, Chanson M, Kwak BR. (2006) Reduced connexin43 expression limits neointima formation after balloon distension injury in hypercholesterolemic mice. Circulation 113:2835–2843 [DOI] [PubMed] [Google Scholar]
- Chadjichristos CE, Morel S, Derouette JP, Sutter E, Roth I, Brisset AC, Bochaton-Piallat ML, Kwak BR. (2008) Targeting connexin 43 prevents platelet-derived growth factor-BB-induced phenotypic change in porcine coronary artery smooth muscle cells. Circ Res 102:653–660 [DOI] [PubMed] [Google Scholar]
- Chadjichristos CE, Scheckenbach KE, van Veen TA, Richani Sarieddine MZ, de Wit C, Yang Z, Roth I, Bacchetta M, Viswambharan H, Foglia B, et al. (2010) Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation 121:123–131 [DOI] [PubMed] [Google Scholar]
- Chalmers S, McCarron JG. (2008) The mitochondrial membrane potential and Ca2+ oscillations in smooth muscle. J Cell Sci 121:75–85 [DOI] [PubMed] [Google Scholar]
- Chalmers S, McCarron JG. (2009) Inhibition of mitochondrial calcium uptake rather than efflux impedes calcium release by inositol-1,4,5-trisphosphate-sensitive receptors. Cell Calcium 46:107–113 [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Shaul PW. (2002) Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 23:665–686 [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. (2000) Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res 87:E44–E52 [DOI] [PubMed] [Google Scholar]
- Charles IG, Palmer RM, Hickery MS, Bayliss MT, Chubb AP, Hall VS, Moss DW, Moncada S. (1993) Cloning, characterization, and expression of a cDNA encoding an inducible nitric oxide synthase from the human chondrocyte. Proc Natl Acad Sci USA 90:11419–11423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasserot-Golaz S, Vitale N, Umbrecht-Jenck E, Knight D, Gerke V, Bader MF. (2005) Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles. Mol Biol Cell 16:1108–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Bakker LM, Edwards DH, Griffith TM. (2005) Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br J Pharmacol 144:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Edwards DH, Bakker LM, Griffith TM. (2003) Distinct hyperpolarizing and relaxant roles for gap junctions and endothelium-derived H2O2 in NO-independent relaxations of rabbit arteries. Proc Natl Acad Sci USA 100:15212–15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Evans WH, Griffith TM. (1997) Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J Physiol 503:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Evans WH, Griffith TM. (1998) Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol 508:561–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Martin PE, Edwards DH, Griffith TM. (2001) Gap junctional communication underpins EDHF-type relaxations evoked by ACh in the rat hepatic artery. Am J Physiol Heart Circ Physiol 280:H2441–H2450 [DOI] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al. (2010) Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467:863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Hashitani H, Suzuki H. (1989) Endothelium-dependent relaxation and hyperpolarization of canine coronary artery smooth muscles in relation to the electrogenic Na-K pump. Br J Pharmacol 98:950–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Suzuki H. (1989) Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J Physiol 410:91–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Suzuki H, Weston AH. (1988) Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol 95:1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Yamamoto Y, Miwa K, Suzuki H. (1991) Hyperpolarization of arterial smooth muscle induced by endothelial humoral substances. Am J Physiol 260:H1888–H1892 [DOI] [PubMed] [Google Scholar]
- Chen GF, Suzuki H. (1990) Calcium dependency of the endothelium-dependent hyperpolarization in smooth muscle cells of the rabbit carotid artery. J Physiol 421:521–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. (2003) Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278:45021–45026 [DOI] [PubMed] [Google Scholar]
- Chen J, Pan L, Wei Z, Zhao Y, Zhang M. (2008) Domain-swapped dimerization of ZO-1 PDZ2 generates specific and regulatory connexin43-binding sites. EMBO J 27:2113–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PF, Tsai AL, Berka V, Wu KK. (1997) Mutation of Glu-361 in human endothelial nitric-oxide synthase selectively abolishes L-arginine binding without perturbing the behavior of heme and other redox centers. J Biol Chem 272:6114–6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VC, Gouw JW, Naus CC, Foster LJ. (2013) Connexin multi-site phosphorylation: mass spectrometry-based proteomics fills the gap. Biochim Biophys Acta 1828:23–34 [DOI] [PubMed] [Google Scholar]
- Chen Y, Medhora M, Falck JR, Pritchard KA, Jr, Jacobs ER. (2006) Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 291:L378–L385 [DOI] [PubMed] [Google Scholar]
- Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. (1999) Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. (1993) Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262:740–744 [DOI] [PubMed] [Google Scholar]
- Cheranov SY, Jaggar JH. (2004) Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries. J Physiol 556:755–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Tostes RC, Webb RC. (2011) S-nitrosylation Inhibits protein kinase C-mediated contraction in mouse aorta. J Cardiovasc Pharmacol 57:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ GJ, Moreno AP, Melman A, Spray DC. (1992) Gap junction-mediated intercellular diffusion of Ca2+ in cultured human corporal smooth muscle cells. Am J Physiol 263:C373–C383 [DOI] [PubMed] [Google Scholar]
- Chu Y, Iida S, Lund DD, Weiss RM, DiBona GF, Watanabe Y, Faraci FM, Heistad DD. (2003) Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circ Res 92:461–468 [DOI] [PubMed] [Google Scholar]
- Clark JH, Kinnear NP, Kalujnaia S, Cramb G, Fleischer S, Jeyakumar LH, Wuytack F, Evans AM. (2010) Identification of functionally segregated sarcoplasmic reticulum calcium stores in pulmonary arterial smooth muscle. J Biol Chem 285:13542–13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary DO, Griff IC, Rothman JE. (1990) SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell 61:709–721 [DOI] [PubMed] [Google Scholar]
- Cleator JH, Zhu WQ, Vaughan DE, Hamm HE. (2006) Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood 107:2736–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sáez JC, Bukauskas FF, Bennett MV. (2003) Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA 100:11388–11393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriu C, Félétou M, Canet E, Vanhoutte PM. (1996) Endothelium-derived factors and hyperpolarization of the carotid artery of the guinea-pig. Br J Pharmacol 119:959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GT, Burt JM. (2001) Heterotypic gap junction channel formation between heteromeric and homomeric Cx40 and Cx43 connexons. Am J Physiol Cell Physiol 281:C1559–C1567 [DOI] [PubMed] [Google Scholar]
- Cowan CL, Palacino JJ, Najibi S, Cohen RA. (1993) Potassium channel-mediated relaxation to acetylcholine in rabbit arteries. J Pharmacol Exp Ther 266:1482–1489 [PubMed] [Google Scholar]
- Crane GJ, Gallagher N, Dora KA, Garland CJ. (2003) Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol 553:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GJ, Garland CJ. (2004) Thromboxane receptor stimulation associated with loss of SKCa activity and reduced EDHF responses in the rat isolated mesenteric artery. Br J Pharmacol 142:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crnich R, Amberg GC, Leo MD, Gonzales AL, Tamkun MM, Jaggar JH, Earley S. (2010) Vasoconstriction resulting from dynamic membrane trafficking of TRPM4 in vascular smooth muscle cells. Am J Physiol Cell Physiol 299:C682–C694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Thomas AP, Hajnóczky G. (1999) Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J 18:96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. (2005) Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105:1950–1955 [DOI] [PubMed] [Google Scholar]
- Dahl G. (2007) Gap junction-mimetic peptides do work, but in unexpected ways. Cell Commun Adhes 14:259–264 [DOI] [PubMed] [Google Scholar]
- Dahl G, Locovei S. (2006) Pannexin: to gap or not to gap, is that a question? IUBMB Life 58:409–419 [DOI] [PubMed] [Google Scholar]
- Dai J, Kuo KH, Leo JM, van Breemen C, Lee CH. (2005a) Rearrangement of the close contact between the mitochondria and the sarcoplasmic reticulum in airway smooth muscle. Cell Calcium 37:333–340 [DOI] [PubMed] [Google Scholar]
- Dai YP, Bongalon S, Hatton WJ, Hume JR, Yamboliev IA. (2005b) ClC-3 chloride channel is upregulated by hypertrophy and inflammation in rat and canine pulmonary artery. Br J Pharmacol 145:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkjaer M, Nielsen G, Bodendiek S, Staehr M, Gramsbergen JB, de Wit C, Jensen BL, Simonsen U, Bie P, Wulff H, et al. (2012) Pharmacological activation of KCa3.1/KCa2.3 channels produces endothelial hyperpolarization and lowers blood pressure in conscious dogs. Br J Pharmacol 165:223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sarma J, Kaplan BE, Willemsen D, Koval M. (2008) Identification of rab20 as a potential regulator of connexin 43 trafficking. Cell Commun Adhes 15:65–74 [DOI] [PubMed] [Google Scholar]
- Das Sarma J, Lo CW, Koval M. (2001) Cx43/beta-gal inhibits Cx43 transport in the Golgi apparatus. Cell Commun Adhes 8:249–252 [DOI] [PubMed] [Google Scholar]
- Davidge ST, Baker PN, Laughlin MK, Roberts JM. (1995) Nitric oxide produced by endothelial cells increases production of eicosanoids through activation of prostaglandin H synthase. Circ Res 77:274–283 [DOI] [PubMed] [Google Scholar]
- Davidson SM, Duchen MR. (2006) Calcium microdomains and oxidative stress. Cell Calcium 40:561–574 [DOI] [PubMed] [Google Scholar]
- De Ceunynck K, De Meyer SF, Vanhoorelbeke K. (2013) Unwinding the von Willebrand factor strings puzzle. Blood 121:270–277 [DOI] [PubMed] [Google Scholar]
- de Leeuw HP, Wijers-Koster PM, van Mourik JA, Voorberg J. (1999) Small GTP-binding protein RalA associates with Weibel-Palade bodies in endothelial cells. Thromb Haemost 82:1177–1181 [PubMed] [Google Scholar]
- De Mey JG, Claeys M, Vanhoutte PM. (1982) Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther 222:166–173 [PubMed] [Google Scholar]
- De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. (2006) Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J 25:34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, Leybaert L. (2007) Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell 18:34–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit C. (2010) Different pathways with distinct properties conduct dilations in the microcirculation in vivo. Cardiovasc Res 85:604–613 [DOI] [PubMed] [Google Scholar]
- de Wit C, Roos F, Bolz SS, Kirchhoff S, Krüger O, Willecke K, Pohl U. (2000) Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86:649–655 [DOI] [PubMed] [Google Scholar]
- Decrock E, Krysko DV, Vinken M, Kaczmarek A, Crispino G, Bol M, Wang N, De Bock M, De Vuyst E, Naus CC, et al. (2012) Transfer of IP₃ through gap junctions is critical, but not sufficient, for the spread of apoptosis. Cell Death Differ 19:947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defamie N, Mesnil M. (2012) The modulation of gap-junctional intercellular communication by lipid rafts. Biochim Biophys Acta 1818:1866–1869 [DOI] [PubMed] [Google Scholar]
- Déglise S, Martin D, Probst H, Saucy F, Hayoz D, Waeber G, Nicod P, Ris HB, Corpataux JM, Haefliger JA. (2005) Increased connexin43 expression in human saphenous veins in culture is associated with intimal hyperplasia. J Vasc Surg 41:1043–1052 [DOI] [PubMed] [Google Scholar]
- del Castillo FJ, Cohen-Salmon M, Charollais A, Caille D, Lampe PD, Chavrier P, Meda P, Petit C. (2010) Consortin, a trans-Golgi network cargo receptor for the plasma membrane targeting and recycling of connexins. Hum Mol Genet 19:262–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca HF, Engstrom GW. (1961) Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci USA 47:1744–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Zink T, Chen HY, Walters D, Liu FT, Liu GY. (2009) Impact of actin rearrangement and degranulation on the membrane structure of primary mast cells: a combined atomic force and laser scanning confocal microscopy investigation. Biophys J 96:1629–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis CV, André P, Saffaripour S, Wagner DD. (2001) Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci USA 98:4072–4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnos C, Schonn JS, Huet S, Tran VS, El-Amraoui A, Raposo G, Fanget I, Chapuis C, Ménasché G, de Saint Basile G, et al. (2003) Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites. J Cell Biol 163:559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deth R, van Breemen C. (1977) Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol 30:363–380 [DOI] [PubMed] [Google Scholar]
- Dewey MM, Barr L. (1964) A study of the structure and distribution of the nexus. J Cell Biol 23:553–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva F, Domi T, Fedrizzi L, Lim D, Carafoli E. (2008) The plasma membrane Ca2+ ATPase of animal cells: structure, function and regulation. Arch Biochem Biophys 476:65–74 [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605 [DOI] [PubMed] [Google Scholar]
- Dinauer MC, Orkin SH, Brown R, Jesaitis AJ, Parkos CA. (1987) The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 327:717–720 [DOI] [PubMed] [Google Scholar]
- Disse J, Vitale N, Bader MF, Gerke V. (2009) Phospholipase D1 is specifically required for regulated secretion of von Willebrand factor from endothelial cells. Blood 113:973–980 [DOI] [PubMed] [Google Scholar]
- Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, López JA. (2002) ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood 100:4033–4039 [DOI] [PubMed] [Google Scholar]
- Dora KA. (2010) Coordination of vasomotor responses by the endothelium. Circ J 74:226–232 [DOI] [PubMed] [Google Scholar]
- Dora KA, Doyle MP, Duling BR. (1997) Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci USA 94:6529–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Gallagher NT, McNeish A, Garland CJ. (2008) Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res 102:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Garland CJ. (2001) Properties of smooth muscle hyperpolarization and relaxation to K+ in the rat isolated mesenteric artery. Am J Physiol Heart Circ Physiol 280:H2424–H2429 [DOI] [PubMed] [Google Scholar]
- Dora KA, Hinton JM, Walker SD, Garland CJ. (2000) An indirect influence of phenylephrine on the release of endothelium-derived vasodilators in rat small mesenteric artery. Br J Pharmacol 129:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Martin PE, Chaytor AT, Evans WH, Garland CJ, Griffith TM. (1999) Role of heterocellular Gap junctional communication in endothelium-dependent smooth muscle hyperpolarization: inhibition by a connexin-mimetic peptide. Biochem Biophys Res Commun 254:27–31 [DOI] [PubMed] [Google Scholar]
- Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, Garland CJ. (2003a) Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res 40:480–490 [DOI] [PubMed] [Google Scholar]
- Dora KA, Xia J, Duling BR. (2003b) Endothelial cell signaling during conducted vasomotor responses. Am J Physiol Heart Circ Physiol 285:H119–H126 [DOI] [PubMed] [Google Scholar]
- Doreian BW, Fulop TG, Smith CB. (2008) Myosin II activation and actin reorganization regulate the mode of quantal exocytosis in mouse adrenal chromaffin cells. J Neurosci 28:4470–4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty JM, Plane F, Langton PD. (1999) Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am J Physiol 276:H1107–H1112 [DOI] [PubMed] [Google Scholar]
- Dow GS, Caridha D, Goldberg M, Wolf L, Koenig ML, Yourick DL, Wang Z. (2005) Transcriptional profiling of mefloquine-induced disruption of calcium homeostasis in neurons in vitro. Genomics 86:539–550 [DOI] [PubMed] [Google Scholar]
- Drummond RM, Fay FS. (1996) Mitochondria contribute to Ca2+ removal in smooth muscle cells. Pflugers Arch 431:473–482 [DOI] [PubMed] [Google Scholar]
- Drummond RM, Tuft RA. (1999) Release of Ca2+ from the sarcoplasmic reticulum increases mitochondrial [Ca2+] in rat pulmonary artery smooth muscle cells. J Physiol 516:139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan DD. (2011) The ClC-3 chloride channels in cardiovascular disease. Acta Pharmacol Sin 32:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy HS. (2012) The molecular mechanisms of gap junction remodeling. Heart Rhythm 9:1331–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy HS, Ashton AW, O’Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. (2004) Regulation of connexin43 protein complexes by intracellular acidification. Circ Res 94:215–222 [DOI] [PubMed] [Google Scholar]
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Südhof TC, Rizo J. (1999) A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J 18:4372–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S. (2013) TRPM4 channels in smooth muscle function. Pflugers Arch 465:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Gonzales AL, Garcia ZI. (2010) A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol 77:612–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Heppner TJ, Nelson MT, Brayden JE. (2005) TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97:1270–1279 [DOI] [PubMed] [Google Scholar]
- Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. (2009) TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297:H1096–H1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Straub SV, Brayden JE. (2007) Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 292:H2613–H2622 [DOI] [PubMed] [Google Scholar]
- Earley S, Waldron BJ, Brayden JE. (2004) Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95:922–929 [DOI] [PubMed] [Google Scholar]
- Eastman SD, Chen TH, Falk MM, Mendelson TC, Iovine MK. (2006) Phylogenetic analysis of three complete gap junction gene families reveals lineage-specific duplications and highly supported gene classes. Genomics 87:265–274 [DOI] [PubMed] [Google Scholar]
- Ebong EE, Depaola N. (2013) Specificity in the participation of connexin proteins in flow-induced endothelial gap junction communication. Pflugers Arch 465:1293–1302 [DOI] [PubMed] [Google Scholar]
- Edwards DH, Li Y, Griffith TM. (2008) Hydrogen peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler Thromb Vasc Biol 28:1774–1781 [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. (1998) K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396:269–272 [DOI] [PubMed] [Google Scholar]
- Edwards G, Félétou M, Gardener MJ, Thollon C, Vanhoutte PM, Weston AH. (1999a) Role of gap junctions in the responses to EDHF in rat and guinea-pig small arteries. Br J Pharmacol 128:1788–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Feletou M, Weston AH. (2010) Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch 459:863-879 [DOI] [PubMed] [Google Scholar]
- Edwards G, Gardener MJ, Feletou M, Brady G, Vanhoutte PM, Weston AH. (1999b) Further investigation of endothelium-derived hyperpolarizing factor (EDHF) in rat hepatic artery: studies using 1-EBIO and ouabain. Br J Pharmacol 128:1064–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek-Vitorin JF, Burt JM. (2013) Structural basis for the selective permeability of channels made of communicating junction proteins. Biochim Biophys Acta 1828:51–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ella SR, Yang Y, Clifford PS, Gulia J, Dora KA, Meininger GA, Davis MJ, Hill MA. (2010) Development of an image-based system for measurement of membrane potential, intracellular Ca(2+) and contraction in arteriolar smooth muscle cells. Microcirculation 17:629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis A, Goto K, Chaston DJ, Brackenbury TD, Meaney KR, Falck JR, Wojcikiewicz RJ, Hill CE. (2009) Enalapril treatment alters the contribution of epoxyeicosatrienoic acids but not gap junctions to endothelium-derived hyperpolarizing factor activity in mesenteric arteries of spontaneously hypertensive rats. J Pharmacol Exp Ther 330:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. (2000) Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 87:474–479 [DOI] [PubMed] [Google Scholar]
- Evans WH, Boitano S. (2001) Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans 29:606–612 [DOI] [PubMed] [Google Scholar]
- Evans WH, Bultynck G, Leybaert L. (2012) Manipulating connexin communication channels: use of peptidomimetics and the translational outputs. J Membr Biol 245:437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WH, Martin PE. (2002) Gap junctions: structure and function (Review). Review Mol Membr Biol 19:121–136 [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. (1972) Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res 31:293–307 [DOI] [PubMed] [Google Scholar]
- Fameli N, Kuo KH, van Breemen C. (2009) A model for the generation of localized transient [Na+] elevations in vascular smooth muscle. Biochem Biophys Res Commun 389:461–465 [DOI] [PubMed] [Google Scholar]
- Fanchaouy M, Serir K, Meister JJ, Beny JL, Bychkov R. (2005) Intercellular communication: role of gap junctions in establishing the pattern of ATP-elicited Ca2+ oscillations and Ca2+-dependent currents in freshly isolated aortic smooth muscle cells. Cell Calcium 37:25–34 [DOI] [PubMed] [Google Scholar]
- Faraci FM. (2002) nNOS-containing perivascular nerves: stranger by the minute. Circ Res 91:7–8 [DOI] [PubMed] [Google Scholar]
- Fasciani I, Temperán A, Pérez-Atencio LF, Escudero A, Martínez-Montero P, Molano J, Gómez-Hernández JM, Paino CL, González-Nieto D, Barrio LC. (2013) Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology 75:479–490 [DOI] [PubMed] [Google Scholar]
- Fawcett DW. (1961) The sarcoplasmic reticulum of skeletal and cardiac muscle. Circulation 24:336–348 [DOI] [PubMed] [Google Scholar]
- Feaver RE, Hastings NE, Pryor A, Blackman BR. (2008) GRP78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells. Arterioscler Thromb Vasc Biol 28:1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M. (2011a) The Endothelium: Part 2: EDHF-Mediated Responses “The Classical Pathway,” Morgan and Claypool Life Sciences Publisher, San Rafael, CA: [PubMed] [Google Scholar]
- Feletou M (2011b) The Endothelium: Part 1: Multiple Functions of the Endothelial Cells-Focus on Endothelium-Derived Vasoactive Mediators Morgan and Claypool Life Sciences Publisher, San Rafael, CA. [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. (2013) Endothelium-dependent hyperpolarization: no longer an f-word! J Cardiovasc Pharmacol 61:91–92 [DOI] [PubMed] [Google Scholar]
- Fernández-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC. (2006) Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol 174:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernhoff NB, Derbyshire ER, Marletta MA. (2009) A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. Proc Natl Acad Sci USA 106:21602–21607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441:179–185 [DOI] [PubMed] [Google Scholar]
- Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103:4150–4156 [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. (2008) Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: electrotonic versus regenerative conduction. Am J Physiol Heart Circ Physiol 295:H2001–H2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, Duling BR. (2003) Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res 92:793–800 [DOI] [PubMed] [Google Scholar]
- Firth AL, Won JY, Park WS. (2013) Regulation of Ca(2+) signaling in pulmonary hypertension. Korean J Physiol Pharmacol 17:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AB. (2009) Redox signaling across cell membranes. Antioxid Redox Signal 11:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I. (2008) Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res 102:1520–1528 [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. (1999) Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401:493–497 [DOI] [PubMed] [Google Scholar]
- Fleischmann BK, Murray RK, Kotlikoff MI. (1994) Voltage window for sustained elevation of cytosolic calcium in smooth muscle cells. Proc Natl Acad Sci USA 91:11914–11918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Busse R. (1999) Signal transduction of eNOS activation. Cardiovasc Res 43:532–541 [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. (2001) Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88:E68–E75 [DOI] [PubMed] [Google Scholar]
- Floyd R, Wray S. (2007) Calcium transporters and signalling in smooth muscles. Cell Calcium 42:467–476 [DOI] [PubMed] [Google Scholar]
- Fowler SL, Akins M, Zhou H, Figeys D, Bennett SA. (2013) The liver connexin32 interactome is a novel plasma membrane-mitochondrial signaling nexus. J Proteome Res 12:2597–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. (1964) Fine structure of sarcoplasmic reticulum and tranverse tubular system in muscle fibers. Fed Proc 23:887–895 [PubMed] [Google Scholar]
- Freund-Michel V, Guibert C, Dubois M, Courtois A, Marthan R, Savineau JP, Muller B. (2013) Reactive oxygen species as therapeutic targets in pulmonary hypertension. Ther Adv Respir Dis 7:175–200 [DOI] [PubMed] [Google Scholar]
- Fu J, Naren AP, Gao X, Ahmmed GU, Malik AB. (2005) Protease-activated receptor-1 activation of endothelial cells induces protein kinase Calpha-dependent phosphorylation of syntaxin 4 and Munc18c: role in signaling P-selectin expression. J Biol Chem 280:3178–3184 [DOI] [PubMed] [Google Scholar]
- Fujimoto T. (1993) Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol 120:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Kuroda TS. (2002) Slac2-c (synaptotagmin-like protein homologue lacking C2 domains-c), a novel linker protein that interacts with Rab27, myosin Va/VIIa, and actin. J Biol Chem 277:43096–43103 [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. (2005) Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25:136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta H, Hashitani H, Yamamoto Y, Suzuki H. (1999) Calcium responses induced by acetylcholine in submucosal arterioles of the guinea-pig small intestine. J Physiol 515:489–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Church JE, Ruan L, Li C, Sood SG, Kemp BE, Jennings IG, Venema RC. (2005) Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J Biol Chem 280:35943–35952 [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. (1999) Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF. (1999) Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep 19:235–251 [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376 [DOI] [PubMed] [Google Scholar]
- Furne J, Saeed A, Levitt MD. (2008) Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295:R1479–R1485 [DOI] [PubMed] [Google Scholar]
- Gairhe S, Bauer NN, Gebb SA, McMurtry IF. (2011) Myoendothelial gap junctional signaling induces differentiation of pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 301:L527–L535 [DOI] [PubMed] [Google Scholar]
- Gairhe S, Bauer NN, Gebb SA, McMurtry IF. (2012) Serotonin passes through myoendothelial gap junctions to promote pulmonary arterial smooth muscle cell differentiation. Am J Physiol Lung Cell Mol Physiol 303:L767–L777 [DOI] [PubMed] [Google Scholar]
- Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, Figeys D, Harrison DG, Berk BC, Aebersold R, et al. (1999) Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem 274:30101–30108 [DOI] [PubMed] [Google Scholar]
- Garant PR. (1972) The demonstration of complex gap junctions between the cells of the enamel organ with lanthanum nitrate. J Ultrastruct Res 40:333–348 [DOI] [PubMed] [Google Scholar]
- García-Cardeña G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. (1997) Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem 272:25437–25440 [DOI] [PubMed] [Google Scholar]
- García-Cardeña G, Oh P, Liu J, Schnitzer JE, Sessa WC. (1996) Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA 93:6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Dorado D, Inserte J, Ruiz-Meana M, González MA, Solares J, Juliá M, Barrabés JA, Soler-Soler J. (1997) Gap junction uncoupler heptanol prevents cell-to-cell progression of hypercontracture and limits necrosis during myocardial reperfusion. Circulation 96:3579–3586 [DOI] [PubMed] [Google Scholar]
- Garcia-Perez C, Roy SS, Naghdi S, Lin X, Davies E, Hajnóczky G. (2012) Bid-induced mitochondrial membrane permeabilization waves propagated by local reactive oxygen species (ROS) signaling. Proc Natl Acad Sci USA 109:4497–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ZI, Bruhl A, Gonzales AL, Earley S. (2011) Basal protein kinase Cδ activity is required for membrane localization and activity of TRPM4 channels in cerebral artery smooth muscle cells. Channels (Austin) 5:210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland CJ, Hiley CR, Dora KA. (2011) EDHF: spreading the influence of the endothelium. Br J Pharmacol 164:839–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland JG, McPherson GA. (1992) Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol 105:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Low B, Liang M, Fu J. (2007) Angiotensin II directly triggers endothelial exocytosis via protein kinase C-dependent protein kinase D2 activation. J Pharmacol Sci 105:168–176 [DOI] [PubMed] [Google Scholar]
- Gehi R, Shao Q, Laird DW. (2011) Pathways regulating the trafficking and turnover of pannexin1 protein and the role of the C-terminal domain. J Biol Chem 286:27639–27653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geletu M, Trotman-Grant A, Raptis L. (2012) Mind the gap; regulation of gap junctional, intercellular communication by the SRC oncogene product and its effectors. Anticancer Res 32:4245–4250 [PubMed] [Google Scholar]
- Gentile C, Muise-Helmericks RC, Drake CJ. (2013) VEGF-mediated phosphorylation of eNOS regulates angioblast and embryonic endothelial cell proliferation. Dev Biol 373:163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T. (2010) Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell 38:280–290 [DOI] [PubMed] [Google Scholar]
- Giepmans BN. (2004) Gap junctions and connexin-interacting proteins. Cardiovasc Res 62:233–245 [DOI] [PubMed] [Google Scholar]
- Giepmans BN. (2006) Role of connexin43-interacting proteins at gap junctions. Adv Cardiol 42:41–56 [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Hengeveld T, Postma FR, Moolenaar WH. (2001a) Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication. J Biol Chem 276:8544–8549 [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Verlaan I, Hengeveld T, Janssen H, Calafat J, Falk MM, Moolenaar WH. (2001b) Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol 11:1364–1368 [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Verlaan I, Moolenaar WH. (2001c) Connexin-43 interactions with ZO-1 and alpha- and beta-tubulin. Cell Commun Adhes 8:219–223 [DOI] [PubMed] [Google Scholar]
- Giles TD. (2006) Aspects of nitric oxide in health and disease: a focus on hypertension and cardiovascular disease. J Clin Hypertens (Greenwich) 8(12, Suppl 4)2–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Edwards G, Weston AH, Vanhoutte PM, Félétou M. (2005) Hydrogen peroxide and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Eur J Pharmacol 513:219–224 [DOI] [PubMed] [Google Scholar]
- Gödecke S, Roderigo C, Rose CR, Rauch BH, Gödecke A, Schrader J. (2012) Thrombin-induced ATP release from human umbilical vein endothelial cells. Am J Physiol Cell Physiol 302:C915–C923 [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Lampe PD, Nicholson BJ. (1999) Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol 1:457–459 [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD. (2002) Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem 277:36725–36730 [DOI] [PubMed] [Google Scholar]
- Golser R, Gorren AC, Leber A, Andrew P, Habisch HJ, Werner ER, Schmidt K, Venema RC, Mayer B. (2000) Interaction of endothelial and neuronal nitric-oxide synthases with the bradykinin B2 receptor. Binding of an inhibitory peptide to the oxygenase domain blocks uncoupled NADPH oxidation. J Biol Chem 275:5291–5296 [DOI] [PubMed] [Google Scholar]
- Gomi H, Mizutani S, Kasai K, Itohara S, Izumi T. (2005) Granuphilin molecularly docks insulin granules to the fusion machinery. J Cell Biol 171:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales AL, Amberg GC, Earley S. (2010a) Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 299:C279–C288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales AL, Earley S. (2012) Endogenous cytosolic Ca(2+) buffering is necessary for TRPM4 activity in cerebral artery smooth muscle cells. Cell Calcium 51:82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales AL, Garcia ZI, Amberg GC, Earley S. (2010b) Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. Am J Physiol Cell Physiol 299:C1195–C1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani A, Bricko V, Carmignani M, Graier WF, Groschner K. (2004) Cholesterol- and caveolin-rich membrane domains are essential for phospholipase A2-dependent EDHF formation. Cardiovasc Res 64:234–242 [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. (1994) Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74:1141–1148 [DOI] [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. (1998) Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93:731–740 [DOI] [PubMed] [Google Scholar]
- Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. (2003) Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 113:343–355 [DOI] [PubMed] [Google Scholar]
- Grosely R, Kopanic JL, Nabors S, Kieken F, Spagnol G, Al-Mugotir M, Zach S, Sorgen PL. (2013) Effects of phosphorylation on the structure and backbone dynamics of the intrinsically disordered connexin43 C-terminal domain. J Biol Chem 288:24857–24870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Carr CM, Novick PJ. (2000) Ordering the final events in yeast exocytosis. J Cell Biol 151:439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg M, Fredriksson S, Taussig M, Jarvius J, Gustafsdottir S, Landegren U. (2003) A sense of closeness: protein detection by proximity ligation. Curr Opin Biotechnol 14:82–86 [DOI] [PubMed] [Google Scholar]
- Guo RW, Wang H, Gao P, Li MQ, Zeng CY, Yu Y, Chen JF, Song MB, Shi YK, Huang L. (2009) An essential role for stromal interaction molecule 1 in neointima formation following arterial injury. Cardiovasc Res 81:660–668 [DOI] [PubMed] [Google Scholar]
- Gurney AM, Drummond RM, Fay FS. (2000) Calcium signalling in sarcoplasmic reticulum, cytoplasm and mitochondria during activation of rabbit aorta myocytes. Cell Calcium 27:339–351 [DOI] [PubMed] [Google Scholar]
- Haberichter SL, Merricks EP, Fahs SA, Christopherson PA, Nichols TC, Montgomery RR. (2005) Re-establishment of VWF-dependent Weibel-Palade bodies in VWD endothelial cells. Blood 105:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. (2006) Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol 291:H2047–H2056 [DOI] [PubMed] [Google Scholar]
- Haddock RE, Grayson TH, Morris MJ, Howitt L, Chadha PS, Sandow SL. (2011) Diet-induced obesity impairs endothelium-derived hyperpolarization via altered potassium channel signaling mechanisms. PLoS ONE 6:e16423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger JA, Nicod P, Meda P. (2004) Contribution of connexins to the function of the vascular wall. Cardiovasc Res 62:345–356 [DOI] [PubMed] [Google Scholar]
- Hajnóczky G, Hager R, Thomas AP. (1999) Mitochondria suppress local feedback activation of inositol 1,4,5-trisphosphate receptors by Ca2+. J Biol Chem 274:14157–14162 [DOI] [PubMed] [Google Scholar]
- Hakim CH, Jackson WF, Segal SS. (2008) Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation 15:503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim TS, Sugimori K, Camporesi EM, Anderson G. (1996) Half-life of nitric oxide in aqueous solutions with and without haemoglobin. Physiol Meas 17:267–277 [DOI] [PubMed] [Google Scholar]
- Haldar SM, Stamler JS. (2013) S-Nitrosylation: integrator of cardiovascular performance and oxygen delivery. J Clin Invest 123:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Clement MV, Long LH. (2000) Hydrogen peroxide in the human body. FEBS Lett 486:10–13 [DOI] [PubMed] [Google Scholar]
- Hamilton KK, Sims PJ. (1987) Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. J Clin Invest 79:600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD. (1998) Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox). J Biol Chem 273:16663–16668 [DOI] [PubMed] [Google Scholar]
- Handy DE, Loscalzo J. (2006) Nitric oxide and posttranslational modification of the vascular proteome: S-nitrosation of reactive thiols. Arterioscler Thromb Vasc Biol 26:1207–1214 [DOI] [PubMed] [Google Scholar]
- Hannah MJ, Hume AN, Arribas M, Williams R, Hewlett LJ, Seabra MC, Cutler DF. (2003) Weibel-Palade bodies recruit Rab27 by a content-driven, maturation-dependent mechanism that is independent of cell type. J Cell Sci 116:3939–3948 [DOI] [PubMed] [Google Scholar]
- Hansen M, Boitano S, Dirksen ER, Sanderson MJ. (1993) Intercellular calcium signaling induced by extracellular adenosine 5′-triphosphate and mechanical stimulation in airway epithelial cells. J Cell Sci 106:995–1004 [DOI] [PubMed] [Google Scholar]
- Hansson GK, Geng YJ, Holm J, Hårdhammar P, Wennmalm A, Jennische E. (1994) Arterial smooth muscle cells express nitric oxide synthase in response to endothelial injury. J Exp Med 180:733–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, van Zoelen EJ, Theuvenet AP. (2001) Fenamates: a novel class of reversible gap junction blockers. J Pharmacol Exp Ther 298:1033–1041 [PubMed] [Google Scholar]
- Harris AL. (2001) Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys 34:325–472 [DOI] [PubMed] [Google Scholar]
- Hasenau AL, Nielsen G, Morisseau C, Hammock BD, Wulff H, Köhler R. (2011) Improvement of endothelium-dependent vasodilations by SKA-31 and SKA-20, activators of small- and intermediate-conductance Ca2+-activated K+ -channels. Acta Physiol (Oxf) 203:117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. (1997) K+ channels which contribute to the acetylcholine-induced hyperpolarization in smooth muscle of the guinea-pig submucosal arteriole. J Physiol 501:319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BJ, Madesh M, Kirkpatrick CJ, Fisher AB. (2007) Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell 18:2002–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. (2000) Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 87:677–682 [DOI] [PubMed] [Google Scholar]
- He DS, Jiang JX, Taffet SM, Burt JM. (1999) Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc Natl Acad Sci USA 96:6495–6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein KR, Straub AC, Best AK, Greyson MA, Looft-Wilson RC, Sharma PR, Meher A, Leitinger N, Isakson BE. (2010) Plasminogen activator inhibitor-1 regulates myoendothelial junction formation. Circ Res 106:1092–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein KR, Straub AC, Isakson BE. (2009) The myoendothelial junction: breaking through the matrix? Microcirculation 16:307–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M, Cattaruzza M, Wagner AH. (1999) Regulation of inducible nitric oxide synthase gene expression in vascular smooth muscle cells. Gen Pharmacol 32:9–16 [DOI] [PubMed] [Google Scholar]
- Hegeman RJ, van den Eijnden-Schrauwen Y, Emeis JJ. (1998) Adenosine 3′:5′-cyclic monophosphate induces regulated secretion of tissue-type plasminogen activator and von Willebrand factor from cultured human endothelial cells. Thromb Haemost 79:853–858 [PubMed] [Google Scholar]
- Herbert SP, Stainier DY. (2011) Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 12:551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsmeyer K, Sturek M. (1986) Membrane potential, Ca2+ influx, and Ca2+ release in single vascular muscle cells. J Cardiovasc Pharmacol 8 (Suppl 8):S38–S41 [DOI] [PubMed] [Google Scholar]
- Hervé JC, Derangeon M. (2013) Gap-junction-mediated cell-to-cell communication. Cell Tissue Res 352:21–31 [DOI] [PubMed] [Google Scholar]
- Hervé JC, Derangeon M, Sarrouilhe D, Giepmans BN, Bourmeyster N. (2012) Gap junctional channels are parts of multiprotein complexes. Biochim Biophys Acta 1818:1844–1865 [DOI] [PubMed] [Google Scholar]
- Hibbs JB, Jr, Taintor RR, Vavrin Z. (1987a) Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science 235:473–476 [DOI] [PubMed] [Google Scholar]
- Hibbs JB, Jr, Vavrin Z, Taintor RR. (1987b) L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol 138:550–565 [PubMed] [Google Scholar]
- Hildebrandt TM, Grieshaber MK. (2008) Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275:3352–3361 [DOI] [PubMed] [Google Scholar]
- Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. (2011) Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol 3:a004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton JM, Langton PD. (2003) Inhibition of EDHF by two new combinations of K+-channel inhibitors in rat isolated mesenteric arteries. Br J Pharmacol 138:1031–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Burt JM, Hirschi KD, Dai C. (2003) Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res 93:429–437 [DOI] [PubMed] [Google Scholar]
- Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, et al. (2001) Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 276:3459–3467 [DOI] [PubMed] [Google Scholar]
- Hoffman BD, Grashoff C, Schwartz MA. (2011) Dynamic molecular processes mediate cellular mechanotransduction. Nature 475:316–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. (2003) Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17:2205–2232 [DOI] [PubMed] [Google Scholar]
- Hogg N, Joseph J, Kalyanaraman B. (1994) The oxidation of alpha-tocopherol and trolox by peroxynitrite. Arch Biochem Biophys 314:153–158 [DOI] [PubMed] [Google Scholar]
- Holton M, Mohamed TM, Oceandy D, Wang W, Lamas S, Emerson M, Neyses L, Armesilla AL. (2010) Endothelial nitric oxide synthase activity is inhibited by the plasma membrane calcium ATPase in human endothelial cells. Cardiovasc Res 87:440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann S, Kukovetz WR, Windischhofer W, Paschke E, Graier WF. (1994) Pharmacologic differentiation between endothelium-dependent relaxations sensitive and resistant to nitro-l-arginine in coronary arteries. J Cardiovasc Pharmacol 23:747–756 [DOI] [PubMed] [Google Scholar]
- Howitt L, Chaston DJ, Sandow SL, Matthaei KI, Edwards FR, Hill CE. (2013) Spreading vasodilatation in the murine microcirculation: attenuation by oxidative stress-induced change in electromechanical coupling. J Physiol 591:2157–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Ting AE, Hazuka CD, Davanger S, Kenny JW, Kee Y, Scheller RH. (1996) The mammalian brain rsec6/8 complex. Neuron 17:1209–1219 [DOI] [PubMed] [Google Scholar]
- Hsyu PH, Gisclon LG, Hui AC, Giacomini KM. (1988) Interactions of organic anions with the organic cation transporter in renal BBMV. Am J Physiol 254:F56–F61 [DOI] [PubMed] [Google Scholar]
- Huber D, Cramer EM, Kaufmann JE, Meda P, Massé JM, Kruithof EK, Vischer UM. (2002) Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endothelial cells both in vitro and in vivo. Blood 99:3637–3645 [DOI] [PubMed] [Google Scholar]
- Huie RE, Padmaja S. (1993) The reaction of no with superoxide. Free Radic Res Commun 18:195–199 [DOI] [PubMed] [Google Scholar]
- Hultquist DE, Passon PG. (1971) Catalysis of methaemoglobin reduction by erythrocyte cytochrome B5 and cytochrome B5 reductase. Nat New Biol 229:252–254 [DOI] [PubMed] [Google Scholar]
- Hume AN, Ushakov DS, Tarafder AK, Ferenczi MA, Seabra MC. (2007) Rab27a and MyoVa are the primary Mlph interactors regulating melanosome transport in melanocytes. J Cell Sci 120:3111–3122 [DOI] [PubMed] [Google Scholar]
- Hunter AW, Barker RJ, Zhu C, Gourdie RG. (2005) Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell 16:5686–5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AW, Jourdan J, Gourdie RG. (2003) Fusion of GFP to the carboxyl terminus of connexin43 increases gap junction size in HeLa cells. Cell Commun Adhes 10:211–214 [DOI] [PubMed] [Google Scholar]
- Hutcheson IR, Chaytor AT, Evans WH, Griffith TM. (1999) Nitric oxide-independent relaxations to acetylcholine and A23187 involve different routes of heterocellular communication. Role of Gap junctions and phospholipase A2. Circ Res 84:53–63 [DOI] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Williams P, Chatterjee M. (1994) Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am J Physiol 266:H952–H958 [DOI] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. (2009a) Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci 29:7092–7097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Spray DC, Scemes E. (2009) Mefloquine blockade of Pannexin1 currents: resolution of a conflict. Cell Commun Adhes 16:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. (2008) P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 295:C752–C760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ. (1991) Heme-dependent activation of guanylate cyclase by nitric oxide: a novel signal transduction mechanism. Blood Vessels 28:67–73 [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. (2002) Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol 53:503–514 [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. (1987) Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84:9265–9269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda U, Yamamoto K, Maeda Y, Shimpo M, Kanbe T, Shimada K. (1997) Endothelin-1 inhibits nitric oxide synthesis in vascular smooth muscle cells. Hypertension 29:65–69 [DOI] [PubMed] [Google Scholar]
- Illi B, Dello Russo C, Colussi C, Rosati J, Pallaoro M, Spallotta F, Rotili D, Valente S, Ragone G, Martelli F, et al. (2008) Nitric oxide modulates chromatin folding in human endothelial cells via protein phosphatase 2A activation and class II histone deacetylases nuclear shuttling. Circ Res 102:51–58 [DOI] [PubMed] [Google Scholar]
- Inai T, Shibata Y. (2009) Heterogeneous expression of endothelial connexin (Cx) 37, Cx40, and Cx43 in rat large veins. Anat Sci Int 84:237–245 [DOI] [PubMed] [Google Scholar]
- Isakson BE. (2008) Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J Cell Sci 121:3664–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Best AK, Duling BR. (2008) Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am J Physiol Heart Circ Physiol 294:H2898–H2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Damon DN, Day KH, Liao Y, Duling BR. (2006a) Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol 290:H1199–H1205 [DOI] [PubMed] [Google Scholar]
- Isakson BE, Duling BR. (2005) Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res 97:44–51 [DOI] [PubMed] [Google Scholar]
- Isakson BE, Kronke G, Kadl A, Leitinger N, Duling BR. (2006b) Oxidized phospholipids alter vascular connexin expression, phosphorylation, and heterocellular communication. Arterioscler Thromb Vasc Biol 26:2216–2221 [DOI] [PubMed] [Google Scholar]
- Isakson BE, Ramos SI, Duling BR. (2007) Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res 100:246–254 [DOI] [PubMed] [Google Scholar]
- Ishii K, Chang B, Kerwin JF, Jr, Huang ZJ, Murad F. (1990) N-omega-nitro-l-arginine: a potent inhibitor of endothelium-derived relaxing factor formation. Eur J Pharmacol 176:219–223 [DOI] [PubMed] [Google Scholar]
- Ishii K, Hirose K, Iino M. (2006) Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep 7:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MR, Uramoto H, Okada T, Sabirov RZ, Okada Y. (2012) Maxi-anion channel and pannexin 1 hemichannel constitute separate pathways for swelling-induced ATP release in murine L929 fibrosarcoma cells. Am J Physiol Cell Physiol 303:C924–C935 [DOI] [PubMed] [Google Scholar]
- Jaggar JH. (2001) Intravascular pressure regulates local and global Ca(2+) signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 281:C439–C448 [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, e S, Cheng X. (2002) Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res 91:610–617 [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. (2005) Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res 97:805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Stevenson AS, Nelson MT. (1998a) Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol 274:C1755–C1761 [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, et al. (1998b) Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand 164:577–587 [DOI] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D. (2012) Molecular machines governing exocytosis of synaptic vesicles. Nature 490:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C, Koh YJ, Lim NK, Kang HJ, Kim DH, Park SK, Lee GM, Jeon CJ, Koh GY. (2009) Angiopoietin-2 exocytosis is stimulated by sphingosine-1-phosphate in human blood and lymphatic endothelial cells. Arterioscler Thromb Vasc Biol 29:401–407 [DOI] [PubMed] [Google Scholar]
- Jenkins GM, Frohman MA. (2005) Phospholipase D: a lipid centric review. Cell Mol Life Sci 62:2305–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Colombari E, Colombari DS, Lavesa M, Talman WT, Nasjletti A. (1997) Role of endogenous carbon monoxide in central regulation of arterial pressure. Hypertension 30:962–967 [DOI] [PubMed] [Google Scholar]
- Johnson RG, Meyer RA, Li XR, Preus DM, Tan L, Grunenwald H, Paulson AF, Laird DW, Sheridan JD. (2002) Gap junctions assemble in the presence of cytoskeletal inhibitors, but enhanced assembly requires microtubules. Exp Cell Res 275:67–80 [DOI] [PubMed] [Google Scholar]
- Johnson RG, Reynhout JK, TenBroek EM, Quade BJ, Yasumura T, Davidson KG, Sheridan JD, Rash JE. (2012) Gap junction assembly: roles for the formation plaque and regulation by the C-terminus of connexin43. Mol Biol Cell 23:71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Nerem RM. (2007) Endothelial connexin 37, connexin 40, and connexin 43 respond uniquely to substrate and shear stress. Endothelium 14:215–226 [DOI] [PubMed] [Google Scholar]
- Johnstone S, Isakson B, Locke D. (2009a) Biological and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol 278:69–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SR, Best AK, Wright CS, Isakson BE, Errington RJ, Martin PE. (2010) Enhanced connexin 43 expression delays intra-mitotic duration and cell cycle traverse independently of gap junction channel function. J Cell Biochem 110:772–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SR, Billaud M, Lohman AW, Taddeo EP, Isakson BE. (2012a) Posttranslational modifications in connexins and pannexins. J Membr Biol 245:319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SR, Kroncke BM, Straub AC, Best AK, Dunn CA, Mitchell LA, Peskova Y, Nakamoto RK, Koval M, Lo CW, et al. (2012b) MAPK phosphorylation of connexin 43 promotes binding of cyclin E and smooth muscle cell proliferation. Circ Res 111:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SR, Ross J, Rizzo MJ, Straub AC, Lampe PD, Leitinger N, Isakson BE. (2009b) Oxidized phospholipid species promote in vivo differential Cx43 phosphorylation and vascular smooth muscle cell proliferation. Am J Pathol 175:916–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. (2012) Computational and structural characterisation of protein associations. Adv Exp Med Biol 747:42–54 [DOI] [PubMed] [Google Scholar]
- Jordan K, Solan JL, Dominguez M, Sia M, Hand A, Lampe PD, Laird DW. (1999) Trafficking, assembly, and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol Biol Cell 10:2033–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju H, Venema VJ, Marrero MB, Venema RC. (1998) Inhibitory interactions of the bradykinin B2 receptor with endothelial nitric-oxide synthase. J Biol Chem 273:24025–24029 [DOI] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. (1997) Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 272:18522–18525 [DOI] [PubMed] [Google Scholar]
- Jung NH, Kim HP, Kim BR, Cha SH, Kim GA, Ha H, Na YE, Cha YN. (2003) Evidence for heme oxygenase-1 association with caveolin-1 and -2 in mouse mesangial cells. IUBMB Life 55:525–532 [DOI] [PubMed] [Google Scholar]
- Kabil O, Banerjee R. (2010) Redox biochemistry of hydrogen sulfide. J Biol Chem 285:21903–21907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam Y, Kim DY, Koo SK, Joe CO. (1998) Transfer of second messengers through gap junction connexin 43 channels reconstituted in liposomes. Biochim Biophys Acta 1372:384–388 [DOI] [PubMed] [Google Scholar]
- Kameritsch P, Khandoga N, Nagel W, Hundhausen C, Lidington D, Pohl U. (2005) Nitric oxide specifically reduces the permeability of Cx37-containing gap junctions to small molecules. J Cell Physiol 203:233–242 [DOI] [PubMed] [Google Scholar]
- Kamishima T, Quayle JM. (2002) Mitochondrial Ca2+ uptake is important over low [Ca2+]i range in arterial smooth muscle. Am J Physiol Heart Circ Physiol 283:H2431–H2439 [DOI] [PubMed] [Google Scholar]
- Kanaporis G, Mese G, Valiuniene L, White TW, Brink PR, Valiunas V. (2008) Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol 131:293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu MY, Loo LW, Simon S, Lau AF, Eckhart W. (1997) Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J Biol Chem 272:22824–22831 [DOI] [PubMed] [Google Scholar]
- Kang-Decker N, Cao S, Chatterjee S, Yao J, Egan LJ, Semela D, Mukhopadhyay D, Shah V. (2007) Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J Cell Sci 120:492–501 [DOI] [PubMed] [Google Scholar]
- Kang EY, Ponzio M, Gupta PP, Liu F, Butensky A, Gutstein DE. (2009) Identification of binding partners for the cytoplasmic loop of connexin43: a novel interaction with β-tubulin. Cell Commun Adhes 15:397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno K, Hirata Y, Imai T, Iwashina M, Marumo F. (1994) Regulation of inducible nitric oxide synthase gene by interleukin-1 beta in rat vascular endothelial cells. Am J Physiol 267:H2318–H2324 [DOI] [PubMed] [Google Scholar]
- Kansui Y, Fujii K, Nakamura K, Goto K, Oniki H, Abe I, Shibata Y, Iida M. (2004) Angiotensin II receptor blockade corrects altered expression of gap junctions in vascular endothelial cells from hypertensive rats. Am J Physiol Heart Circ Physiol 287:H216–H224 [DOI] [PubMed] [Google Scholar]
- Karaki H. (1989) Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci 10:320–325 [DOI] [PubMed] [Google Scholar]
- Karashima T, Kuriyama H. (1981) Electrical properties of smooth muscle cell membrane and neuromuscular transmission in the guinea-pig basilar artery. Br J Pharmacol 74:495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin GJ. (1994) Calcium signaling in restricted diffusion spaces. Biophys J 67:262–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin GJ. (2003) Responses of Ca2+-binding proteins to localized, transient changes in intracellular [Ca2+]. J Theor Biol 221:245–258 [DOI] [PubMed] [Google Scholar]
- Katakowski M, Buller B, Wang X, Rogers T, Chopp M. (2010) Functional microRNA is transferred between glioma cells. Cancer Res 70:8259–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katritch V, Cherezov V, Stevens RC. (2012) Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol Sci 33:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S, Arnold W, Mittal C, Murad F. (1977) Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res 3:23–35 [PubMed] [Google Scholar]
- Kaufmann JE, Oksche A, Wollheim CB, Günther G, Rosenthal W, Vischer UM. (2000) Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J Clin Invest 106:107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausalya PJ, Reichert M, Hunziker W. (2001) Connexin45 directly binds to ZO-1 and localizes to the tight junction region in epithelial MDCK cells. FEBS Lett 505:92–96 [DOI] [PubMed] [Google Scholar]
- Kehl SJ. (2001) Eicosatetraynoic acid (ETYA), a non-metabolizable analogue of arachidonic acid, blocks the fast-inactivating potassium current of rat pituitary melanotrophs. Can J Physiol Pharmacol 79:338–345 [PubMed] [Google Scholar]
- Khan MT, Furchgott RF. (1987) Additional evidence that endothelium-derived relaxing factor is nitric oxide, in Pharmacology (Rand MJ, Raper C. eds) pp 341–344, Elsevier, Amsterdam [Google Scholar]
- Kim HP, Ryter SW, Choi AM. (2006) CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol 46:411–449 [DOI] [PubMed] [Google Scholar]
- Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. (2004) Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J 18:1080–1089 [DOI] [PubMed] [Google Scholar]
- Kinnear NP, Boittin FX, Thomas JM, Galione A, Evans AM. (2004) Lysosome-sarcoplasmic reticulum junctions. A trigger zone for calcium signaling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. J Biol Chem 279:54319–54326 [DOI] [PubMed] [Google Scholar]
- Kinnear NP, Wyatt CN, Clark JH, Calcraft PJ, Fleischer S, Jeyakumar LH, Nixon GF, Evans AM. (2008) Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium 44:190–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Bruhl A, Sullivan MN, Francis M, Dinenno FA, Earley S. (2013) Robust internal elastic lamina fenestration in skeletal muscle arteries. PLoS ONE 8:e54849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427:360–364 [DOI] [PubMed] [Google Scholar]
- Kitamura K, Kuriyama H. (1979) Effects of acetylcholine on the smooth muscle cell of isolated main coronary artery of the guinea-pig. J Physiol 293:119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizana E, Cingolani E, Marbán E. (2009) Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene Ther 16:1163–1168 [DOI] [PubMed] [Google Scholar]
- Knop M, Aareskjold E, Bode G, Gerke V. (2004) Rab3D and annexin A2 play a role in regulated secretion of vWF, but not tPA, from endothelial cells. EMBO J 23:2982–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. (1998) Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Standen NB, Nelson MT. (1998) Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol 508:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RG, Palacios M, Palmer RM, Moncada S. (1989) Formation of nitric oxide from l-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci USA 86:5159–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler R, Hoyer J. (2007) Role of TRPV4 in the mechanotransduction of shear stress in endothelial cells, in TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades (Liedtke WB, Heller S. eds), Taylor & Francis Group, LLC, Boca Raton, FL: [PubMed] [Google Scholar]
- Koide M, Nystoriak MA, Krishnamoorthy G, O'Connor KP, Bonev AD, Nelson MT, Wellman GC. (2011) Reduced Ca2+ spark activity after subarachnoid hemorrhage disables BK channel control of cerebral artery tone. J Cereb Blood Flow Metab 31:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komazaki S, Ito K, Takeshima H, Nakamura H. (2002) Deficiency of triad formation in developing skeletal muscle cells lacking junctophilin type 1. FEBS Lett 524:225–229 [DOI] [PubMed] [Google Scholar]
- Kooistra MR, Corada M, Dejana E, Bos JL. (2005) Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett 579:4966–4972 [DOI] [PubMed] [Google Scholar]
- Kopanic JL, Sorgen PL. (2013) Chemical shift assignments of the connexin45 carboxyl terminal domain: monomer and dimer conformations. Biomol NMR Assign 7:293–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz S, Loganathan S, Mikles B, Radovits T, Barnucz E, Hirschberg K, Li S, Hegedüs P, Páli S, Weymann A, et al. (2013) Nitric oxide- and heme-independent activation of soluble guanylate cyclase attenuates peroxynitrite-induced endothelial dysfunction in rat aorta. J Cardiovasc Pharmacol Ther 18:70–77 [DOI] [PubMed] [Google Scholar]
- Kostin S. (2007) Zonula occludens-1 and connexin 43 expression in the failing human heart. J Cell Mol Med 11:892–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JA, Baker KA, Altenberg GA, Abagyan R, Yeager M. (2007) Molecular modeling and mutagenesis of gap junction channels. Prog Biophys Mol Biol 94:15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval M, Billaud M, Straub AC, Johnstone SR, Zarbock A, Duling BR, Isakson BE. (2011) Spontaneous lung dysfunction and fibrosis in mice lacking connexin 40 and endothelial cell connexin 43. Am J Pathol 178:2536–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval M, Geist ST, Westphale EM, Kemendy AE, Civitelli R, Beyer EC, Steinberg TH. (1995) Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43. J Cell Biol 130:987–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger O, Bény JL, Chabaud F, Traub O, Theis M, Brix K, Kirchhoff S, Willecke K. (2002) Altered dye diffusion and upregulation of connexin37 in mouse aortic endothelium deficient in connexin40. J Vasc Res 39:160–172 [DOI] [PubMed] [Google Scholar]
- Kühberger E, Groschner K, Kukovetz WR, Brunner F. (1994) The role of myoendothelial cell contact in non-nitric oxide-, non-prostanoid-mediated endothelium-dependent relaxation of porcine coronary artery. Br J Pharmacol 113:1289–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H, Suzuki H. (1978) The effects of acetylcholine on the membrane and contractile properties of smooth muscle cells of the rabbit superior mesenteric artery. Br J Pharmacol 64:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. (2002) Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 22:225–230 [DOI] [PubMed] [Google Scholar]
- Kwak BR, Pepper MS, Gros DB, Meda P. (2001) Inhibition of endothelial wound repair by dominant negative connexin inhibitors. Mol Biol Cell 12:831–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak BR, Silacci P, Stergiopulos N, Hayoz D, Meda P. (2005) Shear stress and cyclic circumferential stretch, but not pressure, alter connexin43 expression in endothelial cells. Cell Commun Adhes 12:261–270 [DOI] [PubMed] [Google Scholar]
- Kwak BR, Veillard N, Pelli G, Mulhaupt F, James RW, Chanson M, Mach F. (2003) Reduced connexin43 expression inhibits atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Circulation 107:1033–1039 [DOI] [PubMed] [Google Scholar]
- Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, Yu S, Yao X. (2009) TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res 104:670–678 [DOI] [PubMed] [Google Scholar]
- Lab MJ, Bhargava A, Wright PT, Gorelik J. (2013) The scanning ion conductance microscope for cellular physiology. Am J Physiol Heart Circ Physiol 304:H1–H11 [DOI] [PubMed] [Google Scholar]
- Lacy F, O’Connor DT, Schmid-Schönbein GW. (1998) Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J Hypertens 16:291–303 [DOI] [PubMed] [Google Scholar]
- Lagree V, Brunschwig K, Lopez P, Gilula NB, Richard G, Falk MM. (2003) Specific amino-acid residues in the N terminus and TM3 implicated in channel function and oligomerization compatibility of connexin43. J Cell Sci 116:3189–3201 [DOI] [PubMed] [Google Scholar]
- Laing JG, Chou BC, Steinberg TH. (2005a) ZO-1 alters the plasma membrane localization and function of Cx43 in osteoblastic cells. J Cell Sci 118:2167–2176 [DOI] [PubMed] [Google Scholar]
- Laing JG, Koval M, Steinberg TH. (2005b) Association with ZO-1 correlates with plasma membrane partitioning in truncated connexin45 mutants. J Membr Biol 207:45–53 [DOI] [PubMed] [Google Scholar]
- Laing JG, Manley-Markowski RN, Koval M, Civitelli R, Steinberg TH. (2001) Connexin45 interacts with zonula occludens-1 and connexin43 in osteoblastic cells. J Biol Chem 276:23051–23055 [DOI] [PubMed] [Google Scholar]
- Laird DW. (2006) Life cycle of connexins in health and disease. Biochem J 394:527–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas S, Marsden PA, Li GK, Tempst P, Michel T. (1992) Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci USA 89:6348–6352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfi B, Curci S, Debellis L, Pozzan T, Hofer AM. (1998) Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured In situ in intact cells. J Cell Biol 142:1235–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. (2008) Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol Biol Cell 19:912–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson DM, Seul KH, Berthoud VM, Lau AF, Sagar GD, Beyer EC. (2000) Functional expression and biochemical characterization of an epitope-tagged connexin37. Mol Cell Biol Res Commun 3:115–121 [DOI] [PubMed] [Google Scholar]
- Larson DM, Wrobleski MJ, Sagar GD, Westphale EM, Beyer EC. (1997) Differential regulation of connexin43 and connexin37 in endothelial cells by cell density, growth, and TGF-beta1. Am J Physiol 272:C405–C415 [DOI] [PubMed] [Google Scholar]
- Laskey RE, Adams DJ, Cannell M, van Breemen C. (1992) Calcium entry-dependent oscillations of cytoplasmic calcium concentration in cultured endothelial cell monolayers. Proc Natl Acad Sci USA 89:1690–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. (2002) Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci USA 99:10446–10451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado E, Sanchez-Abarca LI, Tabernero A, Bolaños JP, Medina JM. (1997) Oleic acid inhibits gap junction permeability and increases glucose uptake in cultured rat astrocytes. J Neurochem 69:721–728 [DOI] [PubMed] [Google Scholar]
- Lawrence TS, Beers WH, Gilula NB. (1978) Transmission of hormonal stimulation by cell-to-cell communication. Nature 272:501–506 [DOI] [PubMed] [Google Scholar]
- Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. (2008) Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105:9627–9632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. (2006) Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21:69–78 [DOI] [PubMed] [Google Scholar]
- Lee CH, Poburko D, Kuo KH, Seow CY, van Breemen C. (2002) Ca(2+) oscillations, gradients, and homeostasis in vascular smooth muscle. Am J Physiol Heart Circ Physiol 282:H1571–H1583 [DOI] [PubMed] [Google Scholar]
- Lee CH, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, van Breemen C. (2001) The mechanism of phenylephrine-mediated [Ca(2+)](i) oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol 534:641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N. (1999) Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol 276:H1641–H1646 [DOI] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Basuroy S, Jaggar JH, Umstot ES, Fedinec AL. (2011a) Hydrogen sulfide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol 300:H440–H447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Jaggar JH. (2011b) Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol 301:H1–H11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger AL, Rossi CS, Greenawalt JW. (1963) Respiration-dependent accumulation of inorganic phosphate and Ca ions by rat liver mitochondria. Biochem Biophys Res Commun 10:444–448 [DOI] [PubMed] [Google Scholar]
- Lemos VS, Poburko D, Liao CH, Cole WC, van Breemen C. (2007) Na+ entry via TRPC6 causes Ca2+ entry via NCX reversal in ATP stimulated smooth muscle cells. Biochem Biophys Res Commun 352:130–134 [DOI] [PubMed] [Google Scholar]
- Lenting PJ, Casari C, Christophe OD, Denis CV. (2012) von Willebrand factor: the old, the new and the unknown. J Thromb Haemost 10:2428–2437 [DOI] [PubMed] [Google Scholar]
- Levine JD, Harlan JM, Harker LA, Joseph ML, Counts RB. (1982) Thrombin-mediated release of factor VIII antigen from human umbilical vein endothelial cells in culture. Blood 60:531–534 [PubMed] [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM. (2010) Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol 299:C1308–C1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Meng Q, Yu X, Jing X, Xu P, Luo D. (2012) Regulatory effect of connexin 43 on basal Ca2+ signaling in rat ventricular myocytes. PLoS ONE 7:e36165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ruan L, Sood SG, Papapetropoulos A, Fulton D, Venema RC. (2007) Role of eNOS phosphorylation at Ser-116 in regulation of eNOS activity in endothelial cells. Vascul Pharmacol 47:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang GH, Adebiyi A, Leo MD, McNally EM, Leffler CW, Jaggar JH. (2011) Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am J Physiol Heart Circ Physiol 300:H2088–H2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Regan CP, Manabe I, Owens GK, Day KH, Damon DN, Duling BR. (2007) Smooth muscle-targeted knockout of connexin43 enhances neointimal formation in response to vascular injury. Arterioscler Thromb Vasc Biol 27:1037–1042 [DOI] [PubMed] [Google Scholar]
- Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P. (2011) Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res 71:1550–1560 [DOI] [PubMed] [Google Scholar]
- Lima B, Forrester MT, Hess DT, Stamler JS. (2010) S-nitrosylation in cardiovascular signaling. Circ Res 106:633–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Zhou J, Zelenka PS, Takemoto DJ. (2003) Protein kinase Cgamma regulation of gap junction activity through caveolin-1-containing lipid rafts. Invest Ophthalmol Vis Sci 44:5259–5268 [DOI] [PubMed] [Google Scholar]
- Lin R, Martyn KD, Guyette CV, Lau AF, Warn-Cramer BJ. (2006) v-Src tyrosine phosphorylation of connexin43: regulation of gap junction communication and effects on cell transformation. Cell Commun Adhes 13:199–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15:1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Roberts TH, Hirschberg K. (2000) Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol 16:557–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- List BM, Klösch B, Völker C, Gorren AC, Sessa WC, Werner ER, Kukovetz WR, Schmidt K, Mayer B. (1997) Characterization of bovine endothelial nitric oxide synthase as a homodimer with down-regulated uncoupled NADPH oxidase activity: tetrahydrobiopterin binding kinetics and role of haem in dimerization. Biochem J 323:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TL, Xia J, Duling BR. (1995) Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res 76:498–504 [DOI] [PubMed] [Google Scholar]
- Liu J, García-Cardeña G, Sessa WC. (1995) Biosynthesis and palmitoylation of endothelial nitric oxide synthase: mutagenesis of palmitoylation sites, cysteines-15 and/or -26, argues against depalmitoylation-induced translocation of the enzyme. Biochemistry 34:12333–12340 [DOI] [PubMed] [Google Scholar]
- Liu J, Ormsby A, Oja-Tebbe N, Pagano PJ. (2004) Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. Circ Res 95:587–594 [DOI] [PubMed] [Google Scholar]
- Liu J, Sessa WC. (1994) Identification of covalently bound amino-terminal myristic acid in endothelial nitric oxide synthase. J Biol Chem 269:11691–11694 [PubMed] [Google Scholar]
- Lobysheva I, Rath G, Sekkali B, Bouzin C, Feron O, Gallez B, Dessy C, Balligand JL. (2011) Moderate caveolin-1 downregulation prevents NADPH oxidase-dependent endothelial nitric oxide synthase uncoupling by angiotensin II in endothelial cells. Arterioscler Thromb Vasc Biol 31:2098–2105 [DOI] [PubMed] [Google Scholar]
- Locke D, Harris AL. (2009) Connexin channels and phospholipids: association and modulation. BMC Biol 7:52–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke D, Liu J, Harris AL. (2005) Lipid rafts prepared by different methods contain different connexin channels, but gap junctions are not lipid rafts. Biochemistry 44:13027–13042 [DOI] [PubMed] [Google Scholar]
- Locke D, Stein T, Davies C, Morris J, Harris AL, Evans WH, Monaghan P, Gusterson B. (2004) Altered permeability and modulatory character of connexin channels during mammary gland development. Exp Cell Res 298:643–660 [DOI] [PubMed] [Google Scholar]
- Loesberg C, Gonsalves MD, Zandbergen J, Willems C, van Aken WG, Stel HV, Van Mourik JA, De Groot PG. (1983) The effect of calcium on the secretion of factor VIII-related antigen by cultured human endothelial cells. Biochim Biophys Acta 763:160–168 [DOI] [PubMed] [Google Scholar]
- Lohman AW, Billaud M, Isakson BE. (2012a) Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res 95:269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman AW, Billaud M, Straub AC, Johnstone SR, Best AK, Lee M, Barr K, Penuela S, Laird DW, Isakson BE. (2012b) Expression of pannexin isoforms in the systemic murine arterial network. J Vasc Res 49:405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman AW, Weaver JL, Billaud M, Sandilos JK, Griffiths R, Straub AC, Penuela S, Leitinger N, Laird DW, Bayliss DA, et al. (2012c) S-nitrosylation inhibits pannexin 1 channel function. J Biol Chem 287:39602–39612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo LW, Kanemitsu MY, Lau AF. (1999) In vivo association of pp60v-src and the gap-junction protein connexin 43 in v-src-transformed fibroblasts. Mol Carcinog 25:187–195 [PubMed] [Google Scholar]
- Looft-Wilson RC, Haug SJ, Neufer PD, Segal SS. (2004a) Independence of connexin expression and vasomotor conduction from sympathetic innervation in hamster feed arteries. Microcirculation 11:397–408 [DOI] [PubMed] [Google Scholar]
- Looft-Wilson RC, Payne GW, Segal SS. (2004b) Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol (1985) 97:1152–1158 [DOI] [PubMed] [Google Scholar]
- Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. (2008) Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res 80:445–452 [DOI] [PubMed] [Google Scholar]
- Loot AE, Schreiber JG, Fisslthaler B, Fleming I. (2009) Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med 206:2889–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López D, Rodríguez-Sinovas A, Agulló E, García A, Sánchez JA, García-Dorado D. (2009) Replacement of connexin 43 by connexin 32 in a knock-in mice model attenuates aortic endothelium-derived hyperpolarizing factor-mediated relaxation. Exp Physiol 94:1088–1097 [DOI] [PubMed] [Google Scholar]
- Lu W, Wang J, Peng G, Shimoda LA, Sylvester JT. (2009) Knockdown of stromal interaction molecule 1 attenuates store-operated Ca2+ entry and Ca2+ responses to acute hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 297:L17–L25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Wang J, Shimoda LA, Sylvester JT. (2008) Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 295:L104–L113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui-Roberts WW, Collinson LM, Hewlett LJ, Michaux G, Cutler DF. (2005) An AP-1/clathrin coat plays a novel and essential role in forming the Weibel-Palade bodies of endothelial cells. J Cell Biol 170:627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. (2006) The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol 174:815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurtz MM, Louis CF. (2007) Intracellular calcium regulation of connexin43. Am J Physiol Cell Physiol 293:C1806–C1813 [DOI] [PubMed] [Google Scholar]
- Ma M, Dahl G. (2006) Cosegregation of permeability and single-channel conductance in chimeric connexins. Biophys J 90:151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Hui H, Pelegrin P, Surprenant A. (2009) Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther 328:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Du J, Zhang P, Deng J, Liu J, Lam FF, Li RA, Huang Y, Jin J, Yao X. (2013) Functional role of TRPV4-KCa2.3 signaling in vascular endothelial cells in normal and streptozotocin-induced diabetic rats. Hypertension 62:134–139 [DOI] [PubMed] [Google Scholar]
- Maass K, Ghanem A, Kim JS, Saathoff M, Urschel S, Kirfel G, Grümmer R, Kretz M, Lewalter T, Tiemann K, et al. (2004) Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell 15:4597–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass K, Shibayama J, Chase SE, Willecke K, Delmar M. (2007) C-terminal truncation of connexin43 changes number, size, and localization of cardiac gap junction plaques. Circ Res 101:1283–1291 [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Thompson RJ. (2010) Non-junction functions of pannexin-1 channels. Trends Neurosci 33:93–102 [DOI] [PubMed] [Google Scholar]
- Madesh M, Hajnóczky G. (2001) VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol 155:1003–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madesh M, Hawkins BJ, Milovanova T, Bhanumathy CD, Joseph SK, Ramachandrarao SP, Sharma K, Kurosaki T, Fisher AB. (2005) Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol 170:1079–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R, Bilbe G, Rediske J, Lotz M. (1994) Inducible nitric oxide synthase from human articular chondrocytes: cDNA cloning and analysis of mRNA expression. Biochim Biophys Acta 1208:145–150 [DOI] [PubMed] [Google Scholar]
- Majoul I, Shirao T, Sekino Y, Duden R. (2007) Many faces of drebrin: from building dendritic spines and stabilizing gap junctions to shaping neurite-like cell processes. Histochem Cell Biol 127:355–361 [DOI] [PubMed] [Google Scholar]
- Majoul IV, Onichtchouk D, Butkevich E, Wenzel D, Chailakhyan LM, Duden R. (2009) Limiting transport steps and novel interactions of Connexin-43 along the secretory pathway. Histochem Cell Biol 132:263–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH. (2008) Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am J Physiol Cell Physiol 295:C221–C230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA, Buttle K, Rath BK, Marko M. (1998) Electron microscopic tomography of rat-liver mitochondria and their interaction with the endoplasmic reticulum. Biofactors 8:225–228 [DOI] [PubMed] [Google Scholar]
- Manneville JB, Etienne-Manneville S, Skehel P, Carter T, Ogden D, Ferenczi M. (2003) Interaction of the actin cytoskeleton with microtubules regulates secretory organelle movement near the plasma membrane in human endothelial cells. J Cell Sci 116:3927–3938 [DOI] [PubMed] [Google Scholar]
- Marín N, Zamorano P, Carrasco R, Mujica P, González FG, Quezada C, Meininger CJ, Boric MP, Durán WN, Sánchez FA. (2012) S-nitrosation of β-catenin and p120 catenin: a novel regulatory mechanism in endothelial hyperpermeability. Circ Res 111:553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. (1988) Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry 27:8706–8711 [DOI] [PubMed] [Google Scholar]
- Marrelli SP, O’neil RG, Brown RC, Bryan RM., Jr (2007) PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol 292:H1390–H1397 [DOI] [PubMed] [Google Scholar]
- Marsden PA, Schappert KT, Chen HS, Flowers M, Sundell CL, Wilcox JN, Lamas S, Michel T. (1992) Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett 307:287–293 [DOI] [PubMed] [Google Scholar]
- Martin PE, Hill NS, Kristensen B, Errington RJ, Rachael J TM. (2004) Ouabain exerts biphasic effects on connexin functionality and expression in vascular smooth muscle cells. Br J Pharmacol 141:374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AD, Hayrapetyan V, Moreno AP, Beyer EC. (2002) Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ Res 90:1100–1107 [DOI] [PubMed] [Google Scholar]
- Martínez AD, Sáez JC. (1999) Arachidonic acid-induced dye uncoupling in rat cortical astrocytes is mediated by arachidonic acid byproducts. Brain Res 816:411–423 [DOI] [PubMed] [Google Scholar]
- Martínez MC, Andriantsitohaina R. (2009) Reactive nitrogen species: molecular mechanisms and potential significance in health and disease. Antioxid Redox Signal 11:669–702 [DOI] [PubMed] [Google Scholar]
- Mathai JC, Missner A, Kügler P, Saparov SM, Zeidel ML, Lee JK, Pohl P. (2009) No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci USA 106:16633–16638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. (2005) Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res 97:399–407 [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H. (2003) Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Pharmacol Sci 92:1–6 [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A. (2002) Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 290:909–913 [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Morikawa K, Kubota H, Kunihiro I, Urakami-Harasawa L, Mukai Y, Hirakawa Y, Akaike T, Takeshita A. (2003) Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol 23:1224–1230 [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. (2000) Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106:1521–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O’Rourke B, et al. (2003) Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115:139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Morrell CN, Lowenstein CJ. (2004) Sphingosine 1-phosphate activates Weibel-Palade body exocytosis. Proc Natl Acad Sci USA 101:11483–11487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Rama A, Charolidi N, Dupont E, Severs NJ. (2007) Relationship of connexin43 expression to phenotypic modulation in cultured human aortic smooth muscle cells. Eur J Cell Biol 86:617–628 [DOI] [PubMed] [Google Scholar]
- McCarron JG, Muir TC. (1999) Mitochondrial regulation of the cytosolic Ca2+ concentration and the InsP3-sensitive Ca2+ store in guinea-pig colonic smooth muscle. J Physiol 516:149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron JG, Olson ML, Chalmers S. (2012) Mitochondrial regulation of cytosolic Ca2+ signals in smooth muscle. Pflugers Arch 464:51–62 [DOI] [PubMed] [Google Scholar]
- McDonald KK, Zharikov S, Block ER, Kilberg MS. (1997) A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem 272:31213–31216 [DOI] [PubMed] [Google Scholar]
- McDuffie JE, Coaxum SD, Maleque MA. (1999) 5-Hydroxytryptamine evokes endothelial nitric oxide synthase activation in bovine aortic endothelial cell cultures. Proc Soc Exp Biol Med 221:386–390 [DOI] [PubMed] [Google Scholar]
- McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. (1989) GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest 84:92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt NS. (1975) Ultrastructure of the myocardial sarcolemma. Circ Res 37:1–13 [DOI] [PubMed] [Google Scholar]
- Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. (2008) Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27:3092–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Milner RE, Burns K, Opas M. (1992) Calreticulin. Biochem J 285:681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel JB, Feron O, Sacks D, Michel T. (1997) Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem 272:15583–15586 [DOI] [PubMed] [Google Scholar]
- Michel T. (1999) Targeting and translocation of endothelial nitric oxide synthase. Braz J Med Biol Res 32:1361–1366 [DOI] [PubMed] [Google Scholar]
- Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. (2002) Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem 277:42344–42351 [DOI] [PubMed] [Google Scholar]
- Milkau M, Kohler R, de Wit C. (2010) Crucial importance of the endothelial K+ channel SK3 and connexin40 in arteriolar dilations during skeletal muscle contraction. FASEB J 24:3572–3579 [DOI] [PubMed] [Google Scholar]
- Miller EW, Dickinson BC, Chang CJ. (2010) Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci USA 107:15681–15686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner RE, Famulski KS, Michalak M. (1992) Calcium binding proteins in the sarcoplasmic/endoplasmic reticulum of muscle and nonmuscle cells. Mol Cell Biochem 112:1–13 [DOI] [PubMed] [Google Scholar]
- Mineo C, Shaul PW. (2012) Regulation of eNOS in caveolae. Adv Exp Med Biol 729:51–62 [DOI] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI. (2000) Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 404:355–362 [DOI] [PubMed] [Google Scholar]
- Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. (2003) Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92:e31–e40 [DOI] [PubMed] [Google Scholar]
- Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, Touyz RM. (2011) Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 120:131–141 [DOI] [PubMed] [Google Scholar]
- Montezano AC, Touyz RM. (2012) Molecular mechanisms of hypertension—reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol 28:288–295 [DOI] [PubMed] [Google Scholar]
- Moore DH, Ruska H. (1957) The fine structure of capillaries and small arteries. J Biophys Biochem Cytol 3:457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno AP. (2004) Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc Res 62:276–286 [DOI] [PubMed] [Google Scholar]
- Moreno AP, de Carvalho AC, Verselis V, Eghbali B, Spray DC. (1991) Voltage-dependent gap junction channels are formed by connexin32, the major gap junction protein of rat liver. Biophys J 59:920–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa K, Shimokawa H, Matoba T, Kubota H, Akaike T, Talukder MA, Hatanaka M, Fujiki T, Maeda H, Takahashi S, et al. (2003) Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J Clin Invest 112:1871–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. (2002) The exocyst is a Ral effector complex. Nat Cell Biol 4:66–72 [DOI] [PubMed] [Google Scholar]
- Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, et al. (2008) Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem 283:8014–8022 [DOI] [PubMed] [Google Scholar]
- Muller G, Morawietz H. (2009) Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid Redox Signal 11:1711–1731 [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Aalkjaer C. (1990) Structure and function of small arteries. Physiol Rev 70:921–961 [DOI] [PubMed] [Google Scholar]
- Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. (2007) Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204:2373–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SA, Fletcher WH. (1984) Hormone-induced intercellular signal transfer dissociates cyclic AMP-dependent protein kinase. J Cell Biol 98:1710–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T, Vanhoutte PM. (1992) Characterization of endothelium-dependent relaxations resistant to nitro-L-arginine in the porcine coronary artery. Br J Pharmacol 107:1102–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Oyamada M, Dai P, Nakagami T, Kinoshita S, Takamatsu T. (2008) Connexin43 knockdown accelerates wound healing but inhibits mesenchymal transition after corneal endothelial injury in vivo. Invest Ophthalmol Vis Sci 49:93–104 [DOI] [PubMed] [Google Scholar]
- Naraghi M, Neher E. (1997) Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci 17:6961–6973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan J, Imig M, Roman RJ, Harder DR. (1994) Pressurization of isolated renal arteries increases inositol trisphosphate and diacylglycerol. Am J Physiol 266:H1840–H1845 [DOI] [PubMed] [Google Scholar]
- Nathan C. (1992) Nitric oxide as a secretory product of mammalian cells. FASEB J 6:3051–3064 [PubMed] [Google Scholar]
- Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. (2008) STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol 586:5383–5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navedo MF, Amberg GC, Votaw VS, Santana LF. (2005) Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci USA 102:11112–11117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. (2005) Cross-presentation by intercellular peptide transfer through gap junctions. Nature 434:83–88 [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. (1995) Relaxation of arterial smooth muscle by calcium sparks. Science 270:633–637 [DOI] [PubMed] [Google Scholar]
- Nelson MT, Patlak JB, Worley JF, Standen NB. (1990) Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol 259:C3–C18 [DOI] [PubMed] [Google Scholar]
- Nevin RL. (2011) Mefloquine neurotoxicity and gap junction blockade: critical insights in drug repositioning. Neurotoxicology 32:986–987, author reply 987 [DOI] [PubMed] [Google Scholar]
- Ng LC, Airey JA, Hume JR. (2010a) The contribution of TRPC1 and STIM1 to capacitative Ca(2+) entry in pulmonary artery. Adv Exp Med Biol 661:123–135 [DOI] [PubMed] [Google Scholar]
- Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. (2009) TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol 587:2429–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LC, O’Neill KG, French D, Airey JA, Singer CA, Tian H, Shen XM, Hume JR. (2012) TRPC1 and Orai1 interact with STIM1 and mediate capacitative Ca(2+) entry caused by acute hypoxia in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 303:C1156–C1172 [DOI] [PubMed] [Google Scholar]
- Ng LC, Ramduny D, Airey JA, Singer CA, Keller PS, Shen XM, Tian H, Valencik M, Hume JR. (2010b) Orai1 interacts with STIM1 and mediates capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 299:C1079–C1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen H, Harz H, Bedner P, Krämer K, Willecke K. (2000) Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci 113:1365–1372 [DOI] [PubMed] [Google Scholar]
- Niger C, Hebert C, Stains JP. (2010) Interaction of connexin43 and protein kinase C-delta during FGF2 signaling. BMC Biochem 11, article 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli E, Shirokova N. (2007) A guide to sparkology: the taxonomy of elementary cellular Ca2+ signaling events. Cell Calcium 42:379–387 [DOI] [PubMed] [Google Scholar]
- Nightingale TD, Pattni K, Hume AN, Seabra MC, Cutler DF. (2009) Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood 113:5010–5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale TD, White IJ, Doyle EL, Turmaine M, Harrison-Lavoie KJ, Webb KF, Cramer LP, Cutler DF. (2011) Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J Cell Biol 194:613–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, Ide T, Suzuki K, Inoue K, Nagao T, et al. (2008) P2Y6 receptor-Galpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J 27:3104–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S, Fukaya M, Tsujioka T, Wu D, Watanabe M. (2007) Phospholipase Cbeta3 is distributed in both somatodendritic and axonal compartments and localized around perisynapse and smooth endoplasmic reticulum in mouse Purkinje cell subsets. Eur J Neurosci 25:659–672 [DOI] [PubMed] [Google Scholar]
- Nunoi H, Rotrosen D, Gallin JI, Malech HL. (1988) Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science 242:1298–1301 [DOI] [PubMed] [Google Scholar]
- O’Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG. (2011) A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res 108:704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Firth AL, Smith KA, Maliakal MV, Yuan JX. (2012) PDGF enhances store-operated Ca2+ entry by upregulating STIM1/Orai1 via activation of Akt/mTOR in human pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 302:C405–C411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Akiyama M, Takeda M, Akita N, Yoshida K, Hayashi T, Suzuki K. (2011) Connexin32 protects against vascular inflammation by modulating inflammatory cytokine expression by endothelial cells. Exp Cell Res 317:348–355 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Akiyama M, Takeda M, Gabazza EC, Hayashi T, Suzuki K. (2009) Connexin32 is expressed in vascular endothelial cells and participates in gap-junction intercellular communication. Biochem Biophys Res Commun 382:264–268 [DOI] [PubMed] [Google Scholar]
- Olson KR. (2009) Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta 1787:856–863 [DOI] [PubMed] [Google Scholar]
- Olson ML, Chalmers S, McCarron JG. (2010) Mitochondrial Ca2+ uptake increases Ca2+ release from inositol 1,4,5-trisphosphate receptor clusters in smooth muscle cells. J Biol Chem 285:2040–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. (1998) Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res 83:932–939 [DOI] [PubMed] [Google Scholar]
- Omori Y, Yamasaki H. (1999) Gap junction proteins connexin32 and connexin43 partially acquire growth-suppressive function in HeLa cells by deletion of their C-terminal tails. Carcinogenesis 20:1913–1918 [DOI] [PubMed] [Google Scholar]
- Ong HL, Liu X, Tsaneva-Atanasova K, Singh BB, Bandyopadhyay BC, Swaim WD, Russell JT, Hegde RS, Sherman A, Ambudkar IS. (2007) Relocalization of STIM1 for activation of store-operated Ca(2+) entry is determined by the depletion of subplasma membrane endoplasmic reticulum Ca(2+) store. J Biol Chem 282:12176–12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuka K, Watanabe Y, Yasuda M, Takayasu M. (2012) Adiponectin activates endothelial nitric oxide synthase through AMPK signaling after subarachnoid hemorrhage. Neurosci Lett 514:2–5 [DOI] [PubMed] [Google Scholar]
- Ozaka T, Doi Y, Kayashima K, Fujimoto S. (1997) Weibel-Palade bodies as a storage site of calcitonin gene-related peptide and endothelin-1 in blood vessels of the rat carotid body. Anat Rec 247:388–394 [DOI] [PubMed] [Google Scholar]
- Ozkan MH, Uma S. (2009) Does endothelium-derived hyperpolarizing factor play a role in endothelium-dependent component of electrical field stimulation-induced vasorelaxation of rat mesenteric arterial rings? J Cardiovasc Pharmacol 53:30–37 [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatinus JA, O’Quinn MP, Barker RJ, Harris BS, Jourdan J, Gourdie RG. (2011a) ZO-1 determines adherens and gap junction localization at intercalated disks. Am J Physiol Heart Circ Physiol 300:H583–H594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatinus JA, Rhett JM, Gourdie RG. (2011b) Enhanced PKCε mediated phosphorylation of connexin43 at serine 368 by a carboxyl-terminal mimetic peptide is dependent on injury. Channels (Austin) 5:236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatinus JA, Rhett JM, Gourdie RG. (2012) The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim Biophys Acta 1818:1831–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, Moncada S. (1988) Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 333:664–666 [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526 [DOI] [PubMed] [Google Scholar]
- Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E. (2012) SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell 149:425–438 [DOI] [PubMed] [Google Scholar]
- Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. (2000) A ubiquitous family of putative gap junction molecules. Curr Biol 10:R473–R474 [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. (1997) Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 100:3131–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB. (2008) Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol 586:3043–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. (2009a) STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136:876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Yamada K, Kojo A, Sato S, Onozuka M, Yamamoto T. (2009b) Drebrin (developmentally regulated brain protein) is associated with axo-somatic synapses and neuronal gap junctions in rat mesencephalic trigeminal nucleus. Neurosci Lett 461:95–99 [DOI] [PubMed] [Google Scholar]
- Parkos CA, Allen RA, Cochrane CG, Jesaitis AJ. (1987) Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest 80:732–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos CA, Dinauer MC, Walker LE, Allen RA, Jesaitis AJ, Orkin SH. (1988) Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci USA 85:3319–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons SJ, Hill A, Waldron GJ, Plane F, Garland CJ. (1994) The relative importance of nitric oxide and nitric oxide-independent mechanisms in acetylcholine-evoked dilatation of the rat mesenteric bed. Br J Pharmacol 113:1275–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron M, Raffaello A, Granatiero V, Tosatto A, Merli G, De Stefani D, Wright L, Pallafacchina G, Terrin A, Mammucari C, et al. (2013) The mitochondrial calcium uniporter (MCU): molecular identity and physiological roles. J Biol Chem 288:10750–10758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. (1991) Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 115:1077–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch WK, Grund C, Kuhn C, Schnölzer M, Spring H, Schmelz M, Franke WW. (1999) Drebrin is a widespread actin-associating protein enriched at junctional plaques, defining a specific microfilament anchorage system in polar epithelial cells. Eur J Cell Biol 78:767–778 [DOI] [PubMed] [Google Scholar]
- Peitsch WK, Hofmann I, Bulkescher J, Hergt M, Spring H, Bleyl U, Goerdt S, Franke WW. (2005) Drebrin, an actin-binding, cell-type characteristic protein: induction and localization in epithelial skin tumors and cultured keratinocytes. J Invest Dermatol 125:761–774 [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS, Meda P. (1992) Basic fibroblast growth factor increases junctional communication and connexin 43 expression in microvascular endothelial cells. J Cell Physiol 153:196–205 [DOI] [PubMed] [Google Scholar]
- Pepper MS, Spray DC, Chanson M, Montesano R, Orci L, Meda P. (1989) Junctional communication is induced in migrating capillary endothelial cells. J Cell Biol 109:3027–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C. (1978) Calcium effects on gap junction structure and cell coupling. Nature 271:669–671 [DOI] [PubMed] [Google Scholar]
- Pérez GJ, Bonev AD, Nelson MT. (2001) Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol 281:C1769–C1775 [DOI] [PubMed] [Google Scholar]
- Pfenniger A, Wong C, Sutter E, Cuhlmann S, Dunoyer-Geindre S, Mach F, Horrevoets AJ, Evans PC, Krams R, Kwak BR. (2012) Shear stress modulates the expression of the atheroprotective protein Cx37 in endothelial cells. J Mol Cell Cardiol 53:299–309 [DOI] [PubMed] [Google Scholar]
- Pinto A, Abraham NG, Mullane KM. (1987) Arachidonic acid-induced endothelial-dependent relaxations of canine coronary arteries: contribution of a cytochrome P-450-dependent pathway. J Pharmacol Exp Ther 240:856–863 [PubMed] [Google Scholar]
- Plane F, Garland CJ. (1993) Differential effects of acetylcholine, nitric oxide and levcromakalim on smooth muscle membrane potential and tone in the rabbit basilar artery. Br J Pharmacol 110:651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane F, Garland CJ. (1994) Smooth muscle hyperpolarization and relaxation to acetylcholine in the rabbit basilar artery. J Auton Nerv Syst 49 (Suppl):S15–S18 [DOI] [PubMed] [Google Scholar]
- Plane F, Pearson T, Garland CJ. (1995) Multiple pathways underlying endothelium-dependent relaxation in the rabbit isolated femoral artery. Br J Pharmacol 115:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbati AV, Stein A, Fasshauer D. (2006) N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science 313:673–676 [DOI] [PubMed] [Google Scholar]
- Poburko D, Fameli N, Kuo KH, van Breemen C. (2008) Ca2+ signaling in smooth muscle: TRPC6, NCX and LNats in nanodomains. Channels (Austin) 2:10–12 [DOI] [PubMed] [Google Scholar]
- Poburko D, Kuo KH, Dai J, Lee CH, van Breemen C. (2004) Organellar junctions promote targeted Ca2+ signaling in smooth muscle: why two membranes are better than one. Trends Pharmacol Sci 25:8–15 [DOI] [PubMed] [Google Scholar]
- Poburko D, Liao CH, Lemos VS, Lin E, Maruyama Y, Cole WC, van Breemen C. (2007) Transient receptor potential channel 6-mediated, localized cytosolic [Na+] transients drive Na+/Ca2+ exchanger-mediated Ca2+ entry in purinergically stimulated aorta smooth muscle cells. Circ Res 101:1030–1038 [DOI] [PubMed] [Google Scholar]
- Poburko D, Liao CH, van Breemen C, Demaurex N. (2009) Mitochondrial regulation of sarcoplasmic reticulum Ca2+ content in vascular smooth muscle cells. Circ Res 104:104–112 [DOI] [PubMed] [Google Scholar]
- Pomposiello S, Rhaleb NE, Alva M, Carretero OA. (1999) Reactive oxygen species: role in the relaxation induced by bradykinin or arachidonic acid via EDHF in isolated porcine coronary arteries. J Cardiovasc Pharmacol 34:567–574 [DOI] [PubMed] [Google Scholar]
- Ponsaerts R, De Vuyst E, Retamal M, D’hondt C, Vermeire D, Wang N, De Smedt H, Zimmermann P, Himpens B, Vereecke J, et al. (2010) Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J 24:4378–4395 [DOI] [PubMed] [Google Scholar]
- Popescu LM, Gherghiceanu M, Mandache E, Cretoiu D. (2006) Caveolae in smooth muscles: nanocontacts. J Cell Mol Med 10:960–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. (2009) Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J 23:2425–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. (2006) Orai1 is an essential pore subunit of the CRAC channel. Nature 443:230–233 [DOI] [PubMed] [Google Scholar]
- Presta A, Liu J, Sessa WC, Stuehr DJ. (1997) Substrate binding and calmodulin binding to endothelial nitric oxide synthase coregulate its enzymatic activity. Nitric Oxide 1:74-87 [DOI] [PubMed] [Google Scholar]
- Pueyo ME, Arnal JF, Rami J, Michel JB. (1998) Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am J Physiol 274:C214–C220 [DOI] [PubMed] [Google Scholar]
- Pulido IR, Jahn R, Gerke V. (2011) VAMP3 is associated with endothelial weibel-palade bodies and participates in their Ca(2+)-dependent exocytosis. Biochim Biophys Acta 1813:1038–1044 [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr (1986) A model for receptor-regulated calcium entry. Cell Calcium 7:1–12 [DOI] [PubMed] [Google Scholar]
- Putney JW, Bird GS. (2008) Cytoplasmic calcium oscillations and store-operated calcium influx. J Physiol 586:3055–3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Dahl G. (2004) Accessibility of Cx46 hemichannels for uncharged molecules and its modulation by voltage. Biophys J 86:1502–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo LA, Cortes SF, Alvarez-Leite JI, Lemos VS. (2003) Endothelium dysfunction in LDL receptor knockout mice: a role for H2O2. Br J Pharmacol 138:1215–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeymaekers L, Verbist J, Wuytack F, Plessers L, Casteels R. (1993) Expression of Ca2+ binding proteins of the sarcoplasmic reticulum of striated muscle in the endoplasmic reticulum of pig smooth muscles. Cell Calcium 14:581–589 [DOI] [PubMed] [Google Scholar]
- Rand VE, Garland CJ. (1992) Endothelium-dependent relaxation to acetylcholine in the rabbit basilar artery: importance of membrane hyperpolarization. Br J Pharmacol 106:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R. (2002) Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol 159:613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath G, Saliez J, Behets G, Romero-Perez M, Leon-Gomez E, Bouzin C, Vriens J, Nilius B, Feron O, Dessy C. (2012) Vascular hypoxic preconditioning relies on TRPV4-dependent calcium influx and proper intercellular gap junctions communication. Arterioscler Thromb Vasc Biol 32:2241–2249 [DOI] [PubMed] [Google Scholar]
- Rembold CM, Van Riper DA, Chen XL. (1995) Focal [Ca2+]i increases detected by aequorin but not by fura-2 in histamine- and caffeine-stimulated swine carotid artery. J Physiol 488:549–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal MA, Cortés CJ, Reuss L, Bennett MV, Sáez JC. (2006) S-Nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA 103:4475–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal MA, Schalper KA, Shoji KF, Bennett MV, Sáez JC. (2007) Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc Natl Acad Sci USA 104:8322–8327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee DY, Zhao XQ, Francis RJ, Huang GY, Mably JD, Lo CW. (2009) Connexin 43 regulates epicardial cell polarity and migration in coronary vascular development. Development 136:3185–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM, Gourdie RG. (2012) The perinexus: a new feature of Cx43 gap junction organization. Heart Rhythm 9:619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM, Jourdan J, Gourdie RG. (2011) Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell 22:1516–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MA, Walker BR. (2012) Regulation of endothelial BK channels by heme oxygenase-derived carbon monoxide and caveolin-1. Am J Physiol Cell Physiol 303:C92–C101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280:1763–1766 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Simpson AW, Brini M, Pozzan T. (1992) Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358:325–327 [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Michel T. (1995) Mutagenesis of palmitoylation sites in endothelial nitric oxide synthase identifies a novel motif for dual acylation and subcellular targeting. Proc Natl Acad Sci USA 92:11776–11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo Pulido I, Nightingale TD, Darchen F, Seabra MC, Cutler DF, Gerke V. (2011) Myosin Va acts in concert with Rab27a and MyRIP to regulate acute von-Willebrand factor release from endothelial cells. Traffic 12:1371–1382 [DOI] [PubMed] [Google Scholar]
- Rondaij MG, Bierings R, Kragt A, Gijzen KA, Sellink E, van Mourik JA, Fernandez-Borja M, Voorberg J. (2006a) Dynein-dynactin complex mediates protein kinase A-dependent clustering of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol 26:49–55 [DOI] [PubMed] [Google Scholar]
- Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. (2006b) Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol 26:1002–1007 [DOI] [PubMed] [Google Scholar]
- Rondaij MG, Bierings R, van Agtmaal EL, Gijzen KA, Sellink E, Kragt A, Ferguson SS, Mertens K, Hannah MJ, van Mourik JA, et al. (2008) Guanine exchange factor RalGDS mediates exocytosis of Weibel-Palade bodies from endothelial cells. Blood 112:56–63 [DOI] [PubMed] [Google Scholar]
- Rondaij MG, Sellink E, Gijzen KA, ten Klooster JP, Hordijk PL, van Mourik JA, Voorberg J. (2004) Small GTP-binding protein Ral is involved in cAMP-mediated release of von Willebrand factor from endothelial cells. Arterioscler Thromb Vasc Biol 24:1315–1320 [DOI] [PubMed] [Google Scholar]
- Ronson RS, Nakamura M, Vinten-Johansen J. (1999) The cardiovascular effects and implications of peroxynitrite. Cardiovasc Res 44:47–59 [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169:435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. (1962) Subsurface cisterns and their relationship to the neuronal plasma membrane. J Cell Biol 13:405–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosolowsky M, Campbell WB. (1993) Role of PGI2 and epoxyeicosatrienoic acids in relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol 264:H327–H335 [DOI] [PubMed] [Google Scholar]
- Roth MG. (2008) Molecular mechanisms of PLD function in membrane traffic. Traffic 9:1233–1239 [DOI] [PubMed] [Google Scholar]
- Rotrosen D, Yeung CL, Leto TL, Malech HL, Kwong CH. (1992) Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science 256:1459–1462 [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. (1987) Nature of endothelium-derived relaxing factor: are there two relaxing mediators? Circ Res 61:II61–II67 [PubMed] [Google Scholar]
- Russell FD, Skepper JN, Davenport AP. (1998) Human endothelial cell storage granules: a novel intracellular site for isoforms of the endothelin-converting enzyme. Circ Res 83:314–321 [DOI] [PubMed] [Google Scholar]
- Rusu L, Andreeva A, Visintine DJ, Kim K, Vogel SM, Stojanovic-Terpo A, Chernaya O, Liu G, Bakhshi FR, Haberichter SL, et al. (2014) G protein-dependent basal and evoked endothelial cell vWF secretion. Blood 123:442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler JE. (1998) Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem 67:395–424 [DOI] [PubMed] [Google Scholar]
- Sáez JC, Connor JA, Spray DC, Bennett MV. (1989) Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci USA 86:2708–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV. (2010) Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res 316:2377–2389 [DOI] [PubMed] [Google Scholar]
- Sagar GD, Larson DM. (2006) Carbenoxolone inhibits junctional transfer and upregulates connexin43 expression by a protein kinase A-dependent pathway. J Cell Biochem 98:1543–1551 [DOI] [PubMed] [Google Scholar]
- Saidi Brikci-Nigassa A, Clement MJ, Ha-Duong T, Adjadj E, Ziani L, Pastre D, Curmi PA, Savarin P. (2012) Phosphorylation controls the interaction of the connexin43 C-terminal domain with tubulin and microtubules. Biochemistry 51:4331–4342 [DOI] [PubMed] [Google Scholar]
- Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, et al. (2008) Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117:1065–1074 [DOI] [PubMed] [Google Scholar]
- Samuels SE, Lipitz JB, Wang J, Dahl G, Muller KJ. (2013) Arachidonic acid closes innexin/pannexin channels and thereby inhibits microglia cell movement to a nerve injury. Dev Neurobiol 73:621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Bramich NJ, Bandi HP, Rummery NM, Hill CE. (2003a) Structure, function, and endothelium-derived hyperpolarizing factor in the caudal artery of the SHR and WKY rat. Arterioscler Thromb Vasc Biol 23:822–828 [DOI] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. (2000) Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res 86:341–346 [DOI] [PubMed] [Google Scholar]
- Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. (2003b) Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res 60:643–653 [DOI] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. (2006) Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat 209:689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. (2012) Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation 19:403–415 [DOI] [PubMed] [Google Scholar]
- Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. (2002) Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res 90:1108–1113 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Köhler R, Wulff H. (2009) Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol 75:281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Matsu-Ura T, Enomoto M, Nakamura H, Michikawa T, Mikoshiba K. (2011) Highly cooperative dependence of sarco/endoplasmic reticulum calcium ATPase SERCA2a pump activity on cytosolic calcium in living cells. J Biol Chem 286:20591–20599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter T, Bohnensack R. (1999) Serotonin-induced secretion of von Willebrand factor from human umbilical vein endothelial cells via the cyclic AMP-signaling systems independent of increased cytoplasmic calcium concentration. Biochem Pharmacol 57:1191–1197 [DOI] [PubMed] [Google Scholar]
- Schmidt K, Dubrovska G, Nielsen G, Fesüs G, Uhrenholt TR, Hansen PB, Gudermann T, Dietrich A, Gollasch M, de Wit C, et al. (2010) Amplification of EDHF-type vasodilatations in TRPC1-deficient mice. Br J Pharmacol 161:1722–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt VJ, Jobs A, von Maltzahn J, Wörsdörfer P, Willecke K, de Wit C. (2012) Connexin45 is expressed in vascular smooth muscle but its function remains elusive. PLoS ONE 7:e42287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Jacobson BS, Dvorak AM. (1995) Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca(2+)-ATPase, and inositol trisphosphate receptor. Proc Natl Acad Sci USA 92:1759–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert AL, Schubert W, Spray DC, Lisanti MP. (2002) Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry 41:5754–5764 [DOI] [PubMed] [Google Scholar]
- Scotland RS, Morales-Ruiz M, Chen Y, Yu J, Rudic RD, Fulton D, Gratton JP, Sessa WC. (2002) Functional reconstitution of endothelial nitric oxide synthase reveals the importance of serine 1179 in endothelium-dependent vasomotion. Circ Res 90:904–910 [DOI] [PubMed] [Google Scholar]
- Sedeek M, Montezano AC, Hebert RL, Gray SP, Di Marco E, Jha JC, Cooper ME, Jandeleit-Dahm K, Schiffrin EL, Wilkinson-Berka JL, et al. (2012) Oxidative stress, Nox isoforms and complications of diabetes—potential targets for novel therapies. J Cardiovasc Transl Res 5:509–518 [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. (1986) Flow control among microvessels coordinated by intercellular conduction. Science 234:868–870 [DOI] [PubMed] [Google Scholar]
- Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. (2007) Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res 75:349–358 [DOI] [PubMed] [Google Scholar]
- Senadheera S, Kim Y, Grayson TH, Toemoe S, Kochukov MY, Abramowitz J, Housley GD, Bertrand RL, Chadha PS, Bertrand PP, et al. (2012) Transient receptor potential canonical type 3 channels facilitate endothelium-derived hyperpolarization-mediated resistance artery vasodilator activity. Cardiovasc Res 95:439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa WC. (2004) eNOS at a glance. J Cell Sci 117:2427–2429 [DOI] [PubMed] [Google Scholar]
- Sessa WC, Barber CM, Lynch KR. (1993) Mutation of N-myristoylation site converts endothelial cell nitric oxide synthase from a membrane to a cytosolic protein. Circ Res 72:921–924 [DOI] [PubMed] [Google Scholar]
- Sessa WC, Harrison JK, Barber CM, Zeng D, Durieux ME, D’Angelo DD, Lynch KR, Peach MJ. (1992) Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem 267:15274–15276 [PubMed] [Google Scholar]
- Severs NJ. (2007) The carboxy terminal domain of connexin43: from molecular regulation of the gap junction channel to supramolecular organization of the intercalated disk. Circ Res 101:1213–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VS, Traylor TG, Gardiner R, Mizukami H. (1987) Reaction of nitric oxide with heme proteins and model compounds of hemoglobin. Biochemistry 26:3837–3843 [DOI] [PubMed] [Google Scholar]
- Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. (1996) Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem 271:6518–6522 [DOI] [PubMed] [Google Scholar]
- Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. (2007) Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 128:547–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Wang LH, Zheng LR, Zhu JH, Hu SJ. (2010) Lovastatin inhibits gap junctional communication in cultured aortic smooth muscle cells. J Cardiovasc Pharmacol Ther 15:296–302 [DOI] [PubMed] [Google Scholar]
- Sherman PA, Laubach VE, Reep BR, Wood ER. (1993) Purification and cDNA sequence of an inducible nitric oxide synthase from a human tumor cell line. Biochemistry 32:11600–11605 [DOI] [PubMed] [Google Scholar]
- Shimokawa H. (2010) Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch 459:915–922 [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, et al. (1996) The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28:703–711 [DOI] [PubMed] [Google Scholar]
- Si H, Heyken WT, Wölfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, et al. (2006) Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res 99:537–544 [DOI] [PubMed] [Google Scholar]
- Siddall HK, Yellon DM, Ong SB, Mukherjee UA, Burke N, Hall AR, Angelova PR, Ludtmann MH, Deas E, Davidson SM, et al. (2013) Loss of PINK1 increases the heart’s vulnerability to ischemia-reperfusion injury. PLoS ONE 8:e62400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui MR, Komarova YA, Vogel SM, Gao X, Bonini MG, Rajasingh J, Zhao YY, Brovkovych V, Malik AB. (2011) Caveolin-1-eNOS signaling promotes p190RhoGAP-A nitration and endothelial permeability. J Cell Biol 193:841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl G. (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 295:C761–C767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. (2009) The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem 284:18143–18151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek J, Churko J, Shao Q, Laird DW. (2009) Cx43 has distinct mobility within plasma-membrane domains, indicative of progressive formation of gap-junction plaques. J Cell Sci 122:554–562 [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR. (2003) Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci 116:2223–2236 [DOI] [PubMed] [Google Scholar]
- Singh D, Solan JL, Taffet SM, Javier R, Lampe PD. (2005) Connexin 43 interacts with zona occludens-1 and -2 proteins in a cell cycle stage-specific manner. J Biol Chem 280:30416–30421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TD, Mohankumar A, Minogue PJ, Beyer EC, Berthoud VM, Koval M. (2012) Cytoplasmic amino acids within the membrane interface region influence connexin oligomerization. J Membr Biol 245:221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey CG, Heistad DD, Faraci FM. (1997) Mechanisms of bradykinin-induced cerebral vasodilatation in rats. Evidence that reactive oxygen species activate K+ channels. Stroke 28:2290–2294, discussion 2295 [DOI] [PubMed] [Google Scholar]
- Solan JL, Fry MD, TenBroek EM, Lampe PD. (2003) Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci 116:2203–2211 [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. (2009) Connexin43 phosphorylation: structural changes and biological effects. Biochem J 419:261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP. (1985) Excitation-contraction coupling and the ultrastructure of smooth muscle. Circ Res 57:497–507 [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Devine CE, Somlyo AV, North SR. (1971) Sarcoplasmic reticulum and the temperature-dependent contraction of smooth muscle in calcium-free solutions. J Cell Biol 51:722–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV, Shuman H. (1979) Electron probe analysis of vascular smooth muscle. Composition of mitochondria, nuclei, and cytoplasm. J Cell Biol 81:316–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. (2012) Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen CM, Giese I, Braunstein TH, Brasen JC, Salomonsson M, Holstein-Rathlou NH. (2012) Role of connexin40 in the autoregulatory response of the afferent arteriole. Am J Physiol Renal Physiol 303:F855–F863 [DOI] [PubMed] [Google Scholar]
- Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. (2004a) Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem 279:54695–54701 [DOI] [PubMed] [Google Scholar]
- Sorgen PL, Duffy HS, Spray DC, Delmar M. (2004b) pH-dependent dimerization of the carboxyl terminal domain of Cx43. Biophys J 87:574–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, et al. (2011) Pannexin channels are not gap junction hemichannels. Channels (Austin) 5:193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosunov AA, Hassall CJ, Loesch A, Turmaine M, Burnstock G. (1995) Ultrastructural investigation of nitric oxide synthase-immunoreactive nerves associated with coronary blood vessels of rat and guinea-pig. Cell Tissue Res 280:575–582 [DOI] [PubMed] [Google Scholar]
- Sotkis A, Wang XG, Yasumura T, Peracchia LL, Persechini A, Rash JE, Peracchia C. (2001) Calmodulin colocalizes with connexins and plays a direct role in gap junction channel gating. Cell Commun Adhes 8:277–281 [DOI] [PubMed] [Google Scholar]
- Sparacino-Watkins CE, Lai YC, Gladwin MT. (2012) Nitrate-nitrite-nitric oxide pathway in pulmonary arterial hypertension therapeutics. Circulation 125:2824–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Burt JM. (1990) Structure-activity relations of the cardiac gap junction channel. Am J Physiol 258:C195–C205 [DOI] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. (2006) Functional connexin “hemichannels”: a critical appraisal. Glia 54:758–773 [DOI] [PubMed] [Google Scholar]
- Srikanth S, Jew M, Kim KD, Yee MK, Abramson J, Gwack Y. (2012) Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci USA 109:8682–8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg RB, Fletcher WH. (1990) The hormone-induced regulation of contact-dependent cell-cell communication by phosphorylation. Endocr Rev 11:302–325 [DOI] [PubMed] [Google Scholar]
- Stauch K, Kieken F, Sorgen P. (2012) Characterization of the structure and intermolecular interactions between the connexin 32 carboxyl-terminal domain and the protein partners synapse-associated protein 97 and calmodulin. J Biol Chem 287:27771–27788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Weber G, Wahl MC, Jahn R. (2009) Helical extension of the neuronal SNARE complex into the membrane. Nature 460:525–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10:513–525 [DOI] [PubMed] [Google Scholar]
- Stergiopoulos K, Alvarado JL, Mastroianni M, Ek-Vitorin JF, Taffet SM, Delmar M. (1999) Hetero-domain interactions as a mechanism for the regulation of connexin channels. Circ Res 84:1144–1155 [DOI] [PubMed] [Google Scholar]
- Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, et al. (2011) Compartmentalized connexin 43 S-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vasc Biol 31:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub AC, Johnstone SR, Heberlein KR, Rizzo MJ, Best AK, Boitano S, Isakson BE. (2010) Site-specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium. J Vasc Res 47:277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, et al. (2012) Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 491:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D, Pou S, Rosen GM. (2001) Oxygen reduction by nitric-oxide synthases. J Biol Chem 276:14533–14536 [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Kwon NS, Gross SS, Thiel BA, Levi R, Nathan CF. (1989) Synthesis of nitrogen oxides from l-arginine by macrophage cytosol: requirement for inducible and constitutive components. Biochem Biophys Res Commun 161:420–426 [DOI] [PubMed] [Google Scholar]
- Suematsu M, Kashiwagi S, Sano T, Goda N, Shinoda Y, Ishimura Y. (1994) Carbon monoxide as an endogenous modulator of hepatic vascular perfusion. Biochem Biophys Res Commun 205:1333–1337 [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Hata K, Mizuki K, Ito T, Kage Y, Sakaki Y, Fukumaki Y, Nakamura M, Takeshige K. (1996) Assembly and activation of the phagocyte NADPH oxidase. Specific interaction of the N-terminal Src homology 3 domain of p47phox with p22phox is required for activation of the NADPH oxidase. J Biol Chem 271:22152–22158 [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395:347–353 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Eguchi K, Ohtsu H, Higuchi S, Dhobale S, Frank GD, Motley ED, Eguchi S. (2006) Activation of endothelial nitric oxide synthase by the angiotensin II type 1 receptor. Endocrinology 147:5914–5920 [DOI] [PubMed] [Google Scholar]
- Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. (2012) Hypoxic pulmonary vasoconstriction. Physiol Rev 92:367–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szado T, Kuo KH, Bernard-Helary K, Poburko D, Lee CH, Seow C, Ruegg UT, van Breemen C. (2003) Agonist-induced mitochondrial Ca2+ transients in smooth muscle. FASEB J 17:28–37 [DOI] [PubMed] [Google Scholar]
- Taimor G, Hofstaetter B, Piper HM. (2000) Apoptosis induction by nitric oxide in adult cardiomyocytes via cGMP-signaling and its impairment after simulated ischemia. Cardiovasc Res 45:588–594 [DOI] [PubMed] [Google Scholar]
- Taira J, Sugishima M, Kida Y, Oda E, Noguchi M, Higashimoto Y. (2011) Caveolin-1 is a competitive inhibitor of heme oxygenase-1 (HO-1) with heme: identification of a minimum sequence in caveolin-1 for binding to HO-1. Biochemistry 50:6824–6831 [DOI] [PubMed] [Google Scholar]
- Takano H, Dora KA, Spitaler MM, Garland CJ. (2004) Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization. J Physiol 556:887–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata Y, Kuriyama H. (1980) ATP-induced hyperpolarization of smooth muscle cells of the guinea-pig coronary artery. J Pharmacol Exp Ther 212:519–526 [PubMed] [Google Scholar]
- Takens-Kwak BR, Jongsma HJ, Rook MB, Van Ginneken AC. (1992) Mechanism of heptanol-induced uncoupling of cardiac gap junctions: a perforated patch-clamp study. Am J Physiol 262:C1531–C1538 [DOI] [PubMed] [Google Scholar]
- Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. (2000) Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell 6:11–22 [DOI] [PubMed] [Google Scholar]
- Taylor HJ, Chaytor AT, Edwards DH, Griffith TM. (2001) Gap junction-dependent increases in smooth muscle cAMP underpin the EDHF phenomenon in rabbit arteries. Biochem Biophys Res Commun 283:583–589 [DOI] [PubMed] [Google Scholar]
- Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. (2003) Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res 93:124–131 [DOI] [PubMed] [Google Scholar]
- Taylor SG, Southerton JS, Weston AH, Baker JR. (1988) Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br J Pharmacol 94:853–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SG, Weston AH. (1988) Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci 9:272–274 [DOI] [PubMed] [Google Scholar]
- Tencé M, Ezan P, Amigou E, Giaume C. (2012) Increased interaction of connexin43 with zonula occludens-1 during inhibition of gap junctions by G protein-coupled receptor agonists. Cell Signal 24:86–98 [DOI] [PubMed] [Google Scholar]
- Tenhunen R, Marver HS, Schmid R. (1968) The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 61:748–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. (1996) The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J 15:6483–6494 [PMC free article] [PubMed] [Google Scholar]
- Theis M, Mas C, Döring B, Degen J, Brink C, Caille D, Charollais A, Krüger O, Plum A, Nepote V, et al. (2004) Replacement by a lacZ reporter gene assigns mouse connexin36, 45 and 43 to distinct cell types in pancreatic islets. Exp Cell Res 294:18–29 [DOI] [PubMed] [Google Scholar]
- Thibeault S, Rautureau Y, Oubaha M, Faubert D, Wilkes BC, Delisle C, Gratton JP. (2010) S-nitrosylation of beta-catenin by eNOS-derived NO promotes VEGF-induced endothelial cell permeability. Mol Cell 39:468–476 [DOI] [PubMed] [Google Scholar]
- Thomas T, Jordan K, Simek J, Shao Q, Jedeszko C, Walton P, Laird DW. (2005) Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J Cell Sci 118:4451–4462 [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Zhou N, MacVicar BA. (2006) Ischemia opens neuronal gap junction hemichannels. Science 312:924–927 [DOI] [PubMed] [Google Scholar]
- Török K, Stauffer K, Evans WH. (1997) Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem J 326:479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tour O, Adams SR, Kerr RA, Meijer RM, Sejnowski TJ, Tsien RW, Tsien RY. (2007) Calcium Green FlAsH as a genetically targeted small-molecule calcium indicator. Nat Chem Biol 3:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz RM, Briones AM, Sedeek M, Burger D, Montezano AC. (2011) NOX isoforms and reactive oxygen species in vascular health. Mol Interv 11:27–35 [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. (2001) c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J Biol Chem 276:1780–1788 [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. (1998) Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273:12725–12731 [DOI] [PubMed] [Google Scholar]
- Treves S, Vukcevic M, Griesser J, Armstrong CF, Zhu MX, Zorzato F. (2010) Agonist-activated Ca2+ influx occurs at stable plasma membrane and endoplasmic reticulum junctions. J Cell Sci 123:4170–4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse FW, Tse A, Hille B, Horstmann H, Almers W. (1997) Local Ca2+ release from internal stores controls exocytosis in pituitary gonadotrophs. Neuron 18:121–132 [DOI] [PubMed] [Google Scholar]
- Turner MS, Haywood GA, Andreka P, You L, Martin PE, Evans WH, Webster KA, Bishopric NH. (2004) Reversible connexin 43 dephosphorylation during hypoxia and reoxygenation is linked to cellular ATP levels. Circ Res 95:726–733 [DOI] [PubMed] [Google Scholar]
- Unwin PN, Ennis PD. (1983) Calcium-mediated changes in gap junction structure: evidence from the low angle X-ray pattern. J Cell Biol 97:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. (1998) p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem 273:15022–15029 [DOI] [PubMed] [Google Scholar]
- Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. (1998) Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med 188:1751–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn KM, Sadler JE, Valentijn JA, Voorberg J, Eikenboom J. (2011) Functional architecture of Weibel-Palade bodies. Blood 117:5033–5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, et al. (2005) Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol 568:459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C. (1977) Calcium requirement for activation of intact aortic smooth muscle. J Physiol 272:317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C, Fameli N, Evans AM. (2013) Pan-junctional sarcoplasmic reticulum in vascular smooth muscle: nanospace Ca2+ transport for site- and function-specific Ca2+ signalling. J Physiol 591:2043–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C, Lukeman S, Leijten P, Yamamoto H, Loutzenhiser R. (1986) The role of superficial SR in modulating force development induced by Ca entry into arterial smooth muscle. J Cardiovasc Pharmacol 8 (Suppl 8):S111–S116 [DOI] [PubMed] [Google Scholar]
- Van de Voorde J, Vanheel B, Leusen I. (1992) Endothelium-dependent relaxation and hyperpolarization in aorta from control and renal hypertensive rats. Circ Res 70:1–8 [DOI] [PubMed] [Google Scholar]
- van den Eijnden-Schrauwen Y, Atsma DE, Lupu F, de Vries RE, Kooistra T, Emeis JJ. (1997) Involvement of calcium and G proteins in the acute release of tissue-type plasminogen activator and von Willebrand factor from cultured human endothelial cells. Arterioscler Thromb Vasc Biol 17:2177–2187 [DOI] [PubMed] [Google Scholar]
- Van Eldik LJ, Hertzberg EL, Berdan RC, Gilula NB. (1985) Interaction of calmodulin and other calcium-modulated proteins with mammalian and arthropod junctional membrane proteins. Biochem Biophys Res Commun 126:825–832 [DOI] [PubMed] [Google Scholar]
- van Hooren KW, van Agtmaal EL, Fernandez-Borja M, van Mourik JA, Voorberg J, Bierings R. (2012) The Epac-Rap1 signaling pathway controls cAMP-mediated exocytosis of Weibel-Palade bodies in endothelial cells. J Biol Chem 287:24713–24720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. (2000) Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 87:335–340 [DOI] [PubMed] [Google Scholar]
- Vanslyke JK, Naus CC, Musil LS. (2009) Conformational maturation and post-ER multisubunit assembly of gap junction proteins. Mol Biol Cell 20:2451–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma SD, Devamanoharan PS. (1991) Hydrogen peroxide in human blood. Free Radic Res Commun 14:125–131 [DOI] [PubMed] [Google Scholar]
- Vasington FD, Murphy JV. (1962) Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem 237:2670–2677 [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Beyer EC, Brink PR. (1994a) Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ Res 75:483–490 [DOI] [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Beyer EC, Ramanan SV, Brink PR. (1994b) Connexin37 forms high conductance gap junction channels with subconductance state activity and selective dye and ionic permeabilities. Biophys J 66:1915–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verselis VK, Srinivas M. (2013) Connexin channel modulators and their mechanisms of action. Neuropharmacology 75:517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer UM, Barth H, Wollheim CB. (2000) Regulated von Willebrand factor secretion is associated with agonist-specific patterns of cytoskeletal remodeling in cultured endothelial cells. Arterioscler Thromb Vasc Biol 20:883–891 [DOI] [PubMed] [Google Scholar]
- Vischer UM, Wollheim CB. (1997) Epinephrine induces von Willebrand factor release from cultured endothelial cells: involvement of cyclic AMP-dependent signalling in exocytosis. Thromb Haemost 77:1182–1188 [PubMed] [Google Scholar]
- Vischer UM, Wollheim CB. (1998) Purine nucleotides induce regulated secretion of von Willebrand factor: involvement of cytosolic Ca2+ and cyclic adenosine monophosphate-dependent signaling in endothelial exocytosis. Blood 91:118–127 [PubMed] [Google Scholar]
- Vitale N, Mawet J, Camonis J, Regazzi R, Bader MF, Chasserot-Golaz S. (2005) The Small GTPase RalA controls exocytosis of large dense core secretory granules by interacting with ARF6-dependent phospholipase D1. J Biol Chem 280:29921–29928 [DOI] [PubMed] [Google Scholar]
- Volpp BD, Nauseef WM, Clark RA. (1988) Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science 242:1295–1297 [DOI] [PubMed] [Google Scholar]
- Voorberg J, Fontijn R, Calafat J, Janssen H, van Mourik JA, Pannekoek H. (1993) Biogenesis of von Willebrand factor-containing organelles in heterologous transfected CV-1 cells. EMBO J 12:749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorderwülbecke BJ, Maroski J, Fiedorowicz K, Da Silva-Azevedo L, Marki A, Pries AR, Zakrzewicz A. (2012) Regulation of endothelial connexin40 expression by shear stress. Am J Physiol Heart Circ Physiol 302:H143–H152 [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, et al. (2005) Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 97:908–915 [DOI] [PubMed] [Google Scholar]
- Wagner DD, Marder VJ. (1984) Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol 99:2123–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Saffaripour S, Bonfanti R, Sadler JE, Cramer EM, Chapman B, Mayadas TN. (1991) Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell 64:403–413 [DOI] [PubMed] [Google Scholar]
- Waid DK, Chell M, El-Fakahany EE. (2000) M(2) and M(4) muscarinic receptor subtypes couple to activation of endothelial nitric oxide synthase. Pharmacology 61:37–42 [DOI] [PubMed] [Google Scholar]
- Waldron GJ, Garland CJ. (1994a) Contribution of both nitric oxide and a change in membrane potential to acetylcholine-induced relaxation in the rat small mesenteric artery. Br J Pharmacol 112:831–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron GJ, Garland CJ. (1994b) Effect of potassium channel blockers on l-NAME insensitive relaxations in rat small mesenteric artery. Can J Physiol Pharmacol 72:A115 [Google Scholar]
- Walker SD, Dora KA, Ings NT, Crane GJ, Garland CJ. (2001) Activation of endothelial cell IK(Ca) with 1-ethyl-2-benzimidazolinone evokes smooth muscle hyperpolarization in rat isolated mesenteric artery. Br J Pharmacol 134:1548–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hirase T, Inoue T, Node K. (2006) Atorvastatin inhibits angiotensin II-induced T-type Ca2+ channel expression in endothelial cells. Biochem Biophys Res Commun 347:394–400 [DOI] [PubMed] [Google Scholar]
- Wang HH, Kung CI, Tseng YY, Lin YC, Chen CH, Tsai CH, Yeh HI. (2008) Activation of endothelial cells to pathological status by down-regulation of connexin43. Cardiovasc Res 79:509–518 [DOI] [PubMed] [Google Scholar]
- Wang J, Ma M, Locovei S, Keane RW, Dahl G. (2007) Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol 293:C1112–C1119 [DOI] [PubMed] [Google Scholar]
- Wang L, Yin J, Nickles HT, Ranke H, Tabuchi A, Hoffmann J, Tabeling C, Barbosa-Sicard E, Chanson M, Kwak BR, et al. (2012) Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest 122:4218–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Martínez AD, Berthoud VM, Seul KH, Gemel J, Valiunas V, Kumari S, Brink PR, Beyer EC. (2005) Connexin43 with a cytoplasmic loop deletion inhibits the function of several connexins. Biochem Biophys Res Commun 333:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, Rogiers V, Bukauskas FF, Bultynck G, Leybaert L. (2013) Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta 1828:35–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Wu L, Wang Z. (1997) The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch 434:285–291 [DOI] [PubMed] [Google Scholar]
- Wang SS, Alousi AA, Thompson SH. (1995) The lifetime of inositol 1,4,5-trisphosphate in single cells. J Gen Physiol 105:149–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BK, Magno AL, Walsh JP, Ratajczak T. (2012) The role of the calcium-sensing receptor in human disease. Clin Biochem 45:943–953 [DOI] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Lau AF. (2004) Regulation of gap junctions by tyrosine protein kinases. Biochim Biophys Acta 1662:81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselle L, Coppola T, Fukuda M, Iezzi M, El-Amraoui A, Petit C, Regazzi R. (2003) Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis. Mol Biol Cell 14:4103–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424:434–438 [DOI] [PubMed] [Google Scholar]
- Watts VL, Motley ED. (2009) Role of protease-activated receptor-1 in endothelial nitric oxide synthase-Thr495 phosphorylation. Exp Biol Med (Maywood) 234:132–139 [DOI] [PubMed] [Google Scholar]
- Wayakanon P, Bhattacharjee R, Nakahama K, Morita I. (2012) The role of the Cx43 C-terminus in GJ plaque formation and internalization. Biochem Biophys Res Commun 420:456–461 [DOI] [PubMed] [Google Scholar]
- Weerth SH, Holtzclaw LA, Russell JT. (2007) Signaling proteins in raft-like microdomains are essential for Ca2+ wave propagation in glial cells. Cell Calcium 41:155–167 [DOI] [PubMed] [Google Scholar]
- Wei CJ, Xu X, Lo CW. (2004) Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol 20:811–838 [DOI] [PubMed] [Google Scholar]
- Weibel ER, Palade GE. (1964) New cytoplasmic components in arterial endothelia. J Cell Biol 23:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilinger NL, Tang PL, Thompson RJ. (2012) Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J Neurosci 32:12579–12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart R. (1977) The actions of ouabain on intercellular coupling and conduction velocity in mammalian ventricular muscle. J Physiol 264:341–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P, et al. (2006) Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension 48:934–941 [DOI] [PubMed] [Google Scholar]
- Wellman GC, Nathan DJ, Saundry CM, Perez G, Bonev AD, Penar PL, Tranmer BI, Nelson MT. (2002) Ca2+ sparks and their function in human cerebral arteries. Stroke 33:802–808 [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. (1998) Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol 274:H178–H186 [DOI] [PubMed] [Google Scholar]
- Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, Dauban P, Petrel C, Ruat M, Edwards G. (2005) Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: studies with calindol and Calhex 231. Circ Res 97:391–398 [DOI] [PubMed] [Google Scholar]
- Wever RM, van Dam T, van Rijn HJ, de Groot F, Rabelink TJ. (1997) Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun 237:340–344 [DOI] [PubMed] [Google Scholar]
- Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. (2008) Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol 294:R1930–R1937 [DOI] [PubMed] [Google Scholar]
- Whiteheart SW, Rossnagel K, Buhrow SA, Brunner M, Jaenicke R, Rothman JE. (1994) N-Ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J Cell Biol 126:945–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, et al. (2001) Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J 357:673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer C, Hohl TM, Hughes CA, Müller SA, Söllner TH, Engel A, Rothman JE. (2001) Molecular mass, stoichiometry, and assembly of 20 S particles. J Biol Chem 276:29091–29097 [DOI] [PubMed] [Google Scholar]
- Wójciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. (2001) Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 114:1343–1355 [DOI] [PubMed] [Google Scholar]
- Wolff B, Burns AR, Middleton J, Rot A. (1998) Endothelial cell “memory” of inflammatory stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J Exp Med 188:1757–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfle SE, Schmidt VJ, Hoepfl B, Gebert A, Alcoléa S, Gros D, de Wit C. (2007) Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res 101:1292–1299 [DOI] [PubMed] [Google Scholar]
- Wölfle SE, Schmidt VJ, Hoyer J, Köhler R, de Wit C. (2009) Prominent role of KCa3.1 in endothelium-derived hyperpolarizing factor-type dilations and conducted responses in the microcirculation in vivo. Cardiovasc Res 82:476–483 [DOI] [PubMed] [Google Scholar]
- Wolin MS. (2000) Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 20:1430–1442 [DOI] [PubMed] [Google Scholar]
- Wolvetang EJ, Pera MF, Zuckerman KS. (2007) Gap junction mediated transport of shRNA between human embryonic stem cells. Biochem Biophys Res Commun 363:610–615 [DOI] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. (2006) Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol 174:803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RF, Ma Z, Liu Z, Terada LS. (2010) Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol 30:3553–3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Haynes J, Jr, Taylor JT, Obiako BO, Stubbs JR, Li M, Stevens T. (2003) Cav3.1 (alpha1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circ Res 93:346–353 [DOI] [PubMed] [Google Scholar]
- Wulff H, Gutman GA, Cahalan MD, Chandy KG. (2001) Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J Biol Chem 276:32040–32045 [DOI] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. (2000) Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA 97:8151–8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, Jaggar JH. (2008) IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res 102:1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. (2004) Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of alpha-subunits. Am J Physiol Heart Circ Physiol 286:H610–H618 [DOI] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, et al. (1998) Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395:503–507 [DOI] [PubMed] [Google Scholar]
- Xie HQ, Hu VW. (1994) Modulation of gap junctions in senescent endothelial cells. Exp Cell Res 214:172–176 [DOI] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS. (1998) Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279:234–237 [DOI] [PubMed] [Google Scholar]
- Xu Q, Kopp RF, Chen Y, Yang JJ, Roe MW, Veenstra RD. (2012) Gating of connexin 43 gap junctions by a cytoplasmic loop calmodulin binding domain. Am J Physiol Cell Physiol 302:C1548–C1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Kurihara H, Kinoshita K, Sakai T. (2005) Temporal expression of alpha-smooth muscle actin and drebrin in septal interstitial cells during alveolar maturation. J Histochem Cytochem 53:735–744 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Fukuta H, Nakahira Y, Suzuki H. (1998) Blockade by 18beta-glycyrrhetinic acid of intercellular electrical coupling in guinea-pig arterioles. J Physiol 511:501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Imaeda K, Suzuki H. (1999) Endothelium-dependent hyperpolarization and intercellular electrical coupling in guinea-pig mesenteric arterioles. J Physiol 514:505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Klemm MF, Edwards FR, Suzuki H. (2001) Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J Physiol 535:181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, et al. (2008) H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322:587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Bae L, Zhang L. (2000) Estrogen increases eNOS and NOx release in human coronary artery endothelium. J Cardiovasc Pharmacol 36:242–247 [DOI] [PubMed] [Google Scholar]
- Yeh HI, Lu SK, Tian TY, Hong RC, Lee WH, Tsai CH. (2006) Comparison of endothelial cells grown on different stent materials. J Biomed Mater Res A 76:835–841 [DOI] [PubMed] [Google Scholar]
- Yi XY, Li VX, Zhang F, Yi F, Matson DR, Jiang MT, Li PL. (2006) Characteristics and actions of NAD(P)H oxidase on the sarcoplasmic reticulum of coronary artery smooth muscle. Am J Physiol Heart Circ Physiol 290:H1136–H1144 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. (2006) Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol 2:596–607 [DOI] [PubMed] [Google Scholar]
- Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, et al. (2001) High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med 7:853–857 [DOI] [PubMed] [Google Scholar]
- Zenner HL, Collinson LM, Michaux G, Cutler DF. (2007) High-pressure freezing provides insights into Weibel-Palade body biogenesis. J Cell Sci 120:2117–2125 [DOI] [PubMed] [Google Scholar]
- Zhang J, Xiao P, Zhang X. (2009) Phosphatidylserine externalization in caveolae inhibits Ca2+ efflux through plasma membrane Ca2+-ATPase in ECV304. Cell Calcium 45:177–184 [DOI] [PubMed] [Google Scholar]
- Zhang JH, Kawashima S, Yokoyama M, Huang P, Hill CE. (2006a) Increased eNOS accounts for changes in connexin expression in renal arterioles during diabetes. Anat Rec A Discov Mol Cell Evol Biol 288:1000–1008 [DOI] [PubMed] [Google Scholar]
- Zhang L, Li YM, Jing YH, Wang SY, Song YF, Yin J. (2013) Protective effects of carbenoxolone are associated with attenuation of oxidative stress in ischemic brain injury. Neurosci Bull 29:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Church JE, Jagnandan D, Catravas JD, Sessa WC, Fulton D. (2006b) Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler Thromb Vasc Biol 26:1015–1021 [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437:902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, Hu G, Vincent PA, Zhou J, Barroso M, et al. (2011) Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ Res 109:534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Smith EC, Whiteheart SW. (2012) Requirements for the catalytic cycle of the N-ethylmaleimide-Sensitive Factor (NSF). Biochim Biophys Acta 1823:159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Neeb ZP, Leo MD, Pachuau J, Adebiyi A, Ouyang K, Chen J, Jaggar JH. (2010) Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells. J Gen Physiol 136:283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. (2001) The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20:6008–6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. (2009) Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest 119:2009–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zhou MH, Hu C, Kuo E, Peng X, Hu J, Kuo L, Zhang SL. (2013a) Differential roles of the C and N termini of Orai1 protein in interacting with stromal interaction molecule 1 (STIM1) for Ca2+ release-activated Ca2+ (CRAC) channel activation. J Biol Chem 288:11263–11272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Zinkevich NS, Gebremedhin D, Gauthier KM, Nishijima Y, Fang J, Wilcox DA, Campbell WB, Gutterman DD, Zhang DX. (2013b) Arachidonic acid-induced dilation in human coronary arterioles: convergence of signaling mechanisms on endothelial TRPV4-mediated Ca2+ entry. J Am Heart Assoc 2:e000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Hu QH, Wang EB, Si JQ, Sun ZP, Li ZC, He F. (2012) [Roles of gap junctions in proliferation mediated by Hcy in the spontaneous hypertensive rat vascular smooth muscle cells]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 28:289–293 [PubMed] [Google Scholar]
- Zhou C, Chen H, Lu F, Sellak H, Daigle JA, Alexeyev MF, Xi Y, Ju J, van Mourik JA, Wu S. (2007a) Cav3.1 (alpha1G) controls von Willebrand factor secretion in rat pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 292:L833–L844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yang W, Lurtz MM, Ye Y, Huang Y, Lee HW, Chen Y, Louis CF, Yang JJ. (2007b) Identification of the calmodulin binding domain of connexin 43. J Biol Chem 282:35005–35017 [DOI] [PubMed] [Google Scholar]
- Zhuge R, Fogarty KE, Tuft RA, Walsh JV., Jr (2002) Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca(2+) concentration on the order of 10 microM during a Ca(2+) spark. J Gen Physiol 120:15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZhuGe R, Tuft RA, Fogarty KE, Bellve K, Fay FS, Walsh JV., Jr (1999) The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells. J Gen Physiol 113:215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zografou S, Basagiannis D, Papafotika A, Shirakawa R, Horiuchi H, Auerbach D, Fukuda M, Christoforidis S. (2012) A complete Rab screening reveals novel insights in Weibel-Palade body exocytosis. J Cell Sci 125:4780–4790 [DOI] [PubMed] [Google Scholar]
- Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192:1001–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerbraun BS, Stoyanovsky DA, Sengupta R, Shapiro RA, Ozanich BA, Rao J, Barbato JE, Tzeng E. (2007) Nitric oxide-induced inhibition of smooth muscle cell proliferation involves S-nitrosation and inactivation of RhoA. Am J Physiol Cell Physiol 292:C824–C831 [DOI] [PubMed] [Google Scholar]
- Zupancic G, Ogden D, Magnus CJ, Wheeler-Jones C, Carter TD. (2002) Differential exocytosis from human endothelial cells evoked by high intracellular Ca(2+) concentration. J Physiol 544:741–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Högestätt ED. (1996) Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br J Pharmacol 117:1600–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]