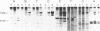Abstract
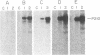
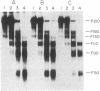
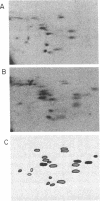
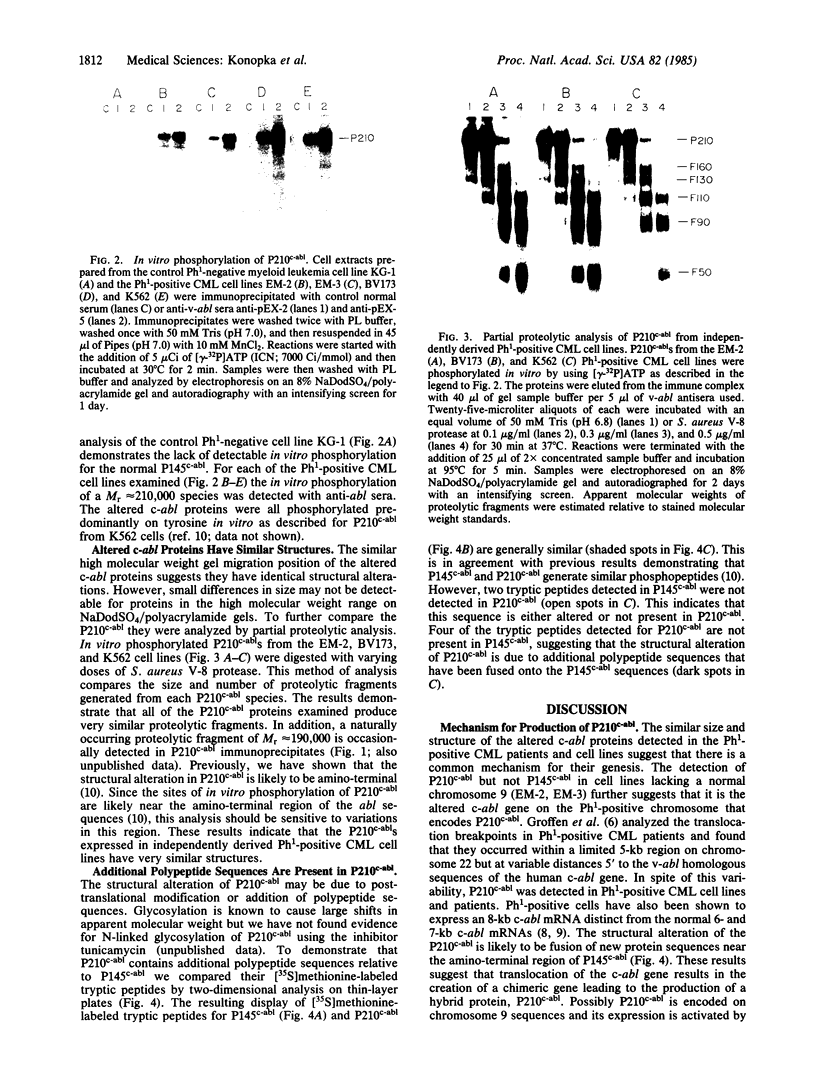
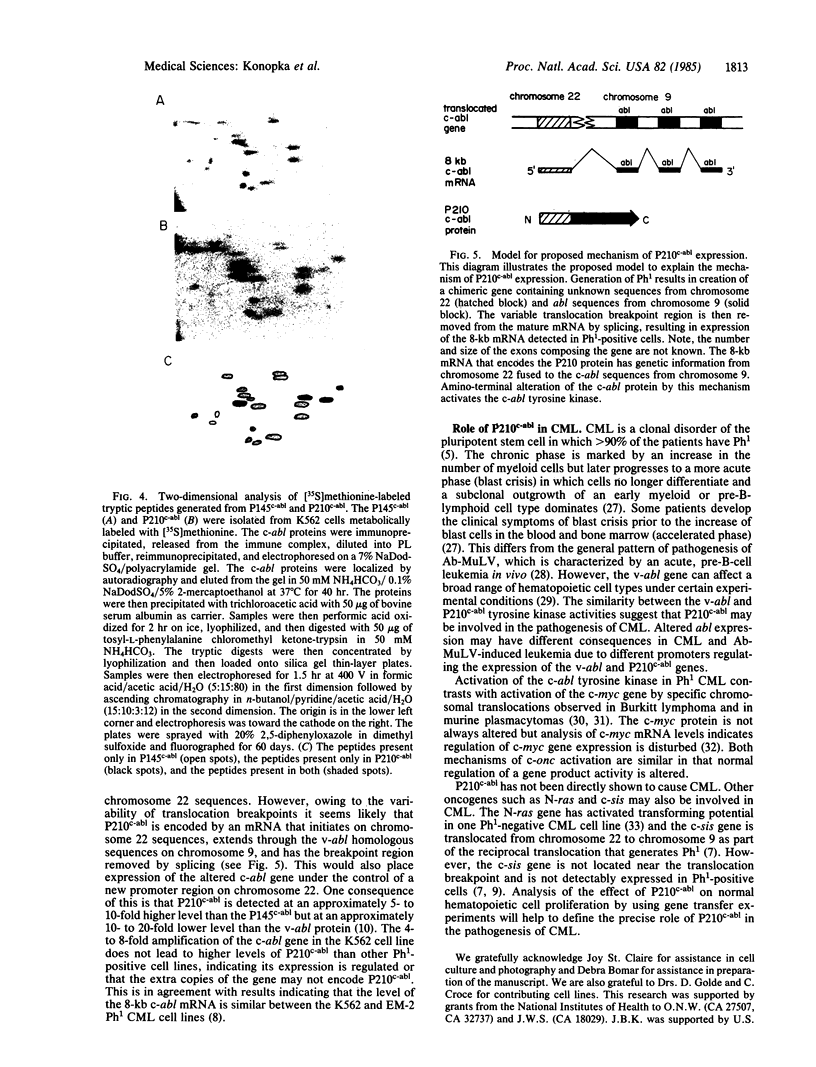
The Philadelphia chromosome (Ph1), observed in greater than 90% of chronic myelogenous leukemia (CML) patients, results from a specific chromosomal translocation involving the c-abl gene. The translocation breakpoint occurs near c-abl and correlates with the production of an altered c-abl mRNA. In the CML-derived cell line K562, Ph1 is accompanied by a structurally altered c-abl protein (P210c-abl) with in vitro tyrosine kinase activity not detected with the normal c-abl protein (P145c-abl). We have examined c-abl proteins in other Ph1-positive CML cell lines and found that they all express P210c-abl. P210c-abl was also detected in bone marrow cells from CML patients with Ph1 in the accelerated and blast crisis phases of the disease. Comparison of the [35S]methionine-labeled tryptic peptides generated from the normal P145c-abl and P210c-abl showed that they have closely related structures, but additional polypeptide sequences are present in P210c-abl. Based on these results we propose that translocation of c-abl in Ph1-positive CML results in the creation of a chimeric gene leading to the production of a structurally altered c-abl protein with activated tyrosine kinase activity. The altered P210 c-abl protein is strongly implicated in the pathogenesis of CML.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Rosenberg N., Witte O. N. Transformation of immature lymphoid cells by Abelson murine leukemia virus. Immunol Rev. 1979;48:3–22. doi: 10.1111/j.1600-065x.1979.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Hardy W. D., Jr, Zuckerman E. E., Bergold P., Lederman L., Snyder H. W., Jr The Hardy-Zuckerman 2-FeSV, a new feline retrovirus with oncogene homology to Abelson-MuLV. Nature. 1983 Jun 30;303(5920):825–828. doi: 10.1038/303825a0. [DOI] [PubMed] [Google Scholar]
- Canaani E., Gale R. P., Steiner-Saltz D., Berrebi A., Aghai E., Januszewicz E. Altered transcription of an oncogene in chronic myeloid leukaemia. Lancet. 1984 Mar 17;1(8377):593–595. doi: 10.1016/s0140-6736(84)90997-8. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Groudine M. T. Rearrangement and amplification of c-abl sequences in the human chronic myelogenous leukemia cell line K-562. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4813–4817. doi: 10.1073/pnas.80.15.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Kubonishi I., Miyoshi I., Groudine M. T. Altered transcription of the c-abl oncogene in K-562 and other chronic myelogenous leukemia cells. Science. 1984 Jul 6;225(4657):72–74. doi: 10.1126/science.6587568. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Konopka J. B., Witte O. N. Activation of the c-abl oncogene by viral transduction or chromosomal translocation generates altered c-abl proteins with similar in vitro kinase properties. Mol Cell Biol. 1985 Jan;5(1):204–213. doi: 10.1128/mcb.5.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Tronick S. R., Gol R. A., Pierce J. H., Aaronson S. A. Transforming genes of human hematopoietic tumors: frequent detection of ras-related oncogenes whose activation appears to be independent of tumor phenotype. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4926–4930. doi: 10.1073/pnas.80.16.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R. The human v-abl cellular homologue. J Mol Appl Genet. 1983;2(1):57–68. [PubMed] [Google Scholar]
- Heisterkamp N., Stephenson J. R., Groffen J., Hansen P. F., de Klein A., Bartram C. R., Grosveld G. Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klein G. Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell. 1983 Feb;32(2):311–315. doi: 10.1016/0092-8674(83)90449-x. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978 Jun 9;200(4346):1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Chronic myelogenous leukemia--new concepts (first of two parts). N Engl J Med. 1981 May 14;304(20):1201–1209. doi: 10.1056/NEJM198105143042004. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Perry R. P. Consequences of myc invasion of immunoglobulin loci: facts and speculation. Cell. 1983 Jul;33(3):647–649. doi: 10.1016/0092-8674(83)90006-5. [DOI] [PubMed] [Google Scholar]
- Ponticelli A. S., Whitlock C. A., Rosenberg N., Witte O. N. In vivo tyrosine phosphorylations of the Abelson virus transforming protein are absent in its normal cellular homolog. Cell. 1982 Jul;29(3):953–960. doi: 10.1016/0092-8674(82)90458-5. [DOI] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Van de Ven W. J., Stephenson J. R. Abelson murine leukemia virus transformation-defective mutants with impaired P120-associated protein kinase activity. J Virol. 1980 Nov;36(2):374–386. doi: 10.1128/jvi.36.2.374-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. Message of myc in context. Nature. 1984 Jun 14;309(5969):585–587. doi: 10.1038/309585a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg N. E., Clark D. R., Witte O. N. Abelson murine leukemia virus mutants deficient in kinase activity and lymphoid cell transformation. J Virol. 1980 Dec;36(3):766–774. doi: 10.1128/jvi.36.3.766-774.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Singer J. W., Adamson J. W., Ernst C., Lin N., Steinmann L., Murphy S., Fialkow P. J. Polycythemia vera. Physical separation of normal and neoplastic committed granulocyte-macrophage progenitors. J Clin Invest. 1980 Oct;66(4):730–735. doi: 10.1172/JCI109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Ledley F., Goff S., Lee R., Groner Y., Baltimore D. The mouse c-abl locus: molecular cloning and characterization. Cell. 1984 Feb;36(2):349–356. doi: 10.1016/0092-8674(84)90228-9. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Goff S., Rosenberg N., Baltimore D. A transformation-defective mutant of Abelson murine leukemia virus lacks protein kinase activity. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4993–4997. doi: 10.1073/pnas.77.8.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Ponticelli A., Gifford A., Baltimore D., Rosenberg N., Elder J. Phosphorylation of the Abelson murine leukemia virus transforming protein. J Virol. 1981 Sep;39(3):870–878. doi: 10.1128/jvi.39.3.870-878.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N. E., Baltimore D. A normal cell protein cross-reactive to the major Abelson murine leukaemia virus gene product. Nature. 1979 Oct 4;281(5730):396–398. doi: 10.1038/281396a0. [DOI] [PubMed] [Google Scholar]