Abstract
Transient receptor potential (TRP) and acid-sensing ion channels (ASIC) are molecular detectors of chemical, mechanical, thermal, and nociceptive stimuli in sensory neurons. They have been identified in the urothelium, a tissue considered part of bladder sensory pathways, where they might play a role in bladder function. This study investigated functional properties of TRP and ASIC channels in cultured urothelial cells from the rat using patch-clamp and fura 2 Ca2+ imaging techniques. The TRPV4 agonist 4α-phorbol-12,13 didecanoate (4α-PDD; 1–5 μM) and the TRPA1/TRPM8 agonist icilin (50–100 μM) elicited transient currents in a high percentage of cells (>70%). 4α-PDD responses were suppressed by the TRPV4 antagonist HC-010961 (10 μM). The TRPV1 agonist capsaicin (1–100 μM) and the TRPA1/TRPM8 agonist menthol (5–200 μM) elicited transient currents in a moderate percentage of cells (∼25%). All of these agonists increased intracellular calcium concentration ([Ca2+]i). Most cells responded to more than one TRP agonist (e.g., capsaicin and 4α-PDD), indicating coexpression of different TRP channels. In the presence of the TRPV1 antagonist capsazepine (10 μM), changes in pH induced by HCl elicited ionic currents (pH 5.5) and increased [Ca2+]i (pH 6.5) in ∼50% of cells. Changes in pH using acetic acid (pH 5.5) elicited biphasic-like currents. Responses induced by acid were sensitive to amiloride (10 μM). In summary, urothelial cells express multiple TRP and ASIC channels, whose activation elicits ionic currents and Ca2+ influx. These “neuron-like” properties might be involved in transmitter release, such as ATP, that can act on afferent nerves or smooth muscle to modulate their responses to different stimuli.
Keywords: bladder, fura 2 calcium imaging, patch clamp
transient receptor potential (TRP) channels are nonselective cation channels that function as sensors for various stimuli, including temperature, mechanical stretch, or chemicals (2, 53). They are highly expressed in primary sensory neurons, where they play a critical role in nociception, mechano-, and thermosensation. In these neurons, distinct sets of TRP channels are coexpressed in different populations of cells. This differential pattern of expression confers functional heterogeneity to primary sensory neurons, and it is the basis for detection of sensory information (8, 16, 31, 32, 53). TRPV1 is a capsaicin/heat/pH-sensitive channel (16, 17); TRPV4 is an osmotic/temperature/mechanical and shear stress detector (24, 36); TRPM8 is a cold (<22–26°C)/menthol/icilin-activated channel (38, 41); and TRPA1 is a noxious cold-sensitive channel (<17°C), also sensitive to cinnamaldehyde, mustard oil, menthol, and icilin (4, 49).
Immunohistochemistry and RT-PCR identified several TRP channels including TRPV1, TRPV4, TRPM8, and TRPA1 in the urothelium, a specialized epithelial tissue believed to be involved in the detection of chemical and mechanical information and the generation of sensory input from the bladder to the spinal cord (2, 3, 12, 13, 22–24, 28, 39, 48, 50). Since the discovery of TRP channels in the urothelium, several studies using intravesical administration of selective agonists (9, 23, 28, 40, 50, 58), genetically engineered mice lacking specific TRP channels (TRP−/− mice) (13, 14, 28), or investigating the changes in the expression of TRP channels in various pathological conditions (1, 22, 37) have attempted to establish a role of these channels in bladder function. Intravesical administration of a TRPV1 agonist (capsaicin) (58), TRPA1 agonists (trans-cinnamaldehyde or allyl isothiocyanate) (50), and a TRPM8/TRPA1 agonist (menthol) (40), have excitatory effects on the reflex bladder activity, increasing micturition frequency, and reducing voided volume. However, these studies often cannot distinguish between actions on urothelial or neuronal TRP channels because the agonists can penetrate through the urothelium and act on the adjacent nerves, which express similar channels.
Knockout mice have provided evidence for a role of TRPV1 (3, 13, 14) and of TRPV4 (28) in bladder function. Using TRPV1−/− mice and cultured urothelial cells, it has been shown that urothelial TRPV1 is involved in stretch-evoked ATP release (13, 14). Also, in cultured urothelial cells activation of TRPV4 with 4α-phorbol-12,13 didecanoate (4α-PDD) (9) releases ATP. These studies suggest that activation of urothelial TRP channels may release transmitters such as ATP, ACh, or nitric oxide (NO) that can act on adjacent afferent nerves to alter their excitability and affect bladder sensations (12, 20, 35, 54).
Ion channels sensitive to pH changes include the acid-sensing ion channels (ASIC) family, which have been implicated in the initiation of inflammation and chronic pain (2, 42). The ASIC family consists of six members, which can assemble in homomeric and heteromeric channels each characterized by different electrophysiological properties, pH thresholds, and sensitivities to amiloride or other blockers (42). Recent studies in the mouse bladder have shown predominant expression of ASIC1 mRNA with modest expression of ASIC2,3 mRNA in the urothelium (33).
In summary, a number of studies suggest that TRP channels are involved in bladder function and/or bladder pathologies (2, 12, 24); however, their properties and physiological role in the urothelium are not well understood. This study was undertaken to investigate urothelial TRP channel properties using electrophysiology and fura 2 Ca2+ imaging.
METHODS
Experimental animals.
Adult male and female Sprague-Dawley rats (200–250 g; Harlan, Indianapolis, IN) were used in this study. Care and handling of the animals were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Rat urothelial cell cultures were prepared as previously described (34). For most of the experiments, bladders from two rats were pooled together to prepare one culture. Data in this paper were obtained from 59 independent cultures. Rats were killed using CO2. The bladder was removed and placed in cold MEM (Invitrogen, Carlsbad, CA) supplemented with HEPES (2.5 g/l, Sigma, St. Louis, MO) and containing penicillin/streptomycin/fungizone (PSF; 1%; Sigma). The bladder was cut open to expose the urothelium, and incubated in dispase (2.5 mg/ml, Worthington Biochemical, Lakewood, NJ) overnight at 4°C. Urothelial cells were gently scraped from the underlying tissue, placed in trypsin (0.25% wt/vol, Sigma) for 10–15 min at 37°C, and dissociated by trituration. Cells were suspended in MEM containing 10% FBS (Invitrogen) and centrifuged at 416 g for 10 min. The supernatant was removed, and cells were suspended in keratinocyte media (Invitrogen) with 1% PSF, centrifuged again, and resuspended in the same media. Cells were plated on collagen-coated glass coverslips (20–24 coverslips/culture) at densities of 50–125 × 104 cells/ml. Media was added after a 4-h incubation period at 37°C and changed every other day. Cells were used 48–96 h after dissociation. During this time in culture, urothelial cells proliferate and form small islands (∼5–30 cells/island) (34).
Calcium imaging.
Urothelial cells were loaded with 5 μM fura 2-AM (Molecular Probes, Eugene, OR) for 30 min at 37°C in an atmosphere of 5% CO2. Fura 2-AM was dissolved in bath solution containing (HBSS; in mM: 138 NaCl, 5 KCl, 0.3 KH2PO4, 4 NaHCO3, 2 CaCl2, 1 MgCl2, 10 HEPES, and 5.6 glucose, pH 7.4, 310 mosmol/l) to which BSA was added (5 mg/ml, Sigma) to promote loading. Coverslips were placed on an inverted epifluorescence microscope (Olympus IX70) or on an upright microscope (Olympus BX61WI) and continuously superfused with HBSS (1.5–2 ml/min), which in some experiments was supplemented with 10 μM Trolox (Sigma) to diminish the effects of photobleaching. Fura 2 Ca2+ imaging was performed as previously described (34). In brief, fura 2 was excited alternately with UV light at 340 and 380 nm, and the fluorescence emission was detected at 510 nm using a computer-controlled monochromator. Image pairs were acquired every 3–30 s using illumination periods between 20 and 50 ms. Wavelength selection, timing of excitation, acquisition, and analysis of images were controlled using C-Imaging software (Compix, Cranberry Township, PA) running on a PC. Digital images were stored on a hard disk for off-line analysis. For image analysis, background was subtracted to minimize camera dark noise and tissue autofluorescence. An area of interest was drawn around each cell, and the average value of all pixels included in this area was taken as one measurement. Data analysis was further performed using Excel (Microsoft, Redmond, WA) and Origin version 7 (OriginLab, Northampton, MA). Baseline intracellular Ca2+ concentration ([Ca2+]i ) was determined from the average of five to eight measurements obtained before drug application. Amplitudes of Ca2+ responses were computed as the difference between the peak value and the baseline value. Only cells responding to ATP (10–100 μM; >95% of urothelial cells responded to ATP), which was used as a control in each experiment, were included in analysis. FAU represents fluorescence arbitrary units, and R is the ratio of the fluorescence signal measured at 340 nm (F340nm) divided by the fluorescence signal measured at 380 nm (F380nm). The results are given as changes in R (F340nm/F380nm) before and after drug application (ΔR) and as percent increase in R above baseline levels of [Ca2+]i (ΔR/R).
Electrophysiological recordings.
Whole-cell recordings were performed under visual guidance using an inverted microscope (Nikon) equipped with a ×40 objective. Cultured urothelial cells form flat monolayers (34). To promote rounding of these cells, before electrophysiological recordings coverslips were incubated (10–15 min at 37°C) in nonenzymatic cell dissociation solution (Sigma) with 1.5 mM EGTA. Patch pipettes (2–4 MΩ) contained (in mM) 120 CsOH, 120 aspartic acid, 10 CsCl, 1 MgCl2, 5 EGTA, and 10 HEPES, adjusted to pH 7.4 with CsOH and to 295 mosmol/l with mannitol. Cells were continuously superfused at 1.5- 2 ml/min with an external solution containing (in mM) 130 sodium aspartate, 6 NaCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, adjusted to pH 7.4 with NaOH and to 305 mosmol/l with mannitol (55). Intracellular solutions contained Cs+ salts to inhibit K+ currents, which could be present in urothelial cells (52). Osmolarity was measured with a vapor pressure osmometer Wescor 5130 (Wescor, Logan, UT). Experiments were performed at room temperature (20–22°C). Unless specified, all salts used for external/internal solutions were purchased from Sigma. Recordings were made in voltage clamp with an Axopatch 200B amplifier and AxoClamp version 8 software (Axon Instruments, Molecular Devices, Sunnyvale, CA). Membrane resistance and membrane capacitance were calculated at the beginning of the experiment using a pClamp built-in protocol. Rseries was recorded every 2–3 min during the entire course of the experiment using a built-in pClamp protocol and in most experiments compensated up to 60%. Voltage-ramp pulses (from −100 mV to +100 mV, 200-ms duration, holding at 0 mV), shown in the inset in Fig. 1C, were delivered every 1–2 s. For each cell, a control period of 2–5 min was recorded in the absence of any drugs, and cells that were not stable during this time were discarded. Data were analyzed using pClamp (Axon Instruments), Origin (Microcal, Northampton, MA), and Excel (Microsoft). Time courses of the whole-cell current were obtained by measuring the current at −80 mV and +80 mV during the voltage-ramp protocol. Current densities were obtained by dividing the current amplitudes by the membrane capacitance. Baseline current was subtracted from the drug-induced current. Rectification ratio was determined by dividing the current measured at +80 mV by the current measured at −80 mV. Data for each agonist were collected from cells in at least three different cultures.
Fig. 1.
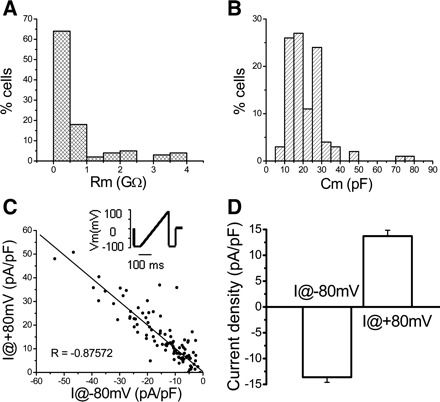
Electrophysiological properties of cultured urothelial cells. A: histogram of membrane resistance (Rm; n = 104 cells). B: histogram of membrane capacitance (Cm) for the same cells. C: plot of background currents measured at −80 (x-axis) and +80 mV (y-axis) for individual cells. Data were fitted with a linear function Y = A + B·X, where A is 0.30419 and B is −0.98577. Pearce coefficient of correlation R is −0.87572. Inset: ramp protocol stepping the cell from −100 to +100 mV in 200 ms, every 2 s, from a holding potential of 0 mV. D: average of background currents measured at −80 and +80 mV.
Drugs.
Drugs from concentrated stock solutions were delivered via a gravity-driven system placed in close proximity to the recorded cells. Ruthenium red (RR; Sigma), a broad-spectrum TRP channel blocker, was dissolved in water at 10–100 mM stock solutions and then further diluted in the external solution to the desired concentration. HC-010961, a selective TRPV4 antagonist from a family of novel antagonists (25), was a gift from Hydra Biosciences. It was prepared in 1% EtOH in water at 10 mM stock solution and subsequently diluted to 10 μM in the external solution. Icilin (Tocris), menthol (Sigma), and 4α-PDD (Sigma) were prepared at 10–100 mM stock solutions in DMSO and subsequently diluted to 1–100 μM in the external solution. Menthol was prepared fresh every 2–3 h and kept on ice until delivered to the cell. Capsaicin (Sigma) was dissolved in 10% ethanol, 10% Tween 80, and 80% physiological saline. Neither the capsaicin vehicle nor DMSO (up to 1%) increased baseline calcium or induced currents. Acetic acid (AA; 0.1–0.25%) was diluted in the external solution to a pH of 5.2–5.5. HCl was also used to change the pH of the external solution to desired pH ranges. All other salts were purchased from Sigma unless specified.
Statistical analysis.
Statistical significance was tested using a paired t-test (significance set at P < 0.05; Prism 4, GraphPad Software, Inc, San Diego, CA). Throughout the text, data are presented as means ± SE.
RESULTS
Whole-cell voltage-clamp recordings were obtained from a total of 104 urothelial cells with average membrane capacitance = 21.42 ± 1.09 pF and membrane resistance = 0.76 ± 0.10 GΩ (Fig. 1, A and B). Background currents were present in urothelial cells (Fig. 1 and Table 1). These currents were sensitive to RR (10 μM), a nonselective TRP channel blocker (data not shown). Fura 2 Ca2+ imaging was performed in 979 cells, the majority of which (>90%) responded to ATP with an increase in [Ca2+]i. Previous studies have shown that in this urothelial culture, most urothelial cells (>95%) stain for cytokeratin 17, a marker of basal/intermediate cells (34).
Table 1.
Summary of electrophysiological and Ca2+ imaging responses of urothelial cells to TRP agonists and pH changes
| Agonist | Electrophysiological Properties |
Ca2+ Imaging Properties |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | I@−80, pA/pF | I@+80, pA/pF | Erev, mV | Rectification ratio, −I@+80/I@−80 | n | ΔR/R, FAU | ||||||
| Background currents | 104 | −13.6±1.0 | −13.7±1.1 | 8.49±1.6 | 1.0±0.1 | |||||||
| 4α−PDD (1–5 μM) | 22 (22) | −32.2±6.6 | 42.4±7.0 | 8.9±2.8 | 1.4±0.1 | 48 (100) | 91.5±17.3 | |||||
| Capsaicin (1–100 μM) | 8 (31) | −31.1±15.2 | 31.5±12.2 | −5.5±6.8 | 0.8±0.1 | 82 (422) | 50.9±5.6 | |||||
| Icilin (50 and 100 μM) | 10 (14) | −54.9±16.2 | 53.4±16.9 | 8.7±1.4 | 0.9±0.1 | 13 (61) | 15.6±4.8 | |||||
| Menthol (5–200 μM) | 3 (7) | −15.3±9.5 | 14.4±8.1 | 10.9±2.1 | 1.0±0.3 | 30 (248) | 48.6±11.2 | |||||
| pH (AA) 5.5 | 10 (10) | −54.7±21.9 | 67.9±23.4 | −1.4±3.1 | 1.2±0.1 | NT | NT | |||||
| pH (HCl) 5.5 and 6.5 | 8 (14) | −16.9±8.1 | 15.2±6.9 | −5.2±0.9 | 0.9±0.1 | 37 (148) | 42.4±10.0 | |||||
Values are means ± SE; n, no. of agonist-responsive cells in patch-clamp and Ca2+ imaging experiments, respectively. Total number of cells tested is given in parentheses. TRP, transient receptor potential; I, current; FAU, fluorescence arbitrary units; NT, not tested; AA, acetic acid; Erev, reversal potential; 4α−PDD, 4α-phorbol-12,13 didecanoate.
TRPV4- and TRPV1-mediated responses.
To determine whether the urothelial TRPV4 channel is functional, we examined the effect of the TRPV4-selective agonist 4α-PDD, a compound with very low activity on protein kinase C (55). In voltage clamp, application of 4α-PDD (1–5 μM) evoked large transient currents in a high percentage of cells (>95%; Table 1 and Fig. 2, A–D). The currents were characterized by slight outward rectification and desensitized in the presence of the agonist (30-s or >1-min applications of the agonist resulted in currents with similar characteristics). A second application of 4α-PDD after a wash of >30 min elicited a small (n = 1/4 cells tested) or no response (n = 3 cells). Application of RR (10 μM) after the 4α-PDD response reached a plateau rapidly reduced both inward and outward currents (inward currents 97.0 ± 15.4% reduction from −60.5 ± 9.5 to −7.8 ± 8.1 pA/pF; n = 7; paired t-test P < 0.05 and outward currents 87.8 ± 11.7% reduction from 92.3 ± 21.1 to 14.5 ± 11.6 pA/pF; n = 7; paired t-test P < 0.05; Fig. 2, C and D). In some cells (n = 3 cells), RR reduced 4α-PDD-induced currents below the baseline before application of 4α-PDD, presumably due to suppression of background currents. RR did not significantly shift the reversal potential (difference between peak 4α-PDD in control and in 4α-PDD plus RR, 7.08 ± 4.05 mV; n = 7 cells; paired t-test P > 0.05), consistent with RR blocking of the pore of the TRPV4 channel. Replacement of permeant extracellular Na+ ions with poorly permeable NMDG ions greatly reduced the 4α-PDD inward current (from −38.2 ± 14.9 to −5.9 ± 5.4 pA/pF; n = 8; paired t-test P < 0.05; Fig. 2, E and F) and significantly shifted the reversal potential in a hyperpolarizing direction (by 30.5 ± 3.01 mV, from 5.15 ± 1.4 to −25.3 ± 3.4 mV; n = 8; paired t-test P < 0.05).
Fig. 2.
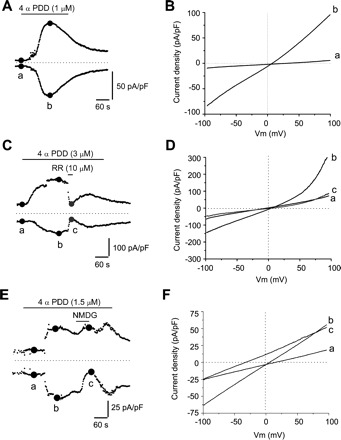
4α-phorbol-12,13 didecanoate (4α-PDD)-elicited currents in cultured urothelial cells. A: representative example of the time course of 4α-PDD-evoked currents measured at ±80 mV using the ramp protocol shown in the inset of Fig. 1C. B: current-voltage (I-V) relationships measured at times indicated by a and b in A, illustrating baseline current (a) and peak current evoked by 4α-PDD (b). C: short application of ruthenium red (RR) during 4α-PDD application reduces both inward and outward currents elicited by 4α-PDD. D: I-V relationships illustrating baseline current (a), peak current evoked by 4α-PDD (b), and the current during RR application (c, grey circles). E: brief replacement of Na+ ions with NMDG during the 4α-PDD exposure reduces the inward current evoked by 4α-PDD. F: I-V relationships recorded at time points indicated in E. In A, C, and E, dotted lines indicate 0 pA/pF.
Fura 2 Ca2+ imaging revealed that 4α-PDD increased [Ca2+]i in 48% of urothelial cells (91.5 ± 17.3 ΔR/R; n = 48/100; Table 1 and Fig. 3). Repetitive applications of 4α-PDD elicited smaller responses or no response (n = 30 cells; Fig. 3A), indicating desensitization of the receptor. The 4α-PDD-elicited responses were blocked by pretreatment with the selective TRPV4 antagonist HC-010961 (10 μM, 10- to 15-min preincubation; Fig. 3B) (25). In the presence of the antagonist, 4α-PDD elicited no responses in 87.5% of cells (14/16) and small responses in the remaining 2 cells (response amplitude 10.1 ± 8.7 ΔR/R). After washout (>15 min) of the antagonist, 4α-PDD elicited large-amplitude Ca2+ transients (response amplitude 75.8 ± 38.3 ΔR/R; n = 16 cells), indicating that 4α-PDD selectively activates TRPV4.
Fig. 3.
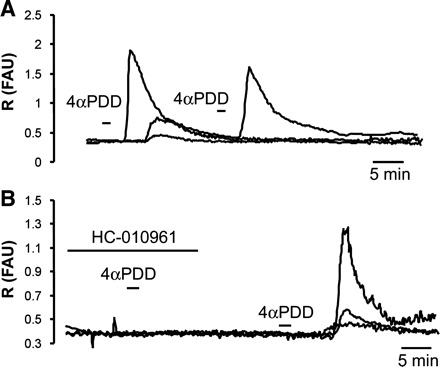
4α-PDD-elicited changes in intracellular Ca2+ concentration ([Ca2+]i) in cultured urothelial cells. A: examples of Ca2+ responses elicited by 2 consecutive applications of 4α-PDD (5 μM). Note desensitization with the second application. B: examples of Ca2+ responses elicited by 4α-PDD (5 μM) in the presence and the absence of the antagonist HC-019061 (10 μM). In A and B, each trace represents data from a single cell. FAU, fluorescence arbitrary units.
The selective TRPV1 agonist capsaicin (1–100 μM applied for 30–60 s) elicited small transient currents in ∼25% of cells (8/31; Fig. 4 and Table 1). The capsaicin-sensitive cells responded to low concentrations (5 and 10 μM) of capsaicin. In nonresponsive cells, even a higher concentration of capsaicin (100 μM) did not elicit responses, indicating that the concentration of capsaicin was not a factor for the absence of responses. Among the eight capsaicin-responsive cells, six responded to the TRPV4 agonist 4α-PDD (3 tested before capsaicin and 3 tested after capsaicin), while the remaining two cells were not tested for 4α-PDD. Among the capsaicin-unresponsive cells, 13 were tested with 4α-PDD and 77% of them (10/13) responded to 4α-PDD whether it was tested before (n = 3) or after (n = 7) capsaicin, demonstrating that cells were able to respond to other chemical stimuli.
Fig. 4.
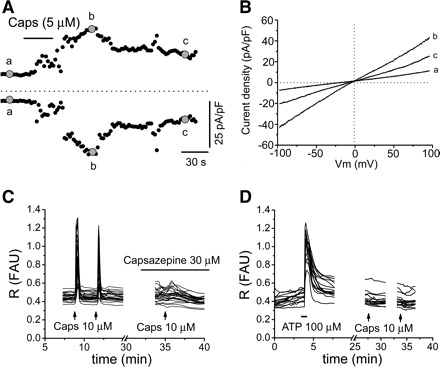
Capsaicin-elicited responses in cultured urothelial cells. A: time course of the capsaicin-elicited current. Currents were measured at +80 (top, outward current) and −80 mV (bottom, inward current) during a ramp test protocol shown in inset in Fig. 1C. B: I-V relationships measured at times indicated by a–c in A and illustrating baseline current (a), peak capsaicin current (b), and partial recovery after capsaicin washout (c). C: examples of capsaicin (10 μM applied at the arrow for 30 s)-elicited Ca2+ responses which are blocked by the TRPV1 antagonist capsazepine (30 μM). Each line represents a cell. FAU, fluorescence arbitrary units; R, ratio of fluorescence signal measured at 340 nm divided by the fluorescence signal measured at 380 nm. D: examples of no responses to capsaicin (10 μM) in cells that respond to ATP (100 μM applied at the black horizontal bar for 30 s) used as a control. Each line represents a cell.
Fura 2 Ca2+ imaging revealed that capsaicin elicited Ca2+ responses in 18.9% (82/422) of cells in 8 of the 23 coverslips tested (10 cultures from 18 rats; Fig. 4, C and D, and Table 1). These responses were repeatable and were abolished by the TRPV1 antagonist capsazepine (n = 28 cells; 10–30 μM; Fig. 4C). There was considerable variability between coverslips in the percentage of cells that responded to capsaicin. In 5 coverslips, all or a high percentage of cells responded (>60%), in 3 other coverslips ∼20% of cells responded, and in the remaining 15 coverslips no cells responded. The nonresponsive cells were healthy, as they responded to ATP or to other stimuli.
TRPA1/TRPM8-mediated responses.
The TRPA1/TRPM8 agonist icilin (50–100 μM applied for 30–60s) elicited transient currents in 71.4% (10/14) of urothelial cells (Table 1 and Fig. 5, A and B). The responses could be repeated after a ∼10-min washout, albeit the second response was smaller in amplitude than the first one (inward current density ∼30% smaller; n = 4; Fig. 5, C and D). Application of the nonselective blocker of TRP channels RR (10 μM for 20 s) after the icilin response reached a plateau markedly reduced the inward and outward currents (Fig. 5E; 88.3 ± 22.6% reduction in inward currents from −40.6 ± 16.6 to −2.6 ± 9.5 pA/pF and 80.6 ± 14.2% reduction in outward currents from 29.3 ± 11.4 to 2.7 ± 3.6 pA/pF; n = 3). Icilin also produced transient increases in [Ca2+]i in 21.3% of cells (n = 13/61 cells; Fig. 5F). The TRPA1 agonist cinnamaldehyde (2–50 μM) applied for 30–60 s elicited currents of various magnitudes that never returned to baseline after washout of the drug (n = 6 cells; Fig. 6, A and B), most likely resulting in cell death, suggesting that cinnamaldehyde was toxic to the cells.
Fig. 5.
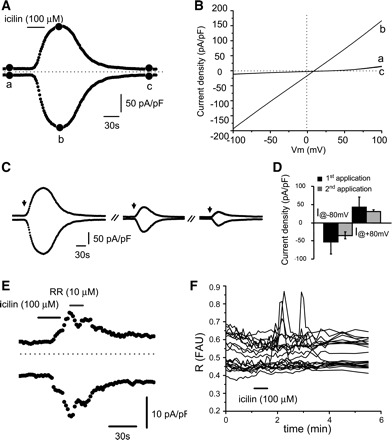
Icilin-elicited responses in cultured urothelial cells. A: time course of the icilin-elicited current. Currents were measured at ±80 mV during a ramp test protocol shown in inset of Fig. 1C. B: I-V relationships measured at times indicated by a–c in A and illustrating baseline current (a), peak current (b), and recovery (c). C: repetitive applications of icilin produce desensitizing responses. Arrows indicate the times when icilin (100 μM, 30s) was applied. D: summary of the amplitude of the inward and outward current densities during 1st and 2nd application of icilin (100 μM). E: short application of RR during icilin application reduces both inward and outward currents elicited by icilin. F: examples of Ca2+ responses elicited by icilin 10 μM (applied at the arrow for 30s) in urothelial cells. Each line represents a cell.
Fig. 6.
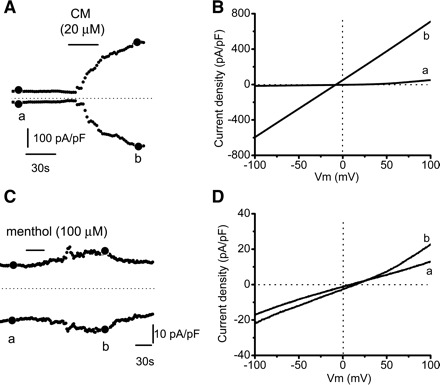
Cinnamaldehyde (CM)- and menthol-elicited currents in cultured urothelial cell. A: time course of the CM-elicited current. Currents were measured at ±80 mV during a ramp test protocol. B: I-V relationships measured at times indicated by a and b in A and illustrating baseline current (a) and peak current (b). C: time course of the menthol-elicited current. Currents were measured at ±80 mV during a ramp test protocol. D: I-V relationships measured at times indicated by a and b in C and illustrating baseline current (a) and peak current (b).
When tested for sensitivity to other stimuli, two of two icilin-responsive cells responded to cinnamaldehyde, three of three cells responded to 4α-PDD, and one of five cells responded to capsaicin. The TRPA1/TRPM8 agonist menthol (5–200 μM applied for 30–60 s) elicited currents in 42.9% of urothelial cells (3/7 cells) (Table 1 and Fig. 6, C and D). Menthol-responsive cells also responded to icilin (3/3). Fura 2 Ca2+ imaging revealed that menthol (100 or 500 μM) elicited Ca2+ responses in 12.1% (30/248) of cells in 3 of the 11 coverslips from 10 rats (Table 1). As in the case of capsaicin, there was great variability between coverslips. In the three responsive coverslips, a high percentage of cells (>90%) responded to menthol while in the remaining eight coverslips no cell responded. The nonresponsive cells were healthy, as they responded to ATP or to other stimuli.
ASIC-mediated responses.
To test for the presence of ASIC channels, whole-cell currents were recorded before and after changes in pH using HCl and/or AA in the presence of the TRPV1 antagonist capsazepine (10 μM). By itself, capsazepine had no effect on background currents or on baseline [Ca2+]i.. When the external solution was adjusted to pH 5.2–5.5 using HCl, currents were induced in 57% of cells (n = 8/14 cells; Table 1 and Fig. 7A). In some cells, the currents had a biphasic-like appearance (3/8; Fig. 7Aii). Regardless of whether the cells responded to HCl-adjusted pH, all cells tested (n = 11) responded to a similar change in pH (5.2–5.5) using AA (0.1–0.25%) with large, repeatable biphasic-like currents (Table 1 and Fig. 7B). In three of four cells tested, the response to AA was sensitive to amiloride (10 μM; Fig. 7B). Using fura 2 Ca2+ imaging, we tested the responses only to pH 6.5 adjusted with HCl because a pH lower than 6.0 affects fura 2 directly. In the presence of capsazepine (10 μM), transient Ca2+ responses were detected in 25% of cells (37/148). Responses were repeatable and reduced by ∼75% in the presence of amiloride (10 μM; from 46.5 ± 17.8 to 12.6 ± 4.6 ΔR/R; n = 14 cells; Fig. 7C).
Fig. 7.
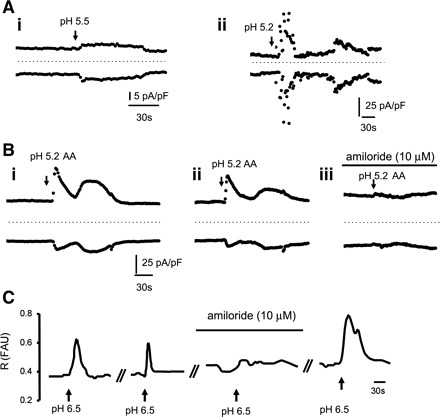
Acid-elicited responses in cultured urothelial cells. A, i and ii: examples of pH (HCl adjusted)-elicited currents in 2 urothelial cells. Currents were measured at ±80 mV during a ramp test protocol shown in the inset of Fig. 1C. In Aii, note the biphasic currents. Arrows indicate the pH change. All applications lasted 30 s, and the start of the application is indicated by arrows. AA, acetic acid. B, i and ii: examples of pH (AA adjusted)-elicited currents in the same cell. Sequential applications with a wash of 7–15 min produces repeatable currents. Preincubation with amiloride (10 μM, 15 min) prevents AA-induced currents. C: examples of Ca2+ responses elicited by pH 6.5 (HCl adjusted) and sensitive to amiloride. Wash time in between applications was 10–15 min.
DISCUSSION
This study revealed that activation of TRPV1, TRPV4, TRPM8, TRPA1, and ASIC-like channels in primary urothelial cells culture induces ionic currents and changes in [Ca2+]i. Single urothelial cells responded to different stimuli, indicating that one cell can express multiple functional TRP channels. These channels might be involved in the mechanisms underlying the release of transmitters from the urothelium and might contribute to the putative sensory function of the urothelium, as well as play a role in homeostatic mechanisms related to cell proliferation and differentiation.
Urothelial cells express multiple TRP channels similar to those in afferent nerves.
The expression of TRP channels in the urothelium was previously examined using mainly immunostaining and/or PCR (3, 9, 12, 13, 22, 23, 39), methods that do not assess the functionality of the channels. Information about the function and role of urothelial TRP channels was inferred from studies using intravesical application of selective TRP agonists (1, 9, 40, 50, 58), a method that cannot entirely distinguish between an effect of the drug on the urothelium or on adjacent afferent nerves as drugs can pass through the urothelial barrier. In this study, we investigated functional TRP channels in individual cells using patch-clamp and Ca2+ imaging techniques in a culture system. The urothelium consists of three layers of cells: basal, intermediate, and the outer layer formed by umbrella cells. Under our culture conditions, urothelial cells predominantly stain for cytokeratin 17 (34), which is expressed in basal and intermediate cells but not in umbrella cells. Thus umbrella cells have not been investigated here. Cells in different layers of the urothelium could express different receptors. In the mouse bladder, immunostaining for TRPV4 was strong in the basal and intermediate cell layers and weak or absent in the umbrella cell layer (28). In mouse and human bladder, M1 muscarinic ACh receptors (mAChRs) were predominantly expressed in the basal cells, M2 mAChRs were restricted to the umbrella cells, and M3, M4, and M5 mAChRs were expressed throughout the urothelium (15, 59).
TRP and ASIC channels are expressed in urothelial cells as well as in primary sensory neurons that terminate in the vicinity of basal and intermediate layers of the urothelium. Immunostaining revealed that TRPV1 is expressed in the majority of urothelial cells (13), while the present study revealed that only a fraction of cells (∼25%) responded to the TRPV1 agonist capsaicin. This difference may be related to the cellular localization of TRPV1 (i.e., in the cytoplasm rather than in the plasma membrane). In contrast to the urothelial cells, capsaicin responses have been detected in a large percentage of bladder primary sensory neurons (>70%) (21, 57). 4α-PDD responses were found in the majority of urothelial cells, consistent with immunostaining illustrating TRPV4 expression throughout the basal and intermediate urothelial layers (9). Immunostaining of TRPV4 was shown in dorsal root ganglion (DRG) neurons innervating visceral organs (e.g., the colon), where 4α-PDD increased [Ca2+]i and elicited currents with characteristics (i.e., outward rectification) similar to those of the urothelial cells (18).
TRPM8 and TRPA1 are expressed in the urothelium (5, 22, 23, 39, 48, 50) and in the sensory neurons (in 10–20%) (38, 41, 43, 49), including the primary sensory neurons innervating the bladder, where ∼50% of neurons stain for TRPA1 (23). In our study, icilin and menthol, which activate both TRPA1 and TRPM8, evoked ionic currents. However, icilin elicited responses in most urothelial cells, while menthol elicited responses in a smaller population of cells (∼40%). TRPM8 responses were reported to be insensitive to RR, a general blocker of TRP channels (7, 41). In our experiments, icilin-evoked currents were sensitive to RR, and thus we attribute icilin responses at least in part to the activation of TRPA1. In addition, cinnamaldehyde, another TRPA1 agonist, elicited currents in urothelial cells (Fig. 6).
Electrophysiological properties of TRPV1, TRPV4, TRPA1/TRPM8, and ASIC-like channels in urothelial cells.
Urothelial cells exhibited small background currents (Fig. 1), sensitive to RR, suggesting that at rest there are some TRP “leak” channels. Further activation of particular TRP channels by specific agonists resulted in ionic currents and changes in [Ca2+]i. TRP channels are nonselective cation channels permeable to Ca2+, Na+, K+, and Cs+ (41, 55, 56). In our experimental conditions (Na+, Ca2+ in the extracellular solution and high Cs+ in the intracellular solution), the inward current was carried in part by Na+ because the substitution of Na+ ions with NMDG+ reduced the TRPV4-mediated inward current and shifted the reversal potential to hyperpolarizing values (Fig. 2). Ca2+ imaging data showing agonist-induced changes in [Ca2+]i provided evidence for Ca2+ influx through TRP channels. The outward currents detected in voltage-clamp recordings were most likely carried by Cs+, which replaced K+. TRPV4-mediated currents showed a slight outward rectification, while the other TRP-mediated currents had weak or no rectification. RR effectively and reversibly suppressed inward and outward currents induced by 4α-PDD and icilin without changing the reversal potential (Figs. 2 and 5), consistent with a block of the channel. These properties were similar to those investigated in HEK-293 cells transfected with TRPV4 (55), mouse 308 keratinocytes (19), and in DRG neurons (18, 46). The properties of menthol- and capsaicin-induced currents were somewhat different from those of the sensory neurons, Chinese hamster ovary cells transfected with TRPM8, or HEK-293 cells transfected with TRPV1 cDNA, where whole-cell recordings showed currents with outward rectification (17, 38, 41).
In addition to TRP channels, urothelial cells also express proton (pH)-sensitive channels, as lowering the pH to 5.2–6.5 with AA or HCl elicited currents and [Ca2+]i changes. AA responses were different from HCl-induced responses, in both the percentage of responding cells and the characteristics of the currents (Table 1). For the same pH range (5.2–5.5), all cells responded to AA, while only ∼50% of cells responded to HCl-adjusted pH. Cells that did not respond to HCl-adjusted pH responded to AA-adjusted pH. Another difference was that AA elicited biphasic-type currents with a fast component followed by a slower component in the majority of cells (80%), whereas HCl elicited biphasic currents in a lower percentage of cells (∼40%). This difference might be due to a distinct action of acetate on other channels than ASIC, such as a calcium-activated K+ channel (29). Based on the sensitivity to amiloride and on recent studies in mouse bladder which show predominant expression of ASIC1 with little expression of ASIC2,3 (33), our results suggest that ASIC1 might underlie the responses to pH, although we cannot rule out the contribution of other subtypes. ASIC currents sensitive to amiloride have also been described in the lumbosacral DRG neurons innervating the bladder, where the majority (∼78%) of neurons responded to pH 5 and in ∼21% of them the currents had a biphasic appearance (21).
Role of multiple TRP and ASIC-like channels in urothelial cells.
One interesting finding of our study is that individual urothelial cells can express multiple TRP channels, similar to DRG neurons (8, 16, 32). 4α-PDD (TRPV4 agonist) and icilin (TRPA1/TRPM8 agonist) elicited responses in a high percentage of urothelial cells, indicating coexpression of TRP channels. In the experiments where we tested multiple chemical stimuli in one cell, we also found coexpression of TRP channels (e.g., capsaicin and 4α-PDD or icilin and 4α-PDD elicited currents in the same cells).
In primary sensory neurons, distinct sets of TRP channels are expressed in subpopulations of neurons and together with other ion channels fine tune that population for the detection of particular sensory information. Histological markers (e.g., myelination or neuropeptides) are further used to characterize these distinct DRG populations (8, 16, 32). Such characterization and subdivision of cells could also exist in the urothelium. Even though the population of urothelial cells studied here seemed homogeneous in regard to markers such as cytokeratin 17 and responses to ATP, we did find differences in TRP responses, which could suggest subpopulations with distinct functions (i.e., cells responsive to chemical stimuli and cells responsive to mechanical stimuli). What would be the reason for different urothelial populations? The sensory innervation of the bladder is denser at the base and sparse at the dome (27); therefore, cells located at the base may have different sets of TRP channels than those of the cells located at the dome of the bladder, which may allow them to better communicate with the underling nerves.
The mechanisms by which activation of urothelial TRP and ASIC channels contributes to modulation of bladder reflexes might involve the ability of these channels to elicit ionic currents and changes in the [Ca2+]i that can result in transmitter release. Urothelial cells release ATP, NO, or ACh, transmitters that can act on afferent neurons to alter their excitability (6, 10, 26, 30, 34, 35, 44, 54). Thus alterations in TRP channel expression or function, which have been reported in pathological conditions (1, 2, 12, 22, 37), might lead to altered extracellular levels of neurotransmitters in bladder mucosa and in turn to altered afferent nerve activity and altered bladder function. Indeed, previous studies reported upregulation of ATP release from bladders that are overactive as a result of inflammation (47) or spinal cord injury (45) and increased ATP release from urothelial cells from human patients (51) or cats (11) with interstitial cystitis (for a review, see Ref. 44).
Another characteristic of TRP channels is high permeability to Ca2+. Changes in [Ca2+]i are involved in processes ranging from cell proliferation to cell death. In vivo, urothelial cells undergo a progressive differentiation from basal to intermediate and to umbrella cells. This process takes 3–6 mo in humans (12), but injury to the urothelium markedly stimulates proliferation. Thus Ca2+ entry through TRP channels might be involved in homeostatic mechanisms underlying cell proliferation and differentiation in normal and pathological conditions.
In summary, urothelial cells express multiple TRP and ASIC channels whose activation produces ionic currents and Ca2+ influx, similar to responses occurring in the primary sensory neurons. These properties might underlie the basic mechanisms for the release of transmitters (e.g., ATP, NO, ACh) or other factors (e.g., prostaglandins) that can act on afferent neurons or smooth muscle cells to communicate changes occurring in the bladder lumen/urine (e.g., infection, noxious chemicals) which could be deleterious to the lower urinary tract. Transmission of this information from the bladder to the central nervous system can induce a defense reaction such as reflex voiding to eliminate noxious substances.
GRANTS
This work was supported by a grant from the American Foundation for Urologic Disease/American Urological Association Research Scholar Program to F. A. Kullmann (Negoita) and National Institutes of Health Grants DK-49430 to W. C. de Groat, DK-54824, and P50-DK-64539 to L. A. Birder.
Acknowledgments
The authors thank M. J. Caterina and M. K. Chung for help with the initial studies on patching urothelial cells. We also thank members of the W. C. de Groat and L. A. Birder laboratories for valuable discussions.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology 65: 400–405, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Araki I, Du S, Kobayashi H, Sawada N, Mochizuki T, Zakoji H, Takeda M. Roles of mechanosensitive ion channels in bladder sensory transduction and overactive bladder. Int J Urol 15: 681–687, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Avelino A, Cruz F. TRPV1 (vanilloid receptor) in the urinary tract: expression, function and clinical applications. Naunyn Schmiedebergs Arch Pharmacol 373: 287–299, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Barrick S, Chopra B, Caterina M, Nealen ML, Lee H, Birder LA. Expression and function of the cold channels in urinary bladder urothelium: TRPM8 and TRPA1. In: American Pain Society Abstracts. Boston, MA: American Pain Society, 2005
- 6.Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol 290: F103–F110, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol 141: 737–745, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belmonte C, Viana F. Molecular and cellular limits to somatosensory specificity. Mol Pain 4: 14, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birder L, Kullmann FA, Lee H, Barrick S, de Groat WC, Kanai A, Caterina M. Activation of urothelial transient receptor potential vanilloid 4 by 4alpha-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther 323: 227–235, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Birder LA, Apodaca G, de Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol Renal Physiol 275: F226–F229, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol 285: F423–F429, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 4: 46–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 98: 13396–13401, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, de Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Bschleipfer T, Schukowski K, Weidner W, Grando SA, Schwantes U, Kummer W, Lips KS. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci 80: 2303–2307, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol 292: R64–R76, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 135: 937–946, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem 278: 32037–32046, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407: 1011–1015, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci 25: 3973–3984, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du S, Araki I, Kobayashi H, Zakoji H, Sawada N, Takeda M. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology 72: 450–455, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Du S, Araki I, Yoshiyama M, Nomura T, Takeda M. Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology 70: 826–831, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Everaerts W, Gevaert T, Nilius B, De Ridder D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn 27: 264–273, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Fanger CM, McNamara CR, Strassmaier T, Witek J, Agueev V, Moran MM, Zhen X. Identification of novel TRPV4 channel modulators. In: Society for Neuroscience. Washington, DC: Society for Neuroscience, Abstract 628 18., 2008
- 26.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes-a possible sensory mechanism? J Physiol 505: 503–511, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol 27: 141–155, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117: 3453–3462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghatta S, Lozinskaya I, Lin Z, Gordon E, Willette RN, Brooks DP, Xu X. Acetic acid opens large-conductance Ca2+-activated K+ channels in guinea pig detrusor smooth muscle cells. Eur J Pharmacol 563: 203–208, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci 80: 2298–2302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci 27: 2435–2443, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 413: 203–210, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi H, Yoshiyama M, Araki I, Zakoji H, Sawada N, Mochizuki T, Tsuchida T, Nomura T, Fukasawa M, Takeda M. Contribution of acid-sensing ion channels to sex differences in mouse bladder response to acetic acid: a novel candidate for interstitial cystitis pathogenesis. J Urol Suppl 179: 129–130, 2008 [Google Scholar]
- 34.Kullmann FA, Artim DE, Beckel JM, Barrick S, de Groat WC, Birder LA. Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol 294: F971–F981, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kullmann FA, Artim DE, Birder LA, de Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci 28: 1977–1987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Mansfield KJ, Kristiana I, Vaux KJ, Millard RJ, Burcher E. The molecular basis of urgency: regional difference of vanilloid receptor expression in the human urinary bladder. Neurourol Urodyn 26: 433–438, 2007 [DOI] [PubMed] [Google Scholar]
- 38.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Mukerji G, Yiangou Y, Corcoran SL, Selmer IS, Smith GD, Benham CD, Bountra C, Agarwal SK, Anand P. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol 6: 6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomoto Y, Yoshida A, Ikeda S, Kamikawa Y, Harada K, Ohwatashi A, Kawahira K. Effect of menthol on detrusor smooth-muscle contraction and the micturition reflex in rats. Urology 72: 701–705, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Reeh PW, Kress M. Molecular physiology of proton transduction in nociceptors. Curr Opin Pharmacol 1: 45–51, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Reid G, Babes A, Pluteanu F. A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction. J Physiol 545: 595–614, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruggieri MR, Sr. Mechanisms of disease: role of purinergic signaling in the pathophysiology of bladder dysfunction. Nat Clin Pract Urol 3: 206–215, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Salas NA, Somogyi GT, Gangitano DA, Boone TB, Smith CP. Receptor activated bladder and spinal ATP release in neurally intact and chronic spinal cord injured rats. Neurochem Int 50: 345–350, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, Liedtke W, Cohen DM, Vanner S, Blackshaw LA, Bunnett NW. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 294: G1288–G1298, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int 47: 291–297, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, Patel AS, Nelson JB, Futrell WJ, Yoshimura N, Chancellor MB, De Miguel F. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol 172: 1175–1178, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Hogestatt ED, Zygmunt PM. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53: 391–399, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol 290: C27–C34, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Sun Y, Chen M, Lowentritt BH, Van Zijl PS, Koch KR, Keay S, Simard JM, Chai TC. EGF and HB-EGF modulate inward potassium current in human bladder urothelial cells from normal and interstitial cystitis patients. Am J Physiol Cell Physiol 292: C106–C114, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 76: 387–417, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21: 5670–5677, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277: 13569–13577, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277: 47044–47051, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura N, Seki S, Erickson KA, Erickson VL, Chancellor MB, de Groat WC. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J Neurosci 23: 4355–4361, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu Y, Fraser MO, de Groat WC. Effects of ZD6169, a K ATP channel opener, on neurally-mediated plasma extravasation in the rat urinary bladder induced by chemical or electrical stimulation of nerves. Brain Res 996: 41–46, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Zarghooni S, Wunsch J, Bodenbenner M, Bruggmann D, Grando SA, Schwantes U, Wess J, Kummer W, Lips KS. Expression of muscarinic and nicotinic acetylcholine receptors in the mouse urothelium. Life Sci 80: 2308–2313, 2007 [DOI] [PubMed] [Google Scholar]


