Abstract
Parkin is a ubiquitin E3 ligase that is implicated in familial Parkinson disease (PD). Previous studies have established its role in mitophagy, a pathway whereby dysfunctional mitochondria are targeted for autophagic degradation. We recently reported that a major function of Parkin in dysfunctional mitochondria is to activate the ubiquitin-proteasome system (UPS) for proteolysis of multiple outer membrane proteins, and that such activation of the UPS is a critical step in Parkin-mediated mitophagy. Here, we discuss the possible roles of the UPS in mitophagy and the pathogenesis of PD.
Key words: Parkinson disease, parkin, ubiquitin-proteasome system, mitophagy, mitochondria
Recent studies have illuminated the role of PINK1 and Parkin in mitochondrial quality control, by mediating the selective degradation of dysfunctional mitochondria via autophagy (mitophagy). Because both proteins are implicated in familial, early-onset PD, an understanding of the molecular mechanism of PINK1-Parkin-mediated mitophagy might provide important insight into the pathogenesis of PD. Parkin is a ubiquitin E3 ligase that normally resides in the cytosol. When cellular insults result in loss of membrane potential in dysfunctional mitochondria, Parkin is recruited to the mitochondrial surface in a PINK1-dependent manner and mediates the ubiquitination of mitochondrial proteins. This selective recruitment leads to the autophagic degradation of dysfunctional mitochondria.
The ubiquitination mediated by Parkin appears essential for its function in mitophagy, because some pathogenic mutants show impaired ubiquitin ligase activity and are unable to induce mitophagy. Several studies have documented the ability of Parkin to mediate K63-linked polyubiquitination of mitochondrial proteins. Given that K63-linked polyubiquitination has an established role in recruiting the autophagy machinery, recent studies have focused on identifying the adaptor proteins responsible for directing mitochondria to the autophagy pathway. In contrast, the possible roles of other types of polyubiquitination, which can signal different cellular outcomes, have not been clear.
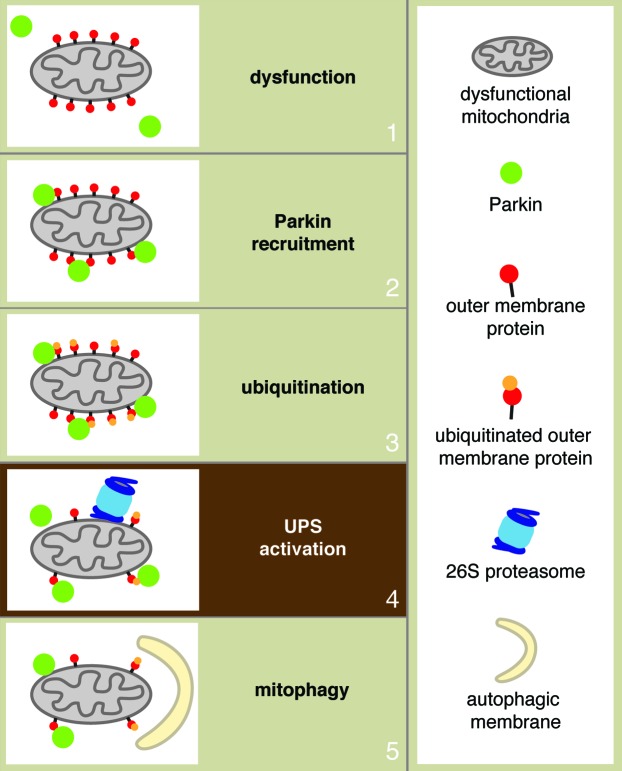
We found that upon recruitment to mitochondria, Parkin activates the ubiquitin-proteasome system. The UPS is a major cellular protein degradation pathway that is classically associated with K48-linked polyubiquitination. Proteins modified with K48-polyubiquitination are targeted for degradation via the 26S proteasome. Using quantitative proteomics, we comprehensively screened for changes in the mitochondrial proteome in response to mitochondrial depolarization in a Parkin-expressing cell line. Besides identifying the expected enrichment of Parkin, K63-linked polyubiquitination and components of the autophagy pathway, we found strong evidence demonstrating activation of the UPS on mitochondria. There is substantial enrichment of K48-linked polyubiquitin and recruitment of the 26S proteasome, as well as the concomitant degradation of multiple outer membrane proteins.
Using inhibitors against the proteasome, our biochemical and cell biological experiments confirmed that the degradation of outer membrane proteins is indeed dependent on the 26S proteasome. Such degradation is not a downstream result of mitophagy, because proteolysis of outer membrane proteins occurs prior to mitophagy and still persists in an autophagy-deficient cell line. Strikingly, we found that inhibition of the UPS is sufficient to abolish mitophagy in a number of cell systems commonly used for studying mitophagy. Therefore, it seems likely that activation of the UPS by Parkin is a critical step within the mitophagy pathway (Fig. 1).
These findings also raise a technical note regarding the analysis of mitophagy. Many studies of mitophagy have used Tom20, a mitochondrial outer membrane protein, as a marker to track mitochondria. However, in the case of Parkin-mediated mitophagy, loss of Tom20 is due to protein degradation by the UPS, rather than mitochondrial elimination. Accordingly, matrix markers such as Hsp60 provide a more accurate evaluation of mitophagy.
How might the UPS promote Parkin-mediated mitophagy? A number of studies have reported that mitofusins are rapidly ubiquitinated and degraded via Parkin upon induction of mitophagy. Because mitofusins mediate the fusion between mitochondria, this observation has led to the proposal that inhibition of fusion facilitates segregation of dysfunctional mitochondria, thereby allowing their selection and degradation by autophagy. Segregation of dysfunctional mitochondria cannot sufficiently explain the role of the UPS, however, because inhibition of the UPS blocks mitophagy in mitofusin-deficient cells. The UPS promotes rapid degradation of a broad group of outer membrane proteins, not simply mitofusins. In doing so, the UPS may prevent the dysfunctional mitochondria from participating in other cellular activities before their eventual uptake by the autophagosome.
Our data suggest two possible hypotheses, not mutually exclusive, for the role of the UPS. First, aggregation of depolarized mitochondria occurs during the induction of mitophagy in many model systems. We speculate that this aggregation is mediated by outer membrane proteins. By degrading outer membrane proteins, the UPS might disperse mitochondria into smaller individual units that are suitable substrates for autophagy. This hypothesis is supported by the broad range of outer membrane proteins that are degraded, and the observation that mitochondrial fragmentation is important for mitophagy. Second, the UPS may remove one or more negative regulators of mitophagy present on the outer membrane.
The recent progress on PINK1 and Parkin suggests that some forms of familial PD involve impairment of mitochondrial quality control. It will be important to determine whether this pathway is relevant to the sporadic form of PD that constitutes 90% of cases. Intriguingly, mitochondrial dysfunction has long been associated with sporadic PD, and the dopaminergic cells of the substantia nigra appear particularly vulnerable to mitochondrial stress.
Figure 1. Model of Parkin-mediated mitophagy. Mitochondrial dysfunction recruits Parkin from the cytosol onto the mitochondrial outer membrane. Parkin mediates ubiquitination of outer membrane proteins. Activation of the UPS on the mitochondrial surface targets K-48-linked polyubiquitinated proteins for degradation. Activation of the UPS is a critical step that mediates subsequent mitophagy.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/15453