Abstract
The underwater sensory world and the sensory systems of aquatic animals have become better understood in recent decades, but typically have been studied one sense at a time. A comprehensive analysis of multisensory interactions during complex behavioral tasks has remained a subject of discussion without experimental evidence. We set out to generate a general model of multisensory information extraction by aquatic animals. For our model we chose to analyze the hierarchical, integrative, and sometimes alternate use of various sensory systems during the feeding sequence in three species of sharks that differ in sensory anatomy and behavioral ecology. By blocking senses in different combinations, we show that when some of their normal sensory cues were unavailable, sharks were often still capable of successfully detecting, tracking and capturing prey by switching to alternate sensory modalities. While there were significant species differences, odor was generally the first signal detected, leading to upstream swimming and wake tracking. Closer to the prey, as more sensory cues became available, the preferred sensory modalities varied among species, with vision, hydrodynamic imaging, electroreception, and touch being important for orienting to, striking at, and capturing the prey. Experimental deprivation of senses showed how sharks exploit the many signals that comprise their sensory world, each sense coming into play as they provide more accurate information during the behavioral sequence of hunting. The results may be applicable to aquatic hunting in general and, with appropriate modification, to other types of animal behavior.
Introduction
The underwater world provides sensory information that in several ways differs from information signals in an aerial environment: underwater light-scatter severely limits visual distance; the dense aquatic medium allows for about five times faster sound propagation and for subtle hydrodynamic imaging; water propagates electric fields including electromagnetic induction; and odor dispersal remains more coherent as a result of aquatic density stratification [1]. The physical dispersal fields are rather well characterized theoretically, but less is known about the manner in which animals use them (sensu Uexküll [2]) in complex tasks and under noisy conditions. Also, sensory perception has been well studied in aquatic animals one sense at a time [3], [4], but the multiple interactions of different senses have remained mostly speculative.
To establish experimentally the multisensory guidance of a complex behavioral task in an aquatic predator, we tested five senses in five phases of hunting behavior in three species of sharks. Because hunting is competitive and strongly correlated with fitness [5], animals are likely to use whichever senses, alone or in combination, that support the best performance [6]. Sensory integration should occur when non-directional signals (odor or sound pressure) can combine with directional signals (hydrodynamic flow). Switching should result when more salient signals appear (e.g. closer to the signal source) as the animal moves from one behavioral phase to the next [7]. If alternate senses can provide information useful to the behavioral task, these may be used when environmental conditions change (e.g. nighttime and turbidity reduce visual resolution [8]), when a sense organ becomes damaged (e.g. by disease or chemical pollutants [9]), or when sensory cues become masked (e.g. by boat noise [10]).
Hunting involves: 1) initially detecting and evaluating cue(s) that alert the hunter to the presence of prey somewhere, 2) tracking the cues to the vicinity of their source, 3) orienting to the prey with direct sensory contact, 4) striking at the prey, and 5) coordinating strike behavior with jaw and/or appendage motion to capture the prey [11]. The timing of these hunting phases accelerates from minutes (tracking) to milliseconds (capture) and various senses guide them. While a single sensory modality may suffice for some behaviors, information from multiple cues can result in shorter latency, greater sensitivity, better spatial and temporal resolution, and improved noise rejection [12].
Sharks capture prey in a variety of ways, such as ram, suction, and biting. In pure suction feeding, the predator remains completely stationary as it rapidly expands the buccopharyngeal cavity to draw the prey into its mouth. In pure ram feeding, the predator accelerates to overtake and engulf a completely stationary prey. Most fishes fall somewhere in the middle of the spectrum. In ram-biting, rather than completely engulfing the prey, the shark will bite into the prey (reviewed in [13]). All capture modalities require sensory control to precisely time and direct jaw movements.
We chose, in a size range (∼1 m total length) conducive to controlled laboratory studies, three species of sharks from different ecological niches and with different capture modalities (Figure 1): ram-feeding blacktip sharks, Carcharhinus limbatus, that rapidly chase down midwater teleost prey [14]; ram-biting bonnetheads, Sphyrna tiburo, that scoop crustaceans off seagrass beds [15]; and benthic, suction-feeding nurse sharks, Ginglymostoma cirratum, that hunt nocturnally for crustaceans and fish, often sucking them out of reef crevices [16]. By blocking various senses, singly and in combination, we learned their involvement in guiding the different hunting phases.
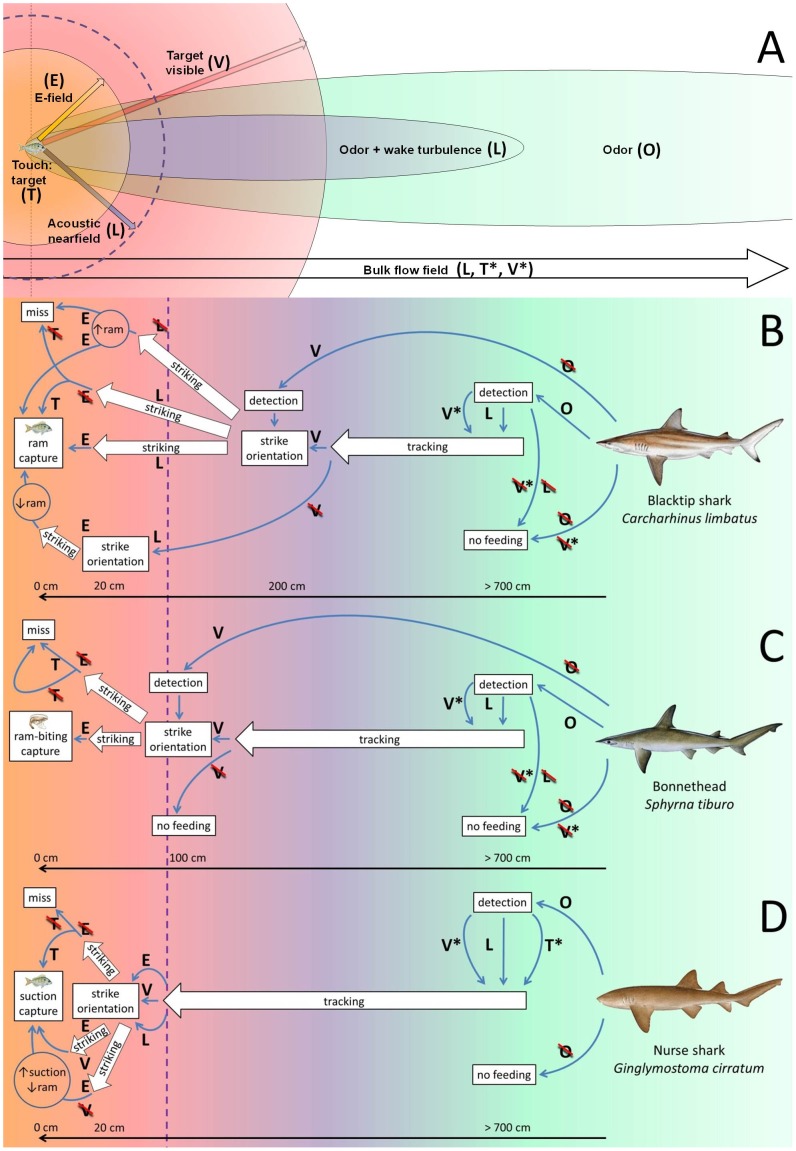
Figure 1. Sensory signals and their use by hunting sharks.
Senses are indicated by capital letters (e.g. V = vision); asterisk (e.g. V*) denotes sense used to measure and orient to the bulk flow (i.e., by detecting environment features); no asterisk (e.g. V) denotes sense used to process prey cues; slashed (e.g. 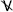 ) indicates sensory block. Background colors in A, B, and C indicate areas of signal availability corresponding to signal dispersal fields in A. Behavioral phases in boxes occur at a discrete distance from the source; behavioral phases in boxed arrows occur over a distance; line arrows indicate transitions from one phase of the behavior to the next. A. Physical model of prey signal fields (After [1]). Prey emit a complex mixture of sensory stimuli that radiate and disperse into the habitat. Animals can detect the bulk flow vector (arrow) by measuring their drift along the walls and substrate, using vision (V*) or touch (T*) of the walls or bottom, or, by detecting turbulence in the bulk flow, with the lateral line (L). Bulk flow disperses prey odor downstream over large distances where it can be detected by olfaction (O, green); closer to the source, prey-generated wake turbulence becomes detectable by the lateral line (L, purple). Close to the source, the prey becomes directly detectable based on vision (V, red), lateral line imaging of the acoustic near field (L, delineated by purple dotted line), electroreception (E, orange), and touch (T, direct tactile contact with prey). B. The blacktip shark,
Carcharhinus limbatus
. From downstream, the blacktip shark detects the presence of prey using O and, during the daytime, tracks the bulk flow upstream using OV* or OL. Seeing the prey, it switches to V to orient and strike from a distance (∼2 m). Near the prey, the strike is adjusted using L. Then it switches to E to ram-capture the prey. With the lateral line blocked (
) indicates sensory block. Background colors in A, B, and C indicate areas of signal availability corresponding to signal dispersal fields in A. Behavioral phases in boxes occur at a discrete distance from the source; behavioral phases in boxed arrows occur over a distance; line arrows indicate transitions from one phase of the behavior to the next. A. Physical model of prey signal fields (After [1]). Prey emit a complex mixture of sensory stimuli that radiate and disperse into the habitat. Animals can detect the bulk flow vector (arrow) by measuring their drift along the walls and substrate, using vision (V*) or touch (T*) of the walls or bottom, or, by detecting turbulence in the bulk flow, with the lateral line (L). Bulk flow disperses prey odor downstream over large distances where it can be detected by olfaction (O, green); closer to the source, prey-generated wake turbulence becomes detectable by the lateral line (L, purple). Close to the source, the prey becomes directly detectable based on vision (V, red), lateral line imaging of the acoustic near field (L, delineated by purple dotted line), electroreception (E, orange), and touch (T, direct tactile contact with prey). B. The blacktip shark,
Carcharhinus limbatus
. From downstream, the blacktip shark detects the presence of prey using O and, during the daytime, tracks the bulk flow upstream using OV* or OL. Seeing the prey, it switches to V to orient and strike from a distance (∼2 m). Near the prey, the strike is adjusted using L. Then it switches to E to ram-capture the prey. With the lateral line blocked ( ) it often misses the prey; successful captures involve increased ram. If
) it often misses the prey; successful captures involve increased ram. If  , it can capture prey using T; if
, it can capture prey using T; if 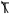 , it will miss. When approaching prey from downstream at night (under moonless conditions;
, it will miss. When approaching prey from downstream at night (under moonless conditions; 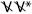 ), it detects (O) and tracks (OL) the prey until it is at close range (∼20 cm), then orients and strikes using L, but captures using less ram. If
), it detects (O) and tracks (OL) the prey until it is at close range (∼20 cm), then orients and strikes using L, but captures using less ram. If 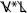 , it detects the prey (O), but cannot track and ceases to feed. When approaching prey from upstream (
, it detects the prey (O), but cannot track and ceases to feed. When approaching prey from upstream (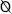 ), it detects the prey using V and orients, strikes, and captures. If it approaches the prey from upstream at night (
), it detects the prey using V and orients, strikes, and captures. If it approaches the prey from upstream at night (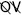 ), it will not detect the prey and will not feed. C. The bonnethead,
Sphyrna tiburo
. From downstream, the bonnethead detects prey using O and, during the daytime, tracks it using OV* or OL; it switches to V to orient and strike, but does so at a closer range (∼1 m) than the blacktip shark, then switches to E to capture using ram-biting. When approaching prey from downstream at night (
), it will not detect the prey and will not feed. C. The bonnethead,
Sphyrna tiburo
. From downstream, the bonnethead detects prey using O and, during the daytime, tracks it using OV* or OL; it switches to V to orient and strike, but does so at a closer range (∼1 m) than the blacktip shark, then switches to E to capture using ram-biting. When approaching prey from downstream at night (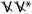 ), it detects (O) and tracks the prey (OL), but cannot orient or strike and ceases to feed. If
), it detects (O) and tracks the prey (OL), but cannot orient or strike and ceases to feed. If 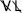 , it detects prey (O), but cannot track, and ceases to feed. When approaching prey from upstream (
, it detects prey (O), but cannot track, and ceases to feed. When approaching prey from upstream (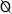 ), it detects prey using V, then orients, strikes, and captures. At night (
), it detects prey using V, then orients, strikes, and captures. At night (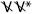 ), it detects (O) and tracks (OL) prey, but cannot orient and strike and ceases to feed. If
), it detects (O) and tracks (OL) prey, but cannot orient and strike and ceases to feed. If  , it misses the prey even when touching it (T or
, it misses the prey even when touching it (T or 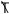 ). D. The nurse shark,
Ginglymostoma cirratum
. From downstream, the nurse shark detects prey using O, then, during the daytime, tracks using OV*, OL, or OT*. At a close range it switches to V, L, or E to orient and strike, then switches to E to suction-capture the prey. At night (
). D. The nurse shark,
Ginglymostoma cirratum
. From downstream, the nurse shark detects prey using O, then, during the daytime, tracks using OV*, OL, or OT*. At a close range it switches to V, L, or E to orient and strike, then switches to E to suction-capture the prey. At night (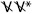 ), it detects (O), tracks (OL or OT*), orients and strikes (L or E) as above, but modulates its capture by increasing suction and decreasing ram. When approaching prey from upstream (
), it detects (O), tracks (OL or OT*), orients and strikes (L or E) as above, but modulates its capture by increasing suction and decreasing ram. When approaching prey from upstream ( ), it does not detect the prey and does not feed. Like the blacktip shark, if
), it does not detect the prey and does not feed. Like the blacktip shark, if  , it can still capture the prey if it touches it (T), but it misses when it does not touch (
, it can still capture the prey if it touches it (T), but it misses when it does not touch ( ) the prey. Nurse shark illustration copyright José Castro, with permission. Pinfish, shrimp, bonnethead, and blacktip shark illustrations copyright Diane Peebles, with permission.
) the prey. Nurse shark illustration copyright José Castro, with permission. Pinfish, shrimp, bonnethead, and blacktip shark illustrations copyright Diane Peebles, with permission.
As a realistic multimodal signal source we used a small (∼8 cm), live prey tethered upstream at 60 cm (blacktip shark), 30 cm (bonnethead), or 10 cm (nurse shark) above the bottom in the center of the (1.2 m deep) water column of a 2 m×7.5 m flume channel. Generalized, prey-generated, aquatic signal fields are diagrammed in Figure 1A. Under natural conditions, bio-electric fields are detectable at a distance of less than one-half meter from the source [17]. The maximum range of detection of hydrodynamic images (the acoustic near-field detected by the lateral line and vestibular organs) is 0.4–2 predator body lengths from the source [18]. Visual detection distance rarely exceeds tens of meters [8]. While far-field sound pressure signals, particularly low frequency signals, may be detectable over distances of (many) kilometers, source direction may be detectable over only tens to hundreds of meters [19]. Wakes with source-generated odor and turbulence signals can be carried by bulk flow over great distances from the source. Beyond that remains a rather non-directional odor far-field that signals the presence of a (food) source. Indeed, odor is often the first cue encountered by aquatic animals searching for food (reviewed in [1]). We characterized the prey odor plume in independent dye tests with similar flow conditions (Figure 2). Tank studies preclude the useful analysis of directional sound. Signal detection distances are dependent on source strength, wavelength and environmental signal-to-noise ratios; these conditions were standardized across all tests.
Figure 2. Experimental flume setup.
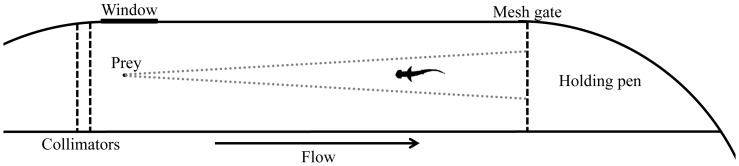
Top view diagram, to scale, with a ∼1 m bonnethead in the test arena, swimming up a plume (approximate outline indicated by gray dotted lines) emanating from the prey. The black dot represents the location of the prey only and is not representative of size. The window allows a side view of the prey area for observation and high speed video recording of the strike-capture sequence. The upstream collimators create uniform, small-scale turbulence and uniform flow through the flume. The downstream gate can be raised to release the shark from the pen used to hold each animal prior to testing. The large remaining part of the oval tank (shown partially) was used to maintain experimental animals.
The goals of this study were to: 1) examine the integration of information from the olfactory, mechanoreceptive, visual, and electroreceptive senses at each stage of the feeding sequence in sharks; 2) investigate sensory switching; and to 3) elucidate the complementary and alternative roles of the senses in each phase of feeding behavior. Examining three shark species from different habitats, with different feeding strategies for different prey types, allowed us to compare, under similar testing conditions, the use of various senses in animals adapted to the ecology of disparate environments.
Materials and Methods
Ethics statement
Shark collections were conducted with permission from the Florida Fish and Wildlife Conservation Commission (07SR-041B and SAL-10-0041-SRP). This study was carried out in accordance with protocols approved by the Institutional Animal Care and Use Committees at the University of South Florida (W3817) and Mote Marine Laboratory (11-03-RH1).
Experimental animals
Eighteen young-of-the-year (YOY) blacktip sharks, Carcharhinus limbatus, 51–65 cm total length (TL), were collected from Terra Ceia Bay on the southwest Florida coast using rod and reel and gillnet gear. Ten juvenile nurse sharks, Ginglymostoma cirratum, 67–94 cm TL, were collected from waters near Long Key in the Florida Keys using rod and reel gear. Sixteen bonnetheads, Sphyrna tiburo, 69–95 cm TL, were collected from Terra Ceia Bay and waters near Sarasota, Florida using gill net gear. Sharks were transported to Mote Marine Laboratory in Sarasota where they were held in a 210,000 L oval tank operated on a closed recirculating life support system with sand filtration and heater/chiller units, maintained 24–26°C on a 12 h:12 h light:dark cycle. Animals were fed fish, shrimp, and squid, supplemented with Mazuri Vita-Zu Sharks/Rays Vitamin Supplement Tablets (PMI Nutrition International, St Louis, MO, USA), to satiation three times per week, except during periods of experimentation, when food was withheld for 48 hours prior to behavioral trials to ensure that the animals were motivated to feed.
Behavioral procedures
Experiments were conducted in a near-laminar flow channel (flume) constructed within the 210,000 liter oval tank; working area (test arena) was 7.5 m long×2 m wide, filled to 120 cm depth, with a flow rate of 2.3 cm/s (Figure 2). Dye tests showed uniform flow with boundary layer shear near the walls and bottom. A 4 m×2 m holding area at the downstream end served as an animal containment area behind a mesh gate. As per Gardiner and Atema [20], for each trial, an individual animal was moved into the flume channel and allowed to acclimate for 30 minutes, then offered a small piece of food to confirm that it was hungry. The animal was then herded into the holding pen. A live prey item from the diet of each species (nurse and blacktip sharks: pinfish, Lagodon rhomboides [21], [22]; bonnethead: pink shrimp, Farfantepenaeus duorarum [23]), was tethered at the upstream end of the flume using a piece of thin, degradable cotton thread, inserted through the musculature. The prey were, therefore, injured from the tether attachment. This tether restricted the prey to the area in front of a window in the side of the tank. Since prey size can affect capture kinematics [24], the prey items were size-matched to the total length of the shark and prey size was consistent across trials. Prey was suspended midwater, at 60 cm (blacktip sharks), 30 cm (bonnetheads) or 10 cm (nurse sharks) above the bottom. Independent dye studies showed the shape and extent of the odor/turbulence plume emanating from the prey (Figure 2). The shark was held in the start box for six minutes to allow a plume of sensory cues emanating from the prey to establish along the length of the flume channel. The shark was then released and a trial proceeded for 10 minutes or until the prey was consumed, during which time the shark's behavior was simultaneously monitored and filmed from above using a series of three overhead cameras (Sony 1/3 inch CCD Camera, Model CUC8752, CIB Security, Sunnyvale, CA, USA). A lateral view of any strikes or bites on the prey was recorded using a fourth camera placed in front of the previously mentioned window in the tank wall (Figure 2). The images from these cameras were combined using a multiplexer (Nuvico EV-8250N, Englewood, NJ, USA) and saved digitally via a computer. Additionally, any bites or strikes were filmed laterally at 250 frames/s using a Photron FASTCAM-X 1024 PCI Model 100 K camera (Photron USA Inc., San Diego, CA, USA), which was also placed in front of the window in the tank wall (Figure 2). Animals were examined intact and after blocking each of the sensory systems (outlined below), alone and in combination.
Sensory deprivation
Olfaction was blocked by inserting pieces of cotton soaked in petroleum jelly into the animal's nares [25]. To block vision, the eyes were covered with small pieces of heavy black plastic, attached to the skin around the margins of the eyes with cyanoacrylate glue. The sensitivity of the electrosensory system was reduced by painting over the pores of the ampullae of Lorenzini with cyanoacrylate glue (blacktip and nurse sharks; The Original Super Glue, Super Glue Corp., Rancho Cucamonga, CA USA) or silicone-rubber paint (bonnetheads; Smooth-On Mold Max Stroke, Smooth-On Inc., Easton, PA USA). The location of the pores in these species has previously been mapped [26], [27]. Prior to use on animals, the insulating nature of these two materials was verified by covering one electrode on the prey-simulating electrical stimulus apparatus described by Kajiura and Holland [17]. The pair of electrodes was then immersed in seawater and a current of up to 200 mA was applied; no current was detected at the multimeter, indicating that the paint and glue break the electrical circuit and are therefore insulating. To minimize any distress, all of these blocks were applied while the animal was under anesthesia with tricaine methanesulfonate (MS-222), with a dose of 100 mg/L in buffered seawater for induction and 50 mg/L for maintenance. Animals were ventilated using a hose attached to a small recirculating pump while the blocks were applied, then revived using fresh seawater. Animals were allowed to recover in approximately 1000 L of seawater in a 244 cm diameter round tank for three hours, then moved to the flume channel and allowed to acclimatize for a further 30 minutes as above, prior to a behavioral trial. MS-222 is a sensory depressant requiring 1.5 hours of recovery [28].
The lateral line system was lesioned by holding the animals in a 0.5 g/L solution of aerated streptomycin sulfate in seawater for three hours [20]. An individual animal was held in approximately 1000 L of this solution in the 244 cm diameter round tank. For combinations of sensory blocks, those requiring anesthesia were first applied, then the animal was moved to the recovery round tank where it was held until it had recovered sufficiently to swim and navigate the tank normally. The streptomycin sulfate was added to the water and the animal was held in this solution for three hours as described above, prior to being moved to the flume channel for behavioral testing. Streptomycin is an ototoxic antibiotic that has been shown to lesion both the surface neuromasts and canal neuromasts in teleosts [29]. In amphibians, treatment with this drug results in an increase in spontaneous firing of the afferent nerves, which is linked to direct effects on the membrane of the hair cell, and a large lag phase in the receptor potentials, which may be caused by interference with the motion of the sensory hairs [30]. It does not affect inner ear function unless applied intralumenally [31]–[33]. The duration of the effects of this drug is not completely understood. Since teleosts treated with this drug return to normal behavior in 20–24 hours [34], all lateral line blocked trials were completed within six hours of application of the drug. However, since physical damage to the hair cells has been found on scanning electron micrographs of streptomycin-treated neuromasts [32], [33], following lateral line lesion treatments, animals were allowed to recover for a minimum of four weeks prior to any other behavioral testing to allow time for the neuromasts to regenerate [35], [36]. While hearing with the inner ear may be contributing to prey localization in sharks [37], [38], the stimulus field in a closed tank environment is very difficult to control due to ambient noise, as well as echoing off the walls, bottom and surface of the tank, and thus it was not specifically examined in this study.
Video analysis
All videos were digitized using MaxTRAQ Lite+ v.2.2.2.2 software (Innovision Systems Inc., Columbiaville, MI, USA). As described above, the behavior of these animals can be divided into five phases: detection, tracking, orientation, striking, and capture. Detection is indicated by the initiation of feeding behavior (i.e. the onset of tracking, or in the absence of tracking, the onset of orientation/striking). Tracking in other shark species typically begins with a rapid turn and descent towards the bottom, followed by tight circles and figure-8 patterns as the animal approaches the prey from downstream [20], [39]–[43]. Orientation, a turn to align the body or head for the strike, is immediately followed by striking. Striking in ram-feeding bony fish is defined as direct, rapid whole-body acceleration towards the prey, often using an S-start [44]. In fishes, capture begins with the onset of jaw depression [13], [45] (i.e. mouth opening) and for comparative purposes, in this study was defined as ending when the center of mass of the prey had passed the anterior margin of the mouth.
In intact animals, detection is typically indicated by the start of tracking behavior, which ends with orientation (a turn) and a directed strike that culminates in capture. From the composite view of the three overhead cameras and lateral camera, the onset time of each of these behaviors was noted. For the tracking phase, the following variables were examined: 1) swim velocity, in body lengths/s; 2) turn velocity, in °/s; 3) frequency of turns, in turns/s; and 4) tracking time, in s, from the start of tracking to the first strike. To account for differences in the distance at which tracking began, tracking time was standardized by dividing it by predator-prey distance at the onset of tracking, expressed as a proportion of the total length of the test arena, i.e., timestd. = time/(distance to prey/test arena length). For the orientation, striking, and capture phases, 5) orientation distance, predator-prey distance at orientation, in cm; 6) strike rate, percentage of trials in which strikes occurred; 7) strike angle, in °, the angle between the midline of the predator and center of mass of the prey; 8) strike velocity, in body lengths/s; 9) number of misses; and 10) capture success rate, percentage of trials resulting in successful capture, were examined.
Statistical Analyses
Data for each species were regressed against total length using the least squares method to remove the effects of size [46] and the standardized residuals were used in all subsequent analyses. All data were tested for normality and equality of variance with Kolmogorov-Smirnov tests and Levene Median tests, respectively [47]. Florida Fish and Wildlife Conservation Commission regulations prohibit the release of any fishes that are held in captivity for more than 30 days or treated with any chemicals and so, in an effort to reduce the number of animals taken from the wild, individual animals were used in more than one, but not all, treatment groups resulting in an unbalanced design. Data for the different treatments were therefore compared within each species using linear mixed models. Non-parametric repeated measures analyses were conducted using Skillings-Mack tests. When significant differences were found, Tukey post hoc tests were then used to perform pairwise comparisons of the treatments. The Benjamini-Hochberg method was used to control the false discovery rate in multiple statistical tests [48]. Analyses were conducted using R (version 3.0.2, [49]), nlme (version 3.1-111, [50]), multcomp (version 1.3-1, [51]), Skillings-Mack (version 1.0-2, [52]), and nparcomp (version 2.0, [53]).
Results and Discussion
To guide the reader through the analysis of results from multiple sensory blocks affecting five phases of hunting in three species (Figure 1), we start with a generalized description. Based on current and previous results, the hunting sequence can be described as follows. The shark cruises out of the gate; it changes swimming behavior (more frequent and faster turns, but no change in swimming velocity) indicating odor detection and the start of tracking. While odor per se is non-directional and concentration gradients in odor plumes are too chaotic to provide useful directional information, odor is eminently suited for prey identification and motivating subsequent behavior [1], [54]. At the farthest detection distance the sharks have only patches of odor available (odor far-field), followed by the addition of wake turbulence (odor near-field). Then, using both odor and lateral line information [20], the shark starts tracking the plume. To stay connected with the plume they steer into concentration patches based on detection of sub-second time differences between bilateral odor encounters [55]. Upon visual contact with the prey, ram-feeding sharks orient and accelerate into a strike, where the prey's hydrodynamic field guides the precise directional and temporal coordination of swimming and mouth positioning. Suction-feeding sharks track until in close proximity, then visual, electrical, hydrodynamic or tactile cues prompt them to strike by raising the head. In all species, electric fields guide the timing of jaw opening with millisecond precision. Experimental evidence below follows the hunting sequence from detection to capture. Data for all treatments are presented in Tables S1–S3.
Detection
Despite their different sensory specializations, when approaching prey from downstream, all three shark species detected the distant presence of prey by olfaction. With olfaction blocks, blacktip sharks and bonnetheads kept cruising and did not track the plume, but at much closer range (2 m and 1 m respectively) detected prey visually and proceeded to strike and capture. With olfaction and vision blocked simultaneously, neither blacktip sharks nor bonnetheads detected the presence of prey and thus failed to feed (Figures 1B–D, green area). Nurse sharks absolutely required odor: blocking olfaction abolished detection and feeding. Olfactory-blocked nurse sharks spent a significantly greater proportion of their time resting on bottom [intact: 0.2±0.2%, olfaction blocked: 50.9±12.2%, P<0.001].
Although the nurse shark possesses retinal areas specialized for enhanced visual acuity [56], [57], and vision is clearly important for other behaviors [58], they do not appear to identify prey visually. This species has been described as a nocturnal hunter, often cornering fish in reef crevices at night [16], [59], [60]. Visual cues may be diminished on a dark night or even unavailable in the case of hidden prey, which may explain why chemical cues are more important than visual cues for feeding in this species. The smooth dogfish, Mustelus canis, a crepuscular hunter, also requires olfaction to detect prey [25]. Many shark species approach their prey from downstream [20], [39], [40] and olfaction has long been thought to be the primary sensory modality for prey detection [61]. However, our results demonstrate that at least some species can detect prey visually, suggesting that they could also approach prey from upstream (odor cues are unavailable as they are carried away from the prey by the flow), provided there is good visibility (i.e., daytime hunting with good to moderate water visibility). While bonnetheads are thought to be diurnal hunters [62] and blacktip sharks primarily crepuscular [63], their inability to detect prey in the absence of odor and vision suggests that if they hunt at night, they likely approach prey from downstream.
Tracking: rheotaxis in bulk flow and eddy chemotaxis in wakes
After olfactory-based detection, navigation to the vicinity of an odor source is based on upstream swimming and wake tracking [20], [59], [60]. Orientation to flow is often referred to as “rheotaxis” [64]. The bulk flow vector (Figure 1A) needed to steer upstream swimming is not source-directed and animals can determine it only by measuring their drift against an external frame of reference, typically by seeing or touching fixed structures such as the walls or bottom [64], or by detecting turbulence contained in the bulk flow [65], such as the shear/turbulence found at boundary layers near the walls and bottom. Famously, moths in pheromone plumes steer by the visual flow field [66]. Tracking the odor-flavored eddies of a source-generated wake has been called “eddy-chemotaxis” [1] to distinguish it from rheotaxis.
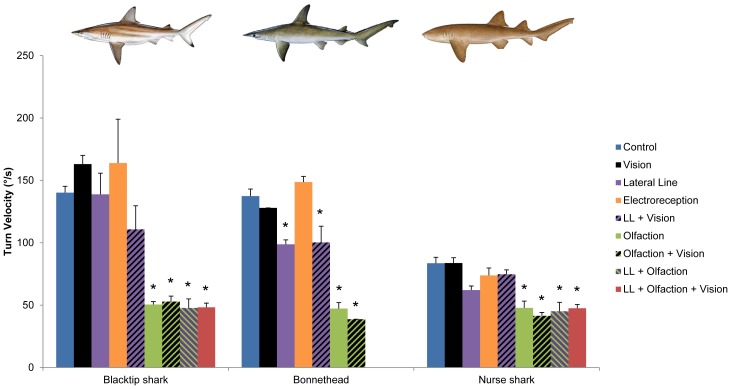
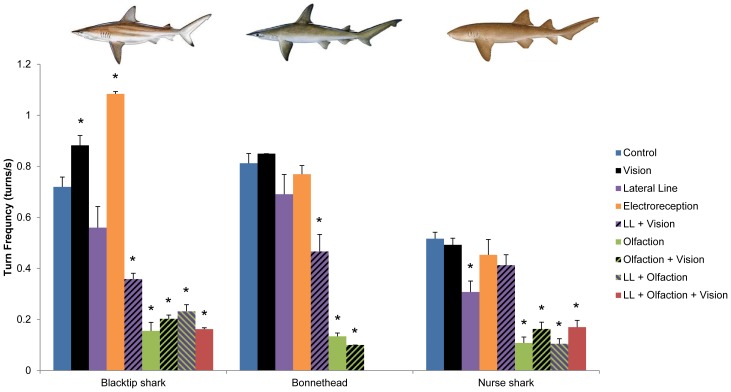
Tracking behavior in other shark species has been described as tight circles and figure-8 patterns [20], [39], [40], [43]. In our study, we have defined it as a period of high-velocity, high-frequency turns (blacktip shark, control: 140.2±2.1°/s, 0.72±0.04 turns/s; bonnethead, control: 137.4±5.7°/s, 0.81±0.04 turns/s; nurse shark, control: 83.6±4.8°/s, 0.52±0.03 turns/s; Figures 3 and 4). Swimming velocity varied slightly among the treatments. Blacktip sharks swam slower when olfaction and vision were blocked (olfaction + vision blocked: 0.72±0.03 BL/s, P<0.001; lateral line + olfaction + vision blocked: 0.73±0.08 BL/s, P = 0.009; lateral line + vision: 0.70±0.04 BL/s, P<0.001). Bonnetheads swam slower with lateral line and vision blocked (0.49±0.03 BL/s, P = 0.009). Nurse sharks swam faster with lateral line blocked, alone or in combination with olfaction (lateral line blocked: 0.76±0.07 BL/s, P<0.001; lateral line + olfaction blocked: 0.73±0.08 BL/s, P = 0.009). In the smooth dogfish, M. canis, tracking requires simultaneous olfactory and hydrodynamic cues [20]. This species can orient to the bulk flow and navigate upstream using either vision or the lateral line, but it requires lateral line input to follow the odor-flavored wake. Sensory blocks in the blacktip shark and bonnethead showed their tracking and upstream swimming were also dependent on olfaction in combination with either vision or the lateral line. In all three species, when olfaction was blocked, alone or in combination with other senses, turns were significantly slower and less frequent compared to the unblocked condition (blacktip shark, olfaction blocked: 54.3±2.3 °/s, P<0.001; 0.16±0.03 turns/s, P<0.001; bonnethead, olfaction blocked: 47.2±4.9 °/s, P<0.001; 0.13±0.01 turns/s, P<0.001; nurse shark, olfaction blocked: 47.8±2.9 °/s, P<0.001; 0.11±0.01 turns/s, P<0.001; Figures 3 and 4); this behavior is similar to that of a shark that simply cruises the tank in the absence of prey and suggests that odor motivates the behavior. When vision and the lateral line were simultaneously blocked, blacktip sharks and bonnetheads turned quickly, but infrequently (blacktip shark: 110.8±18.9 °/s, P>0.05; 0.36±0.03 turns/s, P<0.001; bonnethead: 100.3±13.0 °/s, P = 0.03; 0.47±0.07 turns/s, P<0.001; Figures 3 and 4), and could not locate the prey, indicating that vision or the lateral line provide the directional vector required for source localization, as in M. canis [20]. The nurse shark, on the other hand, could continue to track and successfully locate prey when both vision and the lateral line were blocked (74.8±3.6 °/s, P = 0.984; 0.41±0.04 turns/s, P = 0.900; Figures 3 and 4). Since this species tends to maintain contact with the bottom as it swims, occasionally even using its pectoral fins to propel itself [67], it appears to use tactile substrate cues with free nerve endings in the skin [68] to measure drift and to orient to the flow. Tactile orientation to flow has been described in teleost fish [64], [69], [70] and suggested to be possible for the epaulette shark, Hemiscyllium ocellatum [71], but this is the first evidence of the use of tactile cues for tracking. However, reaching the prey with olfaction and touch is a slow and convoluted process (intact: 98.4±20.6 s, vision and lateral line blocked: 189.8±79.1 s, P<0.001).
Figure 3. Turn velocity during tracking.
The turning velocity, during the tracking phase, in three species of sharks, the blacktip shark, Carcharhinus limbatus, the bonnethead, Sphyrna tiburo, and the nurse shark, Ginglymostoma cirratum, with all senses intact (control) and following blocks of the sensory systems indicated in the figure legend (LL: lateral line). Error bars are ± s.e.m. * denotes treatments that are significantly different from control at α = 0.05; for comparisons among treatments, see Tables S1–S3. Nurse shark illustration copyright José Castro, with permission. Bonnethead, and blacktip shark illustrations copyright Diane Peebles, with permission.
Figure 4. Turn frequency during tracking.
The frequency of turns (turns/s), during the tracking phase, in three species of sharks, the blacktip shark, Carcharhinus limbatus, the bonnethead, Sphyrna tiburo, and the nurse shark, Ginglymostoma cirratum, with all senses intact (control) and following blocks of the sensory systems indicated in the figure legend (LL: lateral line). Error bars are ± s.e.m. * denotes treatments that are significantly different from control at α = 0.05; for comparisons among treatments, see Tables S1–S3. Nurse shark illustration copyright José Castro, with permission. Bonnethead, and blacktip shark illustrations copyright Diane Peebles, with permission.
Strike/orientation
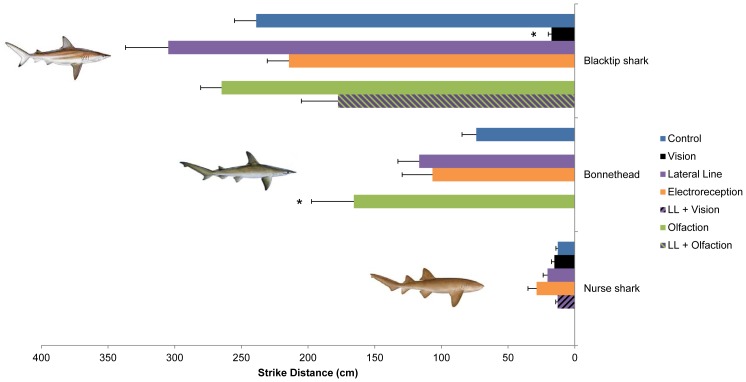
Once an animal had tracked the odor plume to the vicinity of the source, or, in the absence of tracking, upon visual detection (blacktip shark or bonnethead), striking immediately followed. Strikes are fast and need to be precisely oriented. Sensory control of orientation and striking behaviors differed among the three species. Intact blacktip sharks oriented from a distance of a few meters (238.9±16.3 cm; Figure 5) and executed direct (angle of 15.4±2.5° to the prey), rapid strikes (111.0±11.9 cm/s, equivalent to 1.95±0.21 Body Lengths per second, BL/s). Significantly fewer vision-blocked animals struck (60.4±11.1%, P<0.05) and only after lengthy search times (intact: 35.0±9.5 s, vision blocked: 949.3±236.1 s; P<0.001). Vision blocked strikes occurred only from a distance of a few centimeters (17.4±2.5 cm, P<0.001; Figure 5), from greater angles (91.7±15.5°, P<0.001), and at reduced velocity (70.3±17.4 cm/s or 1.25±0.31 BL/s, P = 0.001), indicating that orientation of the long distance strikes is visually guided. In the absence of vision, strikes were guided by the lateral line, as animals with simultaneous vision and lateral line blocks did not orient or strike, even when they were within electrosensory prey detection range [17]. This also suggests that electrical cues alone were not sufficient to prompt a strike. In other studies of the role of electroreception in predation, olfactory stimuli have been required for the initiation of feeding behavior [17], [43], [72]–[80]. Collectively, these results suggest that sharks do not recognize electrical cues alone as prey, but require an additional visual or olfactory cue.
Figure 5. Strike distance.
The distance between the predator and the prey at the initiation of the strike, in cm, in three species of sharks, the blacktip shark, Carcharhinus limbatus, the bonnethead, Sphyrna tiburo, and the nurse shark, Ginglymostoma cirratum, in animals with all senses intact (control) and following blocks of the sensory systems as indicated (LL: lateral line). Treatments in which striking did not occur have been omitted. Error bars are ± s.e.m. * denotes treatments that are significantly different from control at α = 0.05; for comparisons among treatments, see Tables S1–S3. Nurse shark illustration copyright José Castro, with permission. Bonnethead, and blacktip shark illustrations copyright Diane Peebles, with permission.
Intact bonnetheads, compared to blacktip sharks, oriented in closer proximity (73.8±10.7 cm; Figure 5) and a greater angle (28.1±5.1°) to the prey, and struck with lower velocity (59.2±2.34 cm/s, equivalent to 0.70±0.03 BL/s). Bonnethead orientation and striking appeared entirely visually guided: blinded animals never successfully executed a strike, even when lateral line cues, which could guide striking in blacktip sharks, were available. Olfaction-blocked animals began the visually guided strike from a greater distance to the prey, compared to the control treatment (165.7±31.8 cm, P<0.001; Figure 5) but at a similar angle (26.5±8.9°) and with a similar velocity (0.84±0.04 BL/s). Striking behavior was not significantly affected by any other treatment. Both blacktip sharks and bonnetheads are ram-feeders [14], [15]. Ram feeding involves the predator overtaking its prey with the mouth open, which inherently requires that the predator pinpoints its prey from a distance in order to have sufficient room to accelerate [44]. Vision provides the best performance for this task. It allows the animal to precisely localize the prey from a greater distance than the lateral line, which functions over distances of 0.4–2 predator body lengths [18], [81]. Electroreception alone cannot mediate orientation and striking in these species, perhaps because it functions only over distances of tens of centimeters [17], [82]. Hammerhead sharks, such as the bonnethead, possess enhanced binocular vision compared to pointed-nose sharks, such as the blacktip shark [83], which may explain the bonnethead's reliance on vision for striking at prey in the water column.
Intact nurse sharks orient from a close proximity than blacktip sharks or bonnetheads (12.7±1.4 cm; Figure 5), and greater angles (58.4±10.4°), striking with a slower velocity (26.1±3.39 cm/s, equivalent to 0.31±0.04 BL/s). Vision, lateral line, or electroreception blocks did not cause significant changes in either the frequency of orientation and striking [all treatments: 100%], or in striking distance [vision blocked: 15.2±2.2 cm, lateral line blocked: 20.4±3.3 cm, electroreception blocked: 28.6±6.5 cm, P = 0.328; Figure 5]. Strike angle, however, was significantly greater after lateral line + vision blocks or electroreception blocks [lateral line + vision blocked: 91.9±22.8°, electroreception blocked: 93.7±6.3, P = 0.006]. Strike velocity was slower when vision was blocked and faster when the lateral line or electroreception was blocked [vision blocked: 0.23±0.06 BL/s, vision + lateral line blocked: 0.23±0.03 BL/s, lateral line blocked: 0.44±0.06 BL/s, electroreception blocked: 0.44±0.04 BL/s, P = 0.001]. Since suction feeding is only effective over short (cm) distances [16], [84], suction feeders (e.g. nurse sharks) can use any of these senses to successfully align their short-distance strikes, although there are slight differences in the strike, depending on the sensory modality used. The Pacific angel shark, Squatina californica, a lie-in-wait ambush predator that is believed to be a suction feeder, can also use vision or mechanoreception to align short distance (10 cm) strikes; electroreception has not yet been examined [85].
Ram-feeding teleosts typically brake (decelerate) just before capture, which has been suggested to increase capture accuracy by allowing more time for steering and positioning [86], [87]. The lateral line mediates this behavior in largemouth bass, Micropterus salmoides [44], either by providing the animals with information on the position of the prey, just prior to capture, which prompts them to brake, or by aiding in the regulation of swimming speed. Higher swimming velocities have been observed in several species of fish during tracking and striking after the lateral line system has been blocked [44], [88], [89] and detection of self-generated flow fields around the body by the lateral line has been shown to function in other behaviors, such as obstacle avoidance [90]–[93]. Lateral line information also appears to guide the final moments of the strike in blacktip sharks. While intact blacktip sharks rarely missed, blocking the lateral line caused frequent misses associated with high velocity (271±84 cm/s or 3.62±1.10 BL/s) strikes (see Movie S4); successful capture was associated with lower velocity strikes (153±37 cm/s or 1.17±0.26 BL/s, P = 0.03) that may not require braking. In the bonnethead, blocking the lateral line did not affect capture success or strike velocity. Since bonnetheads strike with a lower velocity (59±2 cm/s or 0.75±0.03 BL/s), this species may rely less on feedback from self-generated mechanosensory noise to regulate its swimming speed, as slower swimming speeds have been suggested to create less hydrodynamic noise [94], [95]. Swimming velocity as the bonnethead approaches the prey could instead be mediated by their enhanced binocular vision [83]; swimming speed during other behaviors, such as schooling, is visually regulated in other fishes [96]. Additionally, since slower velocity strikes generate smaller bow waves in front of the head, delaying prey escape responses [97], [98], the lateral line may not be critical for strike precision at slower speeds. Nurse sharks, with their very close proximity strikes, may not need lateral line-mediated strike adjustment. Since suction feeders use little to no forward motion, the prey is primarily alerted to the presence of the predator by the suction flow [99], often too late for a successful escape response.
Capture
High-speed video analysis has demonstrated that the final milliseconds of a strike leading to capture require precisely timed jaw movements [100] (see Movies S1–S3). In all three species, electroreception triggered jaw depression. Without electroreception, blacktip and nurse sharks missed, unless they touched the prey (with the snout, near the mouth) prior to beginning to move the jaws, which then lead to capture (blacktip shark: Pearson Product Correlation, p = 0.00002; nurse shark: Pearson Product Correlation, p = 0.00006; see also Movie S5–S8). While repeated strikes in electroreception-blocked blacktip and nurse sharks eventually resulted in capture in all trials, electroreception-blocked bonnetheads failed to open their jaws, despite repeated strikes, and never made successful captures even when touching the prey (Figure 6; see also Movie S9). This suggests that jaw depression is completely guided by the electrical field surrounding the prey and tactile cues were insufficient to initiate jaw movements. The complete reliance on electroreception for prey capture in bonnetheads may be related to the evolution of wider heads in the hammerhead shark family, as their lateral head expansion supports widely spread electroreceptors sampling a large area of the environment [17]. Other enhanced sensory capabilities, such as olfaction, also have been linked to wider heads in sharks [55].
Figure 6. Prey capture in intact vs. electroreception-blocked bonnetheads.
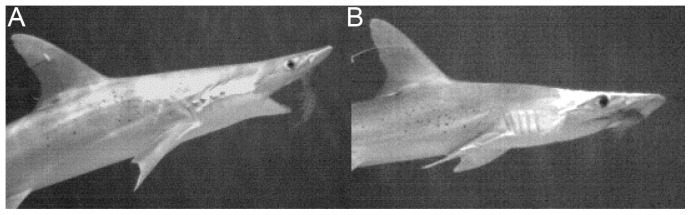
A. A bonnethead, Sphyrna tiburo, with all senses intact opens the mouth to capture shrimp using ram-biting. B. The same bonnethead fails to open the mouth when electroreception is blocked and misses the shrimp, despite making tactile contact with the prey.
When capture occurs, it is altered in response to sensory deprivation. Ram-feeding blacktip sharks use less ram when vision-blocked but do not change the amount of suction, producing an overall ram feeding event [100]. In contrast, a ram-feeding teleost, the largemouth bass (Micropterus salmoides), both decreases ram and actively increases suction when vision-blocked, resulting in a switch from ram to suction feeding [44]. This difference may reflect anatomical limitations on suction generation in the blacktip shark. Unlike many ram-feeding bony fishes, many ram-feeding sharks do not possess the laterally occluded mouth that is characteristic of suction feeders [13]. On the other hand, the nurse shark, a characteristic suction feeder [16], does have the proper mouth morphology to actively increase suction and does so when vision-blocked, while simultaneously decreasing ram [100]. These results suggest that blacktip and nurse sharks modify their capture strategy slightly when using alternate sensory modalities to locate and capture prey. The bonnethead shows little to no change in capture kinematics in response to sensory deprivation, which suggests that capture plasticity varies by species, rather than by capture mechanism [100].
Summary and Conclusions
This study was designed to link the existing physical knowledge of underwater signal dispersal and the behavioral function of individual senses of sharks during their hunting behavior. It is important to realize that our understanding of signal dispersal is typically based on mathematical models developed from physical experiments under idealized, low-noise, single modality conditions [1], [82], [101]. In nature, conditions can be substantially different and cues can be masked by noise, forcing animals to use the best available information rather than mathematically idealized stimulus fields. Using a large laboratory flume allowed us to test animals under standardized naturalistic conditions and with sensory manipulations that would be nearly impossible to accomplish in the field.
As expected, sensory deprivation confirmed that each sense had its optimal range of use; these ranges overlap to form a smooth sequence from detection to capture (Figure 1A). The olfactory range is large but limited to downstream dispersal where directional information in the odor far-field is difficult to extract [1]. When an attractive odor is detected but the source itself is out of direct detection range, the logical response is to swim upstream [59], [60]. The sharks appear to determine upstream directional information from their drift in the bulk flow. Drift can be measured by the lateral line from boundary layer effects on turbulence [65] and in the case of nurse sharks from actual touch of the (sea) floor [67]. In the flume and in near-bottom habitats, drift can also be measured visually [69] as shown by the effects of sensory blocks. Closer to the source, the prey wake can provide simultaneous odor and turbulence information; this odor near-field becomes increasingly directional [1]. In blacktip sharks and bonnetheads, visual detection of the prey itself can direct the strike from any approach angle, upstream and downstream. The timing then changes from minutes of tracking to (sub-)seconds of striking during which the lateral line and electroreception provide precise information on target location. Finally, capture is most dependent on electroreception to coordinate the 10–100 millisecond-scale ram-suction movements. Touch can occasionally lead to capture when electroreception is blocked. Odor may continue to motivate the behavior: nurse sharks do not hunt at all without odor information, while the more pelagic species still hunt after seeing the prey target, allowing them to strike from upstream directions.
In earlier experiments with food odor sources instead of live prey, we noted on several occasions that sharks after locating the source, but not finding actual food, circled back downstream and repeated the search sequence from a distance, over and over [20]. This gave the impression that the hunting sequence was stereotyped. However, the current results using live prey that emit a variety of sensory cues show that there is considerable plasticity in the behavior and use of senses. We conclude that tracking, the olfactory hunting phase, is indeed stereotyped, although different for each species. This may be due to the lack of precise vector information when only odor and flow are available: the observed stereotyped sequences may have evolved as the optimal solution for the difficult task of odor plume tracking. Then, when direct prey contact has been made, the sharks show plasticity in the use of senses.
Other reports have focused on shark attraction to prey sounds [37], [102]. As stated, we deliberately did not include in this laboratory study the use of sound, particularly directional sound. To avoid reverberation, credible analysis of guidance by sound should be done under field conditions and this will remain an interesting challenge. Our results show the lateral line was clearly involved in directing the strike; this suggests that the sound near-field may have provided directional hydrodynamic information that was not extracted by the otolithic (inertial detection) organs. The lateral line can detect both the wake turbulence [103] and the sound near-field (particle displacement) of the prey [104]; mathematical models suggest that the latter would be expected to provide better information on the precise location of the prey [101].
Sensory hierarchies have been developed for a few other vertebrates, but only for one phase of a behavior, such as prey identification in bats [105], detection in lemurs [106], tracking in catfish [103], striking in snakes [107], and capture in frogs [108]. This study is therefore the first to describe the hierarchy of senses engaged in guiding a complete behavioral sequence in a vertebrate, in this case hunting in aquatic vertebrate predators, focusing on sharks as a model group.
Our results demonstrate that sharks are capable of attending to multiple sensory cues simultaneously, switching sensory modalities in a hierarchical fashion as they approach their prey, and substituting alternate sensory cues, when necessary, to accomplish behavioral tasks. This flexibility in behavior suggests that sharks are well adapted to succeed, even in the face of a changing environment and evolutionary advancements in prey defenses, including chemical, visual, and mechanical camouflage [109], [110]. Such flexibility may have contributed to the success of these marine apex predators and may continue to do so as environments and ecosystems change. Sharks, however, are not unique in their sensory guidance of hunting: they exploit information fields available to all marine species. Thus, the results may be seen as a general blueprint for underwater hunting, modifiable by habitat and by the behavioral specializations of many different aquatic animals from lobsters to whales. These results set the stage for neurobiological analysis of sensory integration in the brains of hunting animals and inspire the design of underwater navigation algorithms for autonomous vehicles requiring plasticity in adapting to unpredictable and variable local conditions [111].
Supporting Information
Data summary – blacktip shark, Carcharhinus limbatus . Summary of all variables for the blacktip shark, Carcharhinus limbatus with all senses intact, and following blocks of the senses as indicated. Abbreviations: O = olfaction, V = vision, L = lateral line, E = electroreception. All means are ±s.e.m. The p values are the results of linear mixed effects model analyses or Skillings-Mack tests performed on each variable. Value marked (*) are significant after Benjamini-Hochberg corrections. Tukey Test p values reflect the results of pairwise post-hoc comparisons between treatments. N.A.: Not applicable, parameter was not assessed because behavior did not occur; N.S.: Non-significant at α = 0.05.
(DOCX)
Data summary – bonnethead, Sphyrna tiburo . Summary of all variables for the bonnethead, Sphyrna tiburo with all senses intact, and following blocks of the senses as indicated. Abbreviations: O = olfaction, V = vision, L = lateral line, E = electroreception. All means are ±s.e.m. The p values are the results of linear mixed effects model analyses or Skillings-Mack tests performed on each variable. Value marked (*) are significant after Benjamini-Hochberg corrections. Tukey Test p values reflect the results of pairwise post-hoc comparisons between treatments. N.A.: Not applicable, parameter was not assessed because behavior did not occur; N.R.: Not recorded due to technical difficulties; N.S.: Non-significant at α = 0.05.
(DOCX)
Data summary – nurse shark, Ginglymostoma cirratum . Summary of all variables for the nurse shark, Ginglymostoma cirratum with all senses intact, and following blocks of the senses as indicated. Abbreviations: O = olfaction, V = vision, L = lateral line, E = electroreception. All means are ±s.e.m. The p values are the results of linear mixed effects model analyses or Skillings-Mack tests performed on each variable. Value marked (*) are significant after Benjamini-Hochberg corrections. Tukey Test p values reflect the results of pairwise post-hoc comparisons between treatments. N.A.: Not applicable, parameter was not assessed because behavior did not occur; N.S.: Not significant at α = 0.05.
(DOCX)
Prey capture in an intact blacktip shark, Carcharhinus limbatus , played in slow motion at 30 frames/s (filmed at 250 frames/s). Rapid (mean: 2.0 BL/s) strikes are initiated from a distance (mean: 240 cm) and the prey is overtaken and engulfed using ram-capture (100% capture success rate).
(AVI)
Prey capture in an intact bonnethead, Sphyrna tiburo , played in slow motion at 30 frames/s (filmed at 250 frames/s). Strikes are slower (mean: 0.8 BL/s) and initiated from a closer proximity (mean: 74 cm) than in the blacktip shark. Prey is overtaken and captured between the teeth, using ram-biting (100% capture success rate).
(AVI)
Prey capture in an intact nurse shark, Ginglymostoma cirratum , played in slow motion at 30 frames/s (filmed at 250 frames/s). Slow (mean: 0.3 BL/s) strikes, consisting of raising the head, are initiated from close proximity (mean: 13 cm). The prey is rapidly drawn into the mouth using suction (100% capture success rate).
(AVI)
Prey capture in a blacktip shark, Carcharhinus limbatus , with the lateral line blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When lateral line information is absent, the blacktip sharks frequently missed the prey. These misses were associated with faster strikes (mean: 3.6 BL/s).
(AVI)
Attempted prey capture (miss) in a blacktip shark, Carcharhinus limbatus , with electroreception blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When electrical cues are absent, blacktip sharks frequently missed unless they touched the prey (with the snout, near the mouth) prior to beginning to move the jaws (see Movie S6 for an example of a successful capture).
(AVI)
Prey capture (successful) in a blacktip shark, Carcharhinus limbatus , with electroreception blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When electrical cues are absent, blacktip sharks could successfully capture prey if they touched it (with the snout, near the mouth), prior to beginning to move the jaws. If they did not touch the prey, they frequently missed (see Movie S5 for an example of a miss).
(AVI)
Attempted prey capture (miss) in a nurse shark, Ginglymostoma cirratum , with electroreception blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When electrical cues are absent, nurse sharks frequently missed unless they touched the prey (with the snout, near the mouth) prior to beginning to move the jaws (see Movie S8 for an example of a successful capture).
(AVI)
Prey capture (successful) in a nurse shark, Ginglymostoma cirratum , with electroreception blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When electrical cues are absent, nurse sharks could successfully capture the prey if they touched it (with the snout, near the mouth) prior to beginning to move the jaws. If they did not touch the prey, they frequently missed (see Movie S7 for an example of a miss).
(AVI)
Striking behavior in a bonnethead, Sphyrna tiburo , with electroreception blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When electrical cues are absent, the bonnethead fails to open the mouth and misses the prey (0% capture success rate).
(AVI)
Acknowledgments
We thank J. Morris for assistance with design, construction, and maintenance of the flume system, J. Morris, M. Smith, K. Mara and the staff of Mote Marine Laboratory's Center for Shark Research for assistance with animal collection, handling, and care, A. Stamper and L. Byrd for veterinary support, B. Turffs for assistance with data collection, R. Dryer and B. Leigh for assistance with video analysis, J. Rohr for assistance with statistical analysis, H. Luciano for technical support, D. Minkoff for helpful comments on the figures, and the reviewers for helpful suggestions.
Funding Statement
This work was funded by a collaborative National Science Foundation grant, www.nsf.gov [IOS-0843440 to JA, IOS-0841478 to REH, and IOS-081502 to PJM]; a Lerner-Gray Grant for Marine Research to JMG, www.amnh.org; an American Elasmobranch Society Donald R. Nelson Behavior Research Award to JMG, www.elasmo.org, an American Society for Ichthyologists and Herpetologists Raney Fund Award to JMG, www.asih.org; and by the Porter Family Foundation. JMG was supported by a University of South Florida Presidential Doctoral Fellowship, www.usf.edu. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Atema J (2012) Aquatic odor dispersal fields: opportunities and limits of detection, communication and navigation. In: Bronmark C, Hansson LA, editors. Chemical Ecology in Aquatic Systems. Oxford, U.K.: Oxford University Press. pp. 1–18.
- 2.von Uexküll J (2010) A Foray into the Worlds of Animals and Humans: with a Theory of Meaning. O'Neil JD, translator. Minneapolis, MN: University of Minnesota Press.
- 3.Atema J, Fay RR, Popper AN, Tavolga WN (1988) Sensory Biology of Aquatic Animals. New York, N.Y.: Spinger-Verlag. 936 p.
- 4.Collin SP, Marshall NJ (2003) Sensory Processing in Aquatic Environments. New York: Springer. 480 p.
- 5. Arnold SJ (1983) Morphology, performance and fitness. American Zoologist 23: 347–361. [Google Scholar]
- 6. Vergassola M, Villermaux E, Shraiman BI (2007) “Infotaxis” as a strategy for searching without gradients. Nature 445: 406–409. [DOI] [PubMed] [Google Scholar]
- 7. Raguso RA, LeClere AR, Schlumpberger BO (2005) Sensory flexibility in hawkmoth foraging behavior: lessons from Manduca sexta and other species. Chemical Senses 30 (suppl 1)i295–i296. [DOI] [PubMed] [Google Scholar]
- 8. Duntley SQ (1963) Light in the sea. Journal of the Optical Society of America 53: 214–233. [Google Scholar]
- 9. Tierney KB, Baldwin DH, Hara TJ, Ross PS, Scholz NL, et al. (2010) Olfactory toxicity in fishes. Aquatic Toxicology 96: 2–26. [DOI] [PubMed] [Google Scholar]
- 10. Vasconcelos RO, Amorim MC, Ladich F (2007) Effects of ship noise on the detectability of communication signals in the Lusitanian toadfish. Journal of Experimental Biology 210: 2104–2112. [DOI] [PubMed] [Google Scholar]
- 11.Curio E (1976) The Ethology of Predation. New York: Springer-Verlag. 250 p.
- 12. Stein BE, Stanford TR (2008) Multisensory integration: current issues from the perspective of the single neuron. Nature Reviews Neuroscience 9: 255–266. [DOI] [PubMed] [Google Scholar]
- 13.Motta PJ, Huber DR (2012) Prey capture behavior and feeding mechanics of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of Sharks and Their Relatives, Vol I, 2nd Edition. Boca Raton, FL: CRC Press. pp. 153–209.
- 14. Frazzetta TH, Prange CD (1987) Movements of cephalic components during feeding in some requiem sharks (Carcharhiniformes: Carcharhinidae). Copeia 4: 979–993. [Google Scholar]
- 15. Wilga CD, Motta PJ (2000) Durophagy in sharks: feeding mechanics of the hammerhead Sphyrna tiburo . Journal of Experimental Biology 203: 2781–2796. [DOI] [PubMed] [Google Scholar]
- 16. Motta PJ, Hueter RE, Tricas TC, Summers AP, Huber DR, et al. (2008) Functional morphology of the feeding apparatus, feeding constraint and suction performance in the nurse shark Ginglymostoma cirratum . Journal of Morphology 269: 1041–1055. [DOI] [PubMed] [Google Scholar]
- 17. Kajiura SM, Holland KN (2002) Electroreception in juvenile scalloped hammerhead and sandbar sharks. Journal of Experimental Biology 205: 3609–3621. [DOI] [PubMed] [Google Scholar]
- 18. Palmer LM, Deffenbaugh M, Mensinger AF (2005) Sensitivity of the anterior lateral line to natural stimuli in the oyster toadfish, Opsanus tau (Linnaeus). Journal of Experimental Biology 208: 3441–3450. [DOI] [PubMed] [Google Scholar]
- 19. Mann DA, Casper BM, Boyle KS, Tricas TC (2007) On the attraction of larval fishes to reef sounds. Marine Ecology Progress Series 338: 307–310. [Google Scholar]
- 20. Gardiner JM, Atema J (2007) Sharks need the lateral line to locate odor sources: rheotaxis and eddy chemotaxis. Journal of Experimental Biology 210: 1925–1934. [DOI] [PubMed] [Google Scholar]
- 21. Castro JI (1996) The biology of the blacktip shark, Carcharhinus limbatus, off the southeastern United States. Bulletin of Marine Science 59: 508–522. [Google Scholar]
- 22. Castro JI (2000) The biology of the nurse shark, Ginglymostoma cirratum, off the Florida east coast and the Bahama Islands. Environmental Biology of Fishes 58: 1–22. [Google Scholar]
- 23. Cortés E, Manire CA, Hueter RE (1996) Diet, feeding habits, and diel feeding chronology of the bonnethead shark, Sphyrna tiburo, in southwest Florida. Bulletin of Marine Science 58: 353–367. [Google Scholar]
- 24. Ferry-Graham LA (1998) Effects of prey size and mobility on prey-capture kinematics in leopard sharks Triakis semifasciata . Journal of Experimental Biology 201: 2433–2444. [DOI] [PubMed] [Google Scholar]
- 25. Sheldon RE (1911) The sense of smell in selachians. Journal of Experimental Zoology 10: 51–62. [Google Scholar]
- 26.Cornett AD (2006) Ecomorphology of Shark Electroreceptors. Boca Raton, FL: Florida Atlantic University. 102 p.
- 27. Kajiura SM (2001) Head morphology and electrosensory pore distribution of carcharhinid and sphyrnid sharks. Environmental Biology of Fishes 61: 125–133. [Google Scholar]
- 28. Palmer LM, Mensinger AF (2004) Effect of the anesthetic tricaine (MS-222) on nerve activity in the anterior lateral line of the oyster toadfish, Opsanus tau . Journal of Neurophysiology 92: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 29. Kaus S (1987) The effect of aminoglycoside antibiotics on the lateral line organ of Aplocheilus lineatus (Cyprinodontidae) . Acta Otolaryngologica 103: 291–298. [DOI] [PubMed] [Google Scholar]
- 30. Kroese ABA, van den Bercken J (1982) Effects of ototoxic antibiotics on sensory hair cell functioning. Hearing Research 6: 183–197. [DOI] [PubMed] [Google Scholar]
- 31. Matsuura S, Ikeda K, Furukawa T (1971) Effects of streptomycin, kanamycin, quinine, and other drugs on the microphonic potentials of goldfish sacculus. Japanese Journal of Physiology 21: 579–590. [DOI] [PubMed] [Google Scholar]
- 32. Faucher K, Fichet D, Miramand P, Lagardère JP (2006) Impact of acute cadmium exposure on the trunk lateral line neuromasts and consequences on the “C-start” response behaviour of the sea bass (Dicentrarchus labrax L.; Teleostei, Moronidae). Aquatic Toxicology 76: 278–294. [DOI] [PubMed] [Google Scholar]
- 33. Faucher K, Parmentier E, Becco C, Vandewalle N, Vandewalle P (2010) Fish lateral system is required for accurate control of shoaling behaviour. Animal Behaviour 79: 679–687. [Google Scholar]
- 34.Blaxter JHS, Fuiman LA (1989) Function of the free neuromasts of marine teleost larvae. In: Coombs S, Gorner P, Munz H, editors. The Mechanosensory Lateral Line: Neurobiology and Evolution. New York, N.Y.: Springer-Verlag. pp. 481–499.
- 35. Coombs S, Braun CB, Donovan B (2001) The orienting response of Lake Michigan mottled sculpin is mediated by canal neuromasts. Journal of Experimental Biology 204: 337–348. [DOI] [PubMed] [Google Scholar]
- 36. Faucher K, Dutto G, Covès D, Aubert A, Lagardère JP (2006) No efficiency of the lateral system on nocturnal feeding in the European sea bass (Dicentrarchus labrax L.). Aquaculture 252: 462–475. [Google Scholar]
- 37. Nelson DR, Gruber SH (1963) Sharks: attraction by low-frequency sounds. Science 142: 975–977. [DOI] [PubMed] [Google Scholar]
- 38. Myrberg Jr AA (2001) The acoustical biology of elasmobranchs. Environmental Biology of Fishes 60: 31–45. [Google Scholar]
- 39.Tester AL (1963) Olfaction, gustation, and the common chemical sense in sharks. In: Gilbert PW, editor. Sharks and Survival. Lexington: D.C. Heath and Company. pp. 255–282.
- 40. Tester AL (1963) The role of olfaction in shark predation. Pacific Science 17: 145–170. [Google Scholar]
- 41. Parker GH (1914) The directive influence of the sense of smell in the dogfish. Bulletin of the United States Bureau of Fisheries 33: 61–68. [Google Scholar]
- 42. Hobson ES (1963) Feeding behavior in three species of sharks. Pacific Science 17: 171–194. [Google Scholar]
- 43. Kalmijn AJ (1971) The electric sense of sharks and rays. Journal of Experimental Biology 55: 371–383. [DOI] [PubMed] [Google Scholar]
- 44. Gardiner JM, Motta PJ (2012) Largemouth bass (Micropterus salmoides) switch feeding modalities in response to sensory deprivation. Zoology 115: 78–83. [DOI] [PubMed] [Google Scholar]
- 45. Ferry-Graham LA, Lauder GV (2001) Aquatic prey capture in ray-finned fishes: a century of progress and new directions. Journal of Morphology 248: 99–119. [DOI] [PubMed] [Google Scholar]
- 46. Packard GC, Boardman TJ (1999) The use of percentages and size-specific indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comparative Biochemistry and Physiology Part A, Molecular & Integrative Physiology 122: 37–44. [Google Scholar]
- 47.Sokal RR, Rohlf FJ (1995) Biometry: The Principles and Practices of Statistics in Biological Research. New York: W.H. Freeman and Co. 887 p.
- 48. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 57: 289–300. [Google Scholar]
- 49.Team RC (2013) R: A language and environment for statistical computing. 3.0.2. R Foundation for Statistical Computing, Vienna, Austria.
- 50.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RDC (2013) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–3.111.
- 51. Hothorn T, Bretz F, Westfall P (2008) Simultaneous Inference in General Parametric Models. Biometrical Journal 50: 346–363. [DOI] [PubMed] [Google Scholar]
- 52.Srisuradetchai P, Borkowski JJ (2013) Skillings.Mack: The Skillings-Mack test statistic for block designs with missing observations. R package version 1.0–2. http://CRAN.R-project.org/package=Skillings.Mack
- 53.Konietschke F (2012) nparcomp: Perform multiple comparisons and compute simultaneous confidence intervals for the nonparametric relative contrast effects. R package version 2.0. http://CRAN.R-project.org/package=nparcomp
- 54.Webster DR (2007) Structure of turbulent chemical plumes. In: Woodfin RL, editor. Trace Chemical Sensing of Explosives. New York: John Wiley & Sons. pp. 109–129.
- 55. Gardiner JM, Atema J (2010) The function of bilateral timing differences in olfactory orientation of sharks. Current Biology 20: 1187–1191. [DOI] [PubMed] [Google Scholar]
- 56. Hamasaki DI, Gruber SH (1965) The photoreceptors of the nurse shark, Ginglymostoma cirratum and the sting ray, Dasyatis sayi . Bulletin of Marine Science 15: 1051–1059. [Google Scholar]
- 57.Hueter RE (1991) Adaptations for spatial vision in sharks. Journal of Experimental Zoology Supplement 5: 130–141.
- 58. Aronson LR, Aronson FR, Clark E (1967) Instrumental conditioning and light-dark discrimination in young nurse sharks. Bulletin of Marine Science 17: 249–256. [Google Scholar]
- 59. Hodgson ES, Mathewson RF (1971) Chemosensory orientation in sharks. Annals of the New York Academy of Sciences 188: 175–182. [DOI] [PubMed] [Google Scholar]
- 60. Mathewson RF, Hodgson ES (1972) Klinotaxis and rheotaxis in orientation of sharks toward chemical stimuli. Comparative Biochemistry and Physiology, A: Comparative Physiology 42: 79–84. [DOI] [PubMed] [Google Scholar]
- 61. Bateson W (1890) The sense-organs and perceptions of fishes; with some remarks on the supply of bait. Journal of the Marine Biological Association of the United Kingdom N.S. 1: 225–256. [Google Scholar]
- 62. Myrberg Jr AA, Gruber SG (1974) The behavior of the bonnethead shark, Sphyrna tiburo . Copeia 1974: 358–374. [Google Scholar]
- 63.Barry KP (2002) Feeding habits of blacktip sharks, Carcharhinus limbatus, and Atlantic sharpnose sharks, Rhizoprionodon terraenovae, in Louisiana coastal waters [MS Thesis]. Baton Rouge, LA: Louisiana State University.
- 64. Arnold GP (1974) Rheotropism in fishes. Biological Review 49: 515–576. [DOI] [PubMed] [Google Scholar]
- 65. Chagnaud BP, Brücker C, Hofmann MH, Bleckmann H (2008) Measuring flow velocity and flow direction by spatial and temporal analysis of flow fluctuations. Journal of Neuroscience 28: 4479–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vickers N (2000) Mechanisms of Animal Navigation in Odor Plumes. Biological Bulletin 198: 203–212. [DOI] [PubMed] [Google Scholar]
- 67. Moss SA (1972) Nurse shark pectoral fins: an unusual use. American Midland Naturalist 88: 496–497. [Google Scholar]
- 68.Roberts BL (1978) Mechanoreceptors and the behaviour of elasmobranch fishes with special reference to the acoustico-lateralis system. In: Hodgson ES, Mathewson RF, editors. Sensory Biology of Sharks, Skates, and Rays. Arlington, Virginia: Office of Naval Research. pp. 331–390.
- 69. Lyon EP (1904) On rheotropism. I. Rheotropism in fishes. American Journal of Physiology 12: 149–161. [Google Scholar]
- 70. Baker CF, Montgomery JC (1999) The sensory basis of rheotaxis in the blind Mexican cave fish, Astyanax fasciatus . Journal of Comparative Physiology A, Sensory, Neural, and Behavioral Physiology 184: 519–527. [Google Scholar]
- 71. Peach MB (2003) The behavioral role of pit organs in the epaulette shark. Journal of Fish Biology 62: 793–802. [Google Scholar]
- 72. Wueringer BE, Squire L, Kajiura SM, Tibbetts IR, Hart NS, et al. (2012) Electric field detection in sawfish and shovelnose rays. PLoS ONE 7: e41605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kajiura SM (2003) Electroreception in neonatal bonnethead sharks, Sphyrna tiburo . Marine Biology 143: 603–611. [Google Scholar]
- 74. Kajiura SM, Fitzgerald TP (2009) Response of juvenile scalloped hammerhead sharks to electric stimuli. Zoology 112: 241–250. [DOI] [PubMed] [Google Scholar]
- 75. Kalmijn AJ (1982) Electric and magnetic field detection in elasmobranch fishes. Science 218: 916–918. [DOI] [PubMed] [Google Scholar]
- 76. Jordan LK, Kajiura SM, Gordon MS (2009) Functional consequences of structural differences in stingray sensory systems. Part II: electrosensory system. Journal of Experimental Biology 212: 3044–3050. [DOI] [PubMed] [Google Scholar]
- 77. Haine OS, Ridd PV, Rowe RJ (2001) Range of electrosensory detection of prey by Carcharhinus melanopterus and Himantura granulata . Marine and Freshwater Research 52: 291–296. [Google Scholar]
- 78. McGowan DW, Kajiura SM (2009) Electroreception in the euryhaline stingray, Dasyatis sabina . Journal of Experimental Biology 212: 1544–1552. [DOI] [PubMed] [Google Scholar]
- 79.Bedore CN, Harris LJ, Kajiura SM (2014) Behavioral responses of batoid elasmobranchs to prey-simulating electric fields are correlated to peripheral sensory morphology andecology. Zoology. (In Press) [DOI] [PubMed]
- 80.Kalmijn AJ (1974) The detection of electric fields from inanimate and animate sources other than electric organs. In: Fessard A, editor. Handbook of Sensory Physiology. New York, N.Y.: Springer-Verlag. pp. 147–200.
- 81.Denton EJ, Gray JAB (1988) Mechanical factors in the excitation of the lateral lines of fishes. In: Atema J, Fay RR, Popper AN, Tavolga WN, editors. Sensory Biology of Aquatic Animals. New York, N.Y.: Springer-Verlag. pp. 595–617.
- 82. Kalmijn AJ (1972) Bioelectric fields in seawater and the function of the ampullae of Lorenzini in elasmobranch fishes. Scripps Institute of Oceanography Reference Series 72–83: 1–21. [Google Scholar]
- 83. McComb DM, Tricas TC, Kajiura SM (2009) Enhanced visual fields in hammerhead sharks. Journal of Experimental Biology 212: 4010–4018. [DOI] [PubMed] [Google Scholar]
- 84. Nauwelaerts S, Wilga CD, Sanford CP, Lauder GV (2007) Hydrodynamics of prey capture in sharks: effects of substrate. Journal of the Royal Society Interface 4: 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fouts WR, Nelson DR (1999) Prey capture by the Pacific angle shark, Squatina californica: visually mediated strikes and ambush-site characteristics. Copeia 1999: 304–312. [Google Scholar]
- 86. Higham TE, Day SW, Wainwright PC (2005) Sucking while swimming: evaluating the effects of ram speed on suction generation in bluegill sunfish Lepomis macrochirus using digital particle image velocimetry. Journal of Experimental Biology 208: 2653–2660. [DOI] [PubMed] [Google Scholar]
- 87. Higham TE, Day SW, Wainwright PC (2006) Multidimensional analysis of suction feeding performance in fishes: fluid speed, acceleration, strike accuracy, and the ingested volume of water. Journal of Experimental Biology 209: 2713–2725. [DOI] [PubMed] [Google Scholar]
- 88. Baker CF, Montgomery JC, Dennis TE (2002) The sensory basis of olfactory search behavior in banded kokopu (Galaxias fasciatus). Journal of Comparative Physiology A, Sensory, Neural, and Behavioral Physiology 188: 553–560. [DOI] [PubMed] [Google Scholar]
- 89. Hassan ES, Abdel-Latif H, Biebricher R (1992) Studies on the effects of Ca2+ and Co2+ on the swimming behavior of the blind Mexican cave fish. Journal of Comparative Physiology A, Sensory, Neural, and Behavioral Physiology 171: 413–419. [Google Scholar]
- 90.Gardiner JM, Atema J (2014) Flow sensing in sharks: lateral line contributions to navigation and prey capture. In: Bleckmann H, Mogdans J, Coombs SL, editors. Flow Sensing in Air and Water: Behavioural, Neural, and Engineering Principles of Operation: Springer-Verlag. pp. 127–146.
- 91.Hassan ES (1989) Hydrodynamic imaging of the surroundings by the lateral line of the blind cave fish Anoptichthys jordani. In: Coombs S, Gorner P, Munz H, editors. The Mechanosensory Lateral Line: Neurobiology and Evolution. New York: Springer-Verlag. pp. 217–228.
- 92. Windsor SP, Norris SE, Cameron SM, Mallinson GD, Montgomery JC (2010) The flow fields involved in hydrodynamic imaging by blind Mexican cave fish (Astyanax fasciatus). Part I: open water and heading towards a wall. Journal of Experimental Biology 213: 3819–3831. [DOI] [PubMed] [Google Scholar]
- 93. Windsor SP, Norris SE, Cameron SM, Mallinson GD, Montgomery JC (2010) The flow fields involved in hydrodynamic imaging by blind Mexican cave fish (Astyanax fasciatus). Part II: gliding parallel to a wall. Journal of Experimental Biology 213. [DOI] [PubMed] [Google Scholar]
- 94. Janssen J (1996) Use of the lateral line and tactile senses in feeding in four antarctic nototheniid fishes. Environmental Biology of Fishes 47: 51–64. [Google Scholar]
- 95. Janssen J, Coombs S, Pride S (1990) Feeding and orientation of mottled sculpin to water jets. Environmental Biology of Fishes 29: 43–50. [Google Scholar]
- 96. Torisawa S, Fukuda H, Suzuki K, Takagi T (2011) Schooling behaviour of juvenile Pacific bluefin tuna Thunnus orientalis depends on their vision development. Journal of Fish Biology 79: 1291–1303. [DOI] [PubMed] [Google Scholar]
- 97. Viitasalo M, Kiørboe T, Flinkman J, Pedersen LW, Visser AW (1998) Predation vulnerability of planktonic copepods:consequences of predator foraging strategies and prey sensory abilities. Marine Ecology Progress Series 175: 129–142. [Google Scholar]
- 98. Ferry-Graham LA, Wainwright PC, Lauder GV (2003) Quantification of flow during suction feeding in bluegill sunfishes. Zoology 106: 159–168. [DOI] [PubMed] [Google Scholar]
- 99. Holzman R, Wainwright PC (2009) How to surprise a copepod: strike kinematics reduce hydrodynamic disturbance and increase stealth of suction-feeding fish. Limnology and Oceanography 54: 2201–2212. [Google Scholar]
- 100.Gardiner JM (2012) Multisensory Integration in Shark Feeding Behavior [Dissertation]. Tampa, FL: University of South Florida. 281 p.
- 101.Kalmijn AJ (1988) Hydrodynamic and acoustic field detection. In: Atema J, Fay RR, Popper AN, Tavolga WN, editors. Sensory Biology of Aquatic Animals. New York, NY: Springer-Verlag. pp. 83–130.
- 102. Myrberg Jr AA, Ha SJ, Walewski S, Banbury JC (1972) Effectiveness of acoustic signals in attracting epipelagic sharks to an underwater sound source. Bulletin of Marine Science 22: 926–949. [Google Scholar]
- 103. Pohlmann K, Atema J, Breithaupt T (2004) The importance of the lateral line in nocturnal predation of piscivorous catfish. Journal of Experimental Biology 207: 2971–2978. [DOI] [PubMed] [Google Scholar]
- 104. Coombs S (1994) Nearfield detection of dipole sources by the goldfish (Carassius auratus) and the mottled sculpin (Cottus bairdi). Journal of Experimental Biology 190: 109–129. [DOI] [PubMed] [Google Scholar]
- 105. Page RA, Schnelle T, Kalko EKV, Bunge T, Bernal XE (2012) Sequential assessment of prey through the use of multiple sensory cues by an eavesdropping bat. Naturwissenschaften 99: 505–509. [DOI] [PubMed] [Google Scholar]
- 106. Piep M, Radespiel U, Zimmermann E, Schmidt S, Siemers BM (2008) The sensory basis of prey detection in captive-born grey mouse lemurs, Microcebus murinus . Animal Behaviour 75: 871–878. [Google Scholar]
- 107. Haverly JE, Kardong KV (1996) Sensory deprivation effects on the predatory behavior of the rattlesnake, Crotalus viridis oreganus . Copeia 1996: 419–428. [Google Scholar]
- 108. Monroy JA, Nishikawa KC (2011) Prey capture in frogs: alternative strategies, biomechanical trade-offs, and hierarchical decision making. Journal of Experimental Zoology 315: 61–71. [DOI] [PubMed] [Google Scholar]
- 109. Ruxton GD (2009) Non-visual crypsis: a review of the empirical evidence for camouflage to senses other than vision. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 364: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. McFall-Ngai MJ (1990) Crypsis in the pelagic environment. American Zoologist 30: 175–188. [Google Scholar]
- 111. Grasso FW, Atema J (2002) Integration of flow and chemical sensing for guidance of autonomous marine robots in turbulent flow. Environmental Fluid Mechanics 2: 95–114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data summary – blacktip shark, Carcharhinus limbatus . Summary of all variables for the blacktip shark, Carcharhinus limbatus with all senses intact, and following blocks of the senses as indicated. Abbreviations: O = olfaction, V = vision, L = lateral line, E = electroreception. All means are ±s.e.m. The p values are the results of linear mixed effects model analyses or Skillings-Mack tests performed on each variable. Value marked (*) are significant after Benjamini-Hochberg corrections. Tukey Test p values reflect the results of pairwise post-hoc comparisons between treatments. N.A.: Not applicable, parameter was not assessed because behavior did not occur; N.S.: Non-significant at α = 0.05.
(DOCX)
Data summary – bonnethead, Sphyrna tiburo . Summary of all variables for the bonnethead, Sphyrna tiburo with all senses intact, and following blocks of the senses as indicated. Abbreviations: O = olfaction, V = vision, L = lateral line, E = electroreception. All means are ±s.e.m. The p values are the results of linear mixed effects model analyses or Skillings-Mack tests performed on each variable. Value marked (*) are significant after Benjamini-Hochberg corrections. Tukey Test p values reflect the results of pairwise post-hoc comparisons between treatments. N.A.: Not applicable, parameter was not assessed because behavior did not occur; N.R.: Not recorded due to technical difficulties; N.S.: Non-significant at α = 0.05.
(DOCX)
Data summary – nurse shark, Ginglymostoma cirratum . Summary of all variables for the nurse shark, Ginglymostoma cirratum with all senses intact, and following blocks of the senses as indicated. Abbreviations: O = olfaction, V = vision, L = lateral line, E = electroreception. All means are ±s.e.m. The p values are the results of linear mixed effects model analyses or Skillings-Mack tests performed on each variable. Value marked (*) are significant after Benjamini-Hochberg corrections. Tukey Test p values reflect the results of pairwise post-hoc comparisons between treatments. N.A.: Not applicable, parameter was not assessed because behavior did not occur; N.S.: Not significant at α = 0.05.
(DOCX)
Prey capture in an intact blacktip shark, Carcharhinus limbatus , played in slow motion at 30 frames/s (filmed at 250 frames/s). Rapid (mean: 2.0 BL/s) strikes are initiated from a distance (mean: 240 cm) and the prey is overtaken and engulfed using ram-capture (100% capture success rate).
(AVI)
Prey capture in an intact bonnethead, Sphyrna tiburo , played in slow motion at 30 frames/s (filmed at 250 frames/s). Strikes are slower (mean: 0.8 BL/s) and initiated from a closer proximity (mean: 74 cm) than in the blacktip shark. Prey is overtaken and captured between the teeth, using ram-biting (100% capture success rate).
(AVI)
Prey capture in an intact nurse shark, Ginglymostoma cirratum , played in slow motion at 30 frames/s (filmed at 250 frames/s). Slow (mean: 0.3 BL/s) strikes, consisting of raising the head, are initiated from close proximity (mean: 13 cm). The prey is rapidly drawn into the mouth using suction (100% capture success rate).
(AVI)
Prey capture in a blacktip shark, Carcharhinus limbatus , with the lateral line blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When lateral line information is absent, the blacktip sharks frequently missed the prey. These misses were associated with faster strikes (mean: 3.6 BL/s).
(AVI)
Attempted prey capture (miss) in a blacktip shark, Carcharhinus limbatus , with electroreception blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When electrical cues are absent, blacktip sharks frequently missed unless they touched the prey (with the snout, near the mouth) prior to beginning to move the jaws (see Movie S6 for an example of a successful capture).
(AVI)
Prey capture (successful) in a blacktip shark, Carcharhinus limbatus , with electroreception blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When electrical cues are absent, blacktip sharks could successfully capture prey if they touched it (with the snout, near the mouth), prior to beginning to move the jaws. If they did not touch the prey, they frequently missed (see Movie S5 for an example of a miss).
(AVI)
Attempted prey capture (miss) in a nurse shark, Ginglymostoma cirratum , with electroreception blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When electrical cues are absent, nurse sharks frequently missed unless they touched the prey (with the snout, near the mouth) prior to beginning to move the jaws (see Movie S8 for an example of a successful capture).
(AVI)
Prey capture (successful) in a nurse shark, Ginglymostoma cirratum , with electroreception blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When electrical cues are absent, nurse sharks could successfully capture the prey if they touched it (with the snout, near the mouth) prior to beginning to move the jaws. If they did not touch the prey, they frequently missed (see Movie S7 for an example of a miss).
(AVI)
Striking behavior in a bonnethead, Sphyrna tiburo , with electroreception blocked, played in slow motion at 30 frames/s (filmed at 250 frames/s). When electrical cues are absent, the bonnethead fails to open the mouth and misses the prey (0% capture success rate).
(AVI)