Abstract
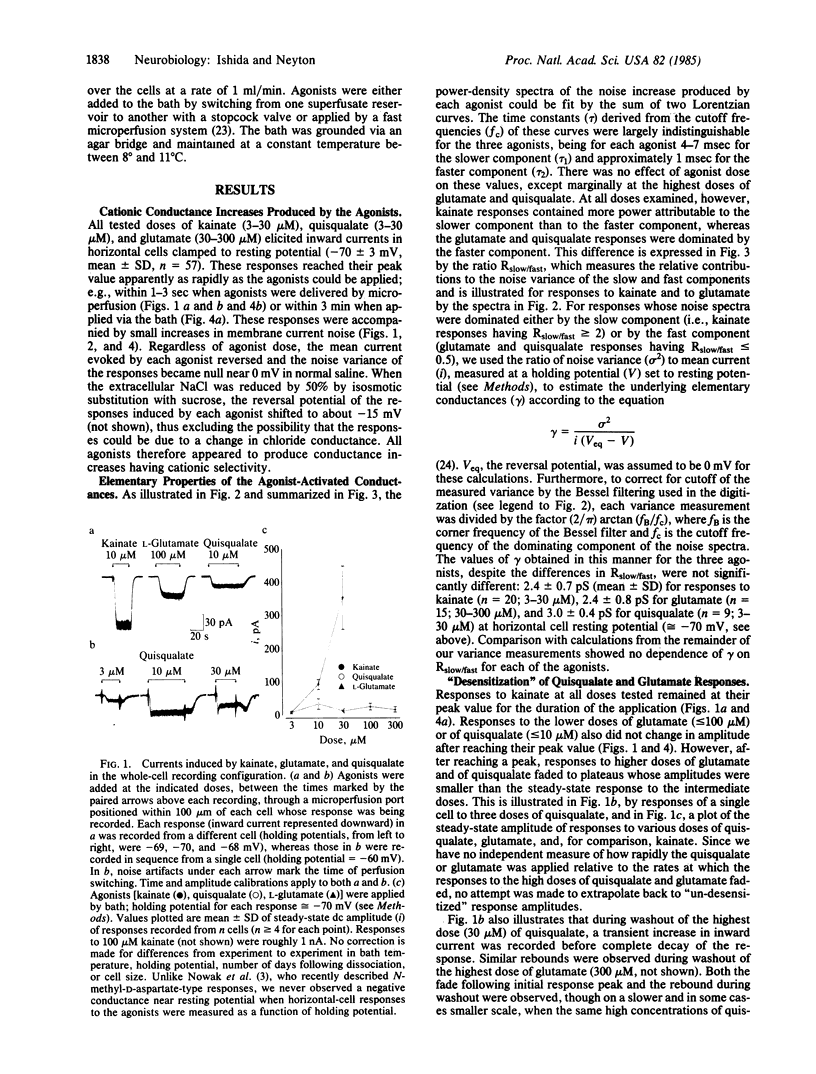
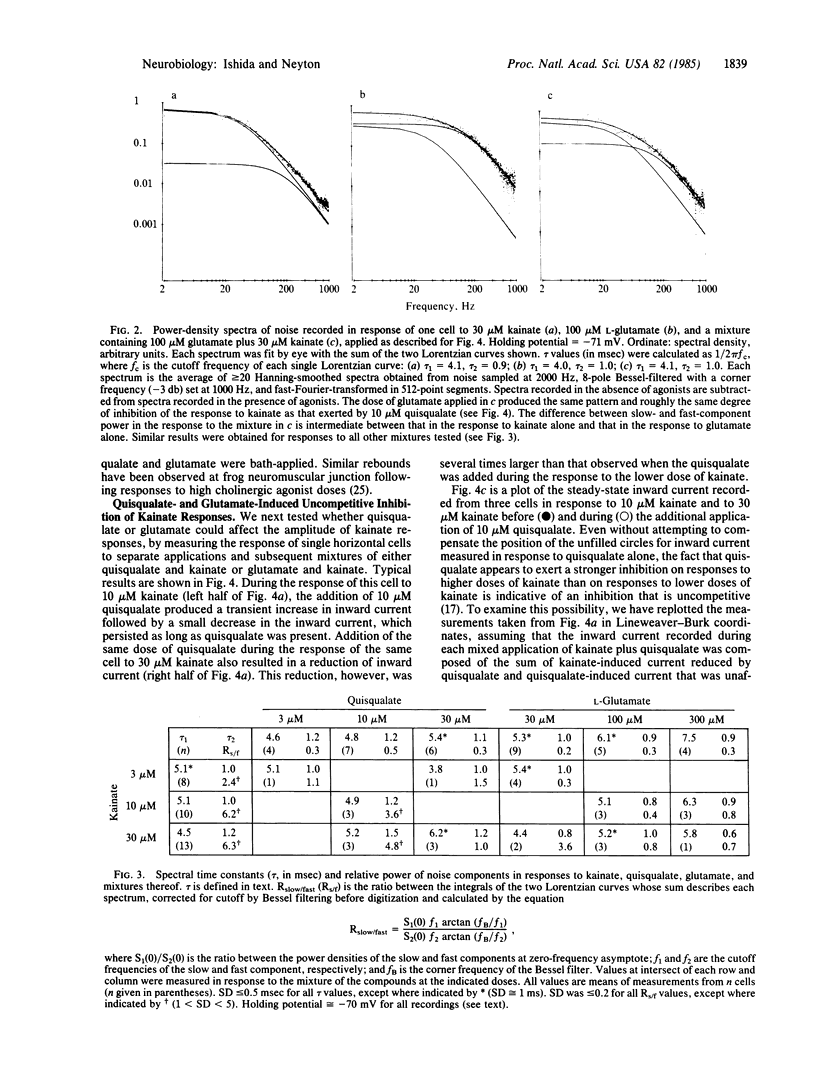
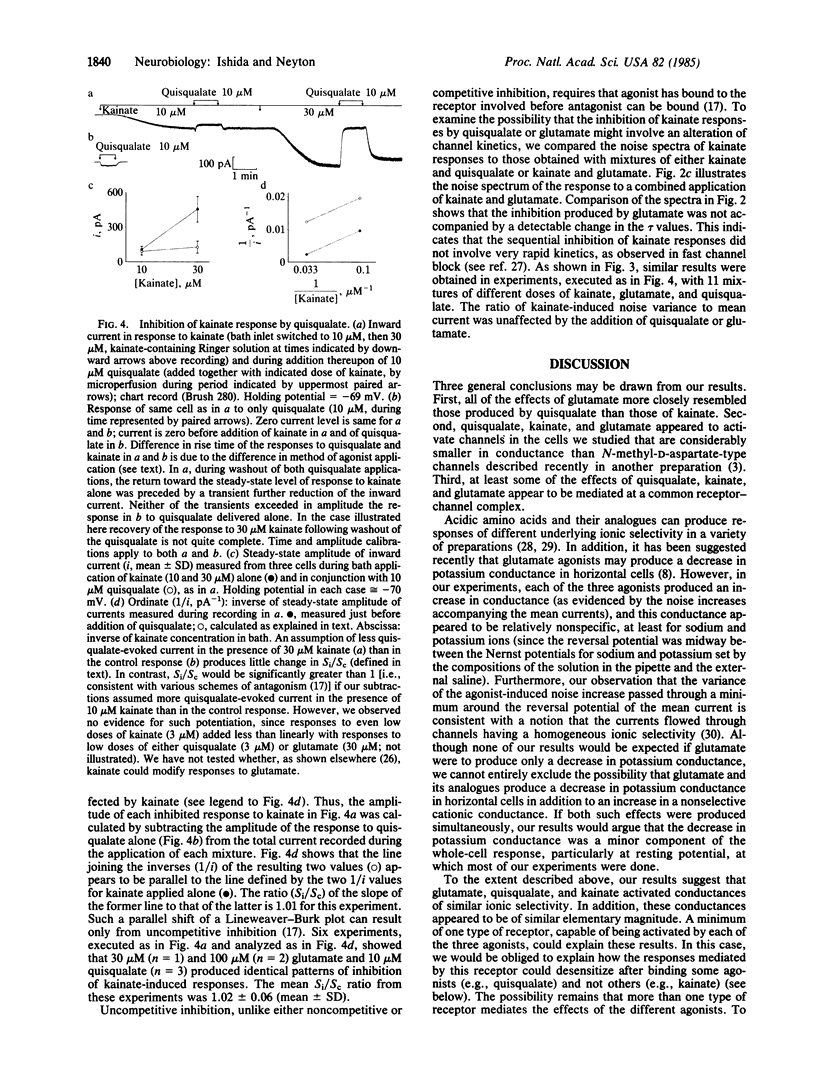
Currents elicited by L-glutamate and the related agonists quisqualate and kainate were analyzed under voltage clamp in isolated goldfish horizontal cells, using the whole-cell recording configuration of the patch-clamp method [Hamill, O.P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. (1981) Pflügers Arch. 391, 85-100]. These currents resulted from an increase in cationic conductance and were indistinguishable from one another in terms of reversal potential (approximately equal to 0 mV) and apparent elementary conductance (2-3 pS). The power-density spectra of the noise increases produced by each agonist were fit by the sum of two Lorentzian curves having similar cutoff frequencies (tau 1 approximately equal to 5 msec, tau 2 approximately equal to 1 msec), but the relative power of these components were different for quisqualate and glutamate than for kainate. Moreover, the responses to high doses of either quisqualate or glutamate rapidly faded, whereas the responses to kainate did not. Finally, quisqualate and glutamate produced an inhibition of responses to kainate which appeared to be uncompetitive. Kainate, quisqualate, and in our preparation, glutamate appear to activate channels different than those activated by N-methyl-D-aspartate in other preparations. At least some of the effects of quisqualate and glutamate appear to be mediated by receptors bound by kainate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. A study of desensitization using voltage clamp. Pflugers Arch. 1975 Oct 28;360(2):135–144. doi: 10.1007/BF00580536. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. Decamethonium both opens and blocks endplate channels. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anis N. A., Clark R. B., Gration K. A., Usherwood P. N. Influence of agonists on desensitization of glutamate receptors on locust muscle. J Physiol. 1981 Mar;312:345–364. doi: 10.1113/jphysiol.1981.sp013632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel M., Lasater E. M., Mangel S. C., Dowling J. E. On the sensitivity of H1 horizontal cells of the carp retina to glutamate, aspartate and their agonists. Brain Res. 1984 Mar 12;295(1):179–183. doi: 10.1016/0006-8993(84)90828-x. [DOI] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G. Two types of extrajunctional L-glutamate receptors in locust muscle fibres. J Physiol. 1976 Feb;255(2):449–464. doi: 10.1113/jphysiol.1976.sp011289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Ruff R. L. Endplate current fluctuations reveal only one channel type at frog neuromuscular junction. Nature. 1977 Mar 17;266(5599):263–265. doi: 10.1038/266263a0. [DOI] [PubMed] [Google Scholar]
- Fagni L., Baudry M., Lynch G. Classification and properties of acidic amino acid receptors in hippocampus. I. Electrophysiological studies of an apparent desensitization and interactions with drugs which block transmission. J Neurosci. 1983 Aug;3(8):1538–1546. doi: 10.1523/JNEUROSCI.03-08-01538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Fain G. L. D-aspartate potentiates the effects of L-glutamate on horizontal cells in goldfish retina. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5890–5894. doi: 10.1073/pnas.78.9.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A. T., Kaneko A., Tachibana M. Responses of solitary retinal horizontal cells from Carassius auratus to L-glutamate and related amino acids. J Physiol. 1984 Mar;348:255–270. doi: 10.1113/jphysiol.1984.sp015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A. T. Responses of solitary retinal horizontal cells to L-glutamate and kainic acid are antagonized by D-aspartate. Brain Res. 1984 Apr 23;298(1):25–32. doi: 10.1016/0006-8993(84)91143-0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Carp horizontal cells in culture respond selectively to L-glutamate and its agonists. Proc Natl Acad Sci U S A. 1982 Feb;79(3):936–940. doi: 10.1073/pnas.79.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Otsu K., Otsuka T. Effects of chemicals on receptors and horizontal cells in the retina. J Physiol. 1972 Dec;227(3):899–913. doi: 10.1113/jphysiol.1972.sp010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Distribution and pharmacological properties of synaptic and extrasynaptic glutamate receptors on crayfish muscle. J Physiol. 1980 Sep;306:233–250. doi: 10.1113/jphysiol.1980.sp013394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J. S., Ruddock K. H. Depolarization of retinal horizontal cells by excitatory amino acid neurotransmitter agonists. Neurosci Lett. 1982 Jun 30;30(3):257–262. doi: 10.1016/0304-3940(82)90409-8. [DOI] [PubMed] [Google Scholar]
- Shiells R. A., Falk G., Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981 Dec 10;294(5841):592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Shingai R., Christensen B. N. Sodium and calcium currents measured in isolated catfish horizontal cells under voltage clamp. Neuroscience. 1983 Nov;10(3):893–897. doi: 10.1016/0306-4522(83)90227-0. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci. 1983 Aug;3(8):1701–1711. doi: 10.1523/JNEUROSCI.03-08-01701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell W. K., Lightfoot D. O. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol. 1975 Feb 15;159(4):473–502. doi: 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Ionic currents of solitary horizontal cells isolated from goldfish retina. J Physiol. 1983 Dec;345:329–351. doi: 10.1113/jphysiol.1983.sp014981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Membrane properties of solitary horizontal cells isolated from goldfish retina. J Physiol. 1981 Dec;321:141–161. doi: 10.1113/jphysiol.1981.sp013976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann A. Curare can open and block ionic channels associated with cholinergic receptors. Nature. 1982 Jul 15;298(5871):272–275. doi: 10.1038/298272a0. [DOI] [PubMed] [Google Scholar]
- Trifonov I. U. Izuchenie sinapticheskoi peredachi mezhdu fotoretseptorom i gorizontal'noi kletkoi pri pomoshchi élektricheskikh razdrazhenii setchatki. Biofizika. 1968 Sep-Oct;13(5):809–817. [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]



