A method was developed to specifically isolate neural progenitor cells (NPCs) from postmortem adult human brains based on the expression of the specific human adult neural stem/progenitor cell marker glial fibrillary acidic protein δ. This study shows that a pure population of NPCs can be isolated from the adult human subventricular zone (SVZ), which is highly instrumental for developing future therapies based on stimulating endogenous SVZ neurogenesis.
Keywords: Glial fibrillary acidic protein delta, Neurodegenerative disorders, Specific isolation, Human adult neural progenitor cells, Subventricular zone
Abstract
Neural progenitor cells (NPCs) in the subventricular zone (SVZ) hold promise for future therapy for neurodegenerative disorders, because the stimulation of adult neurogenesis could potentially restore the function of degenerating neurons and glia. To obtain more knowledge on these NPCs, we developed a method to specifically isolate NPCs from postmortem adult human brains based on the expression of the specific human adult neural stem/progenitor cell marker glial fibrillary acidic protein δ (GFAPδ). An extensive immunophenotyping analysis for cell surface markers resulted in the observation that CD271 was limited to the SVZ-derived GFAPδ-positive cells. CD271+ cells developed into neurospheres and could be differentiated into astrocytes, neurons, and oligodendrocytes. We are the first to show that a pure population of NPCs can be isolated from the adult human SVZ, which is highly instrumental for developing future therapies based on stimulating endogenous SVZ neurogenesis.
Introduction
In the adult human brain, continuous neurogenesis occurs in two regions, which are the subventricular zone (SVZ) [1] and the subgranular zone in the hippocampal dentate gyrus [2]. Neurogenesis begins declining in the first year after birth [3]. However, we have shown that neural progenitor cells (NPCs) are still present and active in the SVZ of elderly subjects [4], including patients suffering from neurodegenerative diseases, such as Alzheimer’s disease (AD) [5] and Parkinson’s disease (PD) [6]. In the elderly human brain, migratory neuroblasts into the olfactory bulb have been identified (reviewed in [7]), although they do not migrate in streams as they do in the rostral migratory stream in the rodent brain. Recently, in the brain of infants, a second stream of migratory neuroblasts has been identified, the medial migratory stream [3].
We have shown that SVZ NPCs in the human brain specifically express the intermediate filament (IF) protein glial fibrillary acidic protein δ (GFAPδ) [8], one of the eight splice variants of GFAP [9, 10], the main IF protein in astrocytes. GFAPδ is also present in neurosphere cultures isolated from postmortem adult human SVZ tissue [4] and is expressed in radial glia and SVZ progenitors of the human fetal brain [11]. There are some indications that GFAPδ is involved in NPC function. GFAPδ can interact with presenilins [12], which are essential partners in the γ-secretase complex. This complex cleaves the transmembrane proteins Notch and amyloid precursor protein (APP) inside the membrane region and therefore is critical for Notch and APP signaling [13]. Notch signaling is required for maintenance of NPC populations in both the developing and adult brain [14], and Notch1 expression can be found in SVZ astrocytes and neuroblasts in the adult rodent brain [15]. Furthermore, the induced expression of GFAPδ in vitro results in an increased phosphorylation of Jnk [16]. In the developing brain, Jnk is involved in NPC proliferation, differentiation [17], and migration [18]. Our previous published data on GFAPδ together with the findings described above suggest that GFAPδ-positive cells in the SVZ may indeed be adult NPCs. A method to study GFAPδ-expressing cells will be essential to uncover the precise function of this protein and will be highly instrumental in understanding adult human SVZ neurogenesis.
To date, no cure is available for neurodegenerative disorders such as PD and AD, illustrating the high need for new therapeutic strategies. Therefore, we are focusing on activating endogenous NPCs in the brains of these patients. To accomplish this, we developed a method to specifically isolate GFAPδ-positive NPCs from postmortem human SVZ material derived from elderly donors. These cells can be used to study the characteristics of NPCs derived from nondemented control donors and donors with a neurodegenerative disorder.
We show that CD271, also known as p75NTR and belonging to the low-affinity neurotrophin receptor and tumor necrosis factor receptor superfamily [19], is specifically expressed on the surface of GFAPδ-positive cells in the human adult SVZ. We isolated CD271+/GFAPδ+ cells derived from the SVZ of elderly subjects and characterized these cells in vitro. This study demonstrates that we are able to specifically isolate GFAPδ-positive cells derived from the adult human brain and that CD271+/GFAPδ+ cells are indeed NPCs. This is the first description of a pure GFAPδ+ NPC culture derived from the human adult brain. Therefore, this isolation protocol is a unique method to study adult human NPCs in vitro and to search for novel therapeutic targets to activate these NPCs and stimulate neurogenesis in the brain of patients suffering from a neurodegenerative disorder.
Materials and Methods
Postmortem Human Brain Material
Tissue from the SVZ was obtained from the Netherlands Brain Bank (NBB, Amsterdam, The Netherlands, http://www.brainbank.nl). The NBB performs brain autopsies with short postmortem intervals, and the brain donors have given informed consent for using the tissue and for accessing the extensive neuropathological and clinical information for scientific research, in compliance with ethical and legal guidelines [20]. Clinico-pathological information of all donors can be found in supplemental online Table 2.
Isolation of Adult Human Postmortem NPCs
For human adult NPC cultures, SVZ tissue was freshly isolated from the anterior horn of the SVZ derived from control donors. NPC cultures were initiated, as described previously [5]. In short, after isolation, the obtained microglia-poor cell pellet was taken up in NPC medium (Neurobasal medium, 1% B27, 0.5% N2, 1% GlutaMax, 1% penicillin/streptomycin (P/S), 2% HEPES [all Invitrogen, Carlsbad, CA, http://www.invitrogen.com]), 1% UltraGlutamin (Cambrex, Walkersville, MD, http://www.cambrex.com), 5 μg/ml heparin (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), 20 ng/ml epidermal growth factor (EGF), and 20 ng/ml fibroblast growth factor (FGF) (both Tebu-Bio, Heerhugowaard, The Netherlands, http://www.tebu-bio.com) and plated in 12-well plates to obtain neurospheres. For adherent cultures, cells were plated in laminin-coated wells in the presence of 0.5% fetal calf serum (FCS). EGF and FGF were added twice per week. Images of the cultures were obtained by using a digital camera (Canon Powershot G6, Tokyo, Japan, http://www.canon.com).
Isolation of Fetal NPCs
For human fetal NPC cultures, fetal brain tissue (gestational weeks 14–17) was obtained from spontaneous or medically induced abortions with appropriate maternal written consent for brain autopsy. Tissue was obtained in accordance with the Declaration of Helsinki and the Amsterdam Medical Center (AMC) Research Code provided by the Medical Ethics Committee of the AMC (Amsterdam, The Netherlands, http://www.amc.nl). All autopsies were performed within 12 h after abortion.
For isolation of NPCs, fetal brain tissue was collected in 10 ml cold Hibernate (Invitrogen), mechanically dissociated into small pieces, and digested with 0.2% trypsin and 0.1% DNase I (Invitrogen) for 5 minutes at 37°C while shaking. Next, 1 ml FCS was added to the mixture, and, subsequently, the cells and pellet were collected by centrifugation. The pellet was taken up in Dulbecco’s modified Eagle’s medium (DMEM) without phenol red containing 10% FCS, 2.5% Hepes, and 1% P/S (all Invitrogen); Percoll (Amersham/GE Healthcare, Piscataway, NJ, http://www.amersham.com) was added (half of cell suspension volume), and this mixture was centrifuged at 4,000 rpm at 4°C for 20 minutes. The second layer (glial cell-containing fraction) was collected and washed with complete DMEM containing 10% FCS, 1% penicillin/streptomycin, 2.5% HEPES, and 1% gentamycin (all Invitrogen). After centrifugation (1,500 rpm, 10 min), the pellet was taken up in complete DMEM and cells were plated in a 6-cm culture dish. After 6 hours at 37°C/5% CO2, the microglia-poor medium containing nonattached cells was taken off and centrifuged, and the pellet was used for neurosphere cultures.
Immunophenotyping
For immunophenotyping, cells were isolated from the postmortem SVZ of three donors. The cells were centrifuged, and the obtained cell pellet was taken up in NPC medium and cultured as adherent cells on laminin-coated (20 μg/ml, 3 hours at 37°C) 16-well chamber slides (5,000 cells per well) and allowed to adhere for 5 days.
Immunophenotyping was performed using the Human Cell Surface Marker Screening panel from BD Lyoplate (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) following the manufacturer’s description. CD133 was added as an additional antibody (supplemental online Table 4), as it was not present in the Lyoplate screening. After immunostaining, cells were fixed with 4% paraformaldehyde (PFA) in PBS for 15 minutes at room temperature (RT). Cells were washed with Tris-buffered saline (TBS) and incubated with TBS containing 0.1% Tween 20 and 0.5% bovine serum albumin (BSA) for 15 minutes. Then the cells were incubated with anti-GFAPδ (supplemental online Table 4) in TBS containing 0.1% Tween 20 plus 0.5% BSA for 2 hours at RT. Cells were washed with TBS and coverslipped with Mowiol (0.1 M Tris, pH 8.5, 25% glycerol, 10% w/v Mowiol 4-88 [Sigma-Aldrich]). Pictures were taken with an Axioplan 2 microscope (Carl Zeiss, Jena, Germany, http://www.zeiss.com).
Magnetic-Activated Cell Sorting Separation
CD271+ cells were isolated using a magnetic separation system (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.om) following the manufacturer’s description. After isolation, cells were collected in NPC medium containing 20 ng/ml EGF and FGF (Tebu-Bio) and used for further experiments. On average, 1,000,000 CD271− and 30,000 CD271+ cells (3%) were obtained from 4 g SVZ tissue per isolation.
Differentiation of NPCs In Vitro
For differentiation of the NPCs, the adherent cells were collected using Accutase (Invitrogen) for 5 min at 37°C and resuspended in NPC medium. Then the cell suspension or neurospheres were centrifuged and replated in complete Dulbecco’s modified Eagle's medium/F12 (DMEM/F12 with GlutaMax containing 5% FCS, 1% P/S; all Invitrogen) on 12-mm laminin-coated (20 μg/ml; Invitrogen) glass coverslips.
RNA Isolation and Reverse Transcription
For RNA isolation, neurospheres were collected and rinsed with phosphate-buffered saline (PBS) or adherent NPCs were rinsed in PBS, scraped from the well in PBS, and centrifuged at 1,200 rpm for 5 min. Supernatant was removed, and 500 μl TRIzol reagent (Invitrogen) was added to the cell pellet. Total RNA was isolated according to the manufacturer’s protocol. The resulting RNA pellet was dissolved in distilled water and stored at −80°C. RNA concentration was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, http://www.nanodrop.com). Subsequently, 0.25 μg RNA was reverse transcribed with the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany, http://www.qiagen.com), according to the manufacturer’s protocol. The cDNA was stored at −20°C for later use in a real-time quantitative polymerase chain reaction (qPCR).
Real-Time Quantitative Polymerase Chain Reaction
qPCR was carried out in 96-well plates, with a final volume of 10 µl per well using the SYBR Green polymerase chain reaction (PCR) kit (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). Each reaction volume contained 5 µl SYBR Green mix, 3.5 µl H2O, 0.5 µl cDNA sample, and 1 µl primer mix (sense and antisense primers, each 2 pmol/µl; see supplemental online Table 3 for primer sequences). The plate was sealed and centrifuged at 1,000 rpm for 1 min before starting the qPCR program on a 7300 Real-Time PCR detection system (Applied Biosystems), with the following cycling conditions: 2 minutes at 50°C; 10 minutes at 95°C; 15 seconds at 95°C; and 1 minute at 60°C for 40 cycles. Cycle threshold values were converted to absolute amounts of cDNA present in the sample. Transcript levels of 18S ribosomal RNA (18S), actin, and elongation factor 1α were most stable and used for normalization. Technical details on quantification and normalization procedures were described previously [21].
Immunocytochemistry on SVZ Tissue Sections
SVZ tissue for immunohistochemistry was sampled as described [4]. SVZ material came from the area beneath the cingulate gyrus and contained only white matter. The donor brain tissue for this part of the study was free from any neurological disease or neuropsychiatric disorder. Paraffin sections (6 μm) were deparaffinized, rehydrated, and washed with Milli-Q water, followed by TBS (0.025 M Tris, 0.14 M NaCl, pH 7.6). The sections were exposed to 20 minutes of heating in a steamer in citrate buffer (10 mM citric acid + 0.05% Tween 20, pH 6.0; 98°C), to provide optimal antigen retrieval. After cooling down on ice to RT, they were preincubated with TBS with 2% normal horse serum, 1% BSA, 0.1% Triton X-100, and 0.05% Tween 20 to block nonspecific staining. Subsequently, they were incubated overnight with CD271 antibody in combination with antibodies against GFAPδ, sex-determining region Y-box 2 (sox2), or nestin (supplemental online Table 4) diluted in TBS plus 1% BSA at 4°C. Then the sections were rinsed and incubated with biotinylated goat anti-mouse at RT for 1 h. The sections were then rinsed and incubated at RT for 45 min with avidin-biotin complex (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) diluted 1:400 in TBS. Subsequently, the slides were washed and then incubated with SA-Alexa Fluor 594 and donkey anti-rabbit Alexa Fluor 488 or donkey anti-chicken Alexa Fluor 488 (1:1,200; Molecular Probes, Eugene, OR, http://www.probes.invitrogen.com) and Hoechst 33258 (1:1,000; Bio-Rad, Hercules, CA, http://www.bio-rad.com). Next, sections were rinsed, incubated in Sudan Black solution (0.3% Sudan Black in 70% ethanol) for 7 minutes to quench autofluorescence, and then washed in 70% ethanol for 1 minute. After an additional wash in TBS, sections were coverslipped in Mowiol (0.1 M Tris, pH 8.5, 25% glycerol, 10% w/v Mowiol 4-88 [Sigma-Aldrich]). For 3, 3′-diaminobenzidine tetrahydrochloride (DAB) staining, after incubation with primary antibodies, sections were incubated with a biotinylated goat anti-mouse or biotinylated goat anti-rabbit diluted in TBS. Then sections were incubated with avidin-biotin complex diluted in TBS. Sections were washed twice with TBS and once with Tris-HCl. Then peroxidase activity was visualized by DAB, and sections were mounted with entellan.
Immunocytochemistry on Human Primary NPC Cultures
Adherent adult SVZ NPCs or NPCs isolated using CD271 magnetic-activated cell sorting (MACS) beads were cultured on laminin-coated (20 μg/ml, 3 hours at 37°C) 16-well chamber slides. Cells were rinsed with PBS and fixed in 4% PFA in PBS for 15 minutes. Following fixation, the cells were rinsed with TBS and preincubated with TBS containing 2% normal horse serum, 0.5% BSA, 0.1% Triton X-100, and 0.05% Tween 20 to block nonspecific staining, and, subsequently, they were incubated at 4°C in TBS containing 0.1% Triton X-100 (TBS-T) overnight with primary antibodies (supplemental online Table 4). Subsequently, the cells were rinsed with TBS following incubation with Cy3- or Cy2-labeled secondary antibodies (1:400; Jackson ImmunoResearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com) in TBS-T at RT for 2 h. Finally, the cells were rinsed three times with TBS and coverslipped in Mowiol (Vector Laboratories).
Statistical Analysis
For statistical analysis, the program GraphPad Prism (GraphPad Software, San Diego, CA, http://www.graphpad.com) was used. When the data were normal distributed, one-way analysis of variance (ANOVA) was performed. Otherwise, the Mann-Whitney test was used for comparison of two groups. Statistical significance was accepted when p < .05.
Results
Immunophenotyping of Primary Adult Human SVZ Cells
For immunophenotyping of adherent NPCs, all cells isolated from the SVZ of three elderly donors were plated onto laminin-coated 16-well chamber slides and allowed to adhere for 5 days. These adherent cell cultures were characterized and compared with neurosphere cultures derived from the same area, and to differentiated human adult NPCs. The adherent cultures before passaging consisted of several cell types, including NPCs based on expression of nestin, astrocytes (glutamine synthetase and GFAPα), and oligodendrocytes (2-3-cyclic nucleotide 3-phosphodiesterase [CNPase]) (supplemental online Figs. 1, 2). The cells were stained for 239 different surface markers (supplemental online Table 1), subsequently fixed, and immunofluorescently stained for GFAPδ. Several markers were expressed on both GFAPδ+ as well as GFAPδ− cells (supplemental online Table 1, Fig. 3). CD271 (p75NTR) was the only marker that was specifically expressed on GFAPδ-positive cells (supplemental online Table 1, Fig. 3).
CD271 Is Present on the Surface of GFAPδ+ Cells in the SVZ of Elderly Subjects
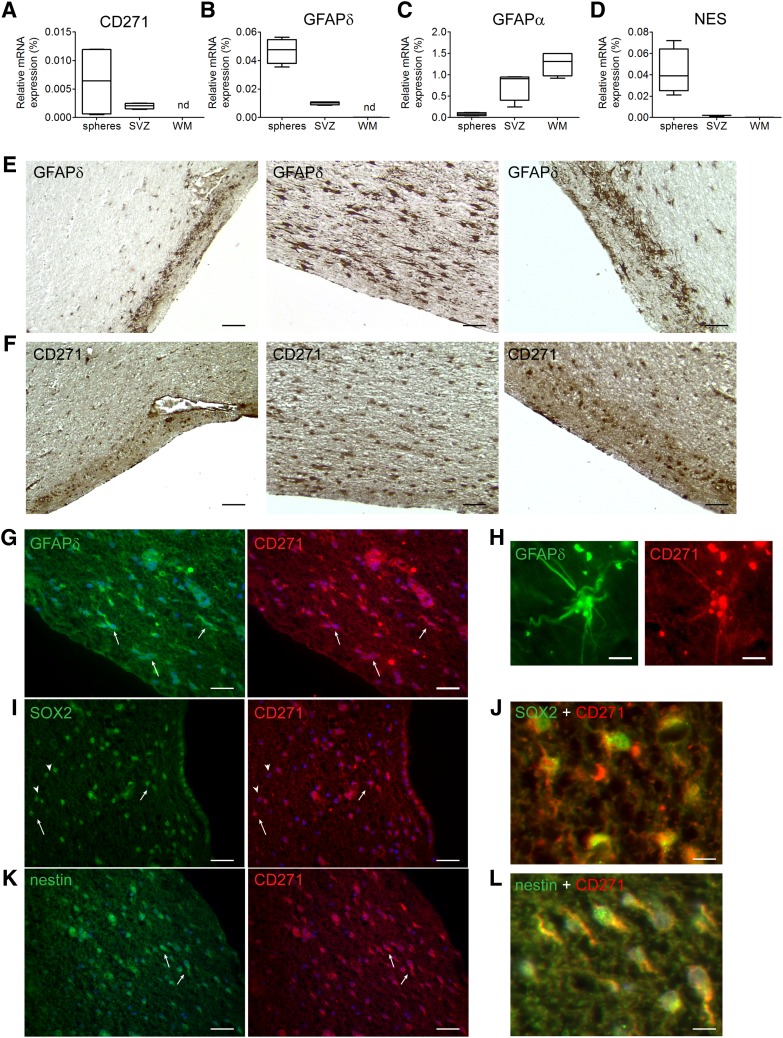
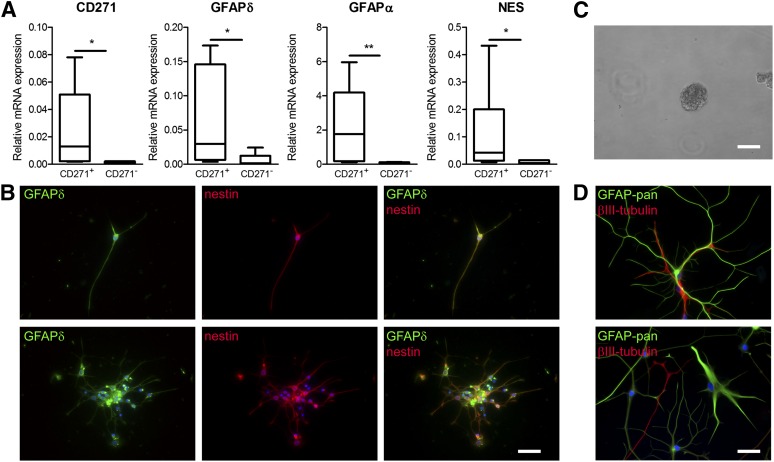
Neurospheres were cultured from the SVZ of elderly subjects, and the mRNA expression level of CD271 in single neurospheres was studied with qPCR. These CD271 levels were compared with the expression of CD271 in SVZ and subcortical white matter (WM) tissue derived from elderly control donors. CD271 was expressed in human adult SVZ neurospheres and in the SVZ, but not in WM tissue (Fig. 1A). GFAPδ and nestin mRNA were both expressed in SVZ tissue and SVZ-derived neurospheres, but were only lowly expressed in WM tissue (Fig. 1B, 1D). GFAPα was highly expressed in WM (Fig. 1C), indicating the presence of astrocytes and the absence of NPCs in the WM.
Figure 1.
CD271 is present in the SVZ of human elderly subjects and in SVZ neurospheres. Quantitative polymerase chain reaction was performed on neurospheres derived from human adult SVZ tissue, human adult SVZ tissue, and subcortical white matter derived from control donors for CD271 (A), GFAPδ (B), GFAPα (C), and nestin (D). Data are expressed as median and interquartile ranges. Analysis of significance was performed by Mann-Whitney U test. n = 4. Postmortem SVZ tissue sections obtained from elderly control subjects were stained for GFAPδ (E) or CD271 (F), and pictures were taken in the same SVZ areas to compare the staining pattern. CD271+ cells in the human adult SVZ are also immunopositive for GFAPδ (G, H), SOX2 (I, J), and nestin (K, L). Scale bar represents 100 μm (E–G, I, K), 10 μm (H), or 25 μm (J, L). Abbreviations: GFAP, glial fibrillary acidic protein; nd, not detectable; NES, nestin; SOX2, sex-determining region Y-box 2; SVZ, subventricular zone; WM, white matter.
Postmortem SVZ tissue sections obtained from elderly control subjects were stained for GFAPδ (Fig. 1E) or CD271 (Fig. 1F), and pictures were taken in the same SVZ areas. GFAPδ and CD271 immunoreactivity showed a similar staining pattern.
To study colocalization, sections were immunofluorescently stained for CD271 together with GFAPδ. As shown in Figure 1G (arrows) and Figure 1H, CD271 was present on GFAPδ+ cells in the SVZ of elderly subjects. The CD271+ cells were also positive for the NPC and neuroblast marker Sox2 (Fig. 1I [arrows], 1J). However, not all Sox2-positive cells were positive for CD271 (Fig. 1I, arrowheads). Furthermore, CD271+ cells were also positive for the NPC marker nestin (Fig. 1K [arrows], 1L).
Direct Isolation of CD271+/GFAPδ+ Cells
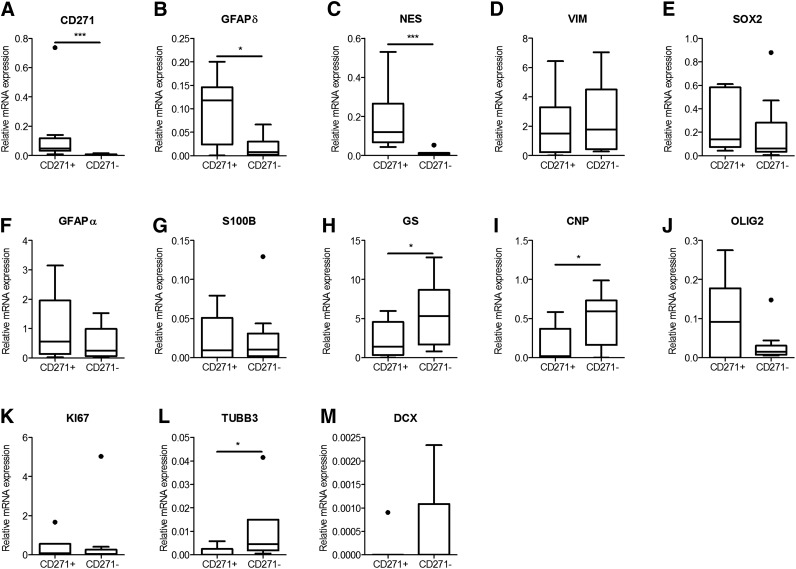
To specifically isolate GFAPδ-positive cells from elderly human postmortem SVZ tissue, we applied MACS with anti-CD271-labeled beads. CD271-positive (CD271+) and CD271-negative (CD271−) cell fractions of 10 donors were collected and used for mRNA analysis directly after isolation. The expression levels of GFAPδ were significantly higher in the CD271+ fraction compared with the CD271− fraction (Fig. 2A, p = .0315). The GFAPα mRNA expression was enriched in the CD271+ fraction (Fig. 2F). The expression of the NPC marker nestin was significantly higher in the CD271+ fraction (Fig. 1C, p = .001), whereas the mRNA levels of vimentin and GFAPα, which are expressed in NPCs as well as in astrocytes (Fig. 2D, 2F), the NPC and neuroblast marker Sox2 (Fig. 2E), and the proliferation marker Ki67 (Fig. 2K) did not significantly differ between both fractions. The expression of S100B, a marker for mature astrocytes, was not different between both fractions (Fig. 2G), whereas the mRNA expression of another marker for mature astrocytes, glutamine synthetase, was higher in the CD271− fraction (p = .0078; Fig. 2H). The neuronal markers βIII-tubulin and doublecortin (Fig. 2L, 2M) were lower in the CD271+ fraction compared with the CD271− fraction (p = .0209 and p = .0454, respectively).
Figure 2.
mRNA analysis of CD271+ and CD271− cell fractions derived from the human adult subventricular zone. Cells were collected directly after isolation using magnetic-activated cell sorting CD271 beads, mRNA was isolated, and real-time quantitative polymerase chain reaction was performed for different stem cell (A–E), astrocyte (F–H), oligodendrocyte (I, J), proliferation (K), and neuronal cell markers (L, M). Data are expressed as median and interquartile ranges. Analysis of significance was performed by Mann-Whitney U test. n = 10 different control donors. *p < .05, ***p < .001. Abbreviations: CNP, 2-3-cyclic nucleotide 3-phosphodiesterase; DCX, doublecortin; GFAP, glial fibrillary acidic protein; GS, glutamine synthetase; NES, nestin; OLIG2, oligodendrocyte lineage transcription factor 2; SOX2, sex-determining region Y-box 2; TUBB3, beta 3 tubulin; VIM, vimentin.
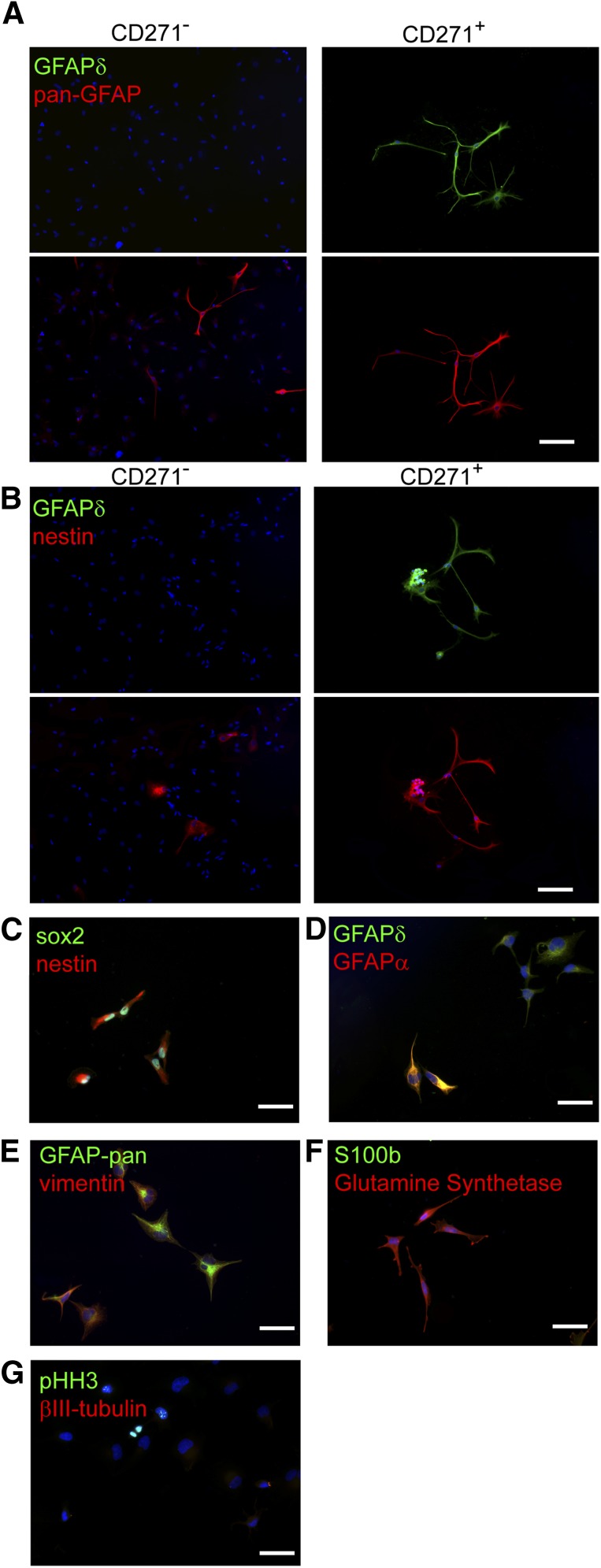
Then the CD271+ and CD271− cell fractions were plated onto laminin-coated wells and were allowed to adhere for 5 days. Immunoreactivity for GFAPδ, pan-GFAP, and nestin was studied (Fig. 3A, 3B). In the CD271− fraction, pan-GFAP (Fig. 3A)- and nestin (Fig. 3B)-positive cells were present, but these cells were not positive for GFAPδ (Fig. 3A, 3B). In contrast, in the CD271+ fraction, GFAPδ-positive cells were present and these cells were also positive for pan-GFAP (Fig. 3A) and nestin (Fig. 3B).
Figure 3.
Specific isolation and characterization of human subventricular zone neural progenitor cells using magnetic beads. (A): In the CD271− fraction, pan-GFAP-positive cells (red) are present, but not GFAPδ-positive cells (green). In the CD271+ fraction, all GFAP-pan (A)- and nestin (B)-positive cells are also positive for GFAPδ. GFAPδ+/CD271+ cells are positive for Sox2 (green) and nestin (red) (C) and GFAPδ (green) (D), and some cells express GFAPα (red), vimentin (red), and pan-GFAP (green) (E). (F): GFAPδ+/CD271+ cells express glutamine synthetase (red) but no S100b (green), and (G) some cells are positive for pHH3 (green), whereas βIII-tubulin (red) is not expressed. Scale bars represent 100 μm. Abbreviations: GFAP, glial fibrillary acidic protein; Sox2, sex-determining region Y-box 2.
To study whether CD271+/GFAPδ+ cells are NPCs, the protein expression of several markers was also studied. The CD271+/GFAPδ+ cells were immunopositive for the NPC markers nestin and sox2 (Fig. 3C), and not all GFAPδ+ cells express high levels of GFAPα (Fig. 3D). CD271+/GFAPδ+ cells were immunopositive for the NPC vimentin, and the proliferation marker pHH3 was present in the nucleus of some cells, which is known to be expressed in NPCs. CD271+/GFAPδ+ cells were not astrocytes because the mature astrocyte marker S100b was not expressed. In contrast, the mature astrocyte and NPC marker glutamine synthetase was expressed (Fig. 3F). The CD271+/GFAPδ+ did not express the neuronal marker βIII-tubulin, showing that neurons were not present in the positive fraction (Fig. 3G). These data, together with the qPCR data, show that CD271+/GFAPδ+ cells do not express neuronal or mature astrocyte markers, but they are positive for NPC and proliferation markers. Together, these data suggest that CD271+/GFAPδ+ cells are NPCs.
CD271+/GFAPδ+ Cells Form Neurospheres and Differentiate Into Neurons, Astrocytes, and Oligodendrocytes
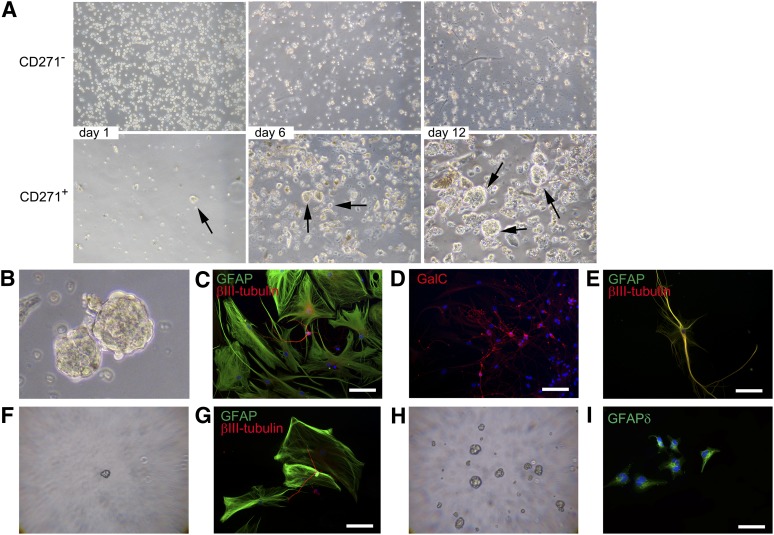
To prove that the CD271+/GFAPδ+ cells were indeed NPCs, neurosphere formation and differentiation of these cells derived from the human adult SVZ were studied. Cells were cultured in uncoated wells in the presence of EGF and FGF and allowed to form neurospheres. Pictures were taken at 1, 6, and 12 days after isolation. At day 1, more CD271−/GFAPδ− cells were present (Fig. 4A). No neurospheres were observed in the CD271− fraction at all time points studied. The CD271+/GFAPδ+ cells started to form neurospheres at day 1, and these spheres increased in size over time, as observed at days 6 and 12 (Fig. 4A, arrows).
Figure 4.
CD271+/GFAPδ+ cells form neurospheres and differentiate into astrocytes, neurons, and oligodendrocytes. (A): CD271+/GFAPδ+ cells were cultured in a neurosphere assay, and pictures were taken at days 1, 6, and 12 after isolation. (B): At day 15 after isolation, neurospheres were collected and allowed to differentiate for 14 days. CD271+/GFAPδ+ cells were able to differentiate into GFAP-positive astrocytes (green) and βIII-tubulin-positive neurons (red) (C) and GalC-positive oligodendrocytes (D), as shown with double-immunofluorescent staining. (E): Some cells are positive for both GFAP and βIII-tubulin. (F): Primary neurospheres were dissociated and plated as single cells per well. Small new spheres were formed after 2 weeks. (G): A single neurosphere differentiated into GFAP-positive astrocytes (green) and βIII-tubulin-positive neurons (red). (H): New neurospheres were formed after dissociation of secondary single neurospheres. (I): These cells were plated onto laminin-coated wells and allowed to adhere. Then cells were immunofluorescently stained for GFAPδ. Scale bars represent 50 μm (B–H) or 100 μm (I). Abbreviations: GalC, galactoceramide; GFAP, glial fibrillary acidic protein.
Neurospheres were collected at day 15 after isolation (Fig. 4B) and plated on laminin-coated wells in the presence of 5% FCS, without growth factors. After 14 days, astrocytes, neurons, and oligodendrocytes were present, as shown with immunofluorescent stainings for pan-GFAP (astrocytes), βIII-tubulin (neurons) (Fig. 4C), and galactoceramide (GalC, oligodendrocytes) (Fig. 4D). Some cells were positive for both GFAP and βIII-tubulin (Fig. 4E).
Neurospheres were dissociated, single cells were plated as one cell per well in a 96-well plate, and small secondary neurospheres were formed (Fig. 4F). When a single neurosphere was plated onto laminin-coated wells, in the presence of 5% FCS without growth factors, the cells differentiated into neurons and astrocytes after 14 days (Fig. 4G). When secondary neurospheres were dissociated, new neurospheres were formed again (Fig. 4H), showing self-renewal. To study whether these cells were still GFAPδ-positive NPCs, neurospheres were plated onto laminin-coated wells in the presence of growth factors and allowed to adhere for 12 hours. As shown in Figure 4I, these cells were indeed immunopositive for GFAPδ, implying that the cells kept on expressing NPC markers.
CD271 Is Also Present on the Surface of NPCs Derived From the SVZ of Alzheimer's and Parkinson's Patients
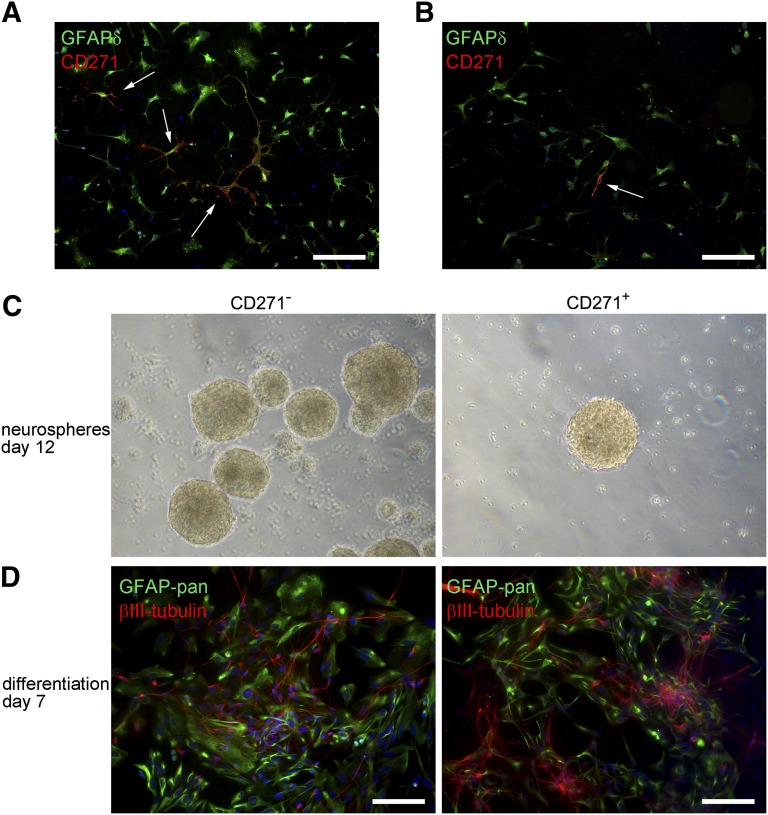
CD271+ and CD271− cells were isolated from the SVZ of three AD and three PD patients, and mRNA expression levels were studied directly after isolation. The mRNA levels of GFAPδ, GFAPα, and nestin were significantly higher (p = .0303, p = .0087, and p = .0260, respectively) in the CD271+ fraction (Fig. 5A). The CD271+/GFAPδ+ cells were also positive for nestin (Fig. 5B). Because the amount of cells obtained per isolation was very low, we used cells isolated from one donor either for qPCR or for in vitro experiments. One donor was used to study the characteristics of CD271+/GFAPδ+ cells derived from the SVZ of an AD patient in vitro and to confirm that these cells had similar characteristics as the cells isolated from the SVZ of elderly subjects. These AD CD271+/GFAPδ+ cells were also capable of forming neurospheres (Fig. 5C) and could be differentiated into GFAP-positive astrocytes and βIII-tubulin-positive neurons (Fig. 5D).
Figure 5.
Isolation of CD271+/GFAPδ+ neural progenitor cells derived from the subventricular zone of patients with a neurodegenerative disorder. (A): mRNA expression levels in CD271+ and CD271− fractions directly after isolation. Data are expressed as median and interquartile ranges. Analysis of significance was performed by Mann-Whitney U test. n = 6 donors, *p < .05, **p < .01. (B): Immunofluorescent double labeling of CD271+ cells 5 days after isolation. (C): CD271+/GFAPδ+ cells derived from a donor with Alzheimer’s disease (AD)-formed neurospheres in vitro. (D): CD271+/GFAPδ+ cells derived from an AD donor were able to differentiate into GFAP-positive astrocytes (green) and βIII-tubulin-positive neurons (red). Scale bar represents 250 μm (C) and 50 μm (B, D). Abbreviations: GFAP, glial fibrillary acidic protein; NES, nestin.
CD271 Is Not a Specific Cell-Surface Marker for Fetal GFAPδ+ Cells
To study whether CD271 can also be used to sort GFAPδ+ cells from the fetal human brain, we studied the presence of CD271 on the surface of primary human fetal brain cells gestational week 14. After isolation, cells were allowed to adhere and were immunofluorescently labeled for CD271 and GFAPδ. The human fetal brain cells were CD271-immunopositive, but the cell surface marker was not specifically present on GFAPδ+ cells (Fig. 6A, 6B). Some cells expressed both CD271 and GFAPδ (Fig. 6A), but not all CD271+ cells were GFAPδ+ (Fig. 6B).
Figure 6.
In adherent human fetal neural progenitor cells (NPCs), CD271 is not specifically expressed on GFAPδ-positive NPCs. (A): Many adherent NPCs derived from human fetal brain, 14 weeks of gestation, are GFAPδ positive. Some of these cells are also positive for CD271 (arrows), whereas other cells expressing CD271 do not express GFAPδ (arrow) (B). (C): Both CD271− and CD271+ human fetal NPCs are able to form neurospheres in vitro. (D): Double staining of GFAP-pan (green) plus βIII-tubulin (red) in CD271− and CD271+ human fetal NPCs that were differentiated for 7 days. Neurospheres were dissociated and plated onto laminin-coated wells without growth factors and in the presence of 2% fetal calf serum and 100 ng/ml brain derived neurotrophic factor (BDNF). Scale bars = 100 μm. Abbreviation: GFAP, glial fibrillary acidic protein.
Both CD271− and CD271+ fetal brain cells formed neurospheres (Fig. 6C), and when CD271+ and CD271− neurospheres were plated onto laminin-coated wells and were allowed to differentiate for 7 days, both fractions contained GFAP-positive astrocytes and βIII-tubulin-positive neurons. However, it should be noted that more βIII-tubulin-positive cells were present in the CD271+ fraction (Fig. 6D).
Discussion
In the present study, we showed for the first time that CD271 is specifically expressed on the surface of adult GFAPδ+ NPCs present in the SVZ of elderly human brains. We made use of the presence of the cell surface marker CD271 to isolate GFAPδ-positive NPCs from the adult human brain. These CD271+/GFAPδ+ cells have all the characteristics of NPCs. CD271+/GFAPδ+ cells proliferate and are able to form (secondary) neurospheres in vitro. Furthermore, they express NPC markers and are multipotent because they can differentiate into neurons, astrocytes, or oligodendrocytes [22]. In contrast, the CD271−/GFAPδ− cells do not form neurospheres and express low levels of the NPC marker nestin. Taken together, these results corroborate our earlier findings and prove that GFAPδ-positive cells in the adult SVZ are indeed NPCs. We also provide a protocol that enables specific isolation of these cells from the human SVZ for culturing and further in-depth analysis. As the human SVZ differs from the rodent SVZ [1], the CD271+/GFAPδ+ cells are a valuable and essential resource for the search for compounds that can stimulate adult neurogenesis in the human brain.
The multipotency of CD271+/GFAPδ+ is of great clinical importance because this allows potential replacement of different subtypes of neurons and glia in the damaged brain. More knowledge on these CD271+/GFAPδ+ cells is therefore essential as they potentially are the founding source of cells that may not only replace neurons in neurodegenerative disorders but also oligodendrocytes in demyelinating disorders. In our in vitro culture of CD271+/GFAPδ+, we, to date, mainly obtain astrocytes, but we also showed that we can differentiate the NPCs in neurons and oligodendrocytes. This current biased differentiation may reflect the culture conditions because cell differentiation is highly dependent on the environment in which these cells grow. More research is necessary to drive these cells into a specific cellular fate, matching the need for replacement in a disease.
After differentiation of CD271+/GFAPδ+ cells, some cells expressed both the astrocyte marker GFAP and the neuronal marker βIII-tubulin. This cell type has previously been described as asterons, having a neuron-to-astrocyte phenotype shifting within differentiating neurospheres [23], as well as in cultures of astrotypic adult human NPCs [24]. A few studies have described such cells with hybrid characteristics, but no extensive analysis has been done. Some GFAP+ cells derived from human embryonic NPCs were shown to display spontaneous neuronal firing patterns [25], and glutamate receptor-expressing astrocytes described in the hippocampus have been proposed to represent an intermediate cell type that possesses glial properties but may have begun to express neuronal genes [26].
In this study, we provide the first evidence that CD271+/GFAPδ+ cells indeed exhibit NPC characteristics, as we and others have previously suggested [4, 8, 11, 27]. Our earlier cultured SVZ neurospheres developed from a mixture of NPCs, astrocytes, oligodendrocytes, neuroblasts, and other cell types all present in the human SVZ. Access to highly enriched populations of NPCs derived from the adult human SVZ through a simple sorting approach will now enable us to study the characteristics of CD271+/GFAPδ+ cells. It also allows development-defined high-throughput drug discovery and toxicology assays, which are required to search for compounds that can activate and protect this population of cells. Furthermore, this method can be used for a phenotypic screen of NPCs derived from the SVZ of patients with a neurodegenerative disorder, to search for disease-specific changes in this population of cells.
Based on GFAPδ and CD271 expression, we showed that human fetal GFAPδ+ brain cells differ from human adult NPCs. Most fetal brain cells are positive for GFAPδ, but CD271 is not specifically expressed on the surface of fetal derived GFAPδ+ cells. After differentiation, there seems to be more neuronal differentiation of CD271+ fetal cells compared with CD271− cells. Indeed, in fetal NPCs, it has been suggested that CD271 is required for neuronal fate decision as well as for differentiation of NPCs [28]. We also observed that CD133, a known marker for human embryonic NPCs [29] and rodent fetal and rodent adult NPCs [30], is not present on cells derived from human postmortem SVZ tissue (supplemental online Fig. 3). Also in our earlier studies, we were not able to detect CD133 on GFAPδ+ cells in the human adult SVZ [4]. Furthermore, the composition of the human SVZ is different from rodent, as there is a hypocellular gap present between the ependymal layer and the dense astrocytic ribbon, which contains the NPCs [31]. Thus, human NPCs derived from the adult SVZ clearly differ from adult NPCs derived from the rodent brain. This implies that our in vitro model of primary human adult NPCs is essential for the development of new therapeutic strategies to stimulate adult neurogenesis in the human brain.
The function of CD271 in human adult NPCs is unknown. CD271 is widely expressed during development on the surface of different cell types, but is restricted in the adult brain [32]. During development, CD271 might be important for neuronal survival and differentiation. Some data are available on the presence and function of CD271 on rodent NPCs. NPCs from P75−/− mice were unable to generate neurons but still had the potential to differentiate into astrocytes and oligodendrocytes [33]. In rats it has been shown that a small population of cells in both the adult and neonatal SVZ was positive for CD271, and these cells also expressed with nestin and cell cycle markers Ki67 and 5-bromo-2′-deoxyuridine (BrdU) [33, 34]. These CD271+ cells were able to form neurospheres, and their neurogenic potential was enhanced by treatment with brain-derived neurotrophic factor or nerve growth factor.
It has also been shown that human gliomas, one of the most common tumors in the human adult central nervous system, contain NPC-like cells [35] and express GFAPδ [36]. It has been suggested that glioblastoma cancer stem cells may arise from SVZ NPCs, which migrate to tumor sites and contribute to glioma growth and recurrence [37]. Interestingly, CD271 is also present on these cells, and it has been shown that CD271 is mediating glioma cell invasion by neurotrophin-dependent regulated intramembrane proteolysis of CD271 [38].
To our knowledge, there is only one study describing restricted CD271 expression in the healthy adult human SVZ. No expression was present in the white matter. However, in the brains of multiple sclerosis (MS) patients, the expression of CD271 in the SVZ was increased and was appearing in a MS lesion [39]. It was suggested that a population of precursor cells induced CD271 expression to subsequently assume an oligodendrocyte phenotype in response to demyelination in the adjacent white matter. In the present study, we show that the oligodendrocyte progenitor marker Olig2 was also expressed in the CD271+/GFAPδ+ cells and that these cells were indeed able to differentiate into oligodendrocytes. However, we showed that the CD271+/GFAPδ+ cells are multipotent.
Conclusion
We have set up a method for the direct and specific isolation of CD271+/GFAPδ+ NPCs from the adult human postmortem SVZ. The next challenge is to immortalize these cells to obtain a sufficient amount of NPCs, which can be used as a continuous source for studying human adult NPCs in vitro. To date, no in vitro model for human adult NPCs is available. Future screens using NPCs derived from the brains of elderly controls and patients with a neurodegenerative disorder may result in the discovery of compounds that can be used for therapeutic strategies to stimulate SVZ neurogenesis in the diseased brain.
Supplementary Material
Acknowledgments
We are grateful to Dr. K. Weijer (Department of Cell Biology and Histology, AMC) for the collection of brain tissue. Postmortem human brain material was obtained from the Netherlands Brain Bank (http://www.brainbank.nl). We thank Simone A. van den Berge for technical assistance. This work was supported by NWO-ALW-Vici 865.09.003 (to E.M.H.); van Leersumfonds (to M.E.v.S.); NIH/National Institute of Neurological Disorders and Stroke Grant NS055165 (to D.A.S.); Maren, Thompson, and McKinney Regeneration Funds (to D.A.S.); and EU FP7 Project DEVELAGE (Grant Agreement N 278486 to E.A.).
Author Contributions
M.E.v.S.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; J.A.S.: collection and assembly of data; B.A.R. and D.A.S.: conception and design; E.A.: provision of study material; E.M.H.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Sanai N, Tramontin AD, Quiñones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 3.Sanai N, Nguyen T, Ihrie RA, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berge SA, Middeldorp J, Zhang CE, et al. Longterm quiescent cells in the aged human subventricular neurogenic system specifically express GFAP-delta. Aging Cell. 2010;9:313–326. doi: 10.1111/j.1474-9726.2010.00556.x. [DOI] [PubMed] [Google Scholar]
- 5.Leonard BW, Mastroeni D, Grover A, et al. Subventricular zone neural progenitors from rapid brain autopsies of elderly subjects with and without neurodegenerative disease. J Comp Neurol. 2009;515:269–294. doi: 10.1002/cne.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Berge SA, van Strien ME, Korecka JA, et al. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain. 2011;134:3249–3263. doi: 10.1093/brain/awr256. [DOI] [PubMed] [Google Scholar]
- 7.van Strien ME, van den Berge SA, Hol EM. Migrating neuroblasts in the adult human brain: A stream reduced to a trickle. Cell Res. 2011;21:1523–1525. doi: 10.1038/cr.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roelofs RF, Fischer DF, Houtman SH, et al. Adult human subventricular, subgranular, and subpial zones contain astrocytes with a specialized intermediate filament cytoskeleton. Glia. 2005;52:289–300. doi: 10.1002/glia.20243. [DOI] [PubMed] [Google Scholar]
- 9.Hol EM, Roelofs RF, Moraal E, et al. Neuronal expression of GFAP in patients with Alzheimer pathology and identification of novel GFAP splice forms. Mol Psychiatry. 2003;8:786–796. doi: 10.1038/sj.mp.4001379. [DOI] [PubMed] [Google Scholar]
- 10.Quinlan RA, Brenner M, Goldman JE, et al. GFAP and its role in Alexander disease. Exp Cell Res. 2007;313:2077–2087. doi: 10.1016/j.yexcr.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middeldorp J, Boer K, Sluijs JA, et al. GFAPdelta in radial glia and subventricular zone progenitors in the developing human cortex. Development. 2010;137:313–321. doi: 10.1242/dev.041632. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen AL, Holm IE, Johansen M, et al. A new splice variant of glial fibrillary acidic protein, GFAP epsilon, interacts with the presenilin proteins. J Biol Chem. 2002;277:29983–29991. doi: 10.1074/jbc.M112121200. [DOI] [PubMed] [Google Scholar]
- 13.Selkoe D, Kopan R. Notch and Presenilin: Regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 14.Alexson TO, Hitoshi S, Coles BL, et al. Notch signaling is required to maintain all neural stem cell populations—irrespective of spatial or temporal niche. Dev Neurosci. 2006;28:34–48. doi: 10.1159/000090751. [DOI] [PubMed] [Google Scholar]
- 15.Givogri MI, de Planell M, Galbiati F, et al. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- 16.Perng MD, Wen SF, Gibbon T, et al. Glial fibrillary acidic protein filaments can tolerate the incorporation of assembly-compromised GFAP-delta, but with consequences for filament organization and alphaB-crystallin association. Mol Biol Cell. 2008;19:4521–4533. doi: 10.1091/mbc.E08-03-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Fu S, Wang Y, et al. Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol Cell Neurosci. 2007;36:343–354. doi: 10.1016/j.mcn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno N, Kokubu H, Sato M, et al. G protein-coupled receptor signaling through Gq and JNK negatively regulates neural progenitor cell migration. Proc Natl Acad Sci USA. 2005;102:12365–12370. doi: 10.1073/pnas.0506101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomson TM, Rettig WJ, Chesa PG, et al. Expression of human nerve growth factor receptor on cells derived from all three germ layers. Exp Cell Res. 1988;174:533–539. doi: 10.1016/0014-4827(88)90323-0. [DOI] [PubMed] [Google Scholar]
- 20.Huitinga I, Rademaker M, Klioueva N. The art of brain banking in Europe: Ethical, legal and practical guidelines for donor recruitment, tissue handling and tissue distribution. Abstract in J Neural Transm. 2008;115:1715. [Google Scholar]
- 21.Kamphuis W, Orre M, Kooijman L, et al. Differential cell proliferation in the cortex of the APPswePS1dE9 Alzheimer’s disease mouse model. Glia. 2012;60:615–629. doi: 10.1002/glia.22295. [DOI] [PubMed] [Google Scholar]
- 22.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 23.Laywell ED, Kearns SM, Zheng T, et al. Neuron-to-astrocyte transition: Phenotypic fluidity and the formation of hybrid asterons in differentiating neurospheres. J Comp Neurol. 2005;493:321–333. doi: 10.1002/cne.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walton NM, Sutter BM, Chen HX, et al. Derivation and large-scale expansion of multipotent astroglial neural progenitors from adult human brain. Development. 2006;133:3671–3681. doi: 10.1242/dev.02541. [DOI] [PubMed] [Google Scholar]
- 25.Gritti A, Rosati B, Lecchi M, et al. Excitable properties in astrocytes derived from human embryonic CNS stem cells. Eur J Neurosci. 2000;12:3549–3559. doi: 10.1046/j.1460-9568.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 26.Matthias K, Kirchhoff F, Seifert G, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollard SM, Yoshikawa K, Clarke ID, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Hosomi S, Yamashita T, Aoki M, et al. The p75 receptor is required for BDNF-induced differentiation of neural precursor cells. Biochem Biophys Res Commun. 2003;301:1011–1015. doi: 10.1016/s0006-291x(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 29.Pfenninger CV, Roschupkina T, Hertwig F, et al. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 2007;67:5727–5736. doi: 10.1158/0008-5472.CAN-07-0183. [DOI] [PubMed] [Google Scholar]
- 30.Fischer J, Beckervordersandforth R, Tripathi P, et al. Prospective isolation of adult neural stem cells from the mouse subependymal zone. Nat Protoc. 2011;6:1981–1989. doi: 10.1038/nprot.2011.412. [DOI] [PubMed] [Google Scholar]
- 31.Quiñones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 32.Yan Q, Johnson EM., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988;8:3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young KM, Merson TD, Sotthibundhu A, et al. p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J Neurosci. 2007;27:5146–5155. doi: 10.1523/JNEUROSCI.0654-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giuliani A, D’Intino G, Paradisi M, et al. p75(NTR)-immunoreactivity in the subventricular zone of adult male rats: Expression by cycling cells. J Mol Histol. 2004;35:749–758. doi: 10.1007/s10735-004-9609-2. [DOI] [PubMed] [Google Scholar]
- 35.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 36.Choi KC, Kwak SE, Kim JE, et al. Enhanced glial fibrillary acidic protein-delta expression in human astrocytic tumor. Neurosci Lett. 2009;463:182–187. doi: 10.1016/j.neulet.2009.07.076. [DOI] [PubMed] [Google Scholar]
- 37.Glantz M, Kesari S, Recht L, et al. Understanding the origins of gliomas and developing novel therapies: Cerebrospinal fluid and subventricular zone interplay. Semin Oncol. 2009;36(suppl 2):S17–S24. doi: 10.1053/j.seminoncol.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Rahn JJ, Lun X, et al. Gamma-secretase represents a therapeutic target for the treatment of invasive glioma mediated by the p75 neurotrophin receptor. PLoS Biol. 2008;6:e289. doi: 10.1371/journal.pbio.0060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petratos S, Gonzales MF, Azari MF, et al. Expression of the low-affinity neurotrophin receptor, p75(NTR), is upregulated by oligodendroglial progenitors adjacent to the subventricular zone in response to demyelination. Glia. 2004;48:64–75. doi: 10.1002/glia.20056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.