The current review describes the different reprogramming strategies that can give rise to cardiomyocytes for regenerative medicine purposes. The advantages and shortcomings of each strategy for cardiac regeneration are discussed, along with the hurdles that need to be overcome on the road to clinical translation.
Keywords: Induced pluripotent stem cells, Direct reprogramming, Cardiomyocytes, Heart failure, Cell therapy, Tissue engineering
Abstract
Myocardial cell-replacement therapies are emerging as novel therapeutic paradigms for myocardial repair but are hampered by the lack of sources of autologous human cardiomyocytes. The recent advances in stem cell biology and in transcription factor-based reprogramming strategies may provide exciting solutions to this problem. In the current review, we describe the different reprogramming strategies that can give rise to cardiomyocytes for regenerative medicine purposes. Initially, we describe induced pluripotent stem cell technology, a method by which adult somatic cells can be reprogrammed to yield pluripotent stem cells that could later be coaxed ex vivo to differentiate into cardiomyocytes. The generated induced pluripotent stem cell-derived cardiomyocytes could then be used for myocardial cell transplantation and tissue engineering strategies. We also describe the more recent direct reprogramming approaches that aim to directly convert the phenotype of one mature cell type (fibroblast) to another (cardiomyocyte) without going through a pluripotent intermediate cell type. The advantages and shortcomings of each strategy for cardiac regeneration are discussed, along with the hurdles that need to be overcome on the road to clinical translation.
Introduction
The endogenous repair mechanisms of the adult heart are usually inadequate in dealing with an extensive myocardial infarction (MI) [1, 2]. The resulting decrease in the contractile mass, which is associated with the loss of approximately 1 billion cardiomyocytes [2], may lead to progressive deterioration in cardiac function and, eventually, to the development of clinical heart failure. Heart failure is a growing epidemic that is associated with significant morbidity and mortality; for example, it is responsible for more hospitalizations than all forms of cancer combined.
It is not surprising, therefore, that the heart has been the target of the emerging discipline of regenerative medicine. Although different approaches were suggested in an attempt to favorably alter the natural history of postinfarction heart failure through a variety of mechanisms [3], the most intriguing are those attempting to replace the lost cardiomyocytes with new ones [4–6]. These include strategies aimed to induce or augment endogenous processes that can potentially give rise to new cardiomyocytes through either the differentiation of resident cardiac progenitor cells (CPCs) or through renewal of pre-existing cardiomyocytes by cell division [4].
Alternatively, transplantation of cardiomyocytes from exogenous sources can be used to replace the lost cells and to repopulate the scar [4–6]. The most attractive candidates for such a task are cardiomyocytes derived from human pluripotent stem cells (hPSCs), namely, from human embryonic stem cells (hESCs) [7] or human induced pluripotent stem cells (hiPSCs) [8, 9] as well as from autologous CPCs [10]. The latter cells can be harvested from the heart (during surgery or using a less-invasive percutaneous biopsy approach), expanded ex vivo, and then transplanted back in an autologous manner. The latter approach has already reached early stage clinical trials (SCIPIO [11] and CADUCEUS [12] trials).
In the current review, we focus on describing the different reprogramming strategies that can be used to give rise to cardiomyocytes for regenerative medicine applications (Fig. 1). Initially, we describe the induced pluripotent stem cell (iPSC) technology [13], a method by which adult somatic cells can be reprogrammed to pluripotent stem cells that could then be coaxed to differentiate into cardiomyocytes. We also describe the more recent direct reprogramming approaches, which aim to directly convert the phenotype of one mature cell type (fibroblast) to another (cardiomyocyte) without going through a pluripotent intermediate cell type [14].
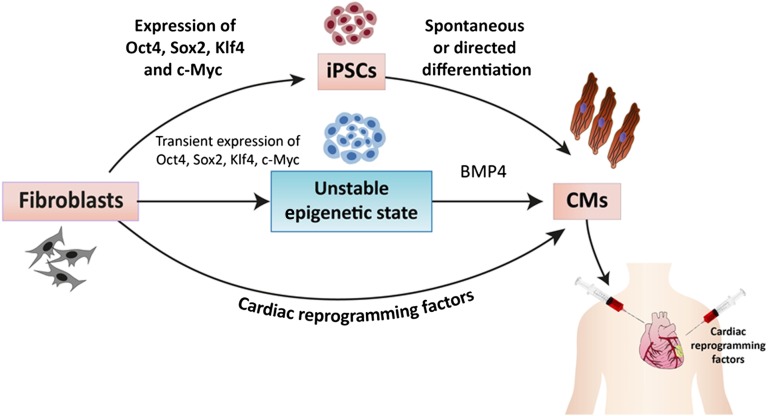
Figure 1.
A schematic representation of the different reprogramming strategies suggested for deriving cardiomyocytes for myocardial regeneration. These include the iPSC approach (top), the partial reprogramming strategy (middle), and the direct reprogramming strategy. The generated cardiomyocytes can be then engrafted into the failing heart. Note that the direct reprogramming strategy could also potentially be used with direct application of the cardiac reprogramming transcription factors into the heart. Abbreviations: CM, cardiomyocyte; iPSC, induced pluripotent stem cell.
iPSCs
The first human stem cell source that could reliably give rise to cardiomyocytes in vitro was hESCs [7]. These pluripotent stem cell lines, derived from human blastocysts, can be propagated in the undifferentiated state and then coaxed to differentiate into a variety of cell lineages, including different types of cardiomyocytes [15–17]. Because of their ability to differentiate into bona fide cardiomyocytes, hESCs were shown to serve as a unique tool for study of early cardiomyocyte differentiation [18–20], for drug discovery [21, 22], and as an attractive cell source for myocardial cell-replacement strategies [23, 24]. Nevertheless, the inability to create patient- or disease-specific hESCs from adult individuals and the anticipated immune rejection associated with such allogeneic cell transplantation raise important hurdles for their clinical use.
The aforementioned obstacles could potentially be overcome with the iPSC technology introduced by Takahashi and Yamanaka in 2006 [13]. The ability to reprogram adult and embryonic murine fibroblast cells to pluripotency by retroviral transduction of four transcription factors (OCT3/4, SOX2, c-MYC, and KLF4) has revolutionized regenerative medicine. The creation, soon after, of hiPSCs [8, 9] and the ability to differentiate them into cardiomyocytes [25, 26] introduced a powerful tool that can potentially be used to develop autologous cell-replacement strategies that can evade the immune system [27], to generate patient- and disease-specific models of inherited cardiac disorders [28–30], and to establish screens for drug testing [31].
The first key step to the potential use of hPSCs for cardiac regenerative medicine applications is the generation of sufficient numbers of heart cells. The initial demonstration that beating cardiomyocytes can be generated from both hESCs [15, 16] and hiPSCs [25, 26] was based on the spontaneous but relatively inefficient serum-dependent embryoid body (EB) differentiation system. This method resulted in the appearance of contracting areas in 8%–25% of hESC-derived EBs [15, 16] (1%–10% in hiPSCs [25, 26]), a finding that translates to only a small percentage of all differentiating cells becoming cardiomyocytes [32]. Following these initial descriptions, several guided cardiomyocyte differentiation systems were developed, inspired by lessons learned from embryology, and resulted in well-defined, serum-free, and reproducible differentiation protocols that are highly efficient and can give rise to >60%–80% cardiomyocytes [32, 33].
One notable method includes culturing high-density hPSC monolayers with activin-A for 24 hours, followed by bone morphogenic protein-4 (BMP4) treatment for 4 days [24]. Another frequently used approach [20, 34] consists of EB formation in BMP4-supplemented media (days 0–1), followed by treatment with BMP4, activin-A, and basic fibroblast growth factor (days 1–4) for induction of a primitive streak-like cell population and mesoderm. Differentiation into cardiac mesoderm and expansion and maturation of CPCs is then induced by inhibition of the canonical Wnt pathway through the application of Dickkopf-related protein 1 (DKK1) and culturing of the cells in a medium supplemented with VEGF (days 4–8). Inhibition of the activin/Nodal/transforming growth factor β and BMP pathways (by SB-431542 and dorsomorphin, respectively) during the cardiac maturation period (days 3–5) further improved cardiomyocyte yield [34].
More recently, a highly effective strategy was introduced for hiPSC cardiomyocyte differentiation in which small molecules are used to manipulate a single signaling pathway (the canonical Wnt pathway) [35]. This method is based on initial activation of the Wnt pathway by CHIR-99021 to facilitate mesoendoderm formation, followed by Wnt inhibition by IWP-2 or IWP-4 to induce cardiomesoderm formation. This method results in a highly efficient cardiomyocyte monolayer differentiating system (80%–98% purity) [35] and yields 3–5 million cardiomyocytes per well of a 12-well plate [35, 36]. This method is also rather cost effective because it eliminates the need for the expensive growth factors used in the previously described protocols.
Consequently, even with existing culturing techniques, it is feasible to obtain clinically relevant numbers of cardiomyocytes (for large-animal and initial clinical studies). Moreover, with recent advancements made in culturing of undifferentiated hPSCs, such as three-dimensional cell clusters in suspension [37–39], as well as in bioreactor-related technologies for stem cell culture and differentiation [38, 40, 41], scaling up these processes to produce relevant numbers of cardiomyocytes for routine clinical use should become feasible.
Ideally, the directed cardiomyocyte-differentiation systems described will result in ∼100% cardiomyocyte-differentiation yield. If this is not achievable, however, then strategies aiming to select only the differentiating cardiomyocytes from the mixed population of differentiating cells may be required. Although mechanical dissection of the beating areas [16, 23] and Percoll gradient centrifugation [24] were demonstrated to enrich the cardiomyocyte population, the relatively low degree of purity and the inadequate ability to scale up may limit their clinical application.
A more effective approach for cardiomyocyte purification involves a transgenic selection strategy using cardiac-specific promoters to drive the expression of selection markers (e.g., fluorescent marker for flow cytometry sorting, antibiotic-resistance gene for antibiotic selection) [42–44]. A major drawback of this strategy, however, is the requirement for genetic manipulation of the cells, which may hamper clinical use. This limitation may be overcome by the recent identification of cardiomyocyte-specific surface markers such as EMILIN2 [45], SIRPA [46], and VCAM [47], which may allow targeted antibody-based cell sorting to isolate CPCs at different developmental stages. Finally, recent reports provide even simpler methods for cardiomyocyte purification that are based on the unique metabolic properties of the differentiating cardiomyocytes. These include the use of mitochondria-specific viable fluorescent dyes for cardiomyocyte selection [48] because these cells are highly enriched with mitochondria. In a similar manner, taking advantage of the unique ability of cardiomyocytes to use lactate as a metabolic fuel, a selection strategy was developed in which the differentiated cells are cultured in a low-glucose, high-lactate medium [49].
Partial Reprogramming
One of the limitations of using the iPSC approach for creation of cardiomyocytes is the issue of speed because it may take a few months to complete the processes involved in fibroblast expansion and reprogramming, expanding the generated hiPSCs colonies, and coaxing their differentiation into the cardiac lineage. Efe et al. [50] suggested an alternative approach that could shorten this procedure and that involves generating partially reprogrammed cells (Fig. 1). The hypothesis underlying this approach is that, during the first days following initiation of reprogramming by Yamanaka factors, the cells enter an epigenetic “activation phase” and that manipulating the environmental cues at this early unstable stage to those favoring cardiogenic differentiation may allow shifting the outcome toward cardiogenesis rather than pluripotency [50].
The successful strategy developed by the investigators included initial overexpression of Oct4, Sox2, and Klf4 (with or without c-Myc) in murine embryonic fibroblasts with a short incubation period in a culture medium favoring reprogramming [50]. The medium is then changed to a chemically defined medium that includes the cardioinductive growth factor (BMP4) and an inhibitor of Janus kinase/signal transducer and activator of transcription that further prevents the cells from reaching a pluripotent state. Using this strategy, the authors showed that fibroblasts could be converted to cardiomyocytes over a brief period of 11–12 days. Nevertheless, the same concepts need to be reproduced for human cells (in which an alternative for the Janus kinase inhibitor would be needed). Importantly, because the generated cardiomyocytes using this strategy—unlike undifferentiated iPSCs—cannot be propagated, this process is less amenable to scaling up, and the derivation of each batch of new cardiomyocytes would require repeating the entire process of cell transduction and reprogramming. Finally, it is not clear how the induced cardiomyocytes compare with those derived from pluripotent stem cell lines in terms of their cardiomyocyte phenotypic properties and their capacity for cardiac repair.
Prospects for Using hiPSC-Derived Cardiomyocytes for Myocardial Repair
The ultimate goal of cardiovascular regenerative medicine is to generate a functional cardiac tissue that will become well integrated structurally and functionally with host myocardium and that will improve myocardial performance. Proof-of-concept studies in animal models of MI demonstrated the feasibility of using hESC-derived cardiomyocytes (hESC-CMs) for such a task [23, 24, 51, 52]. Cell engraftment, in the majority of these studies, led to attenuation of the ventricular remodeling process, delayed heart failure progression, and improved ventricular function when compared with nonmyocyte transplantation or vehicle injection.
As the field of iPSCs progressed, similar myocardial cell-replacement feasibility studies using iPSC-derived cardiomyocytes (iPSC-CMs) were also reported. These studies have demonstrated favorable outcomes in different animal models of MI (mice, rats, guinea pigs, and pigs) following delivery of murine [53, 54] or pig [55] undifferentiated iPSCs, iPSC-derived CPCs [56, 57], or cardiomyocytes derived from hiPSCs (hiPSC-CMs) [58].
Importantly, recent studies have also demonstrated the ability of hiPSC-CMs to couple functionally (electrically) with host cardiomyocytes [59] in a similar manner to hESC-CMs [60–62]. The latter issue may be important with regard to the mechanistic understanding of the beneficial effects of stem cell therapy because direct contribution to contractility would require the electrical coupling of the engrafted cells with host cardiac tissue. The degree of electrical integration may also determine the potential of these strategies to be antiarrhythmic (in the case of well-coupled engrafted cardiomyocytes) [62, 63] or proarrhythmic (when using cells that do not integrate or that integrate poorly) [60, 63].
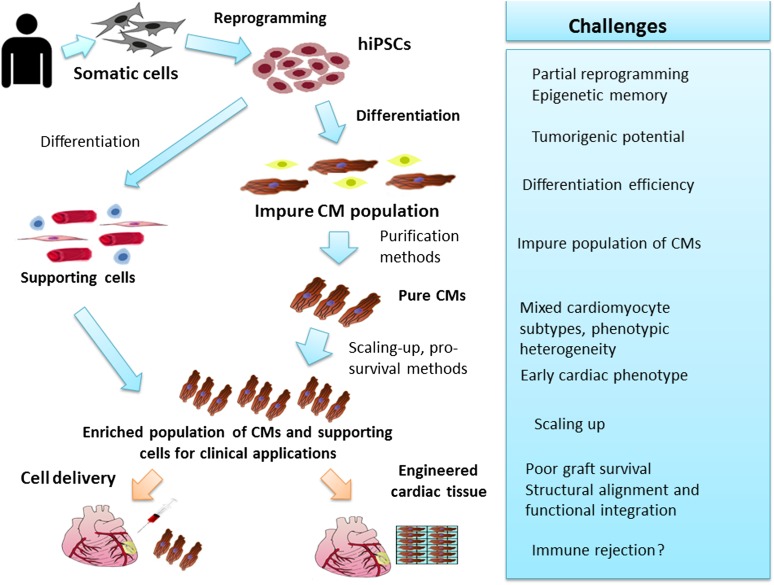
Despite the important progress made since the initial description of the iPSC technology [13], important challenges remain on the road to clinical use (Fig. 2). Some of these challenges include (a) the significant heterogeneity observed in the reprogramming efficacy and in the quality and developmental potential of the derived hiPSCs [27, 64]; (b) the tumorigenic risk associated with the use of hPSCs, in general, and with iPSC technology, specifically [65]; (c) the requisite development of efficient protocols for directed cardiomyocyte differentiation and purification and the need to develop scaling-up procedures to derive clinically relevant numbers of cardiomyocytes, as discussed above; (d) the heterogeneity and relatively immature properties of the hiPSC-CMs; (e) the need to address several important regulatory issues such as stem cell line characterization, good manufacturing practice, and important safety issues; and (f) the relatively poor survival, maturation, and structural alignment and integration of the engrafted cells.
Figure 2.
Schematic representation of the main steps and hurdles on the road to clinical use of the induced pluripotent stem cell technology for myocardial regeneration. Abbreviations: CM, cardiomyocyte; hiPSC, human induced pluripotent stem cell.
Several of the aforementioned issues have already been reviewed in detail elsewhere because either they may be relevant to the use of other types of differentiating hiPSC derivatives for noncardiac regenerative medicine applications [27, 64] or they may deal with similar issues relevant to the use of hESC-CMs [66, 67]. We will further discuss a number of these issues.
Autologous patient-specific hiPSC-derived cardiac tissues are assumed to possess immune-privilege properties and thus may prove superior to hESC derivatives; however, a recent study challenged this assumption by describing the potential immunogenicity of teratomas generated from undifferentiated mouse iPSCs transplanted in syngeneic animals [68]. The resulting T-cell-dependent immune response was believed to result from the expression of new antigens in the mouse iPSCs as a consequence of reprogramming (e.g., Zg6, Hormad1, and Cyp3a11) [68]. More recent studies, however, revealed that differentiated cells derived from mouse iPSCs, in contrast to the undifferentiated cells, may not be rejected following transplantation [69]. Nevertheless, although encouraging, future studies will have to further investigate this important issue in greater detail.
The studies involving hiPSC-CMs to date have used fibroblasts or other cell types derived from either established cell lines or from healthy or young individuals. More recently, Zwi-Dantsis et al. [59] showed the ability to establish hiPSCs from elderly patients with advanced heart failure and multiple comorbidities (representing the candidate patient population for future hiPSC-based cell replacement strategies). Dermal fibroblasts from these patients were reprogrammed to generate hiPSCs by retroviral delivery of Oct4, Sox2, and Klf4 or by using an excisable polycistronic lentiviral vector. Both transgene-containing and transgene-free hiPSCs could later be differentiated into cardiomyocytes portraying similar early stage molecular, structural, and functional properties to those of hiPSC-CMs derived from healthy control foreskin fibroblasts. The generated hiPSC-CMs were able to couple electrically with cardiomyocytes in an in vitro coculture model and to engraft and integrate with host cardiac tissue following in vivo transplantation in the rat heart.
One of the remaining challenges in using hiPSC-CMs for regenerative medicine applications (as well as for cardiac disease modeling and drug discovery) is the phenotypic heterogeneity of the differentiating cardiomyocytes and their relatively immature phenotype. The cardiomyocytes obtained during hiPSC differentiation represent a mixed population of cells with atrial-, ventricular-, and nodal-like action potential morphologies or undetermined properties [25]. Moreover, although the differentiating cardiomyocytes were shown to possess human cardiomyocyte molecular, ultrastructural, metabolic, electrophysiological [25, 26, 70], and excitation-contraction coupling [71] properties, they were all shown to be relatively immature. Because clinical cell-therapy procedures would probably require engraftment of specific cell types (e.g., ventricular “working” cardiomyocytes for infarct repair) with more mature, adult-like, properties, future efforts would have to target both issues. A caveat to this approach is that early stage cardiomyocytes were demonstrated to survive significantly better in the in vivo heart following engraftment compared with mature adult cells [72]. Hence, achieving significant in vitro maturation prior to cell transplantation may actually hinder cell engraftment and survival.
Finally, a major hurdle identified in almost all cardiomyocyte transplantation studies in animal models is the relatively poor short- and long-term survival of the engrafted cardiomyocytes (<10%) within the infarcted region as well as the lack of appropriate alignment and maturation of the cell graft. Because several factors may contribute to the poor survival of the engrafted cells (e.g., initial cell washout, lack of supporting extracellular matrix [anoikis], lack of nonmyocyte supporting cells, and the harsh ischemic environment) a number of potential solutions targeting different mechanisms were suggested to decrease cell loss [73].
One of the more attractive solutions may lie in the emerging field of cardiac tissue engineering, which may allow targeting multiple mechanisms to prevent cell loss as well as controlling graft shape and size, yielding a more organized three-dimensional anisotropic muscle structure [74–76]. Consequently, hPSC-derived cardiomyocytes (hPSC-CMs) were already successfully engrafted to the heart as cell sheets [77, 78] or as cell-seeded fabricated scaffolds or were delivered in situ in hydrogel cell carriers [79]. Some of these studies also highlighted the importance of adding other cell types to the engineered cardiac tissue, such as fibroblasts or vascular progenitor cells (aiming to improve perfusion of the engineered tissue) [80, 81].
Direct Reprogramming
In contrast to the iPSC approach, which seeks to initially reprogram somatic cells to a pluripotent state followed by induction of differentiation of the generated hiPSCs to derive specific cell lineages, the recently described direct reprogramming strategies aim to directly convert the phenotype of one mature cell type (fibroblast) to another. The prototype for such a strategy was described many years ago by the demonstration that MyoD, a master regulator of skeletal muscle formation, can convert murine fibroblasts directly to skeletal muscle [82]. Nevertheless, developing a similar transdifferentiation strategy to achieve a cardiomyocyte fate seemed unlikely for many years because a single master regulatory gene does not exist in the cardiac lineage.
The first reports on using transcription factors to convert the identity of cells into a cardiomyocyte fate took place in the context of embryonic development. This was initially described in zebrafish embryos, in which overexpression of Gata5 was sufficient to generate ectopic regions of beating cardiomyocytes [83]. A similar finding was next described in developing Xenopus embryos using a combination of Gata5 and Gata4 [84] or MesP1 (a transcription factor associated with precardiac mesoderm) [85]. Achieving a similar cardiomyocyte fate in murine cultured embryos proved somewhat more difficult and required the expression of Gata4, Tbx5, and Baf60c to convert noncardiogenic mesoderm into contracting cardiomyocytes [86].
Taking the concept of transcription factor-based transdifferentiation a step further, Ieda et al. [14] were the first to successfully convert cells that were already terminally differentiated into cardiomyocyte-like cells. To this end, they used a similar strategy to Yamanaka in his pioneering iPSC work [13] and examined 14 different cardiac development-related transcription factors and epigenetic remodeling factors in an attempt to determine the optimal factor composition for reprogramming of mouse postnatal fibroblasts into cardiomyocytes [14]. Using the α-myosin heavy chain-green fluorescent protein reporter as a marker for the development of a cardiac fate, they showed that ectopic retroviral expression of three transcription factors Gata4, Mef2c, and Tbx5 (the “GMT” combination) was sufficient to convert isolated cardiac and dermal murine fibroblasts into induced cardiomyocyte-like (iCM-like) cells.
Since this important breakthrough, a number of laboratories have also reported experiences with in vitro direct reprogramming of fibroblasts to iCM-like cells (Table 1). Song et al. [87], for example, stressed the importance of adding a fourth transcription factor (Hand2) to the GMT combination, whereas Protze et al. [88] used myocardin, Mef2c, and Tbx5 to derive iCM-like cells. More recently, a microRNA-based approach emerged as an alternative approach to transcription factor-based reprogramming, and a combination of microRNAs (miR-1, miR-133, miR-208, and miR-499) delivered in combination with a chemical Janus kinase inhibitor was successful in achieving cardiomyocyte transdifferentiation [89]. Finally, a recent study also extended the direct reprogramming concept to neonatal and adult human fibroblasts [90]. To achieve this goal, a combination of four transcription factors (Gata4, Hand2, Tbx5, and myocardin) and two microRNAs (miR-1 and miR-133) was required. The generated human induced cardiomyocytes (iCMs) presented some degree of cardiac gene expression pattern; development of sarcomeric structures; calcium transients in the minority of cells; and, in a small fraction of cells (and only when human cardiac fibroblasts were used for reprogramming), spontaneous contraction.
Table 1.
Summary of the direct reprogramming strategies
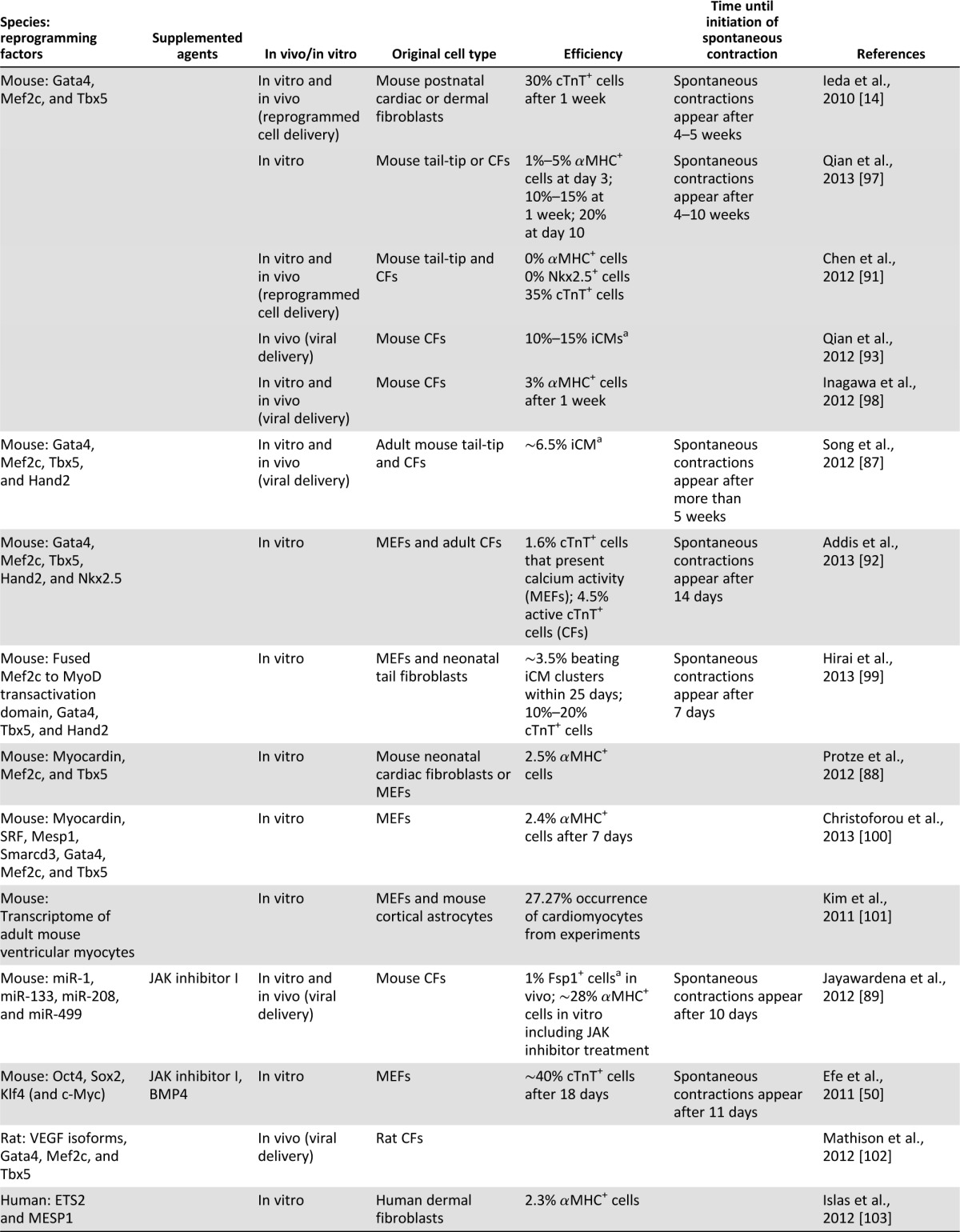
Despite the early success in converting murine fibroblasts to iCM-like cells, several questions and controversies remain regarding the mechanism involved in the reprogramming process, the exact cell type being reprogrammed, the efficiency of the process, and the phenotype of the reprogrammed iCMs (whether they truly represent bona fide cardiomyocytes). These controversies stem, in part, from the variability in the transcription factor combination used for reprogramming, the differences in the vectors used to deliver the transgenes, the variable expression levels of each individual transcription factor, the different sources of fibroblasts used for reprogramming, and the lack of specific fibroblast markers for optimal fate-mapping studies. Importantly, controversies also exist with regard to the definitions used to describe a resulting cardiomyocyte fate (whether it involves only the demonstration of the expression of some cardiac-specific genes at the RNA or protein levels or the activation of a reporter transgene or whether more stringent criteria are to be used such as the development of spontaneous beating or cardiac-specific action potentials and intracellular calcium transients).
The aforementioned differences may lead to variable endpoints. Chen et al., for example, did not find the GMT combination effective, and their lentiviral expression of these factors in adult murine fibroblasts induced only a minimally modified fibroblast phenotype, a partial cardiac gene-expression profile, and no functional cardiomyocytes [91]. More recently, Addis et al. [92] used more stringent criteria for the development of functional cardiomyocytes by reprogramming. Using a transgenic calcium fluorescent reporter driven by a cardiomyocyte-specific gene promoter, they noted that the optimal combination of transcription factors resulting in the most efficient generation of functional cardiomyocytes (identified as possessing spontaneous calcium transients) required adding Nkx2.5 to the combination of GMT and Hand2 [92].
The most exciting potential of cardiomyocyte transcription factor-based reprogramming, however, lies in the possibility of in vivo use of this technology, in which fibroblasts in the infarcted area, for example, may be the target for such cardiogenic reprogramming (Fig. 1). Two recent studies provided proof-of-concept evidence for the validity of this approach. Qian et al. [93] used retroviral delivery of the GMT combination to the murine infarct model, whereas Song et al. [87] added Hand2 to this combination. Newly formed cardiomyocytes appeared in the infarct zone in both studies and comprised 35% and 6% of the cardiomyocytes in the infarcted area, respectively. Lineage-tracing studies (using a Cre recombinase driven by one fibroblast promoter or more to permanently label cardiac fibroblasts and any cells to which they gave rise) suggested that the source of these new cardiomyocytes may be resident cardiac fibroblasts. Finally, in both cases, significant yet modest improvement in cardiac function was observed when compared with controls. Whether this functional improvement is the result of significant contribution to contractility by the transdifferentiated iCMs or indirect effects of the transcription factor delivery on other cellular elements in the infarct has yet to be determined.
Comparison of the Reprogramming Strategies; Future Perspectives
The advent of iPSC technology has provided a powerful tool for the emerging discipline of regenerative medicine. It also opened a new field in biology by highlighting the ability to dramatically alter cell fate through the use of transcription factors. This notion led to a number of studies describing the potential use of lineage-specific development-related transcription factors to convert the fate of one type of terminally differentiated cell to another (including converting fibroblasts into cardiomyocytes).
When comparing the ability to generate ex vivo cardiomyocytes for various applications through the differentiation of iPSCs versus direct reprogramming, it is clear that the former strategy is, by far, more advanced at this stage. This is evident from the marked differences in the efficiency of the cardiomyocyte-differentiation processes of the two strategies, from the potential ability to scale up the differentiation processes to yield clinically relevant number of cardiomyocytes, from the indisputable cardiomyocyte phenotype of the generated hiPSC-CMs, and from the potential to generate other relevant cell types for myocardial repair (e.g., vascular precursor cells). Nevertheless, direct reprogramming also possesses a number of theoretical advantages. These include the potential to significantly shorten the time required to derive the cardiomyocytes in an autologous manner and, importantly, the ability to minimize the risk for teratoma formation. Transcription factor-based transdifferentiation, however, is still at its infancy, and significant efforts will probably be required to close the gap between the two approaches.
Although the hiPSC strategy may currently be superior in producing ex vivo cardiomyocytes for disease modeling, drug development, cell therapy, and tissue engineering applications, the most significant advantage of direct reprogramming may lie in its potential for in vivo use. Consequently, in vivo delivery of the reprogramming factors directly to the heart has the potential to promote myocardial regeneration without the need for any type of cell transplantation, as already shown in initial proof-of-concept studies [87, 93]. This may resolve many of the challenges and shortcomings associated with myocardial cell therapies, as highlighted in this review.
In this regard, direct reprogramming may be more analogous to gene therapy, with the associated advantages, shortcomings, and challenges of this discipline. For transcription factor gene delivery, even more emphasis should be put on designing the appropriate vectors to be used (preferably vectors that do not result in genomic integration), the cell type targeted within the heart, and the magnitude and duration of the expression of the transgenes within the heart. The aforementioned proof-of-concept murine studies, for example, were performed by delivering the transcription factors during the acute stages of myocardial infarction. This setting may differ significantly from the more common chronic clinical scenario in both the type of pathological substrate encountered as well as the “state” of the cells targeted for gene delivery (proliferating fibroblasts in the more acute myocardial infarction stages that maybe transduced selectively using retroviruses vs. nonproliferating cells in the more chronic stages that would require the use of alternative vectors).
Finally, there is very limited information with regard to the potential to convert human fibroblasts into cardiomyocytes using transcription factor reprogramming. In contrast to iPSC technology, the combination of transcription factors required for such a task may differ from those used in the murine model. This may specifically hinder efforts to translate the exciting findings in the murine model to clinically relevant in vivo gene-delivery strategies. Consequently, significant in vitro and in vivo work might be necessary to compare fibroblasts from different large-animal models to human fibroblast in an attempt to determine the best biological model to be used to bridge the gap from animal studies to the clinic.
Conclusion
We have outlined the different reprogramming strategies that are being developed in the field of cardiac regenerative medicine. Although all strategies (iPSC approach, partial reprogramming, and direct reprogramming) have some inherent shortcomings, they all present exciting opportunities for this emerging discipline. In the past decade, we have witnessed the development of different clinical stem cell therapy strategies for heart repair [94, 95]. The vast majority of these clinical trials used adult stem cells (i.e., bone marrow-derived hematopoietic or mesenchymal stem cells) that were delivered to the heart through the coronary circulation in the setting of acute MI.
Although the aforementioned studies were instrumental in demonstrating the feasibility of bringing a stem cell therapy to the stage of late clinical trials, they also highlighted some important limitations: (a) These studies mostly targeted patients during the acute or recent stages of MI; (b) the stem cells used did not transform to generate new myocardial tissue, as originally believed (probably acting through a paracrine mechanism); (c) these cells were only transiently present at the site of delivery; and (d) the result of these studies led to only modest (or neutral) effects on myocardial performance.
Consequently, the huge number of patients with chronic heart failure (caused by dilated or ischemic cardiomyopathy) probably cannot be addressed to a significant extent by the use of the aforementioned cell types. For these patients, cells that could truly remuscularize the heart would probably be required. The most attractive candidate cell types for such a task could be the recently described CPCs [10] or the two strategies described in the current review: exogenously transplanted hPSC-CMs or the recently described directed reprogramming approach.
The aforementioned clinical need coupled with the improvements made in hPSC-CM differentiation and scaling-up processes (giving rise to clinically relevant numbers of cells in a cost-effective manner), the ongoing large-animal trials in a number of laboratories, and the emerging clinical trials using hESCs in other medical fields [96] provide room for optimism regarding the future clinical implementation of these strategies. Nevertheless, as discussed, several obstacles need to be overcome to fully harness the enormous research and clinical potential of these unique technologies.
Acknowledgments
We apologize to authors whose excellent works were not cited due to space restrictions. This work was supported in part by the Israel Science Foundation [1449/10], by the Melvin Berlin research fund for regenerative medicine, by the Lorry Lokey research fund, and by the Nancy and Stephen Grand Philanthropic Fund.
Author Contributions
I.B. and L.G.: conception and design, manuscript writing, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: Observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Ruvinov E, Dvir T, Leor J, et al. Myocardial repair: From salvage to tissue reconstruction. Expert Rev Cardiovasc Ther. 2008;6:669–686. doi: 10.1586/14779072.6.5.669. [DOI] [PubMed] [Google Scholar]
- 4.Garbern JC, Lee RT. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12:689–698. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 6.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 7.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 10.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 11.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He JQ, Ma Y, Lee Y, et al. Human embryonic stem cells develop into multiple types of cardiac myocytes: Action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 16.Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mummery C, Ward-van Oostwaard D, Doevendans P, et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 18.Lev S, Kehat I, Gepstein L. Differentiation pathways in human embryonic stem cell-derived cardiomyocytes. Ann N Y Acad Sci. 2005;1047:50–65. doi: 10.1196/annals.1341.005. [DOI] [PubMed] [Google Scholar]
- 19.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 21.Caspi O, Itzhaki I, Kehat I, et al. In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cells Dev. 2009;18:161–172. doi: 10.1089/scd.2007.0280. [DOI] [PubMed] [Google Scholar]
- 22.Dick E, Rajamohan D, Ronksley J, et al. Evaluating the utility of cardiomyocytes from human pluripotent stem cells for drug screening. Biochem Soc Trans. 2010;38:1037–1045. doi: 10.1042/BST0381037. [DOI] [PubMed] [Google Scholar]
- 23.Caspi O, Huber I, Kehat I, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 24.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zwi L, Caspi O, Arbel G, et al. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513–1523. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]
- 27.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itzhaki I, Maizels L, Huber I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 29.Lan F, Lee AS, Liang P, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12:101–113. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretti A, Bellin M, Welling A, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 31.Liang P, Lan F, Lee AS, et al. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation. 2013;127:1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mummery CL, Zhang J, Ng ES, et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview. Circ Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burridge PW, Keller G, Gold JD, et al. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lian X, Zhang J, Azarin SM, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amit M, Laevsky I, Miropolsky Y, et al. Dynamic suspension culture for scalable expansion of undifferentiated human pluripotent stem cells. Nat Protoc. 2011;6:572–579. doi: 10.1038/nprot.2011.325. [DOI] [PubMed] [Google Scholar]
- 38.Fluri DA, Tonge PD, Song H, et al. Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nat Methods. 2012;9:509–516. doi: 10.1038/nmeth.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steiner D, Khaner H, Cohen M, et al. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat Biotechnol. 2010;28:361–364. doi: 10.1038/nbt.1616. [DOI] [PubMed] [Google Scholar]
- 40.Niebruegge S, Bauwens CL, Peerani R, et al. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102:493–507. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- 41.Zwi-Dantsis L, Mizrahi I, Arbel G, et al. Scalable production of cardiomyocytes derived from c-Myc free induced pluripotent stem cells. Tissue Eng Part A. 2011;17:1027–1037. doi: 10.1089/ten.TEA.2010.0235. [DOI] [PubMed] [Google Scholar]
- 42.Elliott DA, Braam SR, Koutsis K, et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods. 2011;8:1037–1040. doi: 10.1038/nmeth.1740. [DOI] [PubMed] [Google Scholar]
- 43.Huber I, Itzhaki I, Caspi O, et al. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J. 2007;21:2551–2563. doi: 10.1096/fj.05-5711com. [DOI] [PubMed] [Google Scholar]
- 44.van Laake LW, Qian L, Cheng P, et al. Reporter-based isolation of induced pluripotent stem cell- and embryonic stem cell-derived cardiac progenitors reveals limited gene expression variance. Circ Res. 2010;107:340–347. doi: 10.1161/CIRCRESAHA.109.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Hoof D, Dormeyer W, Braam SR, et al. Identification of cell surface proteins for antibody-based selection of human embryonic stem cell-derived cardiomyocytes. J Proteome Res. 2010;9:1610–1618. doi: 10.1021/pr901138a. [DOI] [PubMed] [Google Scholar]
- 46.Dubois NC, Craft AM, Sharma P, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uosaki H, Fukushima H, Takeuchi A, et al. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One. 2011;6:e23657. doi: 10.1371/journal.pone.0023657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattori F, Chen H, Yamashita H, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 49.Tohyama S, Hattori F, Sano M, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Efe JA, Hilcove S, Kim J, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 51.Blin G, Nury D, Stefanovic S, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Laake LW, Passier R, Doevendans PA, et al. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102:1008–1010. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 53.Nelson TJ, Martinez-Fernandez A, Yamada S, et al. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada S, Nelson T, Kane G, et al. iPS cell intervention rescues wall motion disparity achieving biological cardiac resynchronization post-infarction. J Physiol. 2013 doi: 10.1113/jphysiol.2013.252288. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, Zhang F, Song G, et al. Intramyocardial injection of pig pluripotent stem cells improves left ventricular function and perfusion: A study in a porcine model of acute myocardial infarction. PLoS One. 2013;8:e66688. doi: 10.1371/journal.pone.0066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buccini S, Haider KH, Ahmed RP, et al. Cardiac progenitors derived from reprogrammed mesenchymal stem cells contribute to angiomyogenic repair of the infarcted heart. Basic Res Cardiol. 2012;107:301. doi: 10.1007/s00395-012-0301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mauritz C, Martens A, Rojas SV, et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur Heart J. 2011;32:2634–2641. doi: 10.1093/eurheartj/ehr166. [DOI] [PubMed] [Google Scholar]
- 58.Carpenter L, Carr C, Yang CT, et al. Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells Dev. 2012;21:977–986. doi: 10.1089/scd.2011.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zwi-Dantsis L, Huber I, Habib M, et al. Derivation and cardiomyocyte differentiation of induced pluripotent stem cells from heart failure patients. Eur Heart J. 2013;34:1575–1586. doi: 10.1093/eurheartj/ehs096. [DOI] [PubMed] [Google Scholar]
- 60.Gepstein L, Ding C, Rahmutula D, et al. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies. Stem Cells. 2010;28:2151–2161. doi: 10.1002/stem.545. [DOI] [PubMed] [Google Scholar]
- 61.Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 62.Shiba Y, Fernandes S, Zhu WZ, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roell W, Lewalter T, Sasse P, et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 64.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 66.Habib M, Caspi O, Gepstein L. Human embryonic stem cells for cardiomyogenesis. J Mol Cell Cardiol. 2008;45:462–474. doi: 10.1016/j.yjmcc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Shiba Y, Hauch KD, Laflamme MA. Cardiac applications for human pluripotent stem cells. Curr Pharm Des. 2009;15:2791–2806. doi: 10.2174/138161209788923804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 69.Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 70.Ma J, Guo L, Fiene SJ, et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Itzhaki I, Rapoport S, Huber I, et al. Calcium handling in human induced pluripotent stem cell derived cardiomyocytes. PLoS One. 2011;6:e18037. doi: 10.1371/journal.pone.0018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reinecke H, Zhang M, Bartosek T, et al. Survival, integration, and differentiation of cardiomyocyte grafts: A study in normal and injured rat hearts. Circulation. 1999;100:193–202. doi: 10.1161/01.cir.100.2.193. [DOI] [PubMed] [Google Scholar]
- 73.Robey TE, Saiget MK, Reinecke H, et al. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segers VF, Lee RT. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109:910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 75.Vunjak-Novakovic G, Lui KO, Tandon N, et al. Bioengineering heart muscle: A paradigm for regenerative medicine. Annu Rev Biomed Eng. 2011;13:245–267. doi: 10.1146/annurev-bioeng-071910-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmermann WH. Embryonic and embryonic-like stem cells in heart muscle engineering. J Mol Cell Cardiol. 2011;50:320–326. doi: 10.1016/j.yjmcc.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 77.Kawamura M, Miyagawa S, Miki K, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126(suppl 1):S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 78.Miki K, Uenaka H, Saito A, et al. Bioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in rats. Stem Cells Translational Medicine. 2012;1:430–437. doi: 10.5966/sctm.2011-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Habib M, Shapira-Schweitzer K, Caspi O, et al. A combined cell therapy and in-situ tissue-engineering approach for myocardial repair. Biomaterials. 2011;32:7514–7523. doi: 10.1016/j.biomaterials.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 80.Lesman A, Habib M, Caspi O, et al. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010;16:115–125. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 81.Stevens KR, Kreutziger KL, Dupras SK, et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci USA. 2009;106:16568–16573. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 83.Reiter JF, Alexander J, Rodaway A, et al. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13:2983–2995. doi: 10.1101/gad.13.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Latinkić BV, Kotecha S, Mohun TJ. Induction of cardiomyocytes by GATA4 in Xenopus ectodermal explants. Development. 2003;130:3865–3876. doi: 10.1242/dev.00599. [DOI] [PubMed] [Google Scholar]
- 85.David R, Brenner C, Stieber J, et al. MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat Cell Biol. 2008;10:338–345. doi: 10.1038/ncb1696. [DOI] [PubMed] [Google Scholar]
- 86.Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song K, Nam YJ, Luo X, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Protze S, Khattak S, Poulet C, et al. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol. 2012;53:323–332. doi: 10.1016/j.yjmcc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 89.Jayawardena TM, Egemnazarov B, Finch EA, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nam YJ, Song K, Luo X, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci USA. 2013;110:5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen JX, Krane M, Deutsch MA, et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:50–55. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Addis RC, Ifkovits JL, Pinto F, et al. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J Mol Cell Cardiol. 2013;60:97–106. doi: 10.1016/j.yjmcc.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qian L, Huang Y, Spencer CI, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanganalmath SK, Bolli R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: Promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–1206. doi: 10.1093/eurheartj/ehr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trounson A, Thakar RG, Lomax G, et al. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qian L, Berry EC, Fu JD, et al. Reprogramming of mouse fibroblasts into cardiomyocyte-like cells in vitro. Nat Protoc. 2013;8:1204–1215. doi: 10.1038/nprot.2013.067. [DOI] [PubMed] [Google Scholar]
- 98.Inagawa K, Miyamoto K, Yamakawa H, et al. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:1147–1156. doi: 10.1161/CIRCRESAHA.112.271148. [DOI] [PubMed] [Google Scholar]
- 99.Hirai H, Katoku-Kikyo N, Keirstead SA, et al. Accelerated direct reprogramming of fibroblasts into cardiomyocyte-like cells with the MyoD transactivation domain. Cardiovasc Res. 2013;100:105–113. doi: 10.1093/cvr/cvt167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Christoforou N, Chellappan M, Adler AF, et al. Transcription factors MYOCD, SRF, Mesp1 and SMARCD3 enhance the cardio-inducing effect of GATA4, TBX5, and MEF2C during direct cellular reprogramming. PLoS One. 2013;8:e63577. doi: 10.1371/journal.pone.0063577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim TK, Sul JY, Peternko NB, et al. Transcriptome transfer provides a model for understanding the phenotype of cardiomyocytes. Proc Natl Acad Sci USA. 2011;108:11918–11923. doi: 10.1073/pnas.1101223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mathison M, Gersch RP, Nasser A, et al. In vivo cardiac cellular reprogramming efficacy is enhanced by angiogenic preconditioning of the infarcted myocardium with vascular endothelial growth factor. J Am Heart Assoc. 2012;1:e005652. doi: 10.1161/JAHA.112.005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Islas JF, Liu Y, Weng KC, et al. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc Natl Acad Sci USA. 2012;109:13016–13021. doi: 10.1073/pnas.1120299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wada R, Muraoka N, Inagawa K, et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci USA. 2013;110:12667–12672. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]