This study used human junctional adhesion molecule A (JAM-A)-modified human mesenchymal stem cells (MSCs) to repair hair follicle (HF) abnormalities in BALB/c nu/nu mice. Results indicated that JAM-Aov MSCs improved hair formation in nude mice through HF structure remodeling.
Keywords: Mesenchymal stem cells, Cell adhesion molecules, Cell transplantation, Differentiation
Abstract
The junctional adhesion molecule A (JAM-A) has been shown to serve a crucial role in the proliferation, differentiation, and tube-like formation of epithelial cells during angiogenesis. The role of JAM-A in hair follicle (HF) regeneration has not yet been reported. In this study, we used human JAM-A-modified human mesenchymal stem cells (MSCs) to repair HF abnormalities in BALB/c nu/nu mice. The JAM-A gene and JAM-A short hairpin RNA were transfected into cultured human MSCs to generate the JAM-A overexpression MSCs (JAM-Aov MSCs) and JAM-A knockdown MSCs (JAM-Akd MSCs), respectively. These cells were injected intradermally into the skin of nude mice during the first telogen phase of the HF that occurs 21 days postnatally. We found that JAM-Aov MSCs migrated into the HF sheath and remodeled HF structure effectively. The HF abnormalities such as HF curve and HF zigzag were remodeled, and hair formation was improved 7 days following injection in both the JAM-Aov MSC and MSC groups, compared with the JAM-Akd MSC group or negative control group. Furthermore, the JAM-Aov MSC group showed enhanced hair formation in contrast to the MSC group, and the number of curved and zigzagged HFs was reduced by 80% (p < .05). These results indicated that JAM-Aov MSCs improved hair formation in nude mice through HF structure remodeling.
Introduction
Mesenchymal stem cells (MSCs) are multipotent stem cells that can differentiate into a variety of cell types. Recent advances in stem cell research have suggested that MSCs, in fact, have therapeutic potential for tissue repair and organ defects, including genetic disorders, because of their multipotent differentiation abilities and the secretion of a variety of cytokines and growth factors [1]. In our previous study, MSCs had the potential to contribute to the development of hair follicles (HFs) in nude mice in vivo and differentiate into epithelial cells in vitro [2, 3], although the mechanism is unclear.
The development and structure of the HF were demonstrated by Chase and Eaton [4] a half century ago. The HF consists of several concentric epithelial sheaths with the outer root sheath (ORS) forming the outermost layer [5]. In contrast to all other mammalian organs, the hair undergoes continuous cycling throughout adult life [6–9]. In the mouse, a complete hair cycle lasts approximately 25 days and can be divided into three phases: anagen, catagen, and telogen [10]. The 21-day-old mice are in the first telogen stage of the hair cycle [10].
The most striking feature of Foxn1nu/Foxn1nu mice is their lack of fur development [11]. The dorsal skin of mice homozygous for any of the reported allelic mutations in the nude gene is hairless [12, 13]. For nude mice, the hair shafts bend and coil as soon as they enter the hair canal [14]. Histological analyses revealed an increase in epithelial cell proliferation in the hair follicles of nude mice [15]. The hair shaft (HS) bends as it loses support from the hair root sheath [16]. In nude mice, hair growth continues to follow the juvenile pattern with a strict hair growth wave [17].
A considerable number of molecules directly participate in HF development, such as transcription factors and cell growth factors [1, 14, 15]. The role of cell adhesion molecules in HF regeneration and development has been reported too [18]. However, little is known about the role of junctional adhesion molecules during HF development and regeneration.
Junctional adhesion molecule A (JAM-A) is mainly expressed in the tight junctions (TJ) [19, 20]. The dual function of JAM-A is to regulate leukocyte/endothelial cell interactions in the immune system and to form TJ in epithelial cells, which plays a key role in the establishment of cell polarity [21].
Thus, it was thought that overexpression of JAM-A would inhibit endothelial cell migration by enhancing cell-cell contacts [22, 23]. However, previous work has shown that JAM-A overexpression facilitated epithelial cell migration and formation of distinct tube-like structures [24]. The study performed by Thomas et al. [25] has provided new insight into the contribution of JAM-A during epithelial differentiation.
Human MSCs can induce and participate in HF regeneration in nude mice [2, 3]. JAM-A overexpression induces cell migration and tube-like structure formation [24]. Additionally, JAM-A contributes to epithelial cell differentiation and cell polarity [26]. Based on the above rationale, we raise a hypothesis that MSC with JAM-A overexpression can ameliorate nude mice HF abnormality, such as HS bends.
So, we prepared MSCs that overexpressed JAM-A (JAM-Aov MSCs) and MSCs that knocked down JAM-A by RNA interference (JAM-Akd MSCs). We then transplanted these cells into the skin of nude mice, the cells were suspended in 150 μl phosphate-buffered solution (PBS), and equivalent volumes of PBS were injected as negative control. Consistent with our hypothesis, we found hair formation in the nude mice of the JAM-Aov group and MSC group. Tracing experiments revealed that JAM-Aov MSCs effectively migrated into the hair root sheath and strengthened the HF. Morphology results revealed that HF structure in JAM-Aov group mice was remodeled compared with the MSC group and the JAM-Akd MSC group; a decrease in the bending and coiling of the HS and HF was observed in the JAM-Aov MSC group.
Taken together, these data demonstrate that JAM-A overexpression facilitates MSC migration into the HF sheath and remodeling HF structure. JAM-Aov MSCs promote HF architecture reformation and maintain the shape of the HS and HF.
Materials and Methods
Cell Cultures and Intracutaneous Injection
Cultivation of MSCs was from 4–6-week embryo limb bud and somite. The embryo skin was discarded carefully. The MSCs were grown as described previously and stored in the Department of Histology and Embryology of the Second Military Medical University [2, 3]. MSCs involved in the formation of the hair follicle were examined in a previous study [2, 3].
Engraftment was initiated by intracutaneous injection of 2 × 104 MSCs. Before engraftment, the specific markers on the cell surface had been determined by FACS analysis; the expression of specific antigen, such as CD29, CD44, CD90, and CD106, had been determined. Meanwhile, the negative expression of CD34 and CD45 had been determined too (data not shown). All of these antibodies were purchased from BD Pharmingen (San Jose, CA, http://www.bdbiosciences.com).
The intracutaneous injection site was located on the dorsal skin of the 3-week-old BALB/c nu/nu mice. Histological analysis of male BALB/c nu/nu mouse skin was performed using hematoxylin and eosin staining (Richard-Allan Scientific, Kalamazoo, MI, http://www.rallansci.com). We macroscopically observed hair growth at the implantation area every day following the injection.
Vector Design and Transfection
Insertion of the full-length JAM-A gene into the pEGFPN1 expression vector was described previously [27]. All of the JAM-A-pEGFPN1 plasmid transfections were performed using FuGene 6 (catalog no. 1181509100; Roche, Indianapolis, IN, http://www.roche-applied-science.com) in medium, according to the manufacturer’s protocol (DNA:FuGene 6 ratio = 1 μg:3 μl). Pools of stably transfected cells between passages 5 and 9 were used. The level of JAM-A expression was determined by real-time PCR.
The 21-nucleotide JAM-A target sequence 5′-AAAGATGGGATAGTGATGCCT-3′ was reported previously [28]. A GCsilencerTMU6/Neo/GFP/JAM-A-shRNAi plasmid was obtained from Shanghai Genechemat Corp (Shanghai, China, http://www.genechem.com.cn). The recombinant vector was successfully constructed and confirmed by sequence analysis. All of the short hairpin RNA (shRNA) transfections were performed using FuGene 6 in medium according to the manufacturer’s protocol with a final shRNA plasmid (DNA:FuGene 6 ratio = 1 μg:3 μl). EGFPN1 plasmid was transfected into MSCs and served as the negative controls. These cells were named green fluorescent protein (GFP)-MSCs.
Transwell Migration Assay
Migration assays were performed in a six-well Transwell plate containing polycarbonate membranes with 8-μm pores (Corning Costar, Cambridge, MA, http://www.corning.com/lifesciences). GFP-MSCs, JAM-Akd MSCs, and JAM-Aov MSCs at a density of 6 × 105 cells per milliliter in 1000 μl of media were placed in the upper chamber of the Transwell assembly system. After incubation for 10 hours, the upper surface of the membrane was scraped gently to remove nonmigrating cells and washed with phosphate-buffered saline. The membrane was then fixed in 4% paraformaldehyde for 15 minutes and stained with 0.5% crystal violet for 10 minutes. The number of migrating cells was determined by counting five random fields per well under the microscope at ×100 magnification. This was repeated three times.
Animals and Tissue Collection
The studies were approved by the Institutional Animal Care and Use Committee of the Second Military Medical University (Shanghai, China, certificate: SYXK2007-0003 [Shanghai]). Male BALB/c nu/nu mice, 21 days of age and 16–20 g in weight, were randomly assigned to four groups; 15 animals were used for each group. The groups were the PBS injection group, the JAM-Aov MSC group, the JAM-Akd MSC group, and the GFP-MSC group. The skin tissue at the injected site was harvested at 1, 3, 7, 10, and 30 days after intracutaneous injection. All of the tissues were fixed in 10% formalin at room temperature for 12 hours and then embedded in paraffin. The alteration of skin tissue was observed by hematoxylin and eosin staining.
Immunofluorescence
Immunohistochemical analysis was performed, as previously described. The tissues to be fixed and processed were cut to a size no larger than 5 mm in thickness for indirect immunofluorescence and immunohistochemistry. Green fluorescent of GFP was degradation in the process of paraffin section. Microtome-sectioned tissues were stained with mouse anti-mitochondrial antibody (Mit) (ab3298; Abcam, Cambridge, U.K., http://www.abcam.com), rabbit anti-keratin 19 (K19) (ab15463; Abcam), rabbit anti-p-FAK (ab38512; Abcam), goat anti-p-protein kinase C (PKC)ζ (sc-12894-R; Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com), and goat anti-JAM-A (sc-17427; Santa Cruz Biotechnology), respectively, in PBS containing 1% bovine serum albumin (BSA) overnight at 4°C. The sections were washed three times over 10 minutes and incubated with a 1:500 dilution of fluorescein-conjugated secondary antibody (donkey anti-goat, A11055; goat anti-rabbit, A11007; donkey anti-mouse, A21203; donkey anti-goat, A11058; 1:500, Invitrogen, Barcelona, Spain, http://www.invitrogen.com) in PBS containing 1% BSA overnight at 4°C. Before mounting, microtome sections were stained with 4′,6-diamidino-2-phenylindole (sc-3598; Santa Cruz Biotechnology). The results were studied by confocal microscopy using an argon-krypton laser and a Leica (Heerbrugg, Switzerland, http://www.leica.com) TCS-4D confocal imaging system.
Statistical Analysis
The results were expressed as the mean ± SD and analyzed by a paired analysis of variance. p values are described in the figures, and p < .05 was considered statistically significant.
Results
JAM-A Transgene Expression Enhanced MSC Migration
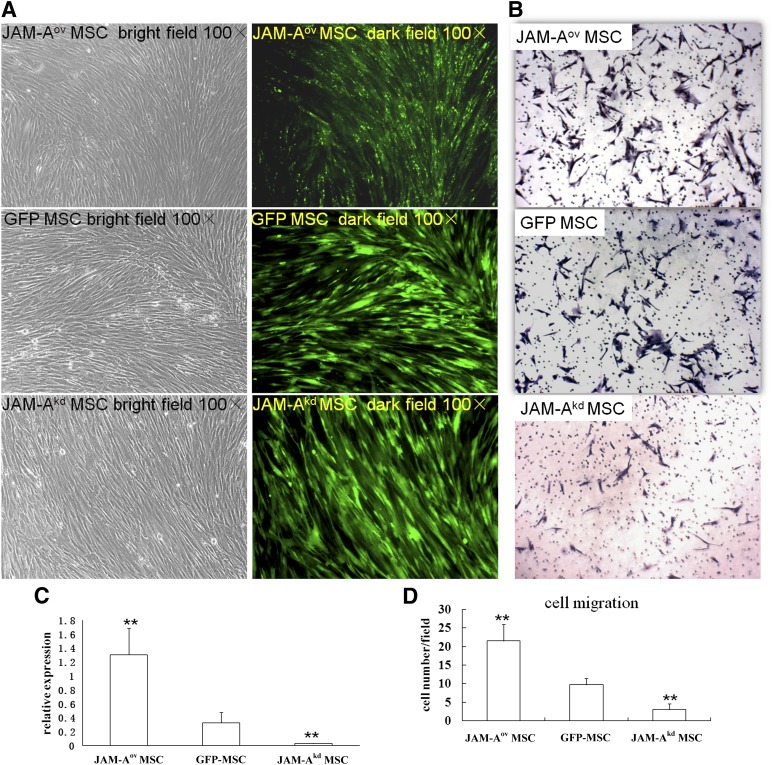
JAM-A expression was upregulated in JAM-A-pEGFPN1 plasmid-transfected MSCs and downregulated in JAM-A shRNA-transfected MSCs (Fig. 1A). Meanwhile, the expression of GFP was monitored using a fluorescent microscope. JAM-A mRNA expression was analyzed by quantitative real-time PCR at different time points (Fig. 1C). Endogenous JAM-A was significantly downregulated in JAM-A shRNA-transfected MSCs, as shown in Figure 1C. We transiently expressed those constructs and engrafted the transfected MSCs (JAM-Aov MSCs, JAM-Akd MSCs, and GFP-MSCs) into nude mice at 48 hours post-transfection.
Figure 1.
Regulation of JAM-A expression in MSC and JAM-A overexpression promoted MSC migration in vitro. (A): A high fluorescence signal was observed after gene transduction. Bright punctiform green fluorescence was observed in JAM-Aov MSCs. In all images, magnification is ×100. (C): JAM-A mRNA levels were confirmed by real-time polymerase chain reaction 48 hours after gene transduction. Marked upregulation of JAM-A expression was detected in JAM-Aov MSCs, whereas low levels of JAM-A expression were observed in JAM-Akd MSCs. ∗∗, p < .01. (B, D): In vitro migration results showed that overexpression of JAM-A enhanced the migration of MSCs. JAM-Aov MSCs significantly increased the number of migrating cells. In all images, magnification is ×100. ∗∗, p < .01. Abbreviations: GFP, green fluorescent protein; JAM-A, junctional adhesion molecule A; MSC, mesenchymal stem cell.
An in vitro migration assay showed that overexpression of JAM-A enhanced the migration of MSCs (Fig. 1B, 1D). The number of migrating JAM-Aov MSCs was more than threefold greater than the number of migrating untransduced MSCs (p < .05) (Fig. 1D), thereby indicating that overexpression of JAM-A enhances the ability of MSCs to migrate. The experiment was repeated three times, with similar results.
JAM-Aov MSCs Promoted Hair Formation in the Skin of Nude Mice
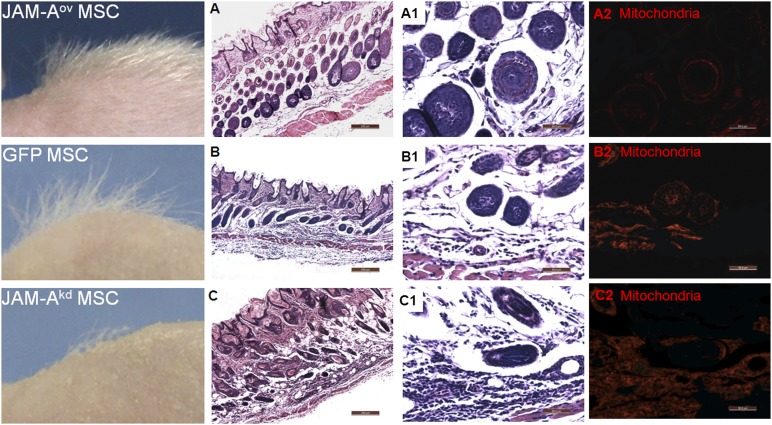
Transfected MSCs were intradermally injected into the dorsal skin of postnatal day 21 nude mice, which was during the first telogen phase of the HF. The number of dorsal hair observed in the JAM-Aov MSC group was significantly greater than the other groups (Figs. 2, 3A). In Figure 2, donor cells labeled with anti-mitochondria antibody show mitochondrial staining in nude mice skin tissue. Hair density, hair length, and hair diameter in the three groups were dissected and assayed. Statistical results suggested that the hair quality of the JAM-Aov MSC group was better than that of the other groups (Figs. 2, 3).
Figure 2.
JAM-A overexpression promoted hair formation in the skin of nude mice. Left column: hair formation in the skin of nude mice. The hair quality in the JAM-Aov MSC group of mice was better than the other groups. (A–C): The number of twisted hair follicles (HFs) in the JAM-Aov MSC group was decreased compared with other groups. A positive signal of mitochondria was observed in three groups. Scale bars = 200 μm (A–C). (A1, B1, C1): Hair diameter in the JAM-Aov MSC group was significantly greater than in the other groups. Meanwhile, on the horizontal plane of the HF in the JAM-Aov MSC group, the hair root is organized into concentric circles of cells from outer to inner. In the JAM-Akd MSC group, the graft could be observed clearly (C1, C2). (C): The funnel-shaped mouths of hair follicles in the skin of JAM-Akd MSC injection mice were ampliate. (A1, A2): JAM-Aov MSCs migrated to HF. (B1, B2): GFP MSCs migrated to HF. The graft could be observed clearly too. But the number of graft under the same amplification was more than in the JAM-Aov MSC group. Scale bars = 50 μm (A1, A2, B1, B2, C1, C2). Abbreviations: GFP, green fluorescent protein; JAM-A, junctional adhesion molecule A; MSC, mesenchymal stem cell.
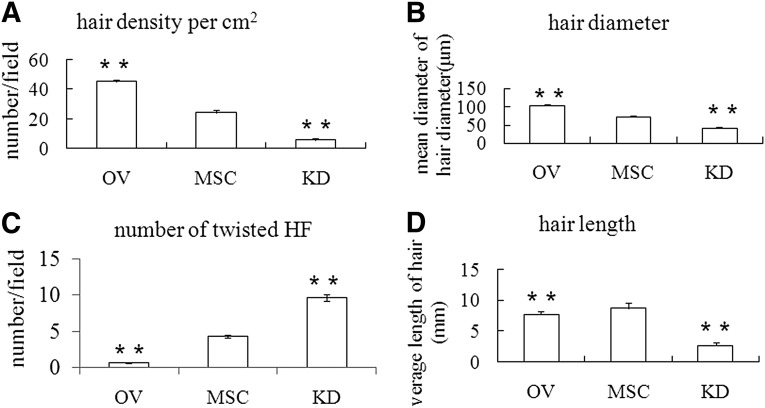
Figure 3.
Overexpression of junctional adhesion molecule A correlates with HF remodeled and hair formation. Hair density (A), hair length (D), the number of twisted HF (C) and hair diameter (B) in the three groups were dissected and assayed. Statistical results revealed a marked difference among the three groups. ∗∗, p < .01. Abbreviations: HF, hair follicle; KD, knockdown; MSC, mesenchymal stem cell; OV, overexpression.
Histological analysis of the skin from nude mice at different stages following engraftment was performed. Seven days after injection, the HF structure was more mature, which was associated with enhanced hair growth in the JAM-Aov MSC group (Fig. 2). The increase of JAM-A expression correlates with remarkable hair formation in nude mice (Fig. 2; supplemental online Fig. 1). In the JAM-Aov MSC group, the number of twisted HF decreased compared with the other groups (Figs. 2A, 2C, 3C). These results indicated that increased expression of JAM-A can result in various levels of hair induction in nude mice.
JAM-Aov MSCs Contribute to HF Structure Remodeling
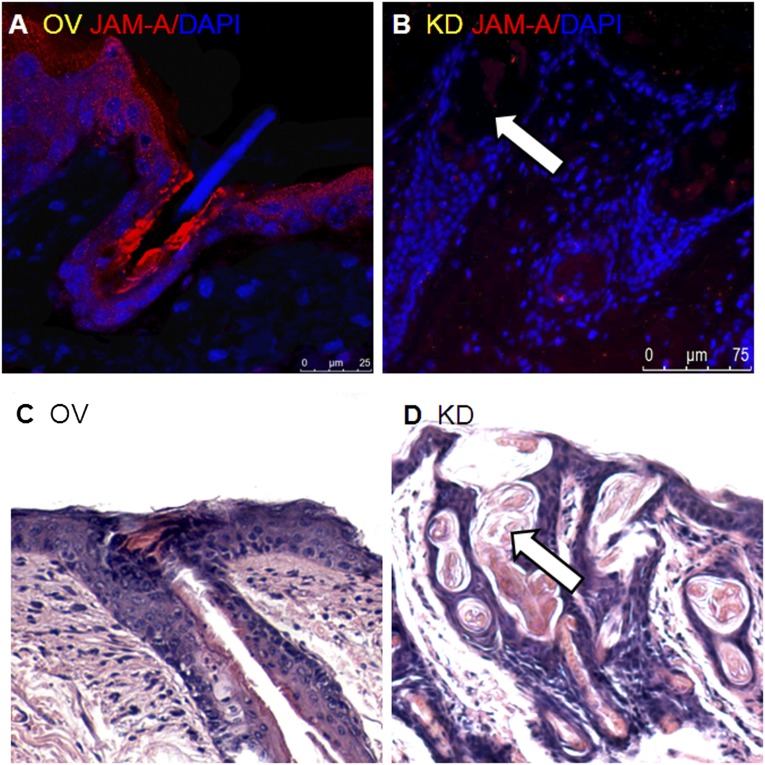
Fourteen days after cell injection, in the junctional zone of the HF and epidermis, high expression of JAM-A was detected and a developing hair canal was observed (Fig. 4A, 4C). In this result, JAM-A expression was associated with the maintenance of an unobstructed hair canal and the growth of the hair root toward the outside. In the JAMAkd MSC injection group, the HF was twisted, the infundibulum was dilated, and the hair root was curled over (Fig. 4B, 4D). The experimental result was different from previous reports [14, 16]. In BALB/c nu/nu mice, JAM-Aov MSC injection led to HF structural change.
Figure 4.
Overexpression of JAM-A correlates with maintenance of funnel tube shape. (A, C): In JAM-Aov mesenchymal stem cell (MSC) injection mice, the funnel-shaped mouths of hair follicles were small and straight. Straight hair root and hair shaft had been seen clearly in the upright funnel tube in JAM-Aov MSC injection mice. JAM-A expression was clearly increased in JAM-Aov MSC injection mice. (B): JAM-A expression was weak in JAM-Akd MSC injection mice (white arrow). (D): Twisted hair root was clearly observed in the ampliate funnel tube in JAM-Akd MSC injection mice (white arrow). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; JAM-A, junctional adhesion molecule A; KD, knockdown; OV, overexpression.
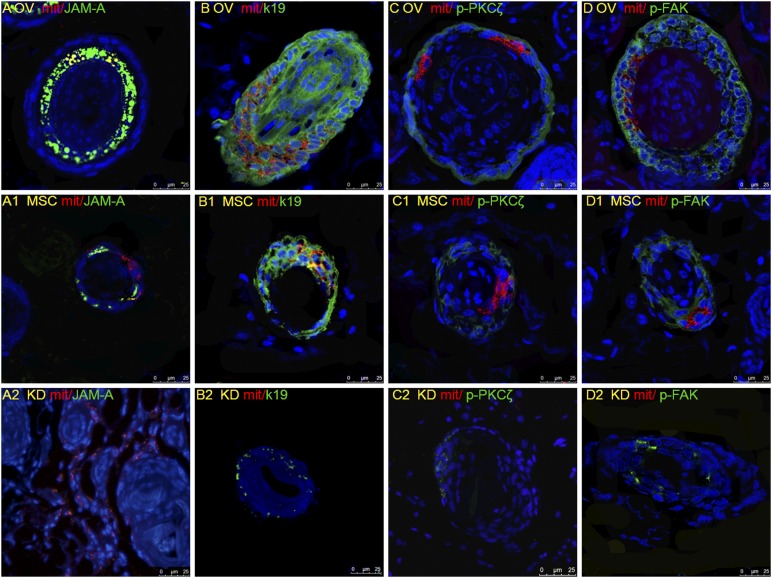
On day 7 after cell injection, to examine how JAM-Aov MSCs in nude mice induce hair formation, we performed immunostaining for mitochondria and JAM-A, K19, p-PKCζ, and p-FAK. The result of mitochondria distribution showed that JAM-Aov MSCs were migrated to the infracted root sheath (Fig. 5A, 5A1, 5A2). JAM-Aov MSCs primarily homed to the HF 7 days after injection (Fig. 5A). A small percentage of JAM-Akd MSCs homed to HF (Fig. 5A2).
Figure 5.
Overexpression of junctional adhesion molecule A (JAM-A) correlates with maintenance of root sheath shape in the skin of nude mice. The skin from experimental day 7 was paraffin embedded and sectioned along the cephalocaudal axis. Green signal represents the positive signal of JAM-A, K19, p-FAK, and p-PKCζ separately. Red signal represents the positive signal of mitochondria, indicating the localization of the engrafted MSCs, which were also 4′,6-diamidino-2-phenylindole stained. (A–D): The increased expression of JAM-A, K19, p-PKCζ, and p-FAK in JAM-Aov MSC injection mice, associated with HF structure improvement. (A1, B1, C1, D1): The expression of JAM-A, K19, p-PKCζ, and p-FAK in MSC injection mice. Hair follicles in this group were smaller than in the JAM-Aov MSC group. (A2, B2, C2, D2): The decreased expression of JAM-A, K19, p-PKCζ, and p-FAK in JAM-Akd MSC injection mice. HF structure was frizzy in this group. Abbreviations: KD, knockdown; MSC, mesenchymal stem cell; OV, overexpression; PKC, protein kinase C.
On day 7 after cell injection, mitochondria and JAM-A double-positive cells were found, especially in the HF sheathes of JAM-Aov MSC and GFP-MSC groups (Fig. 5A, 5A1). On the horizontal plane of the HF in the JAM-Aov MSC group, we can observe that the hair root is organized into concentric circles of cells from outer to inner (Figs. 2A2, 5A).
Compared with the GFP-MSC group and the JAM-Akd MSC group, the expression of p-PKCζ and p-FAK was increased in JAM-Aov MSC group (Fig. 5C, 5C1, 5D, 5D1). The decreased expression of p-PKCζ and p-FAK in the JAM-Akd MSC group was remarkable (Fig. 5C2, 5D2). The increased expression of p-PKCζ and p-FAK is perhaps a great aid in unblocking hair canal. These results indicated that JAM-A expression can enhance MSC participation in HF formation, promote HF structure remodeling, and induce hair regeneration. The successful movement of JAM-Aov MSC to HF sheathes is partly explicable by the function of JAM-A promoting cell migration, as previously reported [24].
Discussion
In the mouse, a complete hair cycle lasts approximately 25 days and can be divided into three phases: anagen, catagen, and telogen [17]. Topical, oral, or parenteral administration of the immunosuppressive immunophilin ligand cyclosporine A induces macroscopically visible hair growth in nude mice [29]. Synthetic analogs of vitamin D3 (calcitriol) also stimulate hair growth in nude mice [30]. Few studies evaluating the role of stem cells in the induction of hair growth have been reported.
Ultrastructural analyses revealed that the cuticle of the inner root sheath (IRS) and the cuticle of the HS are filled with abnormal globular aggregates, the hair cortex is fragmented into irregular cornified material, and the hair medulla is partially lacking [31]. The HS bends as it loses support from the IRS and ORS [16]. Proliferation of stratified squamous epithelium in the root sheath is a compensatory response to lateral hair shaft penetration [32].
In our study, hair formation was observed in the skin of BALB/c nu/nu mice, which was an interesting finding. During the process of HF formation, noticeable morphology alterations occurred. Our present study has now uncovered a crucial role for JAM-A in the migration of MSCs into the hair root sheath and the formation of hair in the skin of nude mice.
JAM-Aov MSCs Remodeled the Root Sheath Structure of BALB/c nu/nu Mice
It is well known that the hair shaft penetrates through the epidermis and through the hair canal that is formed at the distal portion of the HF epithelium [33]. In nude mice, the HS bends as it loses support from the root sheath, causing the hair canal to be filled with a distorted hair shaft [16]. Therefore, for the formation of hair in nude mice, root sheath reconstruction is essential.
These results suggested that JAM-A overexpression in MSCs facilitated cell homing to the HF and helped to maintain the shape of the ORS. The relationship between the expression level of JAM-A and the number of cells that migrated into the ORS needs to be studied further.
JAM-A contains a canonical type-II, PDZ-domain-targeting motif that mediates binding to several proteins, such as ZO-1, p-PKCζ, and p-FAK [34, 35]. JAM-A overexpression in the epithelium resulted in the formation of distinct tube-like structures in a three-dimensional Matrigel assay [24]. P-PKCζ and p-FAK expression are essential for cell migration and rearrangement [34, 35]. In this study, JAM-A overexpression promoted the migration of MSCs into the root sheath.
The reconstructed HF in the JAM-Aov MSC group was remarkable. The ORS, IRS, and hair root showed an ordered shape. By maintaining the proper structure of the ORS and IRS, it is possible to keep the hair canal unblocked.
Analysis of Hair Formation in the Skin of Nude Mice
Seven days after cell transplantation, short and floppy hair appeared around the JAM-Aov MSC and MSC injection sites. The newly formed hair in the JAM-Aov MSC group was denser compared with the other groups. Fourteen days after cell transplantation, twisted HFs were counted in three groups of mice. There was a noticeable difference between the three groups. Meanwhile, hair density, hair diameter, and hair length were detected and analyzed.
Long-term observations revealed that a strict hair cycle of 23 days per cycle developed in the JAM-Aov MSC group. Dense hair formation on the dorsal skin remained for approximately 5 days and then disappeared rapidly.
In addition, using the same engraftment conditions, minor hair formation was observed when the cells were injected into the dorsal skin of a forelimb (supplemental online Fig. 2). This result was consistent with the previous report that the dorsal skin of the forelimb was beneficial for hair formation [12, 13].
Meanwhile, we detected K19 expression in the HFs of JAM-Aov MSC mice. K19, a biochemical marker of skin stem cells in vivo and in vitro, indicates intermediate phenotypes from stromal cells to luminal epithelial cells [36, 37]. The results suggest that JAM-Aov MSCs promote epithelial cell differentiation in the HF.
Does JAM-A overexpression maintain hair canal shape or remodel the structure of HF abnormalities in nude mice? In previous reports, the adhesion of JAM-A to vitronectin is hindered in JAM-A-depleted cells, thus hindering their ability to migrate [24].
Conclusion
Intracutaneous injection of JAM-Aov MSCs into BALB/c nu/nu mice can induce hair formation in the skin of the mice. The overexpression of JAM-A in MSCs promotes cell trafficking to the hair root sheath and helps to remodel HF structure. These results suggest that JAM-A overexpression promotes MSC homing to the root sheath and promotes hair formation in the skin of nude mice.
Supplementary Material
Acknowledgments
This work was supported by National 973 Project of China (2011CB965101) and National Natural Science Foundation of China (81301649).
Author Contributions
M.W. and X.G.: collection and assembly of data, data analysis and interpretation, manuscript writing; L.Y., Y.W., and Y.T.: collection and assembly of data; Y.Y.: data analysis and interpretation; H.L.: conception and design.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Undale AH, Westendorf JJ, Yaszemski MJ, et al. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin Proc. 2009;84:893–902. doi: 10.4065/84.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu M, Yang L, Liu S, et al. Differentiation potential of human embryonic mesenchymal stem cells for skin-related tissue. Br J Dermatol. 2006;155:282–291. doi: 10.1111/j.1365-2133.2006.07357.x. [DOI] [PubMed] [Google Scholar]
- 3.Wu M, Sun Q, Guo X, et al. hMSCs possess the potential to differentiate into DP cells in vivo and in vitro. Cell Biol Int Rep (2010) 2012;19:e00019. doi: 10.1042/CBR20120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chase HB, Eaton GJ. The growth of hair follicles in waves. Ann N Y Acad Sci. 1959;83:365–368. doi: 10.1111/j.1749-6632.1960.tb40912.x. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs E, Merrill BJ, Jamora C, et al. At the roots of a never-ending cycle. Dev Cell. 2001;1:13–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 6.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 7.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 8.Naik UP, Naik MU, Eckfeld K, et al. Characterization and chromosomal localization of JAM-1, a platelet receptor for a stimulatory monoclonal antibody. J Cell Sci. 2001;114:539–547. doi: 10.1242/jcs.114.3.539. [DOI] [PubMed] [Google Scholar]
- 9.Blanpain C, Fuchs E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshima H, Rochat A, Kedzia C, et al. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:223–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 11.Jensen UB, Yan X, Triel C, et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci. 2008;121:609–617. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanagan SP. ‘Nude,’ a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966;8:295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- 13.Huang YC, Chan CC, Lin WT, et al. Scalable production of controllable dermal papilla spheroids on PVA surfaces and the effects of spheroid size on hair follicle regeneration. Biomaterials. 2013;34:442–451. doi: 10.1016/j.biomaterials.2012.09.083. [DOI] [PubMed] [Google Scholar]
- 14.Paus R, Müller-Röver S, Van Der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 15.Prowse DM, Lee D, Weiner L, et al. Ectopic expression of the nude gene induces hyperproliferation and defects in differentiation: Implications for the self-renewal of cutaneous epithelia. Dev Biol. 1999;212:54–67. doi: 10.1006/dbio.1999.9328. [DOI] [PubMed] [Google Scholar]
- 16.Mecklenburg L, Paus R, Halata Z, et al. FOXN1 is critical for onycholemmal terminal differentiation in nude (Foxn1) mice. J Invest Dermatol. 2004;123:1001–1011. doi: 10.1111/j.0022-202X.2004.23442.x. [DOI] [PubMed] [Google Scholar]
- 17.Eaton GJ. Hair growth cycles and wave patterns in “nude” mice. Transplantation. 1976;22:217–222. doi: 10.1097/00007890-197609000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Enshell-Seijffers D, Lindon C, Kashiwagi M, et al. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Severson EA, Parkos CA. Mechanisms of outside-in signaling at the tight junction by junctional adhesion molecule A. Ann N Y Acad Sci. 2009;1165:10–18. doi: 10.1111/j.1749-6632.2009.04034.x. [DOI] [PubMed] [Google Scholar]
- 20.Martìn-Padura I, Lostaglio S, Schneemann M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebnet K, Aurrand-Lions M, Kuhn A, et al. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: A possible role for JAMs in endothelial cell polarity. J Cell Sci. 2003;116:3879–3891. doi: 10.1242/jcs.00704. [DOI] [PubMed] [Google Scholar]
- 22.Bazzoni G, Martinez-Estrada OM, Mueller F, et al. Homophilic interaction of junctional adhesion molecule. J Biol Chem. 2000;275:30970–30976. doi: 10.1074/jbc.M003946200. [DOI] [PubMed] [Google Scholar]
- 23.Kostrewa D, Brockhaus M, D’Arcy A, et al. X-ray structure of junctional adhesion molecule: Structural basis for homophilic adhesion via a novel dimerization motif. EMBO J. 2001;20:4391–4398. doi: 10.1093/emboj/20.16.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naik MU, Vuppalanchi D, Naik UP. Essential role of junctional adhesion molecule-1 in basic fibroblast growth factor-induced endothelial cell migration. Arterioscler Thromb Vasc Biol. 2003;23:2165–2171. doi: 10.1161/01.ATV.0000093982.84451.87. [DOI] [PubMed] [Google Scholar]
- 25.Thomas FC, Sheth B, Eckert JJ, et al. Contribution of JAM-1 to epithelial differentiation and tight-junction biogenesis in the mouse preimplantation embryo. J Cell Sci. 2004;117:5599–5608. doi: 10.1242/jcs.01424. [DOI] [PubMed] [Google Scholar]
- 26.Itoh M, Sasaki H, Furuse M, et al. Junctional adhesion molecule (JAM) binds to PAR-3: A possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Severson EA, Jiang L, Ivanov AI, et al. Cis-dimerization mediates function of junctional adhesion molecule A. Mol Biol Cell. 2008;19:1862–1872. doi: 10.1091/mbc.E07-09-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandell KJ, Babbin BA, Nusrat A, et al. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J Biol Chem. 2005;280:11665–11674. doi: 10.1074/jbc.M412650200. [DOI] [PubMed] [Google Scholar]
- 29.Gafter-Gvili A, Sredni B, Gal R, et al. Cyclosporin A-induced hair growth in mice is associated with inhibition of calcineurin-dependent activation of NFAT in follicular keratinocytes. Am J Physiol Cell Physiol. 2003;284:C1593–C1603. doi: 10.1152/ajpcell.00537.2002. [DOI] [PubMed] [Google Scholar]
- 30.Vegesna V, O’Kelly J, Uskokovic M, et al. Vitamin D3 analogs stimulate hair growth in nude mice. Endocrinology. 2002;143:4389–4396. doi: 10.1210/en.2002-220118. [DOI] [PubMed] [Google Scholar]
- 31.Köpf-Maier P, Mboneko VF, Merker HJ. Nude mice are not hairless: A morphological study. Acta Anat (Basel) 1990;139:178–190. doi: 10.1159/000146996. [DOI] [PubMed] [Google Scholar]
- 32.Rigdon RH, Packchanian AA. Histologic study of the skin of congenitally athymic “nude” mice. Tex Rep Biol Med. 1974;32:711–723. [PubMed] [Google Scholar]
- 33.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 34.Bazzoni G, Martinez-Estrada OM, Orsenigo F, et al. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 2000;275:20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- 35.Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, et al. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275:27979–27988. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- 36.Fradette J, Germain L, Seshaiah P, et al. The type I keratin 19 possesses distinct and context-dependent assembly properties. J Biol Chem. 1998;273:35176–35184. doi: 10.1074/jbc.273.52.35176. [DOI] [PubMed] [Google Scholar]
- 37.Sherwood ER, Theyer G, Steiner G, et al. Differential expression of specific cytokeratin polypeptides in the basal and luminal epithelia of the human prostate. Prostate. 1991;18:303–314. doi: 10.1002/pros.2990180404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.