This study aimed to review the experience with 13 consecutive cases of cranio-maxillofacial hard-tissue defects at four anatomically different sites, namely frontal sinus (3 cases), cranial bone (5 cases), mandible (3 cases), and nasal septum (2 cases). Successful integration of the construct to the surrounding skeleton was noted in 10 of the 13 cases.
Keywords: Adipose stem cells, Bioactive glass, β-Tricalcium phosphate, Bone morphogenetic protein
Abstract
Although isolated reports of hard-tissue reconstruction in the cranio-maxillofacial skeleton exist, multipatient case series are lacking. This study aimed to review the experience with 13 consecutive cases of cranio-maxillofacial hard-tissue defects at four anatomically different sites, namely frontal sinus (3 cases), cranial bone (5 cases), mandible (3 cases), and nasal septum (2 cases). Autologous adipose tissue was harvested from the anterior abdominal wall, and adipose-derived stem cells were cultured, expanded, and then seeded onto resorbable scaffold materials for subsequent reimplantation into hard-tissue defects. The defects were reconstructed with either bioactive glass or β-tricalcium phosphate scaffolds seeded with adipose-derived stem cells (ASCs), and in some cases with the addition of recombinant human bone morphogenetic protein-2. Production and use of ASCs were done according to good manufacturing practice guidelines. Follow-up time ranged from 12 to 52 months. Successful integration of the construct to the surrounding skeleton was noted in 10 of the 13 cases. Two cranial defect cases in which nonrigid resorbable containment meshes were used sustained bone resorption to the point that they required the procedure to be redone. One septal perforation case failed outright at 1 year because of the postsurgical resumption of the patient’s uncontrolled nasal picking habit.
Introduction
Cranio-maxillofacial skeletal defects arise as a consequence of congenital malformations such as cleft lip and palate, are due to traumatic avulsion, result from tumor resection, or follow severe infection. The use of autogenous bone is still considered to be the gold standard for the reconstruction of cranio-maxillofacial skeletal defects [1]. Harvesting bone for grafting, however, is associated with a second potentially unnecessary surgical site with significant donor site morbidity that also requires additional operative and anesthetic time [2–7]. The elimination of autogenous bone graft harvesting would spare patients from the potential suffering related to major donor site morbidity. This realization has led to a search for an ideal bone substitute that could mimic the osteogenic capacity of autogenous bone [8].
An alternative approach is that of tissue engineering. Tissue engineering was defined by Langer and Vacanti in 1993 [9] as an interdisciplinary field of research that applies both the principles of engineering and the processes and phenomena of the life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function. Three components are described as part of the tissue engineering of bone, including vital bone-forming cells, growth factors, and a scaffold to promote the formation of new bone in a desired location and with particular precise dimensions and shape [10].
Growth factors can induce mesenchymal cells to differentiate into osteoprogenitor cells [11]. Whereas several growth factors have been identified, few have been used in a clinical setting [12, 13]. Scaffolds or bone substitutes such as bioactive glass (BAG) and β-tricalcium phosphate (β-TCP) can be used in combination with growth factors [14, 15].
Constructs using stem cells obtained from autogenous adipose tissue, for example, can be made using scaffolds and growth factors to enhance bone regeneration [16–19]. Although major segments of human mandibular defects have been reconstructed with constructs containing growth factors, such as recombinant human bone morphogenetic protein-7 (rhBMP-7) [12, 13], reports regarding cell-seeded constructs are still few in number [15–24]. Whereas there has been much laboratory study of possible techniques [25–27], most clinical reports describe the occasional single-case success with limited follow-up [15–24].
The aim of this study is to review the experience with 13 consecutive cases of cranio-maxillofacial hard-tissue defects reconstructed with a variety of scaffolds seeded with adipose-derived stem cells (ASCs) and in some cases with the addition of the growth factor rhBMP-2.
Materials and Methods
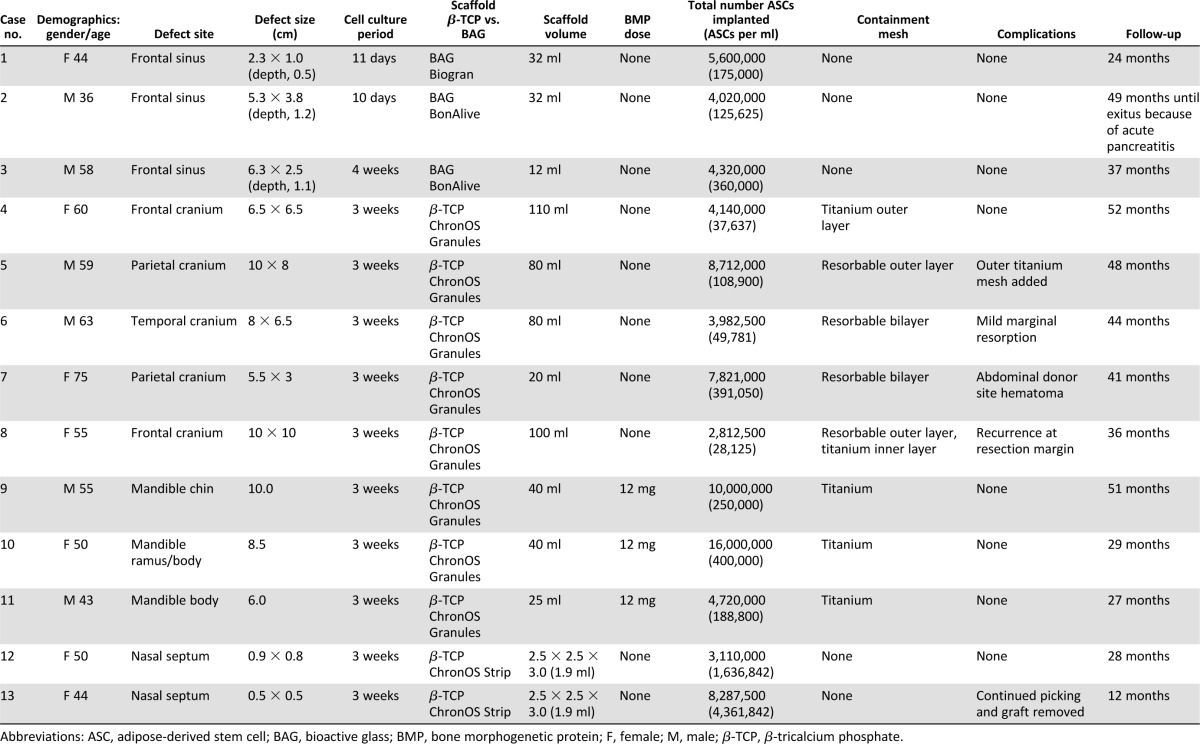
A total of 13 consecutive adult patients with defects in the cranio-maxillofacial skeleton treated at Tampere University Hospital (Tampere, Finland) and the Central Hospital of Central Finland Health Care District (Jyväskylä, Finland) were included in this study. The average age of the patients was 53.2 years (range, 36–75). There were 8 females and 5 males. The patients were otherwise in good health with no history of diabetes or wound-healing problems. None of the patients admitted to smoking. None of the defects required prior treatment with radiation therapy. Although none of the cases assessed for surgery had any of these clinical conditions, the following should be regarded as exclusion criteria for this series: positive HIV, hepatitis, or syphilis tests. The 13 defects were located at four different anatomic sites, namely frontal sinus (3 cases), cranial bone (5 cases), mandible (3 cases), and nasal septum (2 cases) (Table 1). The tissues overlying the hard-tissue defects were noninflamed, with the exception of the frontal sinus patients who had major problems with recurrent infection, and the two nasal septum perforation patients who both had chronic crusted ulcerative wounds. Cases 4–7 and 9–11 (Table 1) have been reported previously [17–19] and are now presented as part of this larger case series with additional long-term follow-up.
Table 1.
Details of 13 cases with cranio-maxillofacial skeletal defects treated with good manufacturing practice-level adipose stem cells
The combination of adipose stem cells and biomaterials is considered to be an advanced therapy-combined medicinal product according to the regulations of the European Medicines Agency. The treatment was carried out with the approval of the Finnish Medicines Agency under hospital exemption of patient-specific treatment (Dnro 615/11.01.06./2010). The approval to use adipose stem cells for research purposes was granted by the Ethics Committee of the Pirkanmaa Hospital District (Tampere, Finland [R03058]) within the ethical principles of the Helsinki declaration. Before surgery, the patients were thoroughly informed about the procedures, which they approved and to which they gave their written consent.
Autogenous Fat Harvesting and Handling
Before bone reconstruction, 50–200 ml of subcutaneous adipose tissue was harvested and was subsequently used for ex vivo cell culturing and future autologous reimplantation. Additionally, approximately 60 ml of autologous serum was obtained for the expansion of the autologous stem cells.
For all 13 patients, the ASCs were isolated and expanded in vitro using clean rooms according to good manufacturing practice (GMP) guidelines and standard operating procedures at the Regea Cell and Tissue Center, BioMediTech, the Institute of Biosciences and Medical Technology, University of Tampere. The isolation and expansion procedures were performed, as reported previously, with minor modifications [16–19]. The adipose tissue was first minced with scissors and then digested with recombinant collagenase NB-6 (GMP grade; Invitrogen, Paisley, U.K., http://www.invitrogen.com; Serva Electrophoresis, Heidelberg, Germany, http://www.serva.de).
The isolated cells were expanded in basal medium containing Dulbecco’s modified Eagle's medium/F-12 (Gibco Invitrogen, Paisley, U.K., http://www.invitrogen.com) with 10%–15% of autologous serum and without antibiotics. Cells were subsequently passaged until confluent, detached mechanically with a cell scraper, and prepared for cell transplantation. Because of a lack of GMP-grade reagents for the first two patients, adipose tissue was digested with collagenase type I (Invitrogen), and passaging was done with TrypLE Select (Gibco). Furthermore, 15% human serum (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com/PAA Laboratories, Pasching, Austria, http://www.paa.at) was used instead of autologous serum. Cell expansion periods varied from 10 days to approximately 1 month depending on the defect volume, amount of harvested fat, and cell growth rate.
Cell sterility and endotoxins were tested by Biovian (Turku, Finland, http://www.biovian.com), according to the methods described in the European Pharmacopoeia (Council of Europe, Strasbourg, France). The cells tested negative for mycoplasma contamination as determined by a mycoplasma polymerase chain reaction kit (VenorGem; Minerva Biolabs, Berlin, Germany, http://www.minerva-biolabs.com/en).
The genomic stability of human ASCs was verified using a standard chromosome G-banding method. Karyotypic evaluation was performed as an outsourcing service in Medix Laboratoriot (Espoo, Finland, https://www.medixbiochemica.com).
The immunophenotype of the cells was analyzed by flow cytometry (FACSAria; BD Biosciences, Erembodegem, Belgium, http://www.bdbiosciences.com). Monoclonal antibodies against CD105-phycoerythrin (PE; R&D Systems, Minneapolis, MN, http://www.rndsystems.com), CD14-PE cyanine 7 (Cy7), CD19-PECy7, CD34-allophycocyanin (APC), CD45RO-APC, CD49d-PE, CD73-PE, CD90-APC, CD106-PECy5 (BD Biosciences), HLA-ABC-PE, and HLA-DR-PE (Immunotools, Friesoythe, Germany, http://www.immunotools.de) were used. Analysis was performed on 10,000 cells per sample, and unstained cell samples were used to compensate for the background autofluorescence levels.
Scaffold biomaterials are listed in Table 1. Before combining biomaterials and cells, biomaterials were incubated in cell growth medium for 24 hours. Cells and biomaterials were combined 24–48 hours before the surgical procedure. If rhBMP-2 (InductOS; Wyeth Europa, Maidenhead, U.K.) was used, the biomaterial was incubated for 48 hours in basal medium containing 12 mg of rhBMP-2. After incubation, the basal medium containing rhBMP-2 was discarded and ASCs were added to the scaffold.
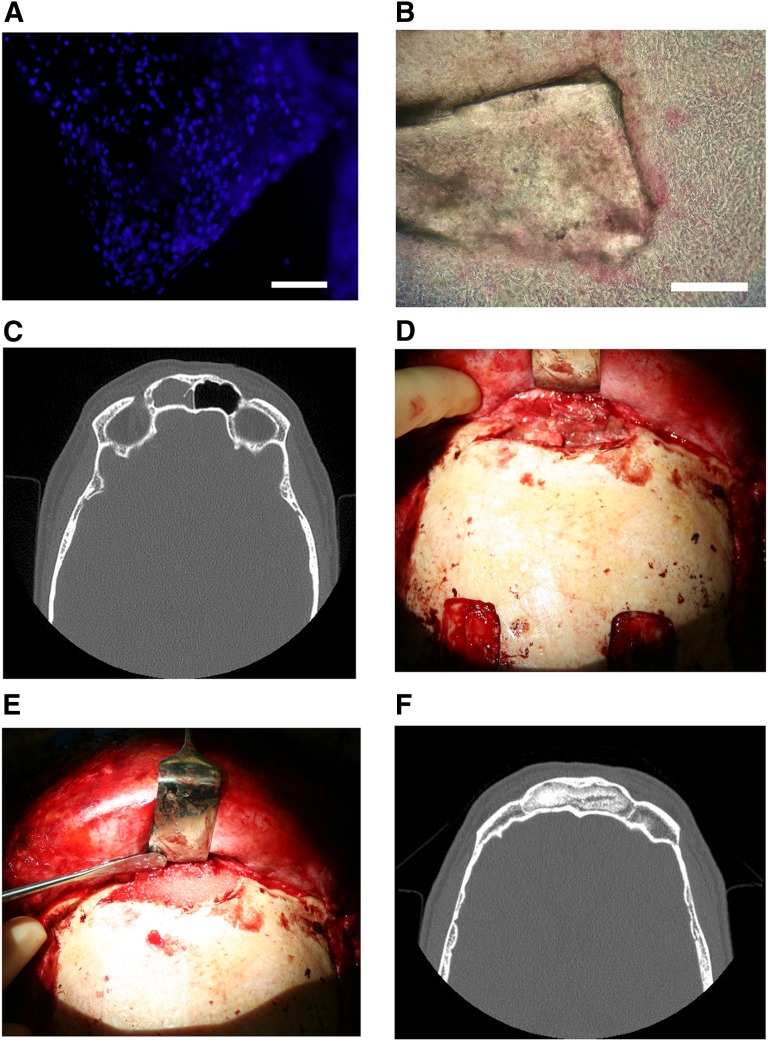
Cell attachment to the material and the cell viability were studied using live/dead staining before cell transplantation. Briefly, the cell-biomaterial combination was incubated with a mixture of Cell-Tracker green (5-chloromethylfluorescein diacetate; Molecular Probes, Eugene, OR, http://probes.invitrogen.com) and ethidium homodimer-1 (Molecular Probes). The viable cells (green fluorescence) and dead cells (red fluorescence) were detected with a fluorescence microscope [16–19]. For the first two patients, 4′,6-diamidino-2-phenylindole nuclear staining (Vector Laboratories, Burlingame, CA, http://www.vedco.com) was used to detect cell attachment on BAG granules (Fig. 1A). The cell-biomaterial constructs were transported in a temperature-controlled sterile container to the operating theater.
Figure 1.
Collage of frontal sinus treatment. (A): 4′,6-diamidino-2-phenylindole nuclear staining image of bioactive glass granules seeded with adipose stem cells (BonAlive). Scale bar = 1 mm. (B): Alkaline phosphatase staining of a bioactive glass granule seeded with adipose stem cells (BonAlive). Scale bar = 1 mm. (C): Preoperative computed tomography (CT) scan of the left frontal sinus demonstrating mucosal changes and frontal sinusitis. (D): Clinical photograph of exposed diseased frontal sinus with purulent mucosa. (E): Clinical photograph of debrided frontal sinus packed with granules of bioactive glass seeded with autologous adipose-derived stem cells. (F): Postoperative CT scan of the frontal sinus obliterated with autologous adipose stem cell-seeded bioactive glass 28 months following surgery with no resorption of the construct.
Because of the limited amount of autologous serum for the subsequent in vitro analyses, basic medium supplemented with 15% human serum of the clot type AB (PAA Laboratories) and 1% antibiotics (100 U/ml penicillin, 0.1 mg/ml streptomycin; Invitrogen) was used. Furthermore, the experiments were done in a standard cell culture laboratory instead of cleanrooms. The surplus biomaterials and cells from the surgical procedures were recovered and maintained in vitro. For the in vitro osteogenic differentiation analyses, osteogenic medium containing the basal medium supplemented with 50 mmol/l l-ascorbic acid 2-phosphate (Sigma-Aldrich), 10 mmol/l β-glycerophosphate (Sigma-Aldrich), and 100 nmol/l dexamethasone (Sigma-Aldrich) was used. After 7–14 days in culture, the cells were fixed with a 4% paraformaldehyde solution and stained with the leukocyte alkaline phosphatase kit (Fig. 1B), according to Sigma-Aldrich procedure 86 (86R-1KT).
Clinical Protocol Frontal Sinus
A total of three patients, one female and two males, with an average age of 46 years (range, 36–58), all with severe chronic frontal sinus infections that failed numerous conventional nonsurgical and surgical treatment, such as frontal sinus trephinations, mucosal stripping, and obliteration with abdominal fat, were included in this case series. The specifics of the three patients follow below.
Frontal sinus patient 1 was a 44-year-old woman who had chronic frontal sinusitis since 1993. Using an external approach, her frontal sinuses were obliterated with abdominal fat in 1993. The patient was asymptomatic for 5 years postoperatively, after which her chronic frontal sinusitis recurred with pain, colored infectious discharge, and edema of the forehead. She underwent an endonasal sinus operation in 2004 without relief. She was reoperated with ASC-seeded Biogran (Biomet, Warsaw, IN, http://www.biomet.com) BAG granules in 2006. The scaffold Biogran granules have a particle size of 300–355 µm and a composition by weight of 45.0% SiO2, 24.5% Na2O, 24.5% CaO, and 6.0% P2O5.
Frontal sinus patient 2 was a 36-year-old man who was previously free from sinusitis until 2005, when he suffered from prolonged pansinusitis. Despite management with conventional antibiotic and surgical treatments, including frontal sinus trephinations and three endonasal sinus surgeries, the patient still had recurrent frontal sinusitis. His symptoms included intense pain of the forehead, colored infectious discharge, and edema. An osteoplastic frontal sinus obliteration with ASC-seeded BonAlive BAG granules (BonAlive Biomaterials, Turku, Finland, http://www.bonalive.com) was performed in 2006. The BonAlive scaffold contains granules with a particle size of 500–800 µm and has a composition by weight of 53.0% SiO2, 23.0% Na2O, 20.0% CaO, and 4.0% P2O5.
Frontal sinus patient 3 was a 58-year-old male with a history of multiple recurrent frontal sinusitis episodes and purulent discharge who was refractory to multiple courses of antibiotic therapy and frontal sinus trephinations. This patient was ultimately treated with an ASC-seeded BAG construct using BonAlive as the scaffold material (Fig. 1C–1F).
All three patients underwent two distinctly separate operations consisting of adipose tissue harvesting and later reconstructive operations that ranged between 10 and 28 days apart. In the first operation, approximately 100–200 ml of abdominal adipose tissue was harvested either through an incision in a pre-existing scar or through a new incision in the lower abdomen under local anesthesia. The adipose tissue samples were transported to the cell culture laboratory for stem cell isolation and expansion.
The second operation was performed between 10 and 28 days following the fat harvesting through a coronal incision. The mucosa was completely removed from the frontal sinus, and the mucosa in the region of the frontonasal ostium was inverted nasally. The drainage opening was sealed with fascia, fibrin glue, and tissudural (Baxter, Deerfield, IL, http://www.baxter.com), a native equine collagen biomatrix to provide a liquid-tight seal to prevent cerebrospinal fluid leakage. This material also restores the local anatomy by adhering to complex wound surfaces and develops into living dura tissue. Bioactive glass granules combined with autologous adipose stem cells were used to obliterate the frontal sinuses (Fig. 1E, 1F).
The three patients were closely followed up, and clinical evaluations were carried out at 1 week; 1, 6, and 12 months after surgery; and annually thereafter. Computed tomography and magnetic resonance images were taken 1, 6, and 12 months postoperatively.
Clinical Protocol Cranial Defects
A total of five patients (four females and one male) with an average age of 61.8 years (range, 54–75 years) underwent cranioplasty, and their bony cranial defects were filled with constructs consisting of granules of β-TCP seeded with autologous ASCs. None of the cranioplasty constructs contained rhBMP-2. All patients had bony cranial defects in the frontal, frontoparietal, or frontotemporal cranial regions. The average defect size was 8.1 × 6.7 cm.
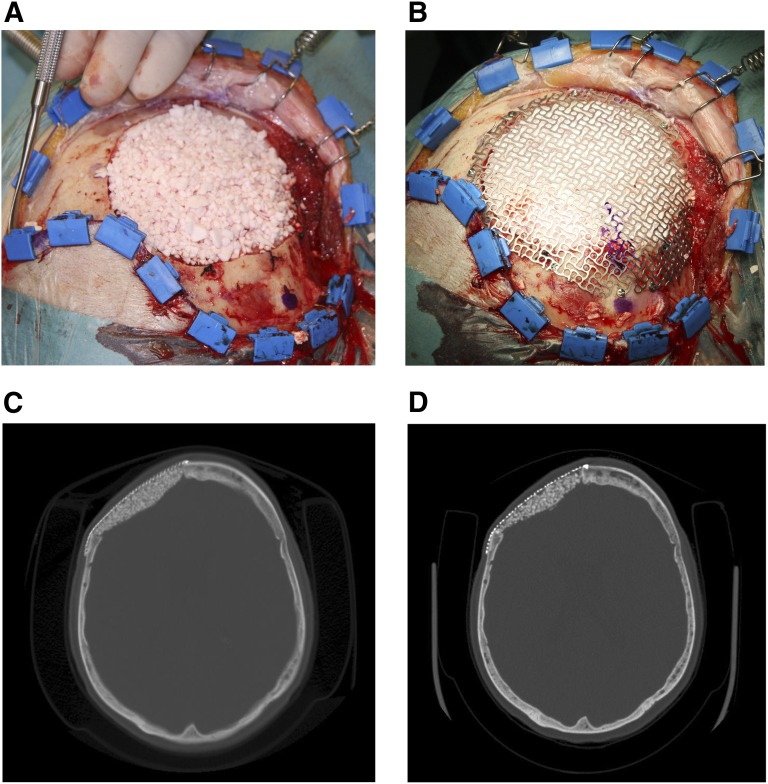
The indications for cranioplasties included cranial hemangioma (cranial patient 1), a previously resected meningioma with late loosening of the acrylic cranioplasty (cranial patient 2), an acute subdural hematoma and decompressive craniectomy with late bone flap infection (cranial patient 3), a previously resected meningioma with bone flap infection (cranial patient 4), and an unresected frontal bone meningioma (cranial patient 5). The hemangioma and meningioma tumors were removed with the outer and inner diploë of bone, and, in the cases of failed acrylic cranioplasty or previously infected cranial bone flaps, the failing reconstructions were removed and the ASC-seeded β-TCP granular cranioplasty constructs were applied to the defects (Fig. 2A).
Figure 2.
Collage of cranial defect cases. (A): Meningioma resection site filled with autologous adipose stem cell-seeded β-tricalcium phosphate (β-TCP) granules. (B): Titanium containment mesh on top of stem cell-seeded β-TCP granules to keep granules from migrating. (C): Immediate postoperative cranial computed tomography (CT) scan showing titanium containment mesh and adipose-derived stem cell-seeded β-TCP granular construct. (D): Postoperative cranial CT taken 28 months following surgery with signs of integration of the cranioplasty construct.
In cranial patient 1, an outer titanium mesh had been used to stabilize the granular cranioplasty reconstruction material and fixated with titanium screws (Fig. 2B–2D). Cranial patient 2 had an outer resorbable mesh, RapidSorb (Synthes, Oberdorf, Switzerland, http://www.synthes.com), that is custom moldable in hot water in the operating room setting. This resorbable mesh was fixated with resorbable screws. Cranial patients 3 and 4 had the same resorbable mesh applied to both the inner and outer aspects of the cranial bone defects, which was also fixated with resorbable screws on the outer surface. Cranial patient 5 had a titanium mesh placed on the inner side of the cranial defect and the resorbable mesh placed and fixated with resorbable screws on the outside surface. The five patients were followed closely, and clinical evaluations were carried out at 1 week; 1, 6, and 12 months after surgery; and annually thereafter.
Clinical Protocol Mandibular Defects
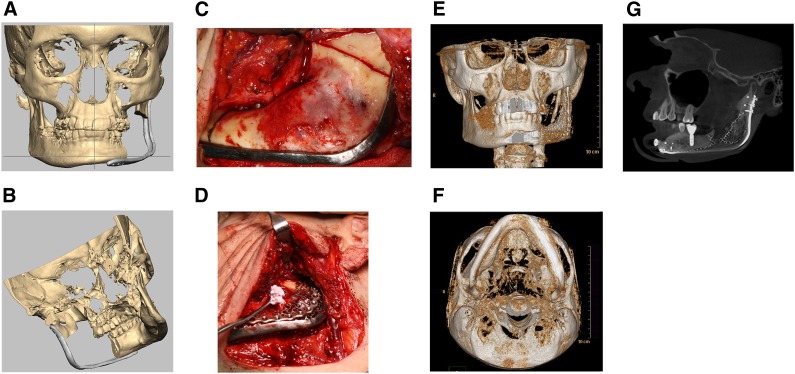
A total of three patients, all with recurrent ameloblastomas requiring segmental mandibular resection, were included in this study. There were two males and one female with an average age of 49.3 years (range, 43-55 years [Table 1]). The planned mandibular segmental resection defects ranged from 6.0 to 10 cm in length, with an average defect size of 8.2 cm. All three cases were managed by harvesting adipose tissue, followed by the reconstructive procedure approximately 3 weeks later with computer-aided surgical planning (Fig. 3A, 3B), using custom-designed hardware and titanium mesh with ASCs, β-TCP granules, and 12 mg of rhBMP-2 (Fig. 3C–3G). The three patients were followed closely, and clinical evaluations were carried out at 1 week; 1, 6, and 12 months after surgery; and annually thereafter.
Figure 3.
Collage of mandibular reconstructions. (A): Virtual preoperative planning using Romexis software with computer-generated image of patient with large mandibular ameloblastoma showing the reconstruction plate over the area planned for resection from an anterior view. (B): Computer-generated image of patient with large mandibular ameloblastoma showing the reconstruction plate over the area planned for resection from a medial view. (C): Intraoperative photograph showing reconstruction plate in position and resection lines on mandibular ramus posteriorly and body anteriorly. (D): Intraoperative photograph with adipose-derived stem cell-seeded β-tricalcium phosphate (β-TCP) granular construct with recombinant human bone morphogenetic protein-2 (rhBMP-2) being placed beneath titanium containment mesh at mandibular resection site. (E): Postoperative three-dimensional (3D) computed tomography (CT) scan anterior view of the regenerated left body and ramus of the mandible 12 months after reconstruction. (F): Postoperative 3D CT scan basilar view of the regenerated left body and ramus of the mandible 12 months after reconstruction. (G): Cone beam CT scan showing mandibular reconstruction using autologous adipose-derived stem cell-seeded β-TCP granular construct with rhBMP-2 restored with successful functionally loaded dental implant in the regenerated bone.
Clinical Protocol Nasal Septum
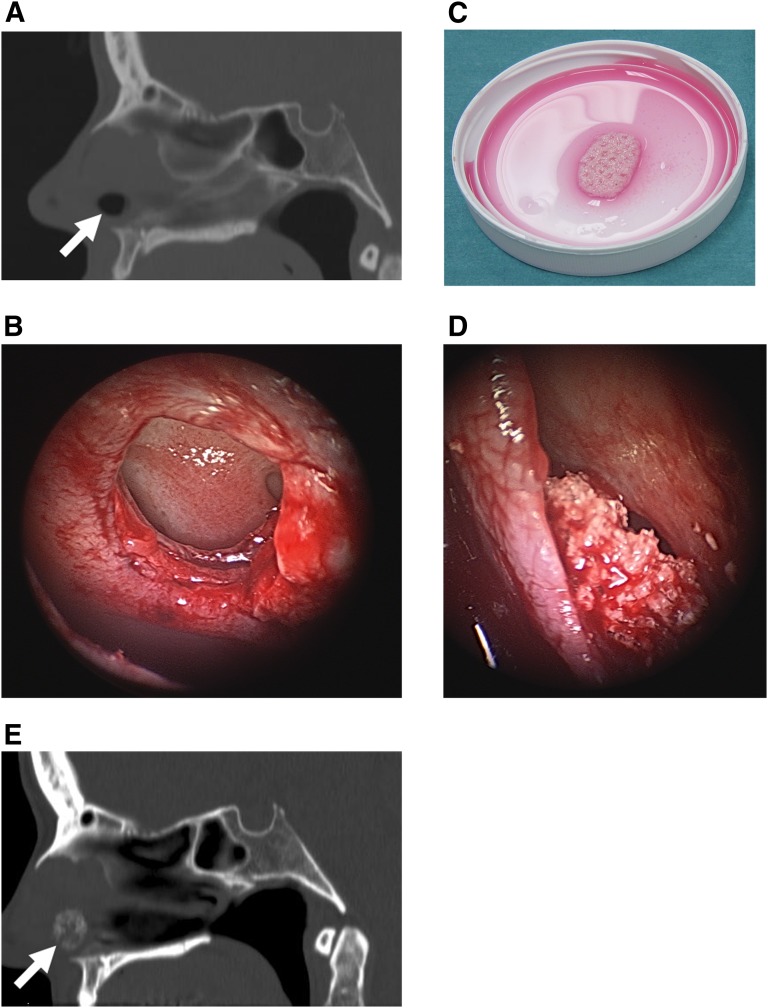
Two female patients aged 44 and 50 years with chronic nasal septum perforations were included in the study. Both patients were troubled by their constantly crusted intranasal ulcers and found this difficult to tolerate (Fig. 4A). Neither patient had a history of cocaine or other drug abuse. In both cases the nasal septal perforations were treated with soft tissue flaps on both sides of the nasal septal perforations (Fig. 4B). An ASC-seeded resorbable ChronOS Strip (Synthes) was used as a scaffold and implanted sandwiched between the two flaps. The flexible ChronOS Strips contain ε-polycaprolactone and differ from ChronOS Granules, which consist solely of β-TCP (Fig. 4C–4E). The two patients were followed closely, and clinical evaluations were carried out at 1 week; 1, 6, and 12 months after surgery; and annually thereafter.
Figure 4.
Collage of nasal septal perforation treatment. (A): Preoperative computed tomography (CT) scan in the sagittal plane revealing chronic nasal septal perforation before repair (arrow). (B): Intraoperative endoscopic view of chronic nasal septal perforation with margins incised. (C): Autologous adipose stem cell-seeded resorbable scaffold composed of β-tricalcium phosphate (β-TCP) and ε-polycaprolactone ready for implantation. (D): Intraoperative endoscopic view of implanted seeded scaffold sandwiched between two flaps closing the nasal septal perforation. (E): Postoperative CT scan showing chronic nasal septal perforation repaired with autologous adipose stem cell-seeded β-TCP strip construct (arrow).
Results
Laboratory Results
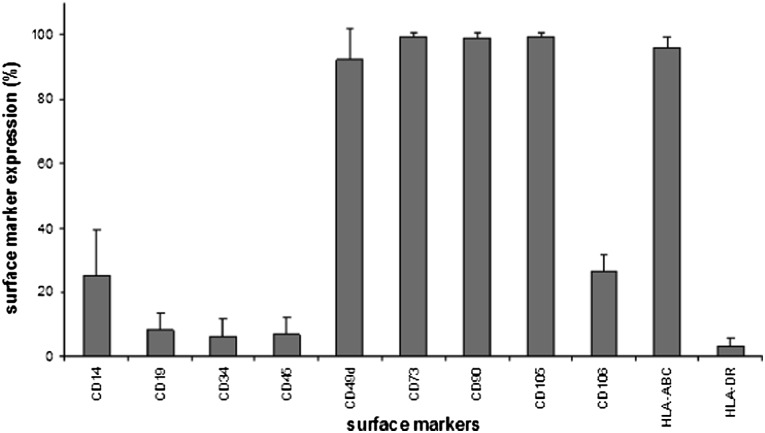
The flow cytometry results of ASCs showed strong expression of markers CD49d, CD73, CD90, CD105, and HLA-ABC; moderate expression of CD14 and CD106; and low expression of CD19, CD34, CD45, and HLA-DR (Fig. 5). However, the immunophenotype showed patient variability, especially in CD14, CD19, CD34, CD45RO, CD49d, and HLA-DR, which has also been reported previously [17].
Figure 5.
Column diagram on flow cytometry data of adipose-derived stem cells. Columns illustrate mean surface marker expression levels with standard deviation error bars of all patient samples.
The alkaline phosphatase staining revealed that biomaterials used in these clinical cases support osteogenic differentiation of adipose stem cells.
Frontal Sinus Clinical and Radiologic Results
All three frontal sinus patients were relieved of their symptoms within 1 month postoperatively and were asymptomatic (Table 1). Radiological examinations in frontal sinus patient 1 showed evidence of new bone formation in the frontal sinus 1 month following placement of the construct, which was even more remarkable at 6 months. After 1 month of follow-up, frontal sinus patient 2 had marked new bone formation in over 95% of the area of the frontal sinus, which remained stable over the subsequent follow-up (Fig. 1F). Clinical examinations did not reveal any signs of residual infection. Frontal sinus patient 1 was asymptomatic for her 2 years of active follow-up. Frontal sinus patient 2 was asymptomatic for 30.5 years until he died of an unrelated condition, namely acute pancreatitis. Frontal sinus patient 3 remained asymptomatic for his 37 months of follow-up.
Cranial Defect Clinical and Radiological Results
The wounds of the five cranial defect patients healed uneventfully following their reconstructions (Table 1). Cranial patients 1 and 5 both underwent hemangioma or meningioma resections before their reconstructions. Both these patients had a titanium mesh: cranial patient 1 on the outside, and cranial patient 5 on the inside with an outer resorbable mesh. These patients showed clinical and radiographic evidence of bony defect healing, although one patient had a recurrence of the meningioma at a resection margin with positive evidence of bone healing of the repaired cranial defects at the second resection.
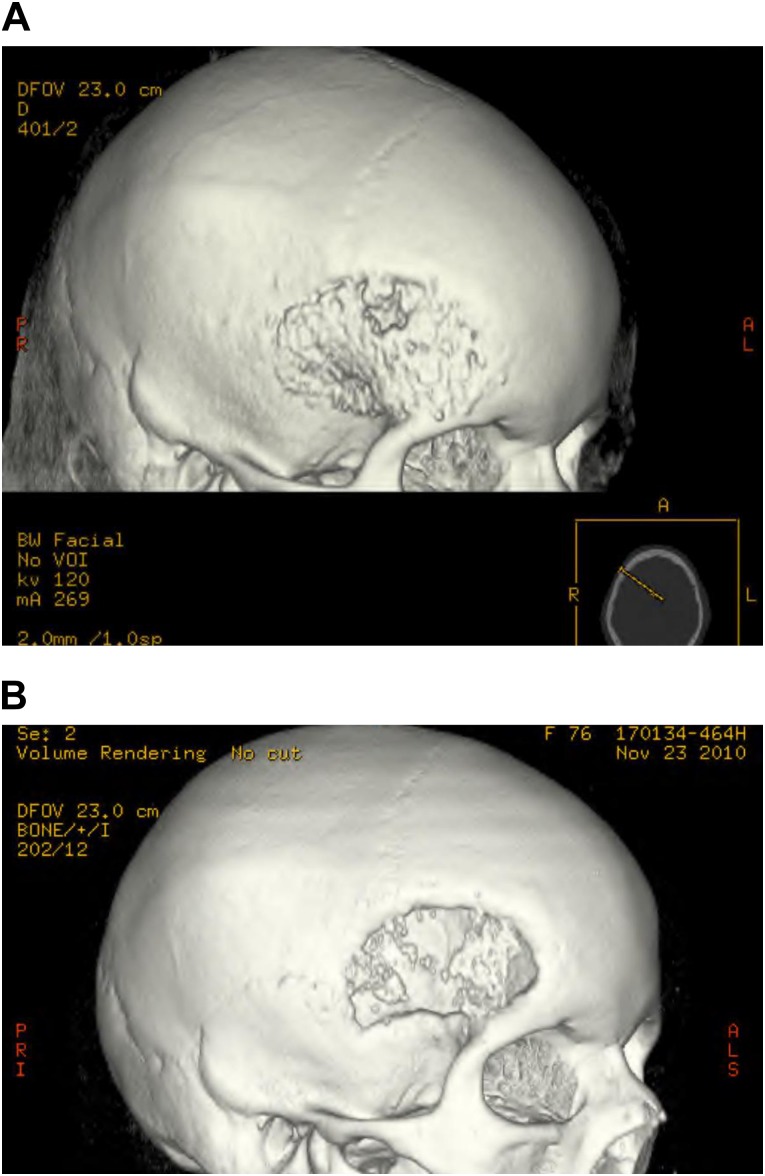
Cranial patient 2 had the loosened acrylic cranioplasty removed before reconstruction with an outer resorbable mesh. This patient had evidence of graft resorption and was reoperated on 1 year later with placement of a titanium mesh on the outer cranium. Cranial patient 3 had a late bone infection before the autologous ASC-seeded β-TCP reconstruction with resorbable meshes on the outer and inner cranium. This patient has shown some mild resorption at the edges of the reconstruction. Cranial patient 4 had a previously resected hemangioma with a bone flap infection before reconstruction. This patient had almost complete resorption of the ASC-seeded β-TCP reconstruction, which also used resorbable meshes on the outer and inner cranium (Fig. 6A, 6B). Cranial patient 4 also sustained an abdominal hematoma at the adipose donor site, which required drainage on the first postoperative day.
Figure 6.
Postoperative cranioplasty radiographs. (A): Computed tomography (CT) image of cranioplasty with resorbable polylactic acid polymer-based mesh on the outer and inner cranium. (B): CT image of cranioplasty with resorbable polylactic acid polymer-based mesh with almost complete resorption of the autologous adipose-derived stem cell-seeded β-tricalcium phosphate-seeded construct.
Clinical and Radiological Results in Mandibular Defects
The reconstruction of the three mandibular defects with autologous ASCs was successful in bridging the large mandibular defects averaging 8.2 cm (range, 6.0–10.0 cm) with uneventful healing (Table 1). Two of the three patients opted for reconstruction with dental implants (Fig. 3E). There was a total of seven dental implant fixtures placed in two patients with six (86%) fixtures being successfully osseointegrated and then loaded in masticatory function. The three patients have been under follow-up for an average of 35 months following their ASC-based mandibular reconstructions (range, 27–51 months).
Nasal Septum Results
The two nasal septum patients healed uneventfully (Table 1). Nasal septum patient 1 was satisfied with her nasal septal perforation repair, and the perforation has not recurred in over 28 months of follow-up (Fig. 4D, 4E). Unfortunately, nasal septum patient 2 began to resume her habitual nasal picking and was unable to stop. This eventually led to recurrent ulceration, crusting, and finally exposure of the ASC seeded ChronOS Strip construct. The construct was removed 12 months after its initial placement.
The success criterion for the grafts at the four different sites, frontal sinus, cranium, mandible, and nasal septum, was functional, that is to say, healed hard-tissue grafts in their recipient bed functioning according to the demands of their new native sites during the follow-up period.
Discussion
This report describes successful reconstruction of cranio-maxillofacial hard-tissue defects in a series of 13 patients at four different anatomic sites using the approach of in situ ossification with adipose stem cells [18] as one nonmorbid source of autogenous mesenchymal stem cells. To our knowledge, this study represents the first GMP compliant nonhematopoietic nonhematologic application for autologous adipose-derived stem cells in the treatment of hard-tissue defects at various sites of the cranio-maxillofacial skeleton.
In this cohort, there was individual variability in cell numbers available for implantation from passages 3 to 4, as the average number of total implanted ASCs was 6,425,246 cells with a range of 2,812,000 to 16,000,000 cells (Table 1). The flow cytometric characterization data of the ASCs expanded in autologous serum correspond to previous results for ASCs [18, 28], with positive expression of certain markers such as CD73, CD90, and CD105 substantiating the mesenchymal origin of cells and low expression of other markers such as CD34 and CD45RO, suggesting the hematopoietic and angiogenic origin of cells [28, 29], albeit very few reports have been published regarding the surface marker expression of ASC expanded in autologous serum [16–19, 30].
ASCs were used in this series of large cranio-maxillofacial defects with the hope that they would favorably alter the wound-healing dynamics of these hard-tissue defects that would otherwise be unlikely to heal when bone replacement scaffolds were used alone. Implanted ASCs, the factors secreted by the ASCs, and the osteoconductivity of the biomaterials act synergistically toward producing a well-ossified construct [19, 31]. The in vitro and in vivo bone-forming capacity of ASCs in combination with various scaffold materials, including β-TCP and BAG, has been reported by our group and others [16–19, 32–35]. Scaffolds containing BAG were considered to be particularly attractive for infected frontal sinus cases because of reported antibacterial and angiogenic properties as well as positive effects on proliferation and osteogenic differentiation of adipose stem cells [33].
Four different scaffolds were used in this case series, including two types of BAG granules in the frontal sinus, β-TCP granules in the cranial and mandibular defects and pliable strips with β-TCP, and held together by ε-polycaprolactone at the nasal septum. Granules were thought to be most advantageous because of the increased surface area they presented when compared with solid scaffolds. Granules also molded well to the complex contours that cranio-maxillofacial defects presented so that β-TCP granules were used in the cranial and mandibular defects and were found to be successful in our earlier cases [16–18]. BAG granules were chosen in infected frontal sinus cases because of their inherent antibacterial properties. In the nasal septum, pliable strips with β-TCP and held together by ε-polycaprolactone were used because containment meshes would be difficult to house in the thin nasal septum wounds.
In mandibular defect cases, the use of osteogenic growth factor rhBMP-2 may have facilitated ossification [32, 33]. However, it was not used in cranial, frontal sinus, or nasal septum cases. Contradictory reports have also been published, including reports of BMP-2 possibly not promoting in vitro osteogenic differentiation of human mesenchymal stem cells [36, 37]. Implanted ASCs, the factors secreted by the ASCs, and the osteoconductivity of the biomaterials act synergistically toward producing a well-ossified construct [19, 31].
Titanium hardware was chosen over resorbable materials in the mandible because at the time the cases were treated, the track record of resorbable hardware for major mandibular reconstruction was still lacking. Because the authors decided to use granules rather than a solid block, a containment mesh was required to house the scaffold. Furthermore, the authors were concerned that with the thin soft tissue coverage of the mandibular alveolus, the wound might not tolerate the additional pH drop associated with the degradation of a resorbable polymer mesh containing polylactic acid.
Resorbable meshes were used initially in cranial patients 2, 3, and 4. These three cases had difficulties requiring replacement of the resorbable meshes with titanium. Patients 3 and 4 also had chronically infected bone flaps removed before the reconstruction operation. Pre-existing infection may be a contraindication for this procedure at the cranial site. The addition of rhBMP-2 at the cranial site like at the mandible might also be helpful.
Whereas a containment mesh was necessary for the β-TCP granules used in the cranial and mandibular defects, no containment mesh was necessary in the frontal sinus sites. Once the frontal sinus contents had been removed, the walls of the frontal sinus acted as a containment mesh (Fig. 1E, 1F). In the nasal septal perforation defects, the β-TCP in the ChronOS Strips was held together by ε-polycaprolactone so that a containment mesh was not required at the nasal septal site either (Fig. 4C–4E).
Titanium mesh was problematic in all three mandibular cases with thin soft tissue coverage, and each of the three patients required resection of the superior borders of the mesh. For this reason, the authors would prefer to use a thin custom-made passive-fitting resorbable mesh once a suitable tested version becomes available. The authors considered many resorbable scaffolds for cranial and mandibular sites such as BAG but chose β-TCP granules because of their more rapid resorption profile [19, 31, 38, 39]. In the future, the authors could consider using scaffolds containing carriers that would release bioactive molecules such as growth factors and other drugs.
Although previous authors have reported single cases of mandibular and maxillary defect reconstruction using bone marrow or adipose-derived stem cells [16, 38], the 13 cases in the current series differ from the previous reports in which an ectopic muscle pouch site was used for bone induction. In the current 13-case series, there was no need for an ectopic bone formation step and there was sufficient soft tissue to cover the construct on site. Therefore, the constructs were placed directly into their final recipient beds from the outset. This obviated the need for an ectopic bone formation site, and this shorter simplified protocol has been called in situ bone formation [17–19].
Preparation of the recipient site by maximizing the contact of the construct with well-vascularized muscle is most important with in situ bone formation [18]. There was no history of prior radiation therapy in any of the 13 cases of this series. Unlike the present cases, the patient in the single case reported by Mesimäki et al. [16] had a very complicated wound with pre-existing oro-nasal and oro-antral fistulae, requiring soft tissue coverage on two sides. In such complex cases requiring soft tissues in addition to bone, or in cases in which there has been prior radiation therapy, the authors feel that it is best to use ectopic ossification as the preferred method over in situ bone formation.
As noted above, the criterion for the success of the grafts at four different sites (frontal sinus, cranium, mandible, and nasal septum) was functional: whether the healed hard-tissue grafts in their recipient bed were functioning according to the demands of their new native sites during the follow-up period. Overall, the results of this technique in the frontal sinus and mandible were good. There was eradication of recalcitrant frontal sinus disease. In the unique situation of the mandible, two of the three patients opted for dental implant reconstruction, and, in those cases, successful dental implant function could be considered as yet another indicator of bone graft success (Fig. 3G). The results were fair at the nasal septum in which only two cases were treated and one failed because of the resumption of the patient’s unremitting nose-picking habit.
The results in the cranial defects were disappointing, as the authors found that the resorption of the constructs was more than expected. The three patients in whom only a resorbable containment mesh was used sustained at least mild resorption of the ASC-seeded β-TCP constructs (Fig. 6A, 6B). The two patients with satisfactory clinical and radiological ossification had either an inner or an outer mesh from titanium. Two revision surgeries were performed in this group of five as a result of resorption of the cranioplasty material. Two patients have shown no resorption during the follow-up period. It seems that either the inner or outer cranial mesh layers in this type of cranial construct must be made of rigid, nonabsorbable material perhaps to sustain the constant dural pulsations to which these cranial wounds are exposed.
Conclusion
This case series assessed the results of 13 patients reconstructed with ASC-seeded resorbable scaffolds at four very different anatomic sites within the cranio-maxillofacial skeleton. The specific requirements for reconstruction of the protective cranial skeleton are very different from reconstructing the masticatory function of the mandible as is the treatment of an infected frontal sinus or the replacement of missing tissue at the site of a chronic nasal septal perforation. Nevertheless, the majority of these challenging defects, 10 of 13, were successfully treated with hard-tissue integration of the ASC-seeded constructs at the defect sites. The rationale to use ASC-seeded constructs to reconstruct major cranio-maxillofacial hard-tissue defects was to provide a safe and predictable reconstruction without the major morbidity associated with the extensive harvesting of a large autogenous bone graft. Further research is needed with animal studies and long-term results from human series in the future.
Acknowledgments
We acknowledge Sari Kalliokoski, Anna-Maija Honkala, Miia Juntunen, Tiia Tallinen, Hanna Kankkonen, and Annika Hakamäki for valuable contributions that made this work possible. This work was supported by EVO (9L100, 9H080, 9P049), the Competitive Research Funding of Tampere University Hospital, and TEKES, the Finnish Funding Agency for Technology and Innovation.
Author Contributions
G.K.S. and S.M.: conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; J.N., T.T., M.R., and J.Ö.: provision of study material, collection and assembly of data, manuscript writing, final approval of manuscript; J.W.: conception and design, provision of study material, collection and assembly of data, manuscript writing, final approval of manuscript; A.M. and V.J.T.: provision of study material, manuscript writing, final approval of manuscript; B.M. and R.S.: collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M.P.: collection and assembly of data, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Bianchi B, Ferri A, Ferrari S, et al. Mandibular resection and reconstruction in the management of extensive ameloblastoma. J Oral Maxillofac Surg. 2013;71:528–537. doi: 10.1016/j.joms.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo DA. Fibula free flap: A new method of mandible reconstruction. Plast Reconstr Surg. 1989;84:71–79. [PubMed] [Google Scholar]
- 3.Sàndor GK, Nish IA, Carmichael RP. Comparison of conventional surgery with motorized trephine in bone harvest from the anterior iliac crest. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:150–155. doi: 10.1067/moe.2003.42. [DOI] [PubMed] [Google Scholar]
- 4.Ling XF, Peng X. What is the price to pay for a free fibula flap? A systematic review of donor-site morbidity following free fibula flap surgery. Plast Reconstr Surg. 2012;129:657–674. doi: 10.1097/PRS.0b013e3182402d9a. [DOI] [PubMed] [Google Scholar]
- 5.Sieg P, Taner C, Hakim SG, et al. Long-term evaluation of donor site morbidity after free fibula transfer. Br J Oral Maxillofac Surg. 2010;48:267–270. doi: 10.1016/j.bjoms.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Nathan SS, Athanasian E, Boland PJ, et al. Valgus ankle deformity after vascularized fibular reconstruction for oncologic disease. Ann Surg Oncol. 2009;16:1938–1945. doi: 10.1245/s10434-009-0485-6. [DOI] [PubMed] [Google Scholar]
- 7.Ling XF, Peng X, Samman N. Donor-site morbidity of free fibula and DCIA flaps. J Oral Maxillofac Surg. 2013;71:1604–1612. doi: 10.1016/j.joms.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Gimbel M, Ashley RK, Sisodia M, et al. Repair of alveolar cleft defects: Reduced morbidity with bone marrow stem cells in a resorbable matrix. J Craniofac Surg. 2007;18:895–901. doi: 10.1097/scs.0b013e3180a771af. [DOI] [PubMed] [Google Scholar]
- 9.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 10.Sándor GK, Suuronen R. Combining adipose-derived stem cells, resorbable scaffolds and growth factors: An overview of tissue engineering. J Can Dent Assoc. 2008;74:167–170. [PubMed] [Google Scholar]
- 11.Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins. J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Moghadam HG, Urist MR, Sándor GK, et al. Successful mandibular reconstruction using a BMP bioimplant. J Craniofac Surg. 2001;12:119–127; discussion 128. doi: 10.1097/00001665-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Clokie CM, Sándor GK. Reconstruction of 10 major mandibular defects using bioimplants containing BMP-7. J Can Dent Assoc. 2008;74:67–72. [PubMed] [Google Scholar]
- 14.Barr T, McNamara AJ, Sándor GK, et al. Comparison of the osteoinductivity of bioimplants containing recombinant human bone morphogenetic proteins 2 (Infuse) and 7 (OP-1) Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:531–540. doi: 10.1016/j.tripleo.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Khojasteh A, Behnia H, Dashti SG, et al. Current trends in mesenchymal stem cell application in bone augmentation: A review of the literature. J Oral Maxillofac Surg. 2012;70:972–982. doi: 10.1016/j.joms.2011.02.133. [DOI] [PubMed] [Google Scholar]
- 16.Mesimäki K, Lindroos B, Törnwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Thesleff T, Lehtimäki K, Niskakangas T, et al. Cranioplasty with adipose-derived stem cells and biomaterial: A novel method for cranial reconstruction. Neurosurgery. 2011;68:1535–1540. doi: 10.1227/NEU.0b013e31820ee24e. [DOI] [PubMed] [Google Scholar]
- 18.Sándor GK, Tuovinen VJ, Wolff J, et al. Adipose stem cell (ASC) tissue engineered construct used to treat large anterior mandibular defect: A case report and review of the clinical application of GMP-level ASCs for bone regeneration. J Oral Maxillofac Surg. 2013;71:938–950. doi: 10.1016/j.joms.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Wolff J, Sándor GK, Miettinen A, et al. GMP-level adipose stem cells combined with computer-aided manufacturing to reconstruct mandibular ameloblastoma resection defects: Experience with 3 cases. Ann Maxillofac Surg. 2013;3:114–125. doi: 10.4103/2231-0746.119216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schliephake H, Zghoul N, Jäger V, et al. Effect of seeding technique and scaffold material on bone formation in tissue-engineered constructs. J Biomed Mater Res A. 2009;90:429–437. doi: 10.1002/jbm.a.32104. [DOI] [PubMed] [Google Scholar]
- 21.Torroni A. Engineered bone grafts and bone flaps for maxillofacial defects: State of the art. J Oral Maxillofac Surg. 2009;67:1121–1127. doi: 10.1016/j.joms.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 23.Warnke PH, Springer IN, Acil Y, et al. The mechanical integrity of in vivo engineered heterotopic bone. Biomaterials. 2006;27:1081–1087. doi: 10.1016/j.biomaterials.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 24.Behnia H, Khojasteh A, Soleimani M, et al. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: A preliminary report. J Craniomaxillofac Surg. 2012;40:2–7. doi: 10.1016/j.jcms.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Terheyden H, Knak C, Jepsen S, et al. Mandibular reconstruction with a prefabricated vascularized bone graft using recombinant human osteogenic protein-1: An experimental study in miniature pigs. Part I: Prefabrication. Int J Oral Maxillofac Surg. 2001;30:373–379. doi: 10.1054/ijom.2001.0032. [DOI] [PubMed] [Google Scholar]
- 26.Terheyden H, Warnke P, Dunsche A, et al. Mandibular reconstruction with prefabricated vascularized bone grafts using recombinant human osteogenic protein-1: An experimental study in miniature pigs. Part II: Transplantation. Int J Oral Maxillofac Surg. 2001;30:469–478. doi: 10.1054/ijom.2000.0008. [DOI] [PubMed] [Google Scholar]
- 27.Terheyden H, Menzel C, Wang H, et al. Prefabrication of vascularized bone grafts using recombinant human osteogenic protein-1-part 3: Dosage of rhOP-1, the use of external and internal scaffolds. Int J Oral Maxillofac Surg. 2004;33:164–172. doi: 10.1054/ijom.2003.0500. [DOI] [PubMed] [Google Scholar]
- 28.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 29.Gimble J, Guilak F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 30.Shahdadfar A, Frønsdal K, Haug T, et al. In vitro expansion of human mesenchymal stem cells: Choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–1366. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- 31.Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82:241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 32.Conejero JA, Lee JA, Parrett BM, et al. Repair of palatal bone defects using osteogenically differentiated fat-derived stem cells. Plast Reconstr Surg. 2006;117:857–863. doi: 10.1097/01.prs.0000204566.13979.c1. [DOI] [PubMed] [Google Scholar]
- 33.Dragoo JL, Lieberman JR, Lee RS, et al. Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat. Plast Reconstr Surg. 2005;115:1665–1673. doi: 10.1097/01.prs.0000161459.90856.ab. [DOI] [PubMed] [Google Scholar]
- 34.Haimi S, Gorianc G, Moimas L, et al. Characterization of zinc-releasing three-dimensional bioactive glass scaffolds and their effect on human adipose stem cell proliferation and osteogenic differentiation. Acta Biomater. 2009;5:3122–3131. doi: 10.1016/j.actbio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Waselau M, Patrikoski M, Juntunen M, et al. Effects of bioactive glass S53P4 or beta tricalcium phosphate and bone morphogenetic proteins 2 and BMP-7 on osteogenic differentiation of human adipose stem cells. J Tissue Eng. 2012;3:2041731412467789. doi: 10.1177/2041731412467789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tirkkonen L, Haimi S, Huttunen S, et al. Osteogenic medium is superior to growth factors in differentiation of human adipose stem cells towards bone-forming cells in 3D culture. Eur Cell Mater. 2013;25:144–158. doi: 10.22203/ecm.v025a10. [DOI] [PubMed] [Google Scholar]
- 37.Kyllönen L, Haimi S, Mannerström B, et al. Effects of different serum conditions on osteogenic differentiation of human adipose stem cells in vitro. Stem Cell Res Ther. 2013;4:17. doi: 10.1186/scrt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leong KF, Cheah CM, Chua CK. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 2003;24:2363–2378. doi: 10.1016/s0142-9612(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 39.Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7:269–291. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]