Abstract
Over 800,000 Americans abuse the psychomotor stimulant, methamphetamine, yet its abuse is without an approved medication. Methamphetamine induces hypermotor activity, and sensitization to this effect is suggested to represent aspects of the addiction process. Methamphetamine’s regulation of 3'-5'-cyclic adenosine monophosphate (cAMP) levels may be partially responsible for its behavioral effects, and compounds that inhibit phosphodiesterase (PDE), the enzyme that degrades cAMP, can alter methamphetamine-induced behaviors. Methamphetamine also activates glial cells and causes a subsequent increase in pro-inflammatory cytokine levels. Modulation of glial cell activation is associated with changes in behavioral responses, and substances that oppose inflammatory activity can attenuate drug-induced behaviors. Ibudilast (aka AV411; 3-isobutyryl-2-isopropylpyrazolo-[1,5-a]pyridine), inhibits both PDE and glial pro-inflammatory activity. Ibudilast’s amino analogue, AV1013, modulates similar glial targets but negligibly inhibits PDE. The present study determined whether ibudilast and AV1013 would attenuate methamphetamine-induced locomotor activity and its sensitization in C57BL/6J mice. Mice were treated b.i.d. with ibudilast (1.8-13 mg/kg), AV1013 (10-56mg/kg) or their vehicles intraperitoneally for 7 days, beginning 48 h before 5 days of daily 1-h locomotor activity tests. Each test was initiated by either a methamphetamine (3 mg/kg) or a saline injection. Ibudilast significantly (P<0.05) reduced the acute, chronic, and sensitization effects of methamphetamine's locomotor activity without significantly affecting activity by itself. AV1013 had similar anti-methamphetamine effects, suggesting that glial cell activity, by itself, can modulate methamphetamine's effects and perhaps serve as a medication target for its abuse.
Keywords: ibudilast, AV411, AV1013, methamphetamine, glial, relapse, sensitization, locomotor activity
1. Introduction
Methamphetamine is a psychomotor stimulant that increases activity and feelings of euphoria, and can lead to drug-seeking and chronic abuse (Everitt and Robbins, 2005; Peachey et al., 1976; Vanderschuren and Everitt, 2005; Winslow et al., 2007). Methamphetamine abuse is associated with many untoward effects such as hallucinations, pulmonary and cardiac problems, dental disease, and suppressed immunity (Hamamoto and Rhodus, 2009; Hauer, 2010; Srisurapanont et al., 2003). Currently, there are no approved pharmacotherapies for treating methamphetamine abuse, and conventional, receptor-mediated approaches have not been successful (Karila et al., 2010). As such, a fuller understanding of less conventional and less studied mechanisms mediating methamphetamine's effects may lead to improved pharmacotherapeutic approaches.
Methamphetamine is well-known for its effects on the monoamine neurotransmitters, dopamine, serotonin, and norepinephrine (Cho and Segal, 1994; Creese, 1983). Methamphetamine impedes the uptake of these monoamines into the pre-synaptic neuron while also reversing their transporter actions (Cho and Segal, 1994; Creese, 1983). Methamphetamine-induced dopamine D1 and D2 receptor activation (Sonsalla et al., 1986) results in alterations of cyclic adenosine 3’,5’-monophosphate (cAMP) levels via coupling to adenylate cyclase (Kebabian et al., 1984). In addition, methamphetamine activates glial cells (both astrocytes and microglia) (Caporaso et al., 2000; Hebert and O'Callaghan, 2000; Iyo et al., 1995) to increase pro-inflammatory cytokine production and immune reaction (Goncalves et al., 2008; Loftis et al., 2010; Nakajima et al., 2004; Yamaguchi et al., 1991). Methamphetamine’s regulation of cAMP levels and induction of inflammation are thought to be involved in its behavioral effects including hyperactivity, sensitization, and its discriminative stimulus effects (Iyo et al., 1996b; Miguel-Hidalgo, 2009; Mori et al., 2000; Niwa et al., 2007; Niwa et al., 2008; Yan et al., 2006; Yan et al., 2007), and substances that oppose its cAMP modulation or inflammatory activity have been reported to attenuate methamphetamine-induced behaviors (Iyo et al., 1996b; Niwa et al., 2007; Yan et al., 2006; Zhang et al., 2006).
Given these observations, both PDE-inhibition and glial cell modulation are potential mechanisms for reducing methamphetamine-induced behaviors. Ibudilast (aka, AV411; 3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine) is a non-selective PDE inhibitor, glial cell modulator and anti-inflammatory agent (Gibson et al., 2006; Kishi et al., 2001). Ibudilast is marketed in Japan to treat bronchial asthma and ischemic stroke (Kishi et al., 2001), and is being clinically evaluated for treating neuropathic pain (Ledeboer et al., 2006) and opioid dependency (Hutchinson et al., 2009). Because other inhibitors of PDE activity and glial activation attenuate methamphetamine's effects (see above), and because we previously reported ibudilast to attenuate stress- and prime-induced reinstatement of methamphetamine drug-seeking in rats (Beardsley et al., 2010), we examined the ability of ibudilast to attenuate the acute and chronic effects of methamphetamine-induced hyperactivity and sensitization in mice. Additionally, we tested the amino analogue of ibudilast, AV1013, which retains ibudilast’s ability to inhibit glial cell activation but has minimal PDE inhibitory effects (Cho et al., 2010), to determine whether PDE inhibition was essential for the initial effects we observed with ibudilast.
2. Materials and methods
2.1 Subjects
Male adult C57BL/6J mice were obtained at approximately 8 weeks of age (The Jackson Laboratory, Bar Harbor, ME) and were allowed to acclimate to the vivarium for approximately one week prior to commencement of testing. The mice were housed at a maximum of four per cage in an AAALAC-accredited animal facility with food (7012 Teklad LM-485 Mouse/Rat Sterilizable Diet, Harlan Laboratories, Inc., Indianapolis, IN) and water available ad libitum under a 12-h/12-h light/dark cycle (lights illuminated from 0600-h to 1800-h) with all testing occurring during the light phase. All procedures were carried out in accordance with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academy Press, 1996) and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
2.2 Apparatus
Locomotor activity tests were conducted in eight commercially obtained, automated activity monitoring devices each enclosed in sound- and light-attenuating chambers that recorded distance travelled in cm in 10-min bins via computer-controlled circuitry (AccuScan Instruments, Columbus OH). The interior of each device was divided into separate 20×20×30 cm arenas permitting the independent and simultaneous measurement of two mice. Sixteen photobeam sensors per axis were spaced 2.5 cm apart along the walls of the chamber and were used to detect movement.
2.3 Locomotor Activity Procedure
One hundred and twenty-eight mice were randomly assigned into 16 groups of eight mice each. Eight groups were treated b.i.d. for 7 days with subcutaneous (s.c.) injections of either 0 (vehicle; VEH1), 1.8, 7.5, or 13 mg/kg ibudilast, with two groups of eight at each dose. Eight other groups were similarly treated but with 0 (vehicle; VEH2), 10, 30, or 56 mg/kg AV1013. Both ibudilast and AV1013 injections occurred twice daily separated approximately 7 hours apart (0900-h and 1600-h). During the last five days of these seven-day regimens (Days 3-7), the mice were given locomotor activity tests. Two, 1-h locomotor activity sessions (Baseline and Test) were given on Days 1 and 5. Single locomotor activity sessions were given on Days 2-4 to minimize the occurrence of extinction of any conditioned locomotor activity effects in methamphetamine treated mice. On days when locomotor activity sessions were administered (Days 3-7), morning ibudilast and AV1013 injections were given one hour prior to the first session. Immediately prior to Baseline and Test sessions on Days 1 and 5, all mice were injected intraperitoneally (i.p.) with saline or 3 mg/kg methamphetamine, respectively. On Days 2-4, half of all mice in the ibudilast and AV1013 groups received 3 mg/kg i.p. methamphetamine (METH) before all locomotor activity sessions (IBUD+METH and AV1013+METH groups), while the other half received saline injections (IBUD+SAL and AV1013+SAL groups). Thus, the mice were distributed across groups as shown in Table 1 and treated as shown in Table 2.
Table 1.
Distribution of mice in chronically and acutely treated methamphetamine (METH) groups.
| Chronic METH | Acute METH |
|---|---|
| Ibudilast Groups | |
| VEH1+METH | VEH1+SAL |
| 1.8 IBUD+METH | 1.8 IBUD+SAL |
| 7.5 IBUD+METH | 7.5 IBUD+SAL |
| 13 IBUD+METH | 13 IBUD+SAL |
| AV1013 Groups | |
| VEH2+METH | VEH2+SAL |
| 10 AV1013+METH | 10 AV1013+SAL |
| 30 AV1013+METH | 30 AV1013+SAL |
| 56 AV1013+METH | 56 AV1013+SAL |
Table 2.
Treatment procedures.
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|
| 1Chronic METH groups | |||||
| Injection #1 Session |
Saline Baseline 1 |
Saline Baseline 5 |
|||
| Injection #2 Session |
METH Test 1 |
METH Test 2 |
METH Test 3 |
METH Test 4 |
METH Test 5 |
|
| |||||
| 2Acute METH groups | |||||
| Injection #1 Session |
Saline Baseline 1 |
Saline Baseline 5 |
|||
| Injection #2 Session |
Saline Test 1 |
Saline Test 2 |
Saline Test 3 |
Saline Test 4 |
METH Test 5 |
Groups include: IBUD+METH, AV1013+METH, VEH1+METH, VEH2+METH
Groups include: IBUD+SAL, AV1013+SAL, VEH1+SAL, VEH2+SAL
2.4 Drugs
(±)-Methamphetamine (National Institute on Drug Abuse, Rockville, MD) was prepared in 0.9% saline stock solutions sterilized by filtration through 0.2 μm filtration disks. Working methamphetamine solutions were dissolved in sterile 0.9% saline and injected i.p. Ibudilast (3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine) and AV1013 ((R)-2-amino-1-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)propan-1-one hydrochloride) were received as a gift from MediciNova, Inc., San Diego, CA). Ibudilast was prepared in 35% polyethylene glycol (PEG) in saline vehicle and administered s.c. (referred to below as "VEH1"). Doses of AV1013 were administered s.c. and prepared in sterile 0.9% saline (referred to below as "VEH2"), with the exception of the highest dose (56 mg/kg) that was solubilized in a 35% PEG in saline vehicle (i.e., VEH1) because of its incomplete dissolution in 0.9% saline. All injections were given in a volume equivalent to 10 ml/kg body weight.
2.5 Data Analysis
Distance travelled (cm) was subjected to analysis by a mixed-model ANOVA (repeated measures on Testday test and between comparisons on drug condition) for the chronically administered methamphetamine and vehicle groups separately for each drug (i.e., 2 drugs × 2 methamphetamine treatment conditions= 4 ANOVAs). Comparisons between ibudilast or AV1013-treated mice to their respective vehicle condition were made using Bonferroni Multiple Comparisons Tests. AD50 (CI) values for attenuating methamphetamine hyperactivity by 50% relative to vehicle controls were estimated by first converting distance travelled scores for each mouse to percent of its respective mean vehicle control, logarithmically transforming dose, and using nonlinear regression assuming a normalized response. All statistical tests were conducted using computer software (Prism 5d for Macintosh, GraphPad Software, Inc., San Diego, CA), and all types of comparisons were considered statistically significant if P<0.05.
3. Results
3.1 Ibudilast and Chronic Methamphetamine
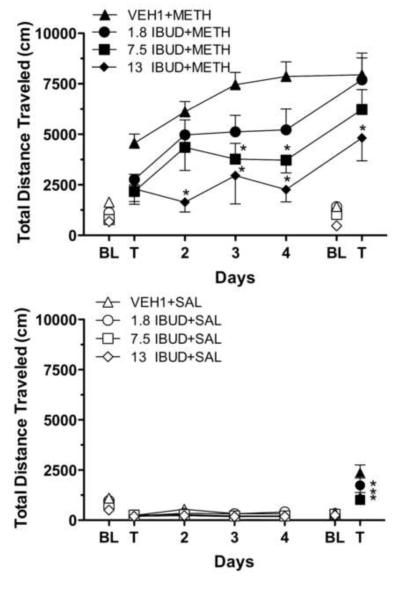
Figure 1 shows the effects of ibudilast on chronic methamphetamine administration (upper frame) and on chronic vehicle administration (lower frame). ANOVA results indicated that drug [F(3,28)=7.093; p=0.0011] and time [F(6,168)=56.64; P<0.0001] and their interaction [F(18,168)=2.479; p=0.0013] significantly affected activity. Methamphetamine induced a significant increase in total distance travelled of over 2900 cm during the Testday 1 test relative to Baseline 1 levels in the VEH1+METH group (t=3.735, df=7, P<0.05) (Figure 1, upper frame; significance not indicated by asterisks). Methamphetamine also induced increases in distance travelled during the Testday 1 test from Baseline 1 levels in the IBUD+METH treated groups, but their levels were non-significantly different, and increases were less than those of the VEH1+METH group. Distance travelled progressively increased in the VEH1+METH group following each subsequent day of methamphetamine administration and was significantly (t=4.325, df=7, P<0.01) greater during the Testday 5 test relative to the Testday 1 test indicative of sensitization. Distance travelled on Testday 5 was significantly greater relative to Testday 1 in the 1.8 IBUD+METH (t=6.316, df=7, P<0.0001) and 7.5 IBUD+METH (t=5.2000, df=7, P<0.0001) groups, but not the 13 IBUD+METH group, indicating that 13 mg/kg ibudilast blocked the induction of sensitization. Ibudilast reduced distance travelled during all test sessions following methamphetamine administration relative to the VEH1+METH treatment group, and significantly so during Testday 2-5 tests at 13 mg/kg ibudilast and during Testday 3 and 4 tests at 7.5 mg/kg ibudilast.
Fig. 1.
Upper frame: Results on distance travelled (cm) by mice treated b.i.d. for seven days with ibudilast (IBUD) or its vehicle (VEH1), beginning two days before five days of treatment with 3 mg/kg methamphetamine. Ibudilast was administered at 1.8, 7.5, or 13 mg/kg. Data points represent group means (±S.E.M.) obtained during 1-h experimental sessions. Filled data points represent sessions preceded by 3 mg/kg i.p. methamphetamine injections. Unfilled data points represent sessions preceded by i.p. saline injections. N=8 for each treatment group. *P<0.05 with respect to mice treated with ibudilast's vehicle.
Lower frame: Results on distance travelled (cm) by mice treated b.i.d. for seven days with ibudilast (IBUD) or its vehicle (VEH1), beginning two days before four days of saline administration and acute treatment with 3 mg/kg methamphetamine on the fifth day. Ibudilast was administered at 1.8, 7.5, or 13 mg/kg. Data points represent group means (±S.E.M.) obtained during 1-h experimental sessions. Other details are as in the upper frame.
3.2 Ibudilast and Acute Methamphetamine
Distance travelled did not differ between the VEH1+SAL group and any of the ibudilast groups following saline administration indicating ibudilast did not affect locomotor behavior in mice without methamphetamine histories (Figure 1, lower frame). However, ibudilast significantly reduced distance travelled following methamphetamine administration during the Testday 5 test, relative to the VEH1+SAL group, at all doses of ibudilast (1.8 mg/kg ibudilast: t=3.278, df=7, P<0.05; 7.5 mg/kg ibudilast: t=6.944, df=7, P<0.0001; 13 mg/kg ibudilast: t=6.374, df=7, P<0.0001) indicating its ability to blunt the acute challenge by methamphetamine.
3.3 AV1013 and Chronic Methamphetamine
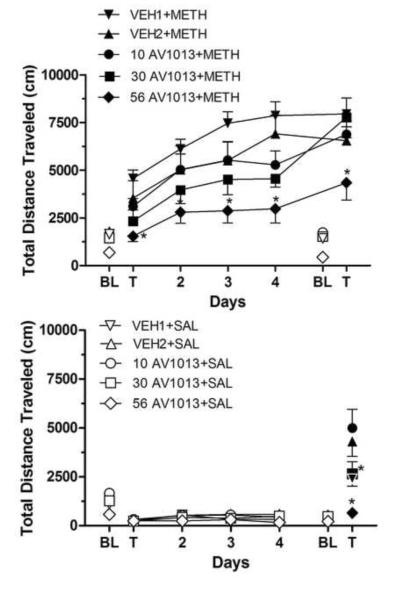
Figure 2 shows the effects of AV1013 on chronic methamphetamine administration (upper frame) and on chronic vehicle administration (lower frame). ANOVA indicated a significant effect of drug [F(4,34)=5.947; p=0.0010], time [F(6,204)=92.28; P<0.0001, and their interaction [F(24,204)=2.282; p=0.0010]. Methamphetamine induced a significant increase in total distance travelled during the Testday 1 test relative to Baseline 1 levels in the VEH1+METH group (t=4.341, df=7, P<0.001). Methamphetamine induced a non-significant mean increase in total distance travelled in the VEH2+METH group during the Testday 1 test relative to Baseline 1 levels, which further increased to significant levels during Testday 2 tests (t=4.530, df=7, P<0.001) (Figure 2, upper frame). Methamphetamine induced increases in total distance travelled by all AV1013 treatment groups during the Testday 1 test relative to Baseline 1 levels; however, these were non-significant increases and were always less than respective control vehicle groups. Total distance travelled generally increased in all groups following each subsequent day of methamphetamine administration and were significantly greater during the Testday 5 test relative to the Testday 1 test in all chronically-treated methamphetamine groups (VEH2+METH: t=4.181, df=7, P<0.001; VEH1+METH: t=5.027, df=7, P<0.001, 10 AV1013+METH: t=5.618, df=7, P<0.001; 30 AV1013+METH: t=8.095, df=7, P<0.001, 56 AV1013+METH: t=4.154, df=7, P<0.001). Total distance travelled was significantly reduced following 56 mg/kg AV1013 administrations relative to its vehicle control group (VEH1+METH) following methamphetamine treatment on all days. (Testday 1: t=3.357, df=7, P<0.01; Testday 2: t=3.681, df=7, P<0.01; Testday 3: t=5.089, df=7, P<0.001; Testday 4: t=5.434, df=7, P<0.001;Testday5:t=4.009, df=7, P<0.01).
Fig. 2.
Upper frame: Results on distance travelled (cm) by mice treated b.i.d. for seven days with AV1013 or its vehicle (VEH2 for 10 and 30 mg/kg and VEH1 for 56 mg/kg), beginning two days before five days of treatment with 3 mg/kg methamphetamine. AV1013 was administered at 10, 30, or 56 mg/kg. *P<0.05 with respect to mice treated with AV1013's vehicle. Other details are as in Figure 1.
Lower frame: Results on distance travelled (cm) by mice treated b.i.d. for seven days with AV1013 or its vehicle (VEH2 for 10 and 30 mg/kg and VEH1 for 56 mg/kg), beginning two days before four days of saline administration and acute treatment with 3 mg/kg methamphetamine on the fifth day. Other details are as in the upper frame.
3.4 AV1013 and Acute Methamphetamine
Total distance travelled did not differ between the VEH2+SAL group and either the 10 AV1013+SAL or 30 AV1013+SAL groups during all test sessions that were preceded by saline administration, indicating that AV1013 did not affect locomotor behavior on its own (Figure 2, lower frame). Similarly, distance travelled did not differ between the VEH1+SAL and 56 AV1013+SAL group indicating 56 mg/kg AV1013 did not affect locomotor behavior in mice without a methamphetamine history. However, following methamphetamine challenge during Testday 5 tests, 30 mg/kg AV1013 significantly reduced levels of total distance travelled relative to its vehicle control group, VEH2+SAL (t=4.683, df=7, P<0.001), as did 56 mg/kg AV1013 relative to its vehicle control group, VEH1+SAL (t=4.900, df=7, P<0.001).
3.5 Ibudilast vs. AV1013
The AD50 (CI) for ibudilast to reduce the hyperactivity effects of acute methamphetamine challenge on Testday 1 was 7.146 (3.763-13.57) mg/kg for groups to be chronically-treated with methamphetamine. By Testday 5 the AD50(CI) increased to 23.23 (9.660-55.86) mg/kg in these groups. In groups whose first exposure to methamphetamine was on Testday 5, but which had received chronic ibudilast up to Testday 5, the AD50 (CI) was 7.092 (3.420-14.71) mg/kg. This AD50 value was non-significantly different from that on Testday 1 in the chronically treated methamphetamine group (i.e., vs. 7.146 mg/kg).
AV1013 attenuated methamphetamine's effects with an AD50(CI) of 43.88 (19.40-99.27) mg/kg on Testday 1 that increased to 201.2 (51.49-786.0) mg/kg on Testday 5 in the chronically treated methamphetamine groups. In groups whose first exposure to methamphetamine was on Testday 5 but which had received chronic AV1013 up to Testday 5 the AD50 (CI) was 48.13 (19.05-121.7) mg/kg. This AD50 value was non-significantly different from that on Testday 1 in the chronically treated methamphetamine group (i.e., vs. 43.88 mg/kg).
When compared to each other, ibudilast produced significantly lower AD50 values than AV1013 on both Testday 1 [F(1,61)=11.32; p=0.0013] and on Testday 5 [F(1,61)=6.978; p=0.0105] in groups chronically-treated with methamphetamine, as well when comparing groups chronically-treated with saline and challenged for the first time on Testday 5 [F(1,62)=10.90; p=0.0016].
4. Discussion
Ibudilast dose-dependently reduced both chronically and acutely administered methamphetamine-induced locomotor activity. Chronic treatment with methamphetamine provided evidence of sensitization as subsequent administrations elicited greater increases in distance travelled. The highest dose of ibudilast (13 mg/kg) tested significantly attenuated these methamphetamine-induced sensitization effects. Ibudilast’s analogue, AV1013, which lacks its potency for inhibiting PDE, but retains its ability to suppress activated glial activity, similarly dose-dependently attenuated methamphetamine’s chronic and acute locomotor activity effects, but was ~6-9 fold less potent in doing so. These later observations suggest that the ability to modulate glial activity is sufficient to attenuate methamphetamine’s locomotor activity effects, although PDE inhibition likely can additionally contribute if present.
Ibudilast is a non-selective PDE inhibitor (Gibson et al., 2006; Kishi et al., 2001), glial cell modulator and anti-inflammatory agent (Mizuno et al., 2004; Suzumura et al., 1999), and an inhibitor of macrophage migration inhibitory factor (Cho et al., 2010). As such, its effects could be a result of any or a combination of all these mechanisms. Some of these effects have already been reported to reduce methamphetamine activity (see below). It is unlikely ibudilast’s effects on methamphetamine are a result of directly affecting conventional mechanisms, for it doesn't have effective activity at ~100 other radioligand binding and enzyme targets (Ledeboer et al., 2006).
PDE inhibition, by itself, significantly reduces some methamphetamine behaviors. PDE inhibitors, such as rolipram and nefiracetam, attenuate methamphetamine-induced locomotor activity, sensitization, and the discriminative stimulus effects of methamphetamine (Iyo et al., 1996a; Iyo et al., 1996b; Iyo et al., 1995; Mori et al., 2000; Yan et al., 2004; Yan et al., 2006). Phosphodiesterase degrades cAMP (Beavo, 1995), and PDE inhibitors, like ibudilast, will increase cAMP levels. Alterations in cAMP levels would likely alter the transduction of receptor signaling dependent upon it, such as with the dopamine D1 and D2 receptors (Kebabian et al., 1984), through which methamphetamine exerts its locomotor and sensitization effects (Kelly et al., 2008).
Methamphetamine increases levels of cytokines and inflammatory factors, such as tumor necrosis factor (TNFα), interleukin 6 (IL-6), interleukin 1β (IL-1β) mRNA levels, monocyte chemo-attractant protein 1 (MCP-1), and cellular adhesion molecule (ICAM-1) (Goncalves et al., 2008; Nakajima et al., 2004; Yamaguchi et al., 1991). Attenuation of glial cell activation and pro-inflammatory signaling, and up-regulation of neuro-protective factors, activities of both ibudilast and AV1013, have also been reported to attenuate some of methamphetamine's effects. For example, induction of GDNF inhibits methamphetamine-induced locomotor sensitization (Niwa et al., 2007). Conversely, reducing GDNF levels potentiates methamphetamine self-administration and reinstatement vulnerability (Yan et al., 2007). Additionally, indomethacin and minocycline, two anti-inflammatory drugs, prevent glial activation (Goncalves et al., 2010) and attenuate methamphetamine hyperlocomotion and sensitization in mice (Zhang et al., 2006). Ibudilast’s anti-inflammatory action reduces glial activation by suppressing TNFα, IL-6, IL-1β, MCP-1, and nitric oxide (NO), while also increasing production of GDNF (Mizuno et al., 2004; Suzumura et al., 1999). Although AV1013 lacks the efficacy of PDE inhibition of ibudilast, it has similar glial cell modulatory activity (Cho et al., 2010). Both ibudilast and AV1013 reduce methamphetamine-induced locomotor behavior, suggesting that AV1013’s modulation of glial cell activation is sufficient to attenuate methamphetamine effects.
How attenuation of glial cell activation and neuroinflammatory activity translates into modulating methamphetamine's behavioral effects is unknown. One proposed mechanism involves the ability of glial cells to regulate neurotransmission and synaptic strength by affecting the cell surface delivery and retention of glutamatergic NMDA and AMPA receptors (Eroglu and Barres, 2010). Interestingly, an up-regulation of TNFα, elicited from activated astrocytes, increases AMPA receptor expression on the cell surface and increases NMDA and AMPA receptor-mediated synaptic currents (Beattie et al., 2002; Stellwagen and Malenka, 2006) that improves synaptic efficacy. Conversely, blockade of TNFα has the opposite effect (Beattie et al., 2002). Therefore, methamphetamine-induced increases in TNFα could indirectly increase the concentration of AMPA receptors and their activation. In contrast, ibudilast and AV1013’s attenuation of TNFα levels would inhibit delivery of these receptors preventing signaling and synaptic change. Excessive activation of both AMPA and metabotropic glutamate receptors may play a role in behavioral sensitization and in the rewarding properties of stimulants such as cocaine and methamphetamine (Wolf, 1998). Thus, the blockade of these processes may be a link to suppressing the effects of stimulant drugs.
An additional way in which TNFα levels may be affected by ibudilast is via cAMP production, which reduces TNFα synthesis (Kast, 2000; Shames et al., 2001). Thus, under ibudilast treatment, PDE inhibition increases cAMP and in an inhibition of TNFα synthesis that reduces further glial activation. If true, perhaps ibudilast’s PDE inhibition and glial modulatory effects are working in conjunction to produce the observed results. AV1013’s minimal PDE inhibitory effects may dampen its attenuation of TNFα synthesis, which could contribute to its lower potency relative to ibudilast. AV1013’s lower potency could also be due to reduced potency at the drugs’ glial targets. Ibudilast and AV1013 are noncompetitive inhibitors of the p-hydroxyphenylpyruvate (HPP) tautomerase activity of the proinflammatory protein, macrophage migration inhibiting factor (MIF), which could be an important mechanism involving their anti-inflammatory effects (Cho et al., 2010). AV1013 is a less potent inhibitor of MIF with a Ki=74.9 (±8.5) uM than is ibudilast, which has a Ki of 30.9 (±2.8) uM. Given this, AV1013 may be less effective in reducing methamphetamine activity due to a combination of decreased potency at both PDE and glial targets.
Lilius (Lilius et al., 2009) reported that ibudilast could induce decreases in spontaneous locomotor activity in rats following its acute administration. Although the Lilius study used rats, and the present study used mice, the possibility of direct locomotor decreasing effects needs to be considered in interpreting ibudilast's and AV1013's modulation of methamphetamine's locomotor activity effects. It is unlikely that these potential locomotor decreasing effects could explain the magnitude of their effects on methamphetamine's activity. Importantly, none of the dosage regimens of ibudilast or AV1013 produced statistically significant reductions in locomotor activity during either baseline test. Perhaps if there had been important locomotor decreasing effects of these drugs initially, tolerance developed to them, for the drugs were given b.i.d beginning two days prior to the initiation of testing, whereas in the Lilius study ibudilast was given acutely. Additionally, acute tolerance may have occurred as well, for in the Lilius study, ibudilast was administered 15 min before testing, whereas in the present study it was given one hour prior to locomotor tests, and sedative-like effects appear to wane within 30 min of its administration (Ledeboer et al., 2006).
Both ibudilast and AV1013 attenuated methamphetamine-induced locomotor activity when administered concurrently with methamphetamine. These results suggest that these drugs potentially could blunt methamphetamine’s stimulatory effects or “value” to chronic users potentially facilitating the effectiveness of other interventions such as psychotherapy. Additionally, ibudilast and AV1013 significantly attenuated the hyperactivity effects following acute methamphetamine challenge. Considering that limited re-exposure to an abused drug can precipitate a longer-termed relapse in an abstinent abuser (Bigelow et al., 1977; Chornock et al., 1992; de Wit, 1996), ibudilast and AV1013’s attenuation of an acute methamphetamine challenge suggests usefulness as a relapse prevention treatment in abstinent abusers, which is consistent with our previous report that ibudilast reduces reinstatement precipitated by methamphetamine primes in rats previously reinforced with methamphetamine (Beardsley et al., 2010). The possibility of clinically useful relapse prevention now extends to AV1013 as well. The AD50 values for reducing the effects of the 3 mg/kg methamphetamine challenge dose did not differ within ibudilast and AV1013 groups between the acutely and chronically treated mice, suggesting that peak ability to blunt methamphetamine’s effects was reached by two days of b.i.d. administration. This speculation requires the qualification that only a single methamphetamine dose (3 mg/kg) was tested, and administration of these drugs was not given for longer than seven days. Similarity between these AD50 values also suggests that tolerance to their effectiveness did not develop, a desirable feature in a potential pharmacotherapeutic.
Several additional observations strengthen the interest in these drugs.. In the present study, repeated administration of methamphetamine-induced sensitization to its locomotor activity effects was significantly attenuated by 13 mg/kg ibudilast. It has been suggested that sensitization plays a key role in drug addiction in humans (Chen et al., 2009; Sax and Strakowski, 2001). For example, three doses of d-amphetamine given to healthy human volunteers produces significant increases in eye-blink and locomotor scores, as well as in reported mood and subjective drug effects (i.e. euphoria) suggestive of sensitization (Strakowski and Sax, 1998). In conjunction with the behavioral effects, the adaptations of specific brain regions implicated in the process of sensitization have been associated with reward pathways linked to drug-seeking and addiction (Robinson and Berridge, 1993). Thus, a compound that blunts sensitization may have additional merit for consideration as a pharmacotherapy for drug abuse. Furthermore, because the neuro-circuitry, neurotransmitter, and neuronal receptor systems activated in reinstatement models of drug abuse are similar to those systems involved in the process of sensitization (Steketee and Kalivas, 2011), the potential usefulness of these drugs in treating methamphetamine relapse is even further enhanced.
5. Conclusion
The present study indentified that both ibudilast and its analog, AV1013, are able to attenuate methamphetamine-induced locomotor activity in mice. Given AV1013's impotency to affect PDE activity, these results suggest that glial cell modulation alone may be sufficient for attenuating these methamphetamine effects. Treatments for stimulant abuse targeting conventional mechanisms have generally proven unsuccessful. The present results are consistent with others suggesting that modulating glial cell activity with drugs could provide a novel, and perhaps fruitful target for treating methamphetamine abuse.
7. Acknowledgements
This research was completed in partial fulfillment of the doctoral requirements in Pharmacology and Toxicology for S. E. S.
This research was supported by Virginia Commonwealth University's Center for Biomarker Research and Personalized Medicine and Institute for Drug and Alcohol Studies.
Footnotes
6. Disclosure Statement
P. M. B. previously served as a consultant to Avigen Inc., a company that at one time was involved with the pharmaceutical development of ibudilast and AV1013.
8. References
- Beardsley P, Shelton K, Hendrick E, Johnson K. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur. J. Pharmacol. 2010;637:102–108. doi: 10.1016/j.ejphar.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol. Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Bigelow GE, Griffiths RR, Liebson IA. Pharmacological influences upon human ethanol self-administration. Adv. Exp. Med. Biol. 1977;85B:523–538. doi: 10.1007/978-1-4615-9038-5_33. [DOI] [PubMed] [Google Scholar]
- Caporaso GL, Bibb JA, Snyder GL, Valle C, Rakhilin S, Fienberg AA, Hemmings HC, Nairn AC, Greengard P. Drugs of abuse modulate the phosphorylation of ARPP-21, a cyclic AMP-regulated phosphoprotein enriched in the basal ganglia. Neuropharmacology. 2000;39:1637–1644. doi: 10.1016/s0028-3908(99)00230-0. [DOI] [PubMed] [Google Scholar]
- Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung medical journal. 2009;32:148–154. [PubMed] [Google Scholar]
- Cho AK, Segal DS. Amphetamine and its analogs : psychopharmacology, toxicology, and abuse. Academic Press; San Diego: 1994. [Google Scholar]
- Cho Y, Crichlow G, Vermeire J, Leng L, Du X, Hodsdon M, Bucala R, Cappello M, Gross M, Gaeta F, Johnson K, Lolis E. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11313–11318. doi: 10.1073/pnas.1002716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chornock WM, Stitzer ML, Gross J, Leischow S. Experimental model of smoking re-exposure: effects on relapse. Psychopharmacology (Berl) 1992;108:495–500. doi: 10.1007/BF02247427. [DOI] [PubMed] [Google Scholar]
- Creese I. Stimulants, neurochemical, behavioral, and clinical perspectives. Raven Press; New York: 1983. [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Gibson LC, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, Mackenzie FL, Nagasawa M, Stevens PA, Mackenzie SJ. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur. J. Pharmacol. 2006;538:39–42. doi: 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Goncalves J, Baptista S, Martins T, Milhazes N, Borges F, Ribeiro CF, Malva JO, Silva AP. Methamphetamine-induced neuroinflammation and neuronal dysfunction in the mice hippocampus: preventive effect of indomethacin. Eur. J. Neurosci. 2010;31:315–326. doi: 10.1111/j.1460-9568.2009.07059.x. [DOI] [PubMed] [Google Scholar]
- Goncalves J, Martins T, Ferreira R, Milhazes N, Borges F, Ribeiro CF, Malva JO, Macedo TR, Silva AP. Methamphetamine-induced early increase of IL-6 and TNF-alpha mRNA expression in the mouse brain. Ann. N. Y. Acad. Sci. 2008;1139:103–111. doi: 10.1196/annals.1432.043. [DOI] [PubMed] [Google Scholar]
- Hamamoto DT, Rhodus NL. Methamphetamine abuse and dentistry. Oral Dis. 2009;15:27–37. doi: 10.1111/j.1601-0825.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- Hauer P. Systemic affects of methamphetamine use. S D Med. 2010;63:285–287. [PubMed] [Google Scholar]
- Hebert MA, O'Callaghan JP. Protein phosphorylation cascades associated with methamphetamine-induced glial activation. Ann. N. Y. Acad. Sci. 2000;914:238–262. doi: 10.1111/j.1749-6632.2000.tb05200.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Lewis S, Coats B, Skyba D, Crysdale N, Berkelhammer D, Brzeski A, Northcutt A, Vietz C, Judd C, Maier S, Watkins L, Johnson K. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain. Behav. Immun. 2009;23:240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyo M, Bi Y, Hashimoto K, Inada T, Fukui S. Prevention of methamphetamine-induced behavioral sensitization in rats by a cyclic AMP phosphodiesterase inhibitor, rolipram. Eur. J. Pharmacol. 1996a;312:163–170. doi: 10.1016/0014-2999(96)00479-7. [DOI] [PubMed] [Google Scholar]
- Iyo M, Bi Y, Hashimoto K, Tomitaka S, Inada T, Fukui S. Does an increase of cyclic AMP prevent methamphetamine-induced behavioral sensitization in rats? Ann. N. Y. Acad. Sci. 1996b;801:377–383. doi: 10.1111/j.1749-6632.1996.tb17458.x. [DOI] [PubMed] [Google Scholar]
- Iyo M, Maeda Y, Inada T, Kitao Y, Sasaki H, Fukui S. The effects of a selective cAMP phosphodiesterase inhibitor, rolipram, on methamphetamine-induced behavior. Neuropsychopharmacology. 1995;13:33–39. doi: 10.1016/0893-133X(94)00133-K. [DOI] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br. J. Clin. Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast RE. Tumor necrosis factor has positive and negative self regulatory feed back cycles centered around cAMP. Int. J. Immunopharmacol. 2000;22:1001–1006. doi: 10.1016/s0192-0561(00)00046-1. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Beaulieu M, Itoh Y. Pharmacological and biochemical evidence for the existence of two categories of dopamine receptor. Can. J. Neurol. Sci. 1984;11:114–117. doi: 10.1017/s0317167100046254. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Low MJ, Rubinstein M, Phillips TJ. Role of dopamine D1-like receptors in methamphetamine locomotor responses of D2 receptor knockout mice. Genes, brain, and behavior. 2008;7:568–577. doi: 10.1111/j.1601-183X.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi Y, Ohta S, Kasuya N, Sakita S, Ashikaga T, Isobe M. Ibudilast: a non-selective PDE inhibitor with multiple actions on blood cells and the vascular wall. Cardiovascular drug reviews. 2001;19:215–225. doi: 10.1111/j.1527-3466.2001.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Liu T, Shumilla JA, Mahoney JH, Vijay S, Gross MI, Vargas JA, Sultzbaugh L, Claypool MD, Sanftner LM, Watkins LR, Johnson KW. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron glia biology. 2006;2:279–291. doi: 10.1017/S1740925X0700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilius TO, Rauhala PV, Kambur O, Kalso EA. Modulation of morphine-induced antinociception in acute and chronic opioid treatment by ibudilast. Anesthesiology. 2009;111:1356–1364. doi: 10.1097/ALN.0b013e3181bdfa11. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine Causes Persistent Immune Dysregulation: A Cross-Species, Translational Report. Neurotox Res. 2010 doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. The Role of Glial Cells in Drug Abuse. Current drug abuse reviews. 2009;2:76–82. doi: 10.2174/1874473710902010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N, Suzumura A. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46:404–411. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Mori T, Baba J, Ichimaru Y, Suzuki T. Effects of rolipram, a selective inhibitor of phosphodiesterase 4, on hyperlocomotion induced by several abused drugs in mice. Jpn. J. Pharmacol. 2000;83:113–118. doi: 10.1254/jjp.83.113. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, He J, Nitta A, Mizuno M, Tran MH, Seto A, Yoshimura M, Kitaichi K, Hasegawa T, Saito K, Yamada Y, Seishima M, Sekikawa K, Kim HC, Nabeshima T. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J. Neurosci. 2004;24:2212–2225. doi: 10.1523/JNEUROSCI.4847-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Yamada K, Nabeshima T. The roles of glial cell line-derived neurotrophic factor, tumor necrosis factor-alpha, and an inducer of these factors in drug dependence. J Pharmacol Sci. 2007;104:116–121. doi: 10.1254/jphs.cp0070017. [DOI] [PubMed] [Google Scholar]
- Niwa M, Yan Y, Nabeshima T. Genes and molecules that can potentiate or attenuate psychostimulant dependence: relevance of data from animal models to human addiction. Ann. N. Y. Acad. Sci. 2008;1141:76–95. doi: 10.1196/annals.1441.024. [DOI] [PubMed] [Google Scholar]
- Peachey E, Rogers B, Brien JF, Maclean A, Rogers D. Measurement of acute and chronic behavioural effects of methamphetamine in the mouse. Psychopharmacology (Berl) 1976;48:271–275. doi: 10.1007/BF00496860. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM. Behavioral sensitization in humans. J. Addict. Dis. 2001;20:55–65. doi: 10.1300/J069v20n03_06. [DOI] [PubMed] [Google Scholar]
- Shames BD, McIntyre RC, Jr., Bensard DD, Pulido EJ, Selzman CH, Reznikov LL, Harken AH, Meng X. Suppression of tumor necrosis factor alpha production by cAMP in human monocytes: dissociation with mRNA level and independent of interleukin-10. J. Surg. Res. 2001;99:187–193. doi: 10.1006/jsre.2001.6178. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Gibb JW, Hanson GR. Roles of D1 and D2 dopamine receptor subtypes in mediating the methamphetamine-induced changes in monoamine systems. The Journal of pharmacology and experimental therapeutics. 1986;238:932–937. [PubMed] [Google Scholar]
- Srisurapanont M, Ali R, Marsden J, Sunga A, Wada K, Monteiro M. Psychotic symptoms in methamphetamine psychotic in-patients. Int J Neuropsychopharmacol. 2003;6:347–352. doi: 10.1017/S1461145703003675. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug Wanting: Behavioral Sensitization and Relapse to Drug-Seeking Behavior. Pharmacol. Rev. 2011 doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Ito A, Yoshikawa M, Sawada M. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 1999;837:203–212. doi: 10.1016/s0006-8993(99)01666-2. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Behavioral and neural mechanisms of compulsive drug seeking. Eur. J. Pharmacol. 2005;526:77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Winslow BT, Voorhees KI, Pehl KA. Methamphetamine abuse. Am. Fam. Physician. 2007;76:1169–1174. [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog. Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kuraishi Y, Minami M, Nakai S, Hirai Y, Satoh M. Methamphetamine-induced expression of interleukin-1 beta mRNA in the rat hypothalamus. Neurosci. Lett. 1991;128:90–92. doi: 10.1016/0304-3940(91)90766-m. [DOI] [PubMed] [Google Scholar]
- Yan Y, Mizuno T, Nitta A, Yamada K, Nabeshima T. Nefiracetam attenuates methamphetamine-induced discriminative stimulus effects in rats. Ann. N. Y. Acad. Sci. 2004;1025:274–278. doi: 10.1196/annals.1316.034. [DOI] [PubMed] [Google Scholar]
- Yan Y, Nitta A, Mizuno T, Nakajima A, Yamada K, Nabeshima T. Discriminative-stimulus effects of methamphetamine and morphine in rats are attenuated by cAMP-related compounds. Behav. Brain Res. 2006;173:39–46. doi: 10.1016/j.bbr.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. FASEB J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog. Neuropsychopharmacol. Bol. Psychiatry. 2006;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]