Abstract
The mRNAs of most inflammatory mediators are short-lived due to AU-rich elements (AREs) in their 3′-untranslated regions. AREs ensure a low basal level of expression during homeostasis and a transient nature of expression during the inflammatory response. Here, we report that the mRNA of the pro-inflammatory chemokine IL-8, which contains an archetypal ARE, is unexpectedly constitutively abundant and highly stable in primary human monocytes and macrophages. Using the pre-monocyte-like THP-1 cell line that can differentiate into macrophage-like cells, we show that a low level of unstable IL-8 mRNA in undifferentiated cells (half-life < 30 min) becomes constitutively elevated and the mRNA is dramatically stabilized in differentiated THP-1 cells with a half-life of more than 15 h similar to primary monocytes and macrophages. In contrast, the level and stability of TNF-α mRNA also containing an ARE is only slightly affected by differentiation; it remains low and unstable in primary macrophages and differentiated THP-1 cells with an estimated half-life of less than 20 min. This differentiation-dependent stabilization of IL-8 mRNA is p38 MAPK-independent and is probably coupled with reduced protein translation. Reporter assays in THP-1 cells suggest that the ARE alone is not sufficient for the constitutive stabilization in macrophage-like cells and imply an effect of the natural biogenesis of the transcript on the stabilization of the mature form. We present a novel, cell type-dependent sustained stabilization of an ARE-containing mRNA with similarities to situations found in disease.
Keywords: mRNA stability, AU-rich elements, IL-8, CXCL-8, monocytes, macrophages
Introduction
Mammalian monocytes are circulating mononuclear blood cells that contribute to antimicrobial innate defense. Under normal homeostatic conditions, monocytes supply tissues with macrophage precursors but they also have the ability to respond directly during encounters with pathogens.1 Both monocytes and macrophages respond to pathogenic stimuli by the induction of a large number of cytokines and chemokines.2 The expression of cytokines is often transient and highly regulated and aberrations lead to chronic inflammation resulting in a host of diseases like high fever, atherosclerosis, and rheumatoid arthritis.3-5 Accordingly, in normal cells, most induced cytokine mRNAs have short half-lives often caused by the presence of AU-rich elements (AREs) in the 3′untranslated region (3′UTR), which promote mRNA decay. The mRNAs that contain AREs are responsive to external stimuli, particularly stress and inflammatory stimuli that are transduced mainly by the p38 MAPK phosphorylation signaling pathway, leading to the phosphorylation and inhibition of the zinc finger ARE-binding protein tristetraprolin (TTP or zfp36).6-9 The regulation of the expression of the chemokine IL-8, also called CXCL-8, at the post-transcriptional level has been used as a human model for the analysis of ARE-dependent regulation of mRNA stability. For instance, in HeLa cells, the ARE of IL-8 mRNA leads to destabilization of a reporter construct and is responsive to p38 MAPK signaling.10,11 Reporter assays have shown that the minimal regulatory element of the 1250 base 3′UTR is located in a 60-nucleotide area with a core domain containing four clustered AUUUA motifs and an auxiliary domain that enhances destabilization exerted by the core domain.12 ARE sequences were traditionally classified in three classes: class I contains scattered pentamers, class II contains clustered often overlapping pentamers, in association with U-rich regions, whereas in the class III, transcripts are only rich in As and Us and the AUUUA pentamer is not present at all.13 Later, ARE sequences were clustered into five groups based on the reiterations of the pentameric repeat.14 The mRNA of IL-8, like that of many other inflammatory response genes, such as TNF-α, IL-6, IL-1β, and COX-2, contain clustered and overlapping AREs.
Here, we investigate the expression and mRNA stability of pro-inflammatory transcripts in primary monocytes and macrophages, and by using the established model of THP-1 pre-monocyte-like cell line that can differentiate into macrophage-like adherent cells by treatment with the phorbol ester PMA.15,16 We show that unlike transient stabilization during the inflammatory response, differentiation can lead to a stronger and sustained stabilization of an inflammatory mRNA.
Results
A transient response phase and a differentiation phase of induction of IL-8 mRNA in PMA-treated THP-1 cells
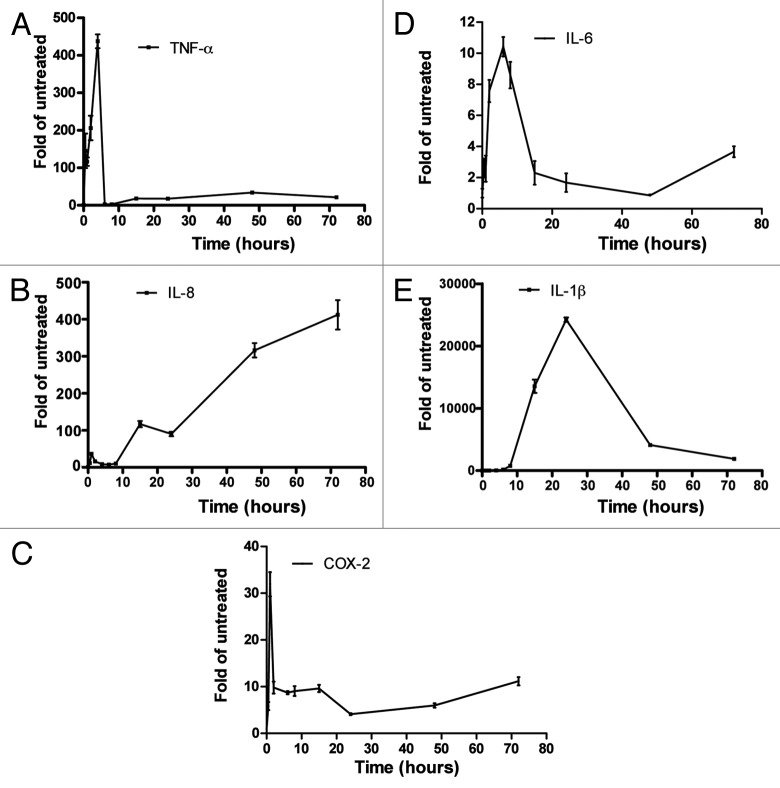
THP-1 cells were treated with 20 ng/ml PMA for up to 72 h (Fig. 1). TNF-α mRNA was strongly induced in an early transient phase of induction reaching a peak of ~400-fold of untreated control at 4 h and dropping dramatically to basal levels at 6 h, after 15 h and in a phase we termed “differentiation phase” the level of TNF-α mRNA was about 20-fold of basal level and remained at this level 72 h post-PMA treatment (Fig. 1A). IL-8 mRNA had a less dramatic transient phase that peaked 1 h post-induction at ~35-fold of untreated control and dropped to basal levels 4 h post-treatment then started rising and reached sustained very high levels of at least 400 times of untreated cells (in some experiments more than 2000-fold) (Fig. 1B). The kinetics of COX-2 and IL-6 mRNAs were similar to that of TNF-α but less dramatic in intensity (Fig. 1C and D). IL-1β mRNA steady-state levels were very low in untreated THP-1 cells, PMA triggered induction was slow but strong and lasted longer than that of TNF-α and peaked at 24 h, similar to a previous observation17 (Fig. 1E). Replacing the cell culture medium that contains PMA after 24 h with fresh medium has little effect on the levels of IL-8 mRNA after 24 additional hours (48 h total), indicating that higher mRNA levels are a consequence of the differentiation status of the cells and not a direct response to PMA.
Figure 1. Time course of TNF-α, IL-8, COX-2, IL-6, and IL-1β mRNA induction after treatment of THP-1 cells with PMA. THP-1 cells were untreated or were differentiated with 20 ng/ml PMA for the indicated time points. Total RNA was extracted and TNF-α (A), IL-8 (B), COX-2 (C), IL-6 (D), and IL-1β (E) mRNA levels were quantified by real-time PCR.
A direct correlation between IL-8 mRNA steady-state level and stability in the course of differentiation of THP-1 cells
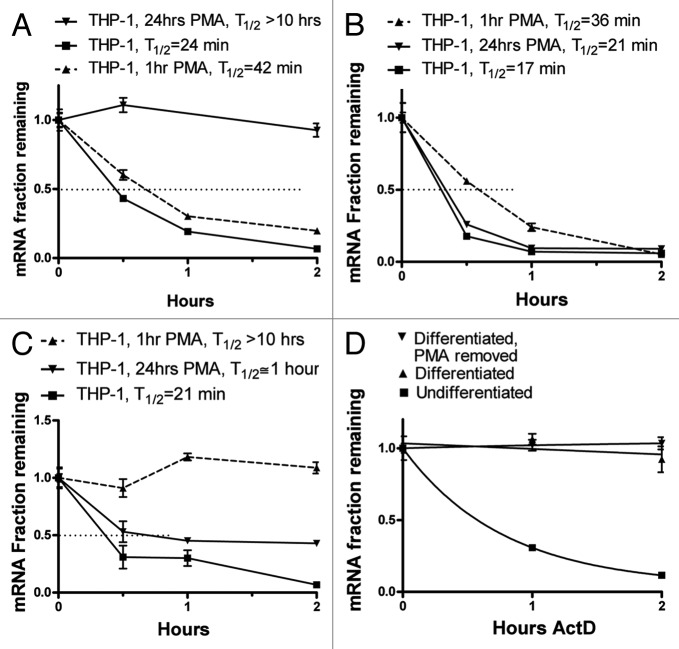
Based on the kinetics of the induction (Fig. 1), we performed actinomycin-D chase experiments to assess the stability of the ARE-containing mRNAs (Fig. 2) without treatment, 1 h or 24 h post-PMA treatment. Interestingly, the level of mRNA stability directly correlated with the mRNA steady-state level. The IL-8 message was unstable in untreated THP-1 cells with an estimated half-life of 24 min and was slightly stabilized in the transient phase of induction 1 h after PMA treatment reaching a half-life of 42 min. Strikingly, 24 h post-PMA treatment, the stability of the IL-8 message was much higher with a half-life of at least 10 h and often more than 15 h (Fig. 2A). For TNF-α mRNA, the highest half-life was in the transient phase (36 min) compared with 17 min in untreated cells, and in the differentiation phase the stability was slightly higher than in untreated cells (21 min) (Fig. 2B). IL-1β mRNA estimated half-life was lowest without PMA treatment (21 min), highest 1 h post-PMA treatment, but remained relatively high 24 h after differentiation with PMA (~1 h) (Fig. 2C). We have attempted to investigate the mRNA stabilities of IL-6 and COX-2; however, the low levels did not allow accurate assessment.
Figure 2. Actinomycin D chase experiments to determine mRNA half-lives in differentiating THP-1 cells. THP-1 cells were left untreated or treated with 20 ng/ml PMA for 1 h or 24 h. Ten µg/ml Actinomycin D was added to block transcription for up to 2 h. Total RNA was extracted and IL-8, TNF-α, and IL-1β mRNA levels were quantified by RT, real time-PCR. (A) IL-8 mRNA decay curves from untreated, 1 h PMA-treated or 24 h PMA-differentiated THP-1 cells (B) TNF-α mRNA decay curves from untreated, 1 h PMA-treated or 24 h PMA-differentiated THP-1 cells. (C) IL-1β mRNA decay curves from untreated, 1 h PMA-treated or 24 h PMA-differentiated THP-1 cells (D) IL-8 mRNA decay curves from untreated THP-1 cells or cells that were differentiated with 20 ng/ml PMA for 24 h or cells that were differentiated with PMA for 24 h, washed with medium four times to remove PMA, and incubated for additional 24 h.
To show that the observed IL-8 mRNA high stability in PMA-differentiated THP-1 is not a direct effect of PMA treatment, we assessed IL-8 mRNA stability in THP-1 cells that were differentiated with PMA for 24 h followed by washing the cells and replacing the medium with fresh PMA-free medium and an additional 24 h incubation period. IL-8 mRNA remained highly stable indicating that the stabilization is a consequence of the differentiated status of the cells and becomes PMA-independent (Fig. 2D).
IL-8 mRNA stability in differentiated cells is stronger than the one induced by endotoxin and is independent of p38 MAPK activity
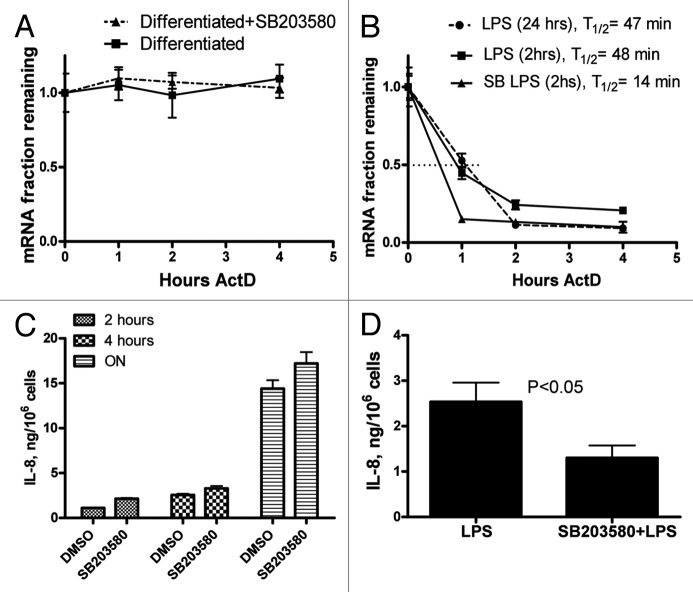
The p38 MAPK signaling cascade plays a major role in ARE-dependent post-transcriptional regulation6,7,18,19 and the ARE of IL-8 was often used as a model to investigate this p38 MAPK function.10,12,20 The bacterial lipopolysaccharide LPS has been widely used to induce p38 signaling in monocytes and macrophages and is a potent stabilizer of ARE-containing mRNAs.8,21 We compared differentiation-dependent stabilization of IL-8 mRNA with the one induced by LPS. When undifferentiated THP-1 cells were treated for 2 h with LPS a 2-fold increase in IL-8 mRNA half-life was observed; the half-life increased from 24 min to 48 min, significantly lower than the increase caused by differentiation (half-life more than 15 h) (Fig. 3A and B). Since the stability of IL-8 mRNA was tested 24 h after PMA treatment, we determined its stability 24 h post-LPS addition; it remained at 47 min significantly lower than in differentiated cells (Fig. 3A and B).
Figure 3. Effect of p38 MAPK inhibition on IL-8 mRNA stability and expression in PMA-differentiated and LPS-treated THP-1 cells. (A) IL-8 mRNA decay curves from cells that were differentiated with 20 ng/ml PMA for 24 h, medium was replaced, and 5 µM SB203580 or DMSO as vehicle control were added for 1 h before actinomycin D chase (B) Actinomycin D chase and IL-8 mRNA decay curves from THP-1 cells that were treated with 1 µg/ml LPS for 2 and 24 h, or with 5 µM SB203580 or DMSO for 1 h followed by 2 h treatment with LPS. (C) ELISA: IL-8 release from PMA differentiated cells for the periods of 2 and 4 h and overnight (ON) with pretreatment with vehicle (DMSO) or 5 µM SB203580. (D) ELISA: IL-8 release from THP-1 pre-monocytes treated with 1 µg/ml LPS for 2 h either with pretreatment with DMSO or 5 µM SB203580.
To assess a possible role of p38 MAPK signaling in the stabilization of IL-8 mRNA in differentiated THP-1 cells, we treated differentiated cells with a 5 µM concentration of p38 inhibitor SB203580 and control cells were treated with vehicle (DMSO) for 1 h prior to Actinomycin D chase experiments. P38 inhibition had no detectable effect on IL-8 mRNA stability in differentiated cells (Fig. 3A), whereas the p38 inhibitor reduced the half-life of IL-8 mRNA from 48 min to 14 min in a parallel experiment in LPS-treated THP-1 cells (Fig. 3B). ELISA assays indicate that SB203580 significantly reduced IL-8 protein release in LPS-treated THP-1 cells but not in PMA differentiated cells (Fig. 3C and D).
Reduced translation of IL-8 mRNA in differentiated THP-1 cells, which are primed for high release of IL-8 in case of endotoxin challenge
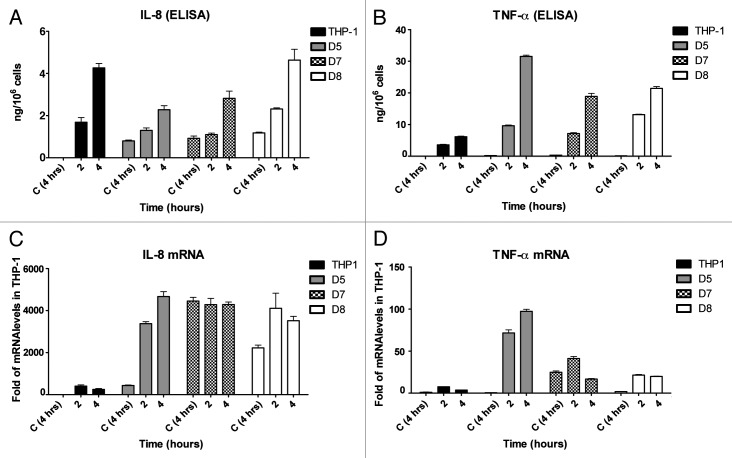
PMA-induced differentiation of THP-1 cells leads to long-term stabilization of IL-8 mRNA. Since resting macrophages are constantly present in human tissues and continuous expression of inflammatory signals without an inflammatory stimulus is not appropriate, we hypothesized that the level of translation of stable IL-8 mRNA in THP-1 macrophages may be reduced. Therefore, the levels of IL-8 protein and mRNA were compared, in parallel, in LPS-treated undifferentiated THP-1 cells and in differentiated cells by ELISA and qPCR (Fig. 4). IL-8 was low in the medium of undifferentiated THP-1 cells (0.059 ng/106 cells) after overnight incubation but when they were treated with LPS, IL-8 was released to levels of ~3 and ~7.5 ng/106 cells after 2 and 4 h, respectively (Fig. 4A). To measure IL-8 release from differentiated cells for the periods of 2 and 4 h, PMA and IL-8-containing medium was thoroughly removed from overnight differentiated cells and they were further incubated for 2 and 4 h in PMA-free medium; IL-8 release was significantly lower than in LPS-treated cells 1.4 ng/106 cells and 3.3 ng/106 cells for 2 and 4 h incubation, respectively (Fig. 4A). A parallel quantification of IL-8 mRNA levels in the same cells used for ELISA shows that the levels of IL-8 mRNA were ~800-fold of untreated in differentiated cells (Fig. 4B, Diff. THP-1, 2 and 4 h), significantly higher than in undifferentiated cells treated with LPS for 2 and 4 h (~350-fold of untreated, THP-1 LPS) (Fig. 4B), keeping in mind that in LPS-treated THP-1 monocytes, IL-8 mRNA starts at basal level compared with sustained 800-fold basal level in the differentiated cells and the levels at 30 min and 1 h post-LPS induction were significantly lower than the levels at 2 h LPS (data not shown). These results indicate that lower levels of IL-8 mRNA in the LPS-induced monocytes are more actively translated than the constantly high level of IL-8 mRNA in the differentiated macrophages.
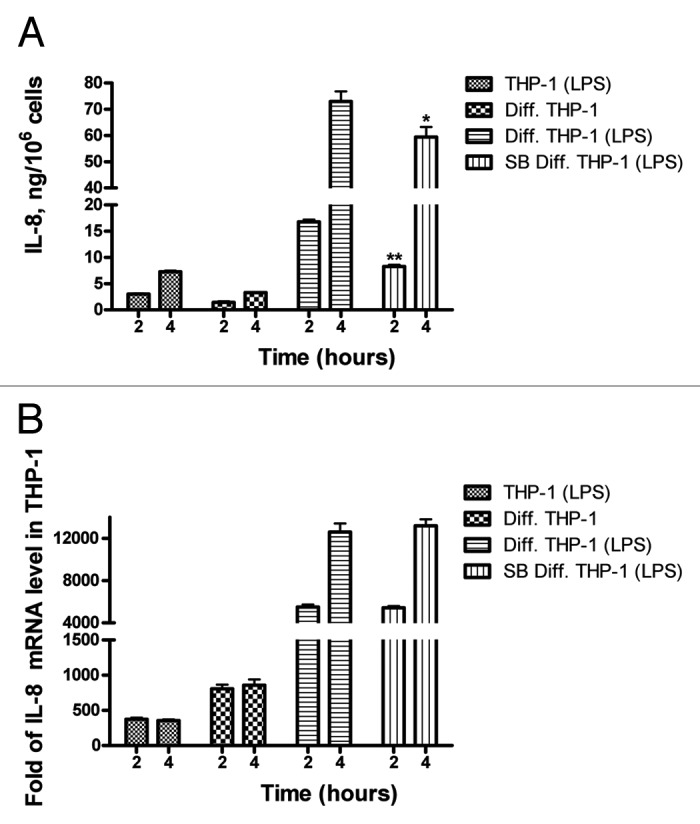
Figure 4. Comparative analysis of IL-8 protein and mRNA levels in undifferentiated and PMA-differentiated THP1 cells and response to LPS and p38 MAPK inhibition. (A) ELISA. 106 undifferentiated pre-monocyte THP1 cells were treated for 2 and 4 h with 1 µg/ml LPS. The same number of cells was differentiated overnight (Diff. THP-1) medium was replaced and the cells were incubated for two or four hours with fresh pre-warmed medium or treated with LPS for 2 and 4 h with or without pretreatment (1 h) with the p38 MAPK inhibitor SB203580. Supernatants were collected and assayed for IL-8 using specific ELISA. (B) qPCR. Total RNA was prepared from the cells in (A) and real-time PCR was performed to quantify IL-8 mRNA levels. * * P < 0.0005, * P = 0.005.
To investigate the response of differentiated cells to LPS, PMA-containing medium was replaced with LPS containing fresh medium; IL-8 mRNA level and protein release were highest reaching 13 000-fold of untreated control for mRNA and ~73 ng/106 cells of protein released after a 4 h incubation period, indicating that the differentiation primes the cells for high IL-8 expression (Fig. 4). Interestingly, if SB203580 is added before LPS stimulation IL-8 protein release but not IL-8 mRNA levels is significantly reduced, suggesting that p38 MAPK signaling may be inducing translation (Fig. 4).
Elevated levels of IL-8 mRNA and spontaneous release of the chemokine in primary human monocytes and macrophages
The level of IL-8 mRNA and protein release was investigated in primary peripheral human monocytes and macrophages from healthy human donors. Under standard cell culturing conditions, IL-8 mRNA levels were strikingly very high in both macrophages (Fig. 5C) and monocytes (Fig. S1). The cDNAs of IL-8 and GAPDH were amplified in duplex for qPCR quantification and had comparable calculated amplification efficiencies; the Ct value of amplification for IL-8 cDNA for all donors was 5–6 values lower than that of GAPDH, suggesting a significantly higher steady-state level of IL-8 mRNA (~50-fold) compared with the housekeeping gene. Since relative IL-8 mRNA levels were lowest in THP-1 cells, we set this level as a fold reference for the IL-8 mRNA levels in primary cells. IL-8 mRNA steady-state levels in monocytes and macrophages from donors 5, 6, 7, and 8 range from 400–4000-fold of levels in THP-1 cells (Fig. 5C; Fig. S1), comparable to the situation in PMA-differentiated THP-1 cells (Fig. 1). Spontaneous release of IL-8 protein occurred and was in the range of 1 ng/106 cells in 4 h for all donors (Fig. 5A). The response to LPS was significant but less dramatic than in differentiated THP-1 cells (Figs. 4, 5A and C). Since a constitutive high level of a proinflammatroy ARE containing mRNA and the subsequent expression and release of the protein is not expected without stimulation, we determined, in parallel, the level of mRNA and protein release of TNF-α. The levels of TNF-α mRNA were low; comparable to the level in THP-1 cells, and protein release was barely detectable without stimulation but is inducible by LPS (Fig. 5B and D). Also, the mRNA levels of COX-2 and IL-6 in primary cells was barely detectable (data not shown). Taken together, our data suggests that the level of IL-8 mRNA is constitutively elevated in unstimulated primary monocytes and macrophages under standard cell culture conditions, leading to a spontaneous release of the chemokine; a situation that is markedly different from other ARE-containing proinflammatory-mediator mRNAs.
Figure 5. Protein release and IL-8 and TNF-α mRNA levels in primary human macrophages and response to LPS. 106 primary macrophages from three healthy donors were seeded overnight, the following day the cells were washed and the medium was replaced, control cells were left untreated for 4 h (C 4 h]), or treated with 1 µg/ml LPS for 2 and 4 h. THP-1 cells were used as control and treated similarly. (A and B) Supernatants were collected and assayed for IL-8 and TNF-α using specific ELISA. (C and D) Total RNA was prepared from the cells and teal-time PCR was performed to quantify IL-8 and TNF-α mRNA levels. D: healthy human donor.
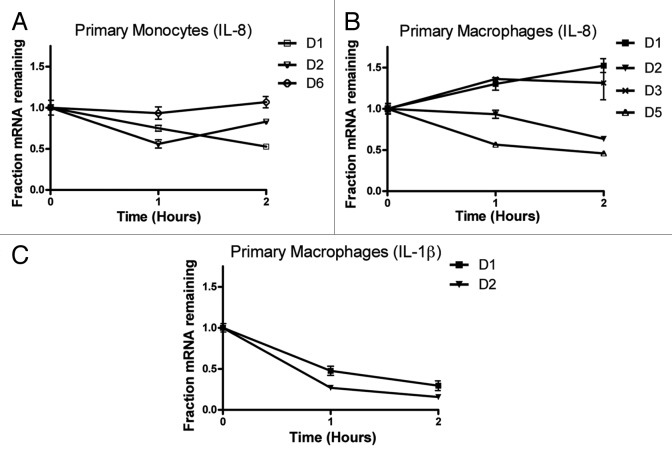
IL-8 mRNA is highly stable in primary human monocytes and macrophages
Most importantly, the stability of the abundant IL-8 mRNA in primary human monocytes and macrophages was assessed by actinomycin-D chase experiments (Fig. 6). IL-8 mRNA was highly stable in primary cells from a total of five healthy human donors with half-lives of at least 2 h. Often, the GAPDH-normalized IL-8 mRNA levels increased after Actinomycin-D treatment, suggesting that IL-8 mRNA is more stable than GAPDH (monocytes donor 6, macrophages Donors 1 and 3) (Fig. 6A and B). The levels of COX-2 and TNF-α were barely detectable by qPCR (ct values > 35) in untreated primary cells and became undetectable after Actinomycin-D treatment, suggesting low levels of transcription and mRNA stability. The mRNA levels of IL-1β were also low but stability could be accessed in the macrophages of only two of the donors (half-lives 30–40 min) (Fig. 6C).
Figure 6. IL-8 mRNA stability in primary human monocytes and macrophages. 106 primary monocytes and macrophages were seeded in three plates 10 µg/ml Actinomycin D was added to block transcription for 1 and 2 h. Total RNA was extracted and IL-8 mRNA levels were quantified by qPCR. (A) IL-8 mRNA decay curves from monocytes three healthy human donors. (B) IL-8 mRNA decay curves from macrophages of four healthy human donors. (C) IL-1β mRNA decay curves from macrophages of two healthy human donors D: healthy human donor.
Reporter experiments suggest that the ARE of IL-8 alone is not sufficient for differentiation-dependent stabilization
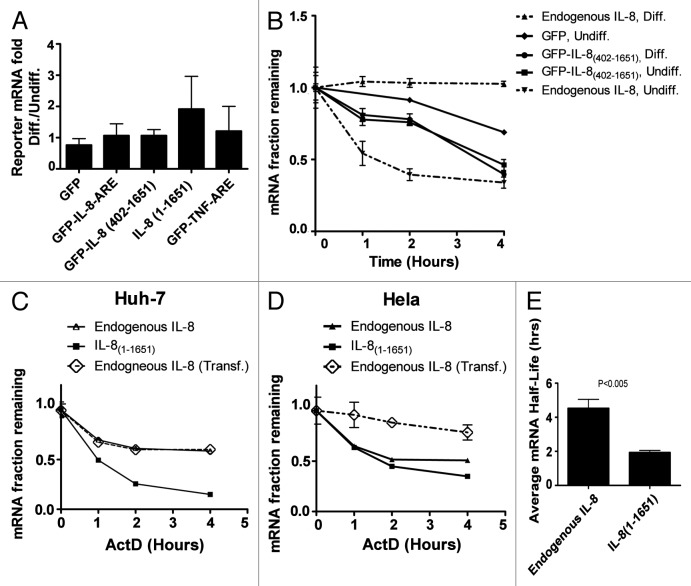
To investigate whether the ARE in the 3′UTR of IL-8 mRNA is responsible for the apparent stabilization in PMA-differentiated THP-1 cells, we cloned the 70 bp ARE of IL-8 and the complete 3′UTR of IL-8(402–1651) in the 3′UTR of the reporter in the RPS30M1-GFP vector. Since TNF-α mRNA remained unstable in differentiated THP-1 cells, we used TNF ARE as control. The non-inducible RPS30M1 ribosomal protein promoter system is suitable to assess a possible stabilization of the constructs due to differentiation without transcriptional induction interference.22 The constructs were transfected into THP-1 monocytes and were left for 24 h with or without PMA-induced differentiation. Having the same expression backbone that includes the non-inducible promoter, stabilization in the differentiated cells is expected to significantly increase the level of reporter mRNA in macrophages compared with monocytes.23 Three independent differentiation experiments were performed with each construct (Fig. 7A). As expected, the macrophage/monocyte mRNA level ratio of the non-ARE GFP control was close to 1, but surprisingly, the macrophage/monocyte mRNA levels ratios of all of the IL8-derived mRNA constructs did not significantly increase, suggesting no or little stabilization. Also, the TNF-α-derived construct led to no significant increase in the macrophage/monocyte ratio of reporter mRNA levels (Fig. 7A). We then replaced the GFP of the reporter vector with the entire IL-8 mRNA (IL-8[1–1651]) again, and surprisingly, the level of the mRNA of this construct did not significantly increase in macrophages compared with monocyte implying no or little stabilization in the differentiated cells (Fig. 7A). Actinomycin-D chase experiments suggest that GFP-IL-8(402–1651) is less stable than the GFP control lacking AREs and differentiation leads to no stabilization (Fig. 7B). It should be noted that reporter experiments in THP-1 cells were hampered by the sensitivity of the cells to transfection. The transfected cells were visibly stressed, and the half-life of endogenous IL-8 mRNA was significantly elevated, even before differentiation (~1.4 h in transfected cells compared with 24 min without transfection [Figs. 2A and 7B]). This sensitivity to transfection might also explain the relatively high stability of GFP-IL-8(402–1651) (~3 h) mRNA in undifferentiated cells (Fig. 7B). Still, differentiation-dependent stabilization could be observed for the endogenous gene but not the transfected ARE-containing reporter in transfected cells (Fig. 7B). Overall, our data suggest a difference in mRNA stability between the transfected reporter IL-8 (IL-8[1–1651]) and endogenous IL-8 mRNAs in differentiated THP-1 cells; the endogenous mRNA becomes highly stable in THP-1 macrophages, while the stability of the transfected transcript is lower. Interestingly, constitutive expression and high mRNA stability of IL-8 mRNA has been reported in many established cells lines and cancer cells,24,25 while others report a highly unstable transfected full-length cDNA-derived IL-8 mRNA in HeLa cells.12 Therefore, we assessed, in parallel, the stability of the endogenous and transfected forms of IL-8 mRNA in cell lines such as HeLa and Huh-7 that have been used as models for the investigation of post-transcriptional regulation. Endogenous IL-8 mRNA was more stable than the transfected forms in both cell types (Fig. 7C–E).
Figure 7. GFP reporter assays in THP-1, Huh-7, and HeLa cells. (A) THP-1 cells were transfected with gWIZ-RPS30M1-GFP reporter vectors with ARE insertions as indicated, the ARE label represents the 70 base ARE regions of IL8 and TNF-α mRNAs, otherwise the numbers indicate the position in the mRNAs of the cytokines. In IL-8(1–1651) the GFP reporter was replaced with the entire IL-8 mRNA sequence. Cells were left untreated or differentiated with PMA for 24 h. The macrophage/monocyte reporter mRNA level ratio of three independently performed experiments is shown. (B) Actinomycin-D chase experiment to assess the half-life of the mRNA of the GFP-IL8(402–1651) reporter along with endogenous IL-8 mRNA in THP-1 monocytes and macrophages. (C and D) Actinomycin-D chase experiment to assess the half-lives of the mRNAs of the transfected IL-8(1–1651) and endogenous IL-8 in untransfected, as well as in cells transfected with the gWIZ-RPS30M1-GFP vector (Endogneous Transf.) in Huh-7 and HeLa cells. (E) The average half-lives of endogenous IL-8 and transfected IL-8(1–1651) in three independent experiments in HeLa cells.
Discussion
Most inflammatory cytokine mRNAs have by default short half-lives that can be transiently elevated by cellular signaling cascades during the onset of inflammation.18,26 The high rate of decay is caused mainly by AREs and is crucial for the termination of the response; otherwise prolonged expression may lead to chronic inflammatory diseases.27,28 The mRNAs of TNF-α and IL-8 have been extensively used as models for the investigation of ARE-dependent post-transcriptional regulation, they behave similarly by causing strong default destabilization and by being stabilized in the early phase of the inflammatory response by the p38 MAPK signaling pathway.6,10,12,20,29-31 TNF-α mRNA is transiently stabilized in LPS-stimulated macrophages, its apparent half-life increases from an estimated 26 to 60 min.21 Winzen et. al. have shown that an mRNA reporter containing IL-8 ARE is stabilized for no more than 1 h after IL-1 treatment and its half-life increases 3-fold reaching ~50 min.10 This is similar to our observation in LPS-treated THP-1 cells for endogenous IL-8 mRNA where the half-life increased from 24 to 48 min (Fig. 3B). This is also similar to the “transient phase” of induction with PMA where the mRNA half-life reached 42 min after 1 h of treatment (Fig. 2A).
However, in the later differentiation phase of PMA-treated THP-1 cells, we observed a much stronger and sustained novel type of stabilization of an ARE-containing mRNA. IL-8 mRNA half-life reached and remained at a very high level of more than 15 h, whereas TNF-α mRNA half-life remained very low (21 min) (Fig. 2A and B). A comparable situation was found in cultured primary human monocytes and macrophages; IL-8 mRNA was abundant and stable (Figs. 5 and 6). Our data also shows that the mRNAs of COX-2 and IL-6 remain low in differentiated cells. These findings are surprising since all these mRNAs contain class II AREs of clustered and overlapping AUUUA pentamers that would be expected to behave similarly at the post-transcriptional level and may suggest an additional effect targeting especially IL-8 mRNA in an ARE- independent manner. In fact, reporter-based experiments suggest that the ARE in the 3′UTR of IL-8 mRNA alone may not be sufficient for the immense stabilization in differentiated cells (Fig. 7). Also, in PMA-differentiated cells, mRNA and protein levels as well as cellular localization of the ARE-binding proteins HuR and TTP did not change significantly (Fig. S2). At this stage, however, a possible ARE role in the stabilization cannot be completely ruled out since IL-1β mRNA remains significantly more stable in differentiated cells than in untreated THP-1 cells (1 h vs. 21 min), also TNF-α mRNA is slightly more stable in differentiated cells (21 vs. 17 min) (Fig. 2).
Sirenko et. al. reported that monocyte adherence rapidly stabilizes the ARE-containing mRNAs of GRO-α and interleukin IL-1β.32 The stabilization of IL-8 mRNA observed here may be different in several aspects: (1) IL-8 mRNA was abundant and stable in primary monocytes seeded in non-adherent plates (Fig. 6; Fig. S1). (2) IL-8 mRNA was abundant and stable in PMA-differentiated THP-1 cells grown in non-adherent plates (data not shown). (3) Unlike the adherence-stimulated stabilization of GRO-α and IL-1β mRNAs, IL-8 mRNA stability in differentiated THP-1 cells is p38 MAPK-independent (Fig. 3).
IL-8 but not TNF-α is unexpectedly spontaneously released at significant levels (~1 ng/106 cells in 4 h) from cultured primary monocytes and macrophages and differentiated THP-1 cells. The spontaneous release of this inflammatory chemokine may have not been the focus of previous studies, which generally investigate the response to stimuli and consider spontaneous release as a negligible background, still this phenomenon has been reported in alveolar macrophages and blood monocytes.33,34
High constitutive levels and stability of IL-8 mRNA have been found in many established cells lines and cancer cells.24,25 Endogenous IL-8 mRNA was also relatively stable in HeLa and Huh-7 cells (Fig. 7C and D). These findings are in contrast with a previous report showing that a cDNA-derived transfected complete IL-8 mRNA is unstable in HeLa cells.12 Therefore, we decided to compare the stabilities of transfected cDNA-derived and endogenous forms of IL-8 mRNA in HeLa and Huh-7 cells. Unexpectedly, the endogenous transcript was more stable in both cell lines (Fig. 7C-E). A similar finding was observed by others in HeLa cells by northern blot analysis (Helmut Holtmann, personal communication). Our data suggests that the biogenesis of endogenous IL-8 in its natural context, which includes the induction of its natural promoter and the processing of its pre-mRNA, may influence the stability of the mature form.
Interestingly, recent reports suggest that RNA sequence elements in pre-mRNA introns can influence the stability of mature cytoplasmic mRNAs. The ARE-binding protein HuR can bind introns as well as 3′UTRs of many transcripts and the combinatorial binding in the same pre-mRNA in introns and 3′UTR can lead to an amplification of the mRNA-stabilizing effect in the mature form.35,36 IL-8 pre-mRNA is one of the few cytokines that contains several HuR binding sites in its last intron and in its 3′UTR, as evidenced by the PAR-CLIP technique.36 However, PMA-induced differentiation in THP-1 cells did not alter HuR level or cellular localization and siRNA knockdown of HuR had no significant effect on the stability of IL-8 mRNA in differentiated THP-1 cells (Figs. S2 and S3).
IL-8 mRNA and protein levels are elevated in many disease conditions; a recent review even suggests the use of IL-8 as a universal biomarker for disease.37 IL-8 mRNA is stable in many cell lines and cancer cells as reported here and by others. If in some cell types a sustained stable IL-8 mRNA is considered an aberration; the PMA/THP-1 differentiation system could be used as a simple and easy tool for in the depth molecular investigation of this novel form of sustained stabilization of a chemokine mRNA.
Cell lines, vectors, and reagents
THP-1 and HeLa cells were obtained from American Type Culture Collection (ATCC) and cultured in RPMI 1640 and DMEM, respectively (Invitrogen), supplemented with 10% FBS and antibiotics. Huh-7 cell line was obtained from Dr Stephen J Polyak (University of Washington), and was propagated in DMEM medium with 10% FBS and antibiotics.38 The gWIZ-RPS30M1-GFP vector with the non-inducible promoter was described previously.22 AU-rich elements inserts were made using two annealed synthetic complementary oligonucleotides with BamHI and XbaI overhangs and cloned into the same sites in the BGH 3′UTR of the vector. The sequence of IL-8 ARE used is: GATCCGTGTA ACTTATTAAC CTATTTATTA TTTATGTATT TATTTAAGCA TCAAATATTT GTGCAAGAAT and that of TNFα is GATCCTTGTG ATTATTTATT ATTTATTTAT TATTTATTTA TTTACAGATG AATGTATTTA TTTGGGAGAT. The complete 3′UTR of IL-8(402–1651) was cloned using PCR into the BamHI and XbaI sites of the vector. Total IL-8 mRNA (NM_000584) IL-8(1–1651) was cloned from differentiated THP-1 cells using the forward primer GTCGACCTCC ATAAGGCACA AACTT and the reverse primer ATCTAGAACT TTGACAACAA ATTATATTT and replaced GFP into SalI and XbaI sites of the vector. All clones sequences were confirmed by DNA sequencing. SB203580 was purchased from Promega. Phorpol 12-myristate 13-acetate (PMA) and DMSO were purchased from sigma. IL-8 ELISA kits were purchased from Abcam and were used according to manufacturer’s protocol.
Preparation and differentiation of primary monocytes and macrophages
Peripheral venous blood drawn from a single volunteer was collected in Heparin bags, the blood was diluted 1 to 2 with PBS. The diluted blood was layered on a Ficoll-Paque in a 50 ml Falcon tube and centrifuged for 40 min at 400 × g at room temperature without brake. The Buffy coat was aspirated and washed with PBS. For donors 5, 6, 7, and 8 Primary Human “untouched” monocytes were isolated using the indirect magnetic labeling system from MACS Miltenyi Monocyte Isolation Kit II (Gladbach Germany) according to manufacturer’s protocol. For experiments with monocytes, cells were seeded on non-adherent plates overnight in RPMI 1640 medium supplemented with 10% serum and antibiotics before treatment. To prepare macrophages, the monocytes were resuspended in medium allowed to adhere on polystyrene plates for 1 h before replacing medium, then the cells were allowed to differentiate on the adherent plates for 8–10 d, the medium was replaced every second day.39,40 Alternatively, monocytes from the washed buffy were directly allowed to adhere on polystyrene plates for 1 h at 37 °C, after washing non-adherent cells, the enriched monocyte population was detached by placing the cells on ice and gentle scraping (donors 1, 2, 3, and 4). The study is approved by the research advisory council ethics committee of King Faisal Specialist Hospital and Research Center (Rac#2100 030) and is in accordance with the Helsinki Declaration.
THP-1, Huh-7, and HeLa transfection
For THP-1, 107 cells were resuspended in 2 ml Optimem medium and transfected with 2.5 µg plasmid DNA and 20 µl Lipofectamin LTX. The cells were left in transfection mix overnight. Next day, the cells were washed and resuspended in RPMI 1640 supplemented with 10% FBS and antibiotics and split into appropriate plates. Eighty to 90% confluent HeLa and Huh-7 cells were transfected in 10 cm cell culture dishes with 2 ug vector DNA and 10 µl Lipofectamin 2000 following manufacture’s protocol. For mRNA stability experiments, cells were split 1 d after transfection.
RNA isolation, reverse transcription, and real-time PCR
Total RNA was extracted with Tri reagent (Sigma). Reverse transcription was performed using Superscript II and Oligo dT primer (Invitrogen). Real-time PCR taqman primer sets, including the Vic-labeled GAPDH internal control as well as Fam-labeled primer sets for IL-8, TNFα, COX-2, IL-6, GMCSF, were ordered from Applied Biosystems. The taqman primer sets for IL-1β were designed manually and ordered from Metabion (Martinsried). (Fw: TCAGCCAATC TTCATTGCTC Rev: TGGAAGGAGC ACTTCATCTG, Taq: FAM TCTGCCATGGC TGCTTCAGAC A BHQ-1). The Fam-labeled Taqman primer set for the spliced GFP reporter was custom designed as previously reported.22 The taqman primer set that recognizes transfected full-length IL-8 mRNA IL-8(1–1651) but not endogenous IL-8 mRNA was manually designed and ordered from Metabion. The forward primer anneals to a short stretch of the 5′UTR from the vector of the spliced mRNA the reverse primer anneals to the 5′UTR of IL-8 mRNA, the Taqman primer (Fam-labeled and quenched with BHQ1) anneals to the exon–exon junction of the spliced product and is unable to detected transfected DNA. Forward primer sequence ctccatcttc gcggtagct, reverse primer sequence gaagcttgtg tgctctgctg, taqman primer sequence ccgccgttca gtcgccgt. The specificity of the primer set was tested on non-transfected cells; it failed to detect endogenous IL-8 mRNA. Real-time PCR was performed using the CFX96 cycler (BioRad). All qPCR experiments were performed in duplex and normalized with the internal control GAPDH. Relative mRNA levels were calculated using a standard curve of a total cDNA dilution series starting with an arbitrary number and using the CFX96 software. Only samples having the exponential phase within the linear range of the standard curve were considered. For mRNA half-life determination, cells were treated with Actinomycin-D (10 µg/ml) to shut-off transcription. Decay curves were plotted using GraphPad Prism software. mRNA half-lives were calculated as the intercept on the x-axis at 50% decay of the reporter mRNA.
Supplementary Material
Materials and Methods
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This Project was supported by King Faisal Specialist Hospital and Research Center intramural funding and by King Abdulaziz City of Science and Technology (KACST) under the Long-Term Comprehensive National Science, Technology, and Innovation Plan (NSTIP) (KACST Project No. 10-BIO953-20). The authors would like to thank Dr Monther Al-Alwan for technical advice and Mr Walid Moghrabi for critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/27863
References
- 1.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–52. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter M, Wang Y, Eubank T, Baran C, Nana-Sinkam P, Marsh C. Survival of monocytes and macrophages and their role in health and disease. Front Biosci (Landmark Ed) 2009;14:4079–102. doi: 10.2741/3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khabar KS. The AU-rich transcriptome: more than interferons and cytokines, and its role in disease. J Interferon Cytokine Res. 2005;25:1–10. doi: 10.1089/jir.2005.25.1. [DOI] [PubMed] [Google Scholar]
- 4.Seko Y, Cole S, Kasprzak W, Shapiro BA, Ragheb JA. The role of cytokine mRNA stability in the pathogenesis of autoimmune disease. Autoimmun Rev. 2006;5:299–305. doi: 10.1016/j.autrev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Fan J, Heller NM, Gorospe M, Atasoy U, Stellato C. The role of post-transcriptional regulation in chemokine gene expression in inflammation and allergy. Eur Respir J. 2005;26:933–47. doi: 10.1183/09031936.05.00120204. [DOI] [PubMed] [Google Scholar]
- 6.Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol. 2006;26:2399–407. doi: 10.1128/MCB.26.6.2399-2407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark AR, Dean JL, Saklatvala J. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 2003;546:37–44. doi: 10.1016/S0014-5793(03)00439-3. [DOI] [PubMed] [Google Scholar]
- 8.Frevel MA, Bakheet T, Silva AM, Hissong JG, Khabar KS, Williams BR. p38 Mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol Cell Biol. 2003;23:425–36. doi: 10.1128/MCB.23.2.425-436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1:94–7. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 10.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Müller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–80. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–55. [PubMed] [Google Scholar]
- 12.Winzen R, Gowrishankar G, Bollig F, Redich N, Resch K, Holtmann H. Distinct domains of AU-rich elements exert different functions in mRNA destabilization and stabilization by p38 mitogen-activated protein kinase or HuR. Mol Cell Biol. 2004;24:4835–47. doi: 10.1128/MCB.24.11.4835-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CY, Shyu AB. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–70. doi: 10.1016/S0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 14.Bakheet T, Frevel M, Williams BR, Greer W, Khabar KS. ARED: human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 2001;29:246–54. doi: 10.1093/nar/29.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 16.Abrink M, Gobl AE, Huang R, Nilsson K, Hellman L. Human cell lines U-937, THP-1 and Mono Mac 6 represent relatively immature cells of the monocyte-macrophage cell lineage. Leukemia. 1994;8:1579–84. [PubMed] [Google Scholar]
- 17.Wilson GM, Lu J, Sutphen K, Sun Y, Huynh Y, Brewer G. Regulation of ARE-directed mRNA Turnover Involving Reversible Phosphorylation of AUF1. J Biol Chem. 2003;19:19. doi: 10.1074/jbc.M305772200. [DOI] [PubMed] [Google Scholar]
- 18.Dean JL, Sully G, Clark AR, Saklatvala J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal. 2004;16:1113–21. doi: 10.1016/j.cellsig.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Ronkina N, Menon MB, Schwermann J, Tiedje C, Hitti E, Kotlyarov A, Gaestel M. MAPKAP kinases MK2 and MK3 in inflammation: complex regulation of TNF biosynthesis via expression and phosphorylation of tristetraprolin. Biochem Pharmacol. 2010;80:1915–20. doi: 10.1016/j.bcp.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Winzen R, Thakur BK, Dittrich-Breiholz O, Shah M, Redich N, Dhamija S, Kracht M, Holtmann H. Functional analysis of KSRP interaction with the AU-rich element of interleukin-8 and identification of inflammatory mRNA targets. Mol Cell Biol. 2007;27:8388–400. doi: 10.1128/MCB.01493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brook M, Sully G, Clark AR, Saklatvala J. Regulation of tumour necrosis factor alpha mRNA stability by the mitogen-activated protein kinase p38 signalling cascade. FEBS Lett. 2000;483:57–61. doi: 10.1016/S0014-5793(00)02084-6. [DOI] [PubMed] [Google Scholar]
- 22.Hitti E, Al-Yahya S, Al-Saif M, Mohideen P, Mahmoud L, Polyak SJ, Khabar KS. A versatile ribosomal protein promoter-based reporter system for selective assessment of RNA stability and post-transcriptional control. RNA. 2010;16:1245–55. doi: 10.1261/rna.2026310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59:423–50. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le X, Shi Q, Wang B, Xiong Q, Qian C, Peng Z, Li XC, Tang H, Abbruzzese JL, Xie K. Molecular regulation of constitutive expression of interleukin-8 in human pancreatic adenocarcinoma. J Interferon Cytokine Res. 2000;20:935–46. doi: 10.1089/10799900050198372. [DOI] [PubMed] [Google Scholar]
- 25.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–91. doi: 10.1016/S1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 26.Beisang D, Bohjanen PR. Perspectives on the ARE as it turns 25 years old. Wiley Interdiscip Rev RNA. 2012;3:719–31. doi: 10.1002/wrna.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/S1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 28.Khabar KS. Post-transcriptional control during chronic inflammation and cancer: a focus on AU-rich elements. Cell Mol Life Sci. 2010;67:2937–55. doi: 10.1007/s00018-010-0383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dean JL, Wait R, Mahtani KR, Sully G, Clark AR, Saklatvala J. The 3′ untranslated region of tumor necrosis factor alpha mRNA is a target of the mRNA-stabilizing factor HuR. Mol Cell Biol. 2001;21:721–30. doi: 10.1128/MCB.21.3.721-730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–5. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 31.Gowrishankar G, Winzen R, Dittrich-Breiholz O, Redich N, Kracht M, Holtmann H. Inhibition of mRNA deadenylation and degradation by different types of cell stress. Biol Chem. 2006;387:323–7. doi: 10.1515/BC.2006.043. [DOI] [PubMed] [Google Scholar]
- 32.Sirenko OI, Lofquist AK, DeMaria CT, Morris JS, Brewer G, Haskill JS. Adhesion-dependent regulation of an A+U-rich element-binding activity associated with AUF1. Mol Cell Biol. 1997;17:3898–906. doi: 10.1128/mcb.17.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom WN. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 1995;95:586–92. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Losa García JE, Rodríguez FM, Martín de Cabo MR, García Salgado MJ, Losada JP, Villarón LG, López AJ, Arellano JL. Evaluation of inflammatory cytokine secretion by human alveolar macrophages. Mediators Inflamm. 1999;8:43–51. doi: 10.1080/09629359990711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lebedeva S, Jens M, Theil K, Schwanhäusser B, Selbach M, Landthaler M, Rajewsky N. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol Cell. 2011;43:340–52. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee N, Corcoran DL, Nusbaum JD, Reid DW, Georgiev S, Hafner M, Ascano M, Jr., Tuschl T, Ohler U, Keene JD. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol Cell. 2011;43:327–39. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahzad A, Knapp M, Lang I, Köhler G. Interleukin 8 (IL-8) - a universal biomarker? Int Arch Med. 2010;3:11. doi: 10.1186/1755-7682-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halees AS, Hitti E, Al-Saif M, Mahmoud L, Vlasova-St Louis IA, Beisang DJ, Bohjanen PR, Khabar K. Global assessment of GU-rich regulatory content and function in the human transcriptome. RNA Biol. 2011;8:681–91. doi: 10.4161/rna.8.4.16283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visioli F, Marangoni F, Moi D, Risè P, Galli C. In vitro differentiation of human monocytes to macrophages results in depletion of antioxidants and increase in n-3 fatty acids levels. FEBS Lett. 2000;471:75–7. doi: 10.1016/S0014-5793(00)01361-2. [DOI] [PubMed] [Google Scholar]
- 40.Gantner F, Kupferschmidt R, Schudt C, Wendel A, Hatzelmann A. In vitro differentiation of human monocytes to macrophages: change of PDE profile and its relationship to suppression of tumour necrosis factor-alpha release by PDE inhibitors. Br J Pharmacol. 1997;121:221–31. doi: 10.1038/sj.bjp.0701124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.