Summary
In double fertilization, a reproductive system unique to flowering plants, two immotile sperm are delivered to an ovule by a pollen tube. One sperm fuses with the egg to generate a zygote, the other with the central cell to produce endosperm[1]. A mechanism preventing multiple pollen tubes from entering an ovule would ensure that only two sperm are delivered to female gametes. We use live-cell imaging[1, 2] and a novel mixed-pollination assay that can detect multiple pollen tubes and multiple sets of sperm within a single ovule to show that Arabidopsis efficiently prevents multiple pollen tubes from entering an ovule. However, when gamete-fusion defective hap2(gcs1) or duo1 sperm are delivered to ovules as many as three additional pollen tubes are attracted. When gamete fusion fails, one of two pollen tube-attracting synergid cells persists, enabling the ovule to attract more pollen tubes for successful fertilization. This mechanism prevents the delivery of more than one pair of sperm to an ovule, provides a means of salvaging fertilization in ovules that have received defective sperm, and ensures maximum reproductive success by distributing pollen tubes to all ovules.
Keywords: fertilization, gamete fusion, supernumerary pollen tubes, GCS1, HAP2, sperm, pollen tube reception, polytubey, polyspermy
Results and Discussion
‘Polytubey’ increases when sperm incapable of gamete fusion are delivered to ovules
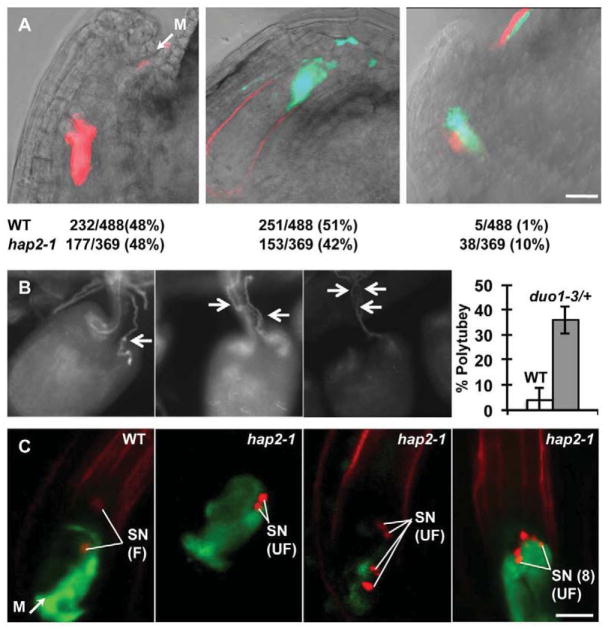
We use the term ‘polytubey’ (many pollen tubes) to describe the attraction of multiple pollen tubes to a single ovule. We chose this term to facilitate comparison with ‘polyspermy’ (the fusion of more than one sperm with a female gamete), which is distinct and also needs to be prevented to maximize reproductive success[3]. We developed a mixed pollination assay that unambiguously detects polytubey (Figure 1A). Half of a male sterile1-1 (ms1-1) stigma was hand-pollinated with pollen expressing GFP from the pollen-specific LAT52 promoter[4] (LAT52:GFP); the other half with pollen expressing DsRed[5] (LAT52:DsRed). These markers define two nearly isogenic populations of pollen tubes and allow pollen tube growth, guidance, and burst to be monitored in the pistil. Twenty-one hours after pollination 48% of ovules had been targeted by a LAT52:DsRed pollen tube (Figure 1A, left) and 51% by a LAT52:GFP pollen tube (Figure 1A, middle). This slight discrepancy from equality reflects the difficulty of placing an equal load of pollen from each genotype on the stigma. Nonetheless, the ~1% of ovules with both the GFP and DsRed signal were obvious and unambiguously indicate that these ovules had been targeted by two pollen tubes (Figure 1A, right).
Figure 1.
Polytubey is rare in wild type, but increases significantly when gamete fusion fails. (A) hap2(gcs1) enhances polytubey. Differentially marked pollen tubes can be tracked as they grow into an ovule at the micropylar end (M). Representative images of ovules targeted by a DsRed+ pollen tube (left), a GFP+ pollen tube (center), or by two different pollen tubes (right). The number of each type of targeting event observed is also shown (bottom). WT, LAT52:GFP pollen mixed with LAT52:DsRed (both types of pollen are wild type) or hap2-1 (hap2-1/HAP2(GCS1), LAT52:GFP mixed with wild-type, LAT52:DsRed pollen). Scale bars, 20μm. (B) duo1 enhances polytubey. duo1-3/+ heterozygous plants were manually self-pollinated and pollen tubes were analyzed 24 hours later using aniline blue staining[14]. Ovules are shown that have attracted a single pollen tube (left panel), two pollen tubes (center panel), or three pollen tubes (right panel). The percentage of ovules with two or more pollen tubes is plotted for No-0 (wild type, 9 pistils, 260 targeted ovules) and duo1-3/+ (8 pistils, 275 ovules targeted). Error bars represent standard deviation (each pistil is one trial). Rates of polytubey are higher in the No-0 accession (4%) than in ms-1 (Landsberg accession, 1%, Figure 1A) and are significantly enhanced by duo1-3 (36%). (C) Multiple hap2-1 sperm are released into a single ovule. The first panel shows an ovule targeted by a wild-type HTR10:HTR10:mRFP, LAT52:GFP pollen tube. Green signal is released from the burst pollen tube; sperm nuclei (SN, red signal) have fused (F) with the egg cell and central cell nuclei. The second panel shows an ovule targeted by a hap2-1, HTR10:HTR10:mRFP, LAT52:GFP pollen tube. The pollen tube has burst (green signal), but sperm cell nuclei remain unfused (UF). The third panel shows an ovule containing four unfused hap2-1, HTR10:HTR10:mRFP sperm. The last panel shows an ovule containing eight unfused hap2-1, HTR10:HTR10:mRFP sperm. Five sperm nuclei are clearly visible, the others are outside the plane of this image. In this ovule, pollen tube cytoplasm (GFP) appears to fill the area occupied by both synergids. Scale bars, 10μm.
Analysis of pollen tube growth to ovules in vitro showed that late-arriving additional pollen tubes were actively repelled[6]. Furthermore, analysis of several female gametophyte mutants (e.g. feronia) that fail to signal to the pollen tube to stop growing, burst, and release sperm indicated that these all attract multiple pollen tubes[7–11]. These studies suggested an active mechanism that blocks polytubey and indicate that any of the steps in double fertilization that occur after the pollen tube stops growing and burst could trigger the block. We tested whether gamete fusion is a potential trigger using Arabidopsis hap2-1 mutants[12, 13]. Arabidopsis hap2(gcs1) mutant pollen tubes target ovules, burst, and release sperm, but mutant sperm do not fuse with female gametes [2, 14]. We performed mixed pollination assays using pollen homozygous for LAT52:GFP and heterozygous for hap2-1/HAP2(GCS1) and pollen homozygous for LAT52:DsRed and HAP2(GCS1). In this experiment, half of the GFP-expressing pollen tubes carry wild-type sperm and half carry hap2-1 mutant sperm. All DsRed-expressing pollen tubes carry wild-type sperm. The rate of polytubey increased 10 fold over wild-type (Figure 1A) even though only ¼ of the pollen on the stigma carried hap2-1 mutant sperm.
We found that like hap2(gcs1) mutants, duo1-3 (Figure S1) mutants enhance polytubey ~10 fold over levels observed in the No-0 accession (wild type, genetic background of duo1-3, Figure 1B). duo1 mutant pollen tubes carry a single sperm-like cell that is incapable of fertilization[15, 16]. These data support the hypothesis that gamete fusion, or an event shortly thereafter is the trigger that prevents multiple pollen tubes from entering an ovule.
To closely monitor pollen tube burst and sperm release, we generated hap2-1 and wild-type pollen tubes expressing LAT52:GFP along with the sperm nuclear marker HTR10:HTR10:mRFP. Twenty-one hours after pollination, wild-type pollen tubes had burst and sperm nuclei had fused with the egg and central cell nuclei (Figure 1C, left panel). The HTR10:mRFP signal diffuses after nuclear fusion as it incorporates into the egg or central cell nucleus[17]. hap2-1 mutant pollen tubes also burst, but sperm nuclei remained compact at the site where gamete plasma membrane fusion would normally occur (Figure 1C second panel, [2]). In many cases, we observed multiple pairs of sperm nuclei (Figure 1C, third and fourth panels) and as many as four pairs of sperm were detected indicating that four pollen tubes had been attracted to one ovule (1 pair of unfused sperm, n=118; 2 pairs of unfused sperm, n=10; 3 pairs of unfused sperm, n=2; four pairs of unfused sperm, n=1). These data suggest that when gametes do not fuse, the block to polytubey fails and ovules continue to attract multiple pollen tubes.
The block to polytubey enhances reproductive success
To determine whether fertilization can be salvaged in an ovule that has attracted a pollen tube with defective sperm, we performed a mixed pollination experiment to analyze interactions between sperm and female gametes. Two types of differentially marked pollen were placed on the stigma at the same time: 1) hap2-1/HAP2(GCS1) pollen marked with HTR10:HTR10:mRFP; 2) wild-type pollen marked with LAT52:GFP. In addition to the expected cases where a single pollen tube was attracted (Table 1, Figure S2), a significant number of polytubey events were observed (Table 1, 39/432 targeted ovules). These included ovules containing multiple pairs of non-functional sperm (Figure S2). Importantly, these also included ovules that contained a pair of unfused RFP+ sperm nuclei and a wild-type, GFP+ pollen tube; and ovules containing a pair of nonfunctional RFP+ sperm (hap2-1) along with a pair of RFP+ wild-type sperm that had fused with female nuclei (Figure S2). We did not observe ovules that were targeted by two pollen tubes carrying wild-type sperm (e.g. wild-type GFP+ pollen tube and functional RFP+ sperm). As expected, when this mixed pollination experiment was conducted with wild-type pollen, polytubey was very rare (2/411 targeted ovules, Table 1). These data indicate that ovules first targeted by defective sperm can attract additional pollen tubes; but when wild-type sperm are attracted subsequent pollen tubes are blocked.
Table 1.
Ovules that have attracted defective sperm can attract functional sperm and polytubey increases if pistils are first pollinated with hap2-1/HAP2 pollen before wild-type pollen is added
| Simultaneous Mixed Pollination | Single pollen tube (signal observed) | Polytubey (signals observed) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| RFP+F | RFP+U | GFP+ | RFP+F GFP+ |
RFP+U GFP+ |
RFP+U RFP+U |
RFP+U RFP+F |
||||
| N | Polytubey | |||||||||
|
hap2-1/HAP2 HTR10:RFP |
wild type LAT52:GFP |
34 | 70 | 289 | 0 | 33 | 3 | 3 | 432 | 39/432 (9%) |
|
| ||||||||||
| wild type LAT52:GFP |
wild type LAT52:GFP |
178 | 0 | 231 | 1 | 1 | 0 | 0 | 411 | 2/411 (0.4%) |
|
| ||||||||||
| Staggered Mixed Pollination | ||||||||||
| Added first | Added 1.5 hours later | |||||||||
|
| ||||||||||
|
hap2-1/HAP2 HTR10:RFP |
wild type LAT52:GFP |
87 | 58 | 93 | 0 | 51 | 7 | 14 | 310 | 72/310 (23%) |
|
| ||||||||||
| wild type LAT52:GFP |
hap2-1/HAP2 HTR10:RFP |
12 | 4 | 353 | 0 | 1 | 0 | 0 | 370 | 1/370 (0.3%) |
Pollen genotypes for mixed pollinations are given in the first two columns.(h), time in hours of pollination; HTR10:RFP, HTR10:HTR10:RFP; RFP+F, ovule contains RFP+ sperm nuclei that have undergone karyogamy; RFP+U, RFP positive sperm that remain unfused; GFP+, GFP positive pollen tube has burst in the ovule; N, total number of ovules targeted.
If gamete fusion is required to trigger the block to polytubey, we would predict that polytubey would by exacerbated if hap2-1/HAP2(GCS1) pollen tubes were allowed to begin growth before addition of wild-type (GFP+) pollen to the stigma. In this scenario, the number of ovules that receive defective sperm would be increased. When mixed pollinations were staggered by 1.5 hours and hap2-1/HAP2(GCS1) pollen was applied first, the rate of polytubey further increased to 23% (Table 1). However, if wild type pollen was applied first, polytubey decreased to well below 1% and the vast majority of ovules were targeted by a wild-type GFP+ pollen tube.
Pollen tube-attracting synergid cells persist in ovules that have received defective sperm
We observed that ovules receiving defective sperm attracted up to four pollen tubes (Figure 1C). In Torenia fournieri, synergid cells secrete pollen tube attractants called LUREs[18] and at least one of the two synergids must be intact for pollen tube attraction[19]. In Arabidopsis, synergids are also required for pollen tube attraction[20] and degeneration of one of two synergids is correlated with arrival of a pollen tube [21]. We propose that in the absence of gamete fusion, ovules are able to continue to attract pollen tubes because one of the synergids persists and continues to secrete attractants. This would potentially explain the adaptive significance for the occurrence of two synergids in many species of flowering plants. We determined the relationship between synergid degeneration, gamete fusion, and pollen tube attraction in ovules receiving wild type or hap2(gcs1) mutant sperm.
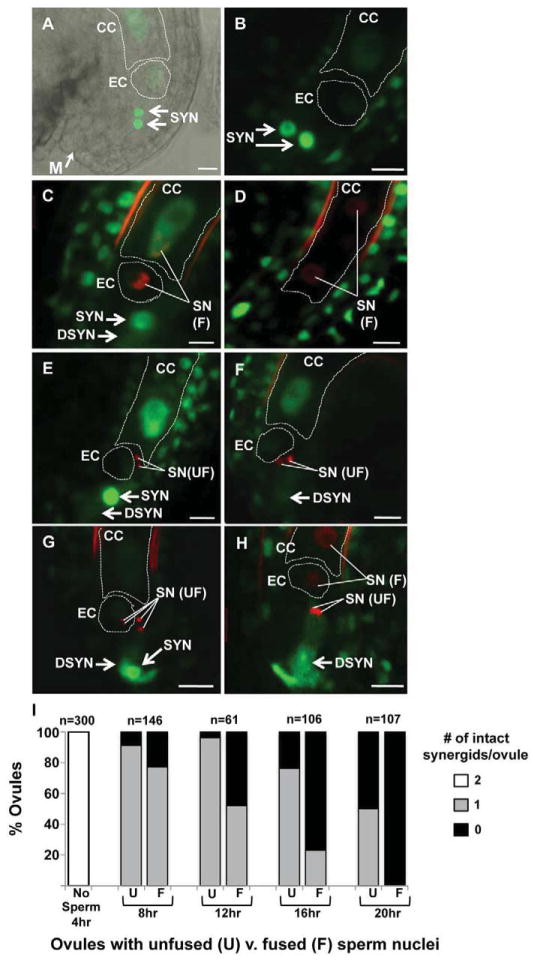
Upon synergid degeneration, ACT11:MSI1:GFP signal is no longer concentrated in the synergid nucleus and becomes diffuse throughout the cytoplasm, providing a clear marker for this important event[17]. We found that all ovules that had received either wild-type sperm (fused HTR10:HTR10:mRFP pattern, Figure 2C,D) or hap2-1 mutant sperm (unfused, Figure 2E,F) had one degenerated synergid (Figure 2C,E), or degeneration of both synergids (Figure 2D,F). Therefore, arrival and burst of a hap2-1 mutant pollen tube triggers degeneration of one synergid cell as in wild type. Ovules that had attracted multiple pairs of sperm also had either one or two degenerated synergids (Figure 2G,H). This result shows that arrival of a second pollen tube does not trigger degeneration of the persistent synergid and suggests that a downstream event like gamete fusion may be required.
Figure 2.
The remaining synergid cell persists longer in ovules targeted by nonfunctional sperm. (A) An unfertilized ovule expressing ACT11:MSI1:GFP. DIC and confocal images are overlaid to highlight the position of the micropyle (M, same in all panels) and GFP accumulation in the egg (EC), central (CC) and synergid (SYN) nuclei. (B–H) Pistils were pollinated with hap2-1/HAP2(GCS1), HTR10:HTR10:mRFP. Representative confocal micrographs are shown of an unfertilized ovule (B), ovules that have received sperm and have a persistent synergid (SYN) (C,E,G), or ovules that have received sperm and both synergids have degenerated (DSYN) (D,F,H). (D) ACT11:MSI1:GFP signal was not detectable in synergids because this image was obtained later in development (note dividing primary endosperm) and significantly after synergid degeneration. (I) Ovules were scored for the number of synergids that remain intact (2, white bar; 1 gray bar; 0 black bar) and whether they had received sperm whose nuclei fused with female nuclei (fused, F) or sperm that remained unfused (U). Data are reported as the percentage of the total number of ovules analyzed (n) at each time point following pollination (hours, hr). Scale bar, 10μm.
To begin to address this hypothesis, we analyzed synergid degeneration over time. At four hours after pollination, no ovules had been targeted and all ovules contained two intact synergid cells (Figure 2I). At each subsequent time point (8, 12, 16, 20 hours), we found that ovules with unfused sperm were more likely to have an intact second synergid than ovules with fused sperm (Figure 2I U, unfused hap2-1, v. F, fused wild type). Twelve hours after pollination, 97% of ovules with unfused sperm had a persistent synergid compared with 48% that had been fertilized by wild-type sperm. By 20 hours after pollination both synergids had degenerated in all ovules with fused sperm, whereas 50% of ovules with unfused sperm still had one intact synergid. These data show that if gamete fusion fails, a pollen tube-attracting synergid cell persists and can attract additional pollen tubes.
Conclusions
We have shown that the attraction of multiple pollen tubes to one ovule is rare in Arabidopsis (Figure 1B), but polytubey increases dramatically when sperm incapable of gamete fusion are deposited (Figure 1, Table 1). In these cases, one synergid persists and can continue to attract multiple pollen tubes until fertile sperm are delivered or the synergid senesces. Interestingly, in wild type, one synergid cell persists well after gamete fusion[21], but multiple pollen tubes are rarely attracted. This suggests that gamete fusion itself, or an event soon thereafter (e.g. nuclear fusion, initiation of development), triggers a mechanism that rapidly blocks additional pollen tubes from entering a fertilized ovule.
The cases of polytubey observed in Arabidopsis mutants can all be accounted for by defects in a block to polytubey that requires gamete fusion. Female mutants defective in pollen tube reception (e.g. feronia) fail to instruct pollen tubes to burst, sperm are not released, gamete fusion does not occur, and consequently, they attract multiple pollen tubes [7–11]. We predict that any mutation disrupting gamete fusion will result in polytubey.
The block to polytubey was likely a significant evolutionary innovation allowing flowering plants to maximize reproductive success by promoting fertilization of all ovules and ensuring that only two sperm are delivered to each ovule. Pollen tubes bypassing fertilized ovules can continue to grow to unfertilized ovules. If the block to polytubey were initiated by any earlier step in the process (pollen tube entry, pollen tube contact with the female gametophyte, pollen tube burst), then ovules would go unfertilized in cases where pollen tubes failed to burst or sperm were defective. The block to polytubey is therefore a final opportunity for the flower to scrutinize pollen, reject unworthy suitors and replace them with productive mates.
Supplementary Material
Highlights.
A ‘block to polytubey’ is triggered by gamete fusion or an event soon thereafter
If defective sperm are deposited, additional pollen tubes are attracted
Pollen tube-attracting synergid cells persist when defective sperm are delivered
Acknowledgments
We thank Frederic Berger, Gregory Copenhaver, and Daphne Preuss for generously providing transgenic plants expressing markers. This work was supported by the National Science Foundation through grant no. IOS-1021917 to MAJ and a Graduate Research Fellowship to KMB. KMB was initially supported by a National Institute of Health Brown University Initiative to Maximize Student Development grant (NIGMS Grant:R25GM083270 & R25GM083270-S1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berger F, Hamamura Y, Ingouff M, Higashiyama T. Double fertilization - caught in the act. Trends Plant Sci. 2008;13:437–443. doi: 10.1016/j.tplants.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Hamamura Y, Saito C, Awai C, Kurihara D, Miyawaki A, Nakagawa T, Kanaoka MM, Sasaki N, Nakano A, Berger F, et al. Live-Cell Imaging Reveals the Dynamics of Two Sperm Cells during Double Fertilization in Arabidopsis thaliana. Curr Biol. 2011;21:497–502. doi: 10.1016/j.cub.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Wong JL, Wessel GM. Defending the zygote: search for the ancestral animal block to polyspermy. Curr Top Dev Biol. 2006;72:1–151. doi: 10.1016/S0070-2153(05)72001-9. [DOI] [PubMed] [Google Scholar]
- 4.Twell D, Yamaguchi J, McCormick S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development (Cambridge, England) 1990;109:705–713. doi: 10.1242/dev.109.3.705. [DOI] [PubMed] [Google Scholar]
- 5.Francis KE, Lam SY, Harrison BD, Bey AL, Berchowitz LE, Copenhaver GP. Pollen tetrad-based visual assay for meiotic recombination in Arabidopsis. Proc Natl Acad Sci U S A. 2007;104:3913–3918. doi: 10.1073/pnas.0608936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palanivelu R, Preuss D. Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol. 2006;6:7. doi: 10.1186/1471-2229-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Current Biology. 2003;13:432–436. doi: 10.1016/s0960-9822(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 8.Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development (Cambridge, England) 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- 9.Capron A, Gourgues M, Neiva LS, Faure JE, Berger F, Pagnussat G, Krishnan A, Alvarez-Mejia C, Vielle-Calzada JP, Lee YR, et al. Maternal Control of Male-Gamete Delivery in Arabidopsis Involves a Putative GPI-Anchored Protein Encoded by the LORELEI Gene. Plant Cell. 2008;20:3038–3049. doi: 10.1105/tpc.108.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukamoto T, Qin Y, Huang Y, Dunatunga D, Palanivelu R. A role for LORELEI, a putative glycosylphosphatidylinositol-anchored protein, in Arabidopsis thaliana double fertilization and early seed development. Plant J. 2010;62:571–588. doi: 10.1111/j.1365-313X.2010.04177.x. [DOI] [PubMed] [Google Scholar]
- 11.Boisson-Dernier A, Frietsch S, Kim TH, Dizon MB, Schroeder JI. The peroxin loss-of-function mutation abstinence by mutual consent disrupts male-female gametophyte recognition. Curr Biol. 2008;18:63–68. doi: 10.1016/j.cub.2007.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MA, von Besser K, Zhou Q, Smith E, Aux G, Patton D, Levin JZ, Preuss D. Arabidopsis hapless mutations define essential gametophytic functions. Genetics. 2004;168:971–982. doi: 10.1534/genetics.104.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Besser K, Frank AC, Johnson MA, Preuss D. Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development (Cambridge, England) 2006;133:4761–4769. doi: 10.1242/dev.02683. [DOI] [PubMed] [Google Scholar]
- 14.Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol. 2006;8:64–71. doi: 10.1038/ncb1345. [DOI] [PubMed] [Google Scholar]
- 15.Rotman N, Durbarry A, Wardle A, Yang WC, Chaboud A, Faure JE, Berger F, Twell D. A novel class of MYB factors controls sperm-cell formation in plants. Curr Biol. 2005;15:244–248. doi: 10.1016/j.cub.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Brownfield L, Hafidh S, Borg M, Sidorova A, Mori T, Twell D. A plant germline-specific integrator of sperm specification and cell cycle progression. PLoS Genet. 2009;5:e1000430. doi: 10.1371/journal.pgen.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingouff M, Hamamura Y, Gourgues M, Higashiyama T, Berger F. Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr Biol. 2007;17:1032–1037. doi: 10.1016/j.cub.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–361. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- 19.Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T. Pollen tube attraction by the synergid cell. Science. 2001;293:1480–1483. doi: 10.1126/science.1062429. [DOI] [PubMed] [Google Scholar]
- 20.Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN. MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell. 2005;17:2981–2992. doi: 10.1105/tpc.105.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faure JE, Rotman N, Fortune P, Dumas C. Fertilization in Arabidopsis thaliana wild type: developmental stages and time course. Plant J. 2002;30:481–488. doi: 10.1046/j.1365-313x.2002.01305.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.