Abstract
In this study, we determined how rosiglitazone (RSG) differentially affected hippocampal neurogenesis in mice fed a low-fat diet (LFD) or high-fat diet (HFD; 60% fat). LFD and HFD were given to the mice for 8 weeks. Four weeks after initiating the LFD and HFD feeding, vehicle or RSG was administered orally once a day to both groups of mice. We measured cell proliferation and neuroblast differentiation in the subgranular zone of the dentate gyrus using Ki67 and doublecortin (DCX), respectively, as markers. In addition, we monitored the effects of RSG on the levels of DCX and brain-derived neurotrophic factor (BDNF) in hippocampal homogenates. At 8 weeks after the LFD feeding, the numbers of Ki67- and DCX-positive cells as well as hippocampal levels of DCX and BDNF were significantly decreased in the RSG-treated group compared to the vehicle-treated animals. In contrast, the numbers of Ki67- and DCX-positive cells along with hippocampal levels of DCX and BDNF in the HFD fed mice were significantly increased in the RSG-treated mice compared to the vehicle-treated group. Our data demonstrate that RSG can modulate the levels of BDNF, which could play a pivotal role in cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus.
Keywords: brain-derived neurotrophic factor, dentate gyrus, high-fat diet, rosiglitazone
Introduction
Increased consumption of a high-fat diet (HFD) results in obesity and neurocognitive disorders [1]. A HFD is a major factor of metabolic disorder development and can contribute to the incidence of neurodegenerative diseases, long-term memory loss, and cognitive impairment [5,10]. This type of diet also induces increased levels of malondialdehyde (MDA) and reduced levels of brain-derived neurotrophic factor (BDNF) in the hippocampus [13,33]. In addition, hyperglycemia caused by a HFD accelerates the deposition of advanced glycation end-products that can promote neuronal damage [25,37].
In the mammalian brain, neurogenesis occurs throughout life [21,22]. The subgranular zone of the hippocampal dentate gyrus, which is associated with learning and memory functions [15], is one of two major neurogenic regions in the adult brain [6]. Neurogenesis in the dentate gyrus is known to be highly plastic, and many studies have focused on identifying factors that regulate this process in adults [29,30,31].
Peroxisome proliferator-activated receptor γ (PPARγ) is a glitazone receptor, and regulates glucose metabolism along with fatty acid storage by stimulating lipid uptake and adipogenesis [17]. It has been reported that adipose tissue is not synthesized in PPARγ-knockout mice fed a HFD [17]. Rosiglitazone (RSG), a synthetic agonist of PPARγ, is widely used as an anti-diabetic drug for treating patients with type 2 diabetes. Recently, it was reported that PPARγ is also involved in modulating the proliferation and differentiation of neural stem cells [18,26,37]. In addition, PPARγ activation was found to help mitigate neuroinflammation induced by acute or chronic insults [19]. PPARγ agonists could be beneficial for ameliorating some neurological disorders including Parkinson's disease [32], ischemia [9,20], and Alzheimer's disease [34]. However, few investigations have addressed the effect PPARγ on adult hippocampal neurogenesis in a model of diet-induced obesity.
In the present study, we investigated the effect of RSG on cell proliferation and neuroblast differentiation in the hippocampus of mice fed a low-fat diet (LFD) or HFD. For this, Ki67 and doublecortin (DCX) levels were measured. Ki67 is a marker of cell proliferation expressed during the active phases of the cell cycle [4] while DCX is a neuroblast marker [2].
Materials and Methods
Experimental animals
Male C57BL/6J mice (n = 68) were purchased from the Jackson Laboratory (USA). The animals were used at 8 weeks of age, and housed at 22℃ with 60% humidity and a 12-h light/dark cycle. All mice had free access to food and tap water. Animal handling and care conformed to guidelines that comply with current international laws and policies (National Institutes of Health [NIH] Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996), and the experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University (Approval No. SNU-110412-2). All experiments were conducted to minimize both the number of mice used and suffering due to the procedures performed in the present study.
HFD feeding and drug treatment
Six-week-old mice were individually caged and allowed to adapt to a chow diet for 1 week. After this time, the mice were fed a commercial LFD (D12450Bi used as a control diet for D12492, n = 34; Research Diets, USA) or HFD (D12492i, n = 34; Research Diets) for 8 weeks. Four weeks after initiating the LFD and HFD feeding, vehicle (0.1% methyl cellulose) or 2 mg/kg RSG (Avandia; GlaxoSmithKline, USA) was orally administered to mice in both groups using a feeding needle (Kent Scientific, USA) once a day for 4 weeks. The RSG dose was chosen because it is equivalent to the doses clinically used in humans. With this concentration, we previously observed a reduction of cell proliferation and neuroblast differentiation in normal mice [26]. The experimental schedule was adopted because a previous study detected an HFD-induced reduction of cell proliferation and neuroblast differentiation at 4 weeks after the start of HFD consumption [12]. In addition, DCX is exclusively expressed in immature neurons in cells 1 to 28 days old [2].
Tissue processing for histology
Eight weeks after starting LFD or HFD feeding (4 weeks after vehicle or RSG treatment), the mice (n = 7 in each group) were anesthetized with 30 mg/kg Zoletil 50 (Virbac, France) and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The brains were removed and postfixed by 4% paraformaldehyde in 0.1 M PB for 12 h. The brain tissues were cryoprotected by overnight infusion with 30% sucrose, and 30-µm-thick coronal sections were serially cut using a cryostat (Leica, Germany). The sections were transferred to six-well plates containing PBS for further processing.
Immunohistochemistry specific for Ki67 and DCX
Immunohistochemistry was performed under the same conditions for tissues from each group to obtain consistent results. We selected brain sections collected between 1.6 mm and 1.8 mm posterior to the bregma as determined by a mouse brain atlas [8]. The sections were incubated with goat anti-DCX antibodies (1 : 50 dilution; Santa Cruz Biotechnology, USA) or rabbit anti-Ki67 (1 : 1,000; Abcam, UK) overnight at room temperature, and subsequently exposed to biotinylated donkey anti-goat or goat anti-rabbit IgG (1 : 200; Vector Laboratories, USA) for 2 h and streptavidin peroxidase complex (1 : 200, Vector Laboratories) for 30 min at room temperature. Antibody binding was detected using 0.05% 3,3'-diaminobenzidine tetrachloride (Sigma, USA) in 0.1 M Tris-HCl buffer (pH 7.2), and the sections were mounted on gelatin-coated slides.
The numbers of Ki67- and DCX-positive cells in samples from all groups were determined using an image analysis system equipped with a computer-based charge-coupled device (CCD) camera (Optimas 6.5; Media Cybernetics, USA). In addition, images of all DCX-immunoreactive structures in the dentate gyrus were obtained with a BX51 light microscope (Olympus, Japan) equipped with a digital camera (DP71; Olympus) connected to a PC monitor. Ki67- and DCX-positive cells in the dentate gyrus of each section were counted using Optimas 6.5 software (Media Cybernetics). Cell counts for all the sections from every mouse were averaged and are presented as a percentage.
Western blot analysis
To confirm the effects of RSG on neuroblast differentiation, five mice from each group were sacrificed and used for Western blot analysis [39]. After the brains were removed, the dentate gyrus was removed with a surgical blade. The dentate gyrus was homogenized in 50 mM PBS (pH 7.4) containing 0.1 mM ethylene glycol bis-(2-aminoethyl ether)-N,N,N',N' tetraacetic acid (EGTA, pH 8.0; Sigma), 0.2% Nonidet P-40 (Sigma), 10 mM ethylendiamine-tetraacetic acid (EDTA, pH 8.0; Sigma), 15 mM sodium pyrophosphate (Sigma), 100 mM β-glycerophosphate (Sigma), 50 mM NaF (Sigma), 150 mM NaCl (Sigma), 2 mM sodium orthovanadate (Sigma), 1 mM phenylmethylsulfonyl fluoride (PMSF, Sigma), and 1 mM dithiothreitol (DTT; Sigma). After centrifugation, protein concentration of the supernatants was determined using a Micro BCA protein assay kit (Pierce Chemical, USA) with bovine serum albumin as the standard. Aliquots containing 50 µg of total protein were boiled in a loading buffer that contained 150 mM Tris (pH 6.8), 3 mM DTT, 6% sodium dodecylsulfate (SDS), 0.3% bromophenol blue (Sigma), and 30% glycerol. The aliquots were then loaded onto a 8% polyacrylamide gel (Sigma). After electrophoresis, the proteins were transferred to nitrocellulose membranes (Pall Corporation, USA). To reduce background signals, the membranes were blocked with 5% non-fat dry milk (Sigma) in PBS containing 0.1% Tween 20 for 45 min. Next, the blots were incubated with with goat anti-DCX (1 : 100) and then peroxidase-conjugated anti-goat IgG (Vector Laboratories). Antibody binding was detected with an enhanced luminol-based chemiluminescent (ECL) kit (Pierce Chemical). The bands was densitometrically scanned to quantify the relative optical density (ROD) using Scion Image software (Scion Corporation, USA). The obtained data were normalized against that for β-actin.
Measurement of BDNF levels
In order to confirm changes in BDNF levels in the dentate gyrus [38], five mice from each group were anesthetized with 100 mg/kg Zoletil 50 (Virbac) and decapitated. The hippocampus was removed from the brain and stored in liquid nitrogen. BDNF levels in the hippocampus were measured using a BDNF Emax immunoassay kit (Promega, USA). The tissue samples were weighed and 300 µL of lysis buffer was added to each sample. The samples were then sonicated for 30 sec and centrifuged at 4℃ for 20 min. The supernatant was stored at -20℃ until it was analyzed. All samples were assayed in duplicate and the absorbance was read with an enzyme-linked immunosorbent assay (ELISA) plate reader (Bio Tek, USA). The concentration for each sample was calculated by plotting the absorbance values on a standard curve with known concentrations generated by the assay.
Statistical analysis
Data are presented as the mean for each experiment. Differences between the mean values were analyzed with a one-way analysis of variance followed by Tukey's multiple range test. Statistical significance was considered at p < 0.05.
Results
Effect of RSG on cell proliferation
In all groups, Ki67-positive nuclei were detected in the subgranular zone of the dentate gyrus. Among all mice, the LFD-fed vehicle-treated group had the highest number of Ki67-positive cells (Figs. 1A and E). The number of Ki67-positive cells was decreased in the LFD-fed RSG-treated group compared to the LFD-fed vehicle-treated animals (Figs. 1B and E). Ki67-positive nuclei were rarely detected in the dentate gyrus of the HFD-fed vehicle-treated group, and the number of Ki67-positive cells was significantly decreased in these mice compared to the LFD-fed vehicle-treated animals (Figs. 1C and E). The number of Ki67-positive cells was markedly increased in the HFD-fed RSG-treated mice compared to the HFD-fed vehicle-treated group (Figs. 1D and E).
Fig. 1.
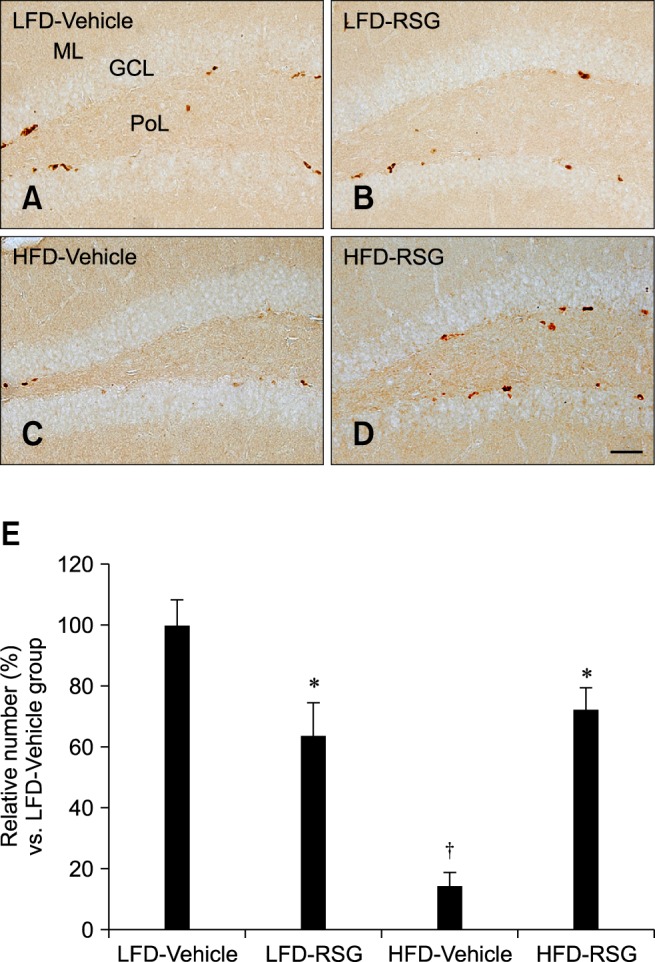
Immunohistochemistry specific for Ki67 in the dentate gyrus. Ki67-positive nuclei were detected in the subgranular zone of the dentate gyrus. The number of Ki67-immunoreactive nuclei was decreased in the LFD-RSG group compared to the LFD-Vehicle group. Ki67-positive nuclei were rarely seen in the HFD-Vehicle group unlike the LFD-Vehicle group. The number of Ki67-positive nuclei was significantly increased in the HFD-RSG group compared to the HFD-Vehicle group. (A) low-fat diet (LFD)-fed vehicle-treated group (LFD-Vehicle). (B) LFD-fed rosiglitazone (RSG)-treated group (LFD-RSG). (C) high-fat diet (HFD)-fed vehicle-treated group (HFD-Vehicle). (D) HFD-fed RSG-treated (HFD-RSG) groups. (E) Relative numbers of Ki67-immunoreactive nuclei in the LFD-Vehicle, LFD-RSG, HFD-Vehicle, and HFD-RSG groups (n = 7 per group; *p < 0.05, Vehicle versus RSG groups; †p < 0.05, LFD versus HFD groups). All data are expressed as the mean ± standard error of the mean (SEM). ML: molecular layer, GCL: granule cell layer, PoL: polymorphic layer. Scale bar = 50 µm.
Effect of RSG on neuroblast differentiation
In all groups, DCX-immunoreactive neuroblasts were detected in the subgranular zone of the dentate gyrus. The dendrites extended into the molecular layer of the dentate gyrus. Compared to the other three groups, the LFD-fed vehicle-treated mice had a greater number of DCX-positive neuroblasts in the dentate gyrus (Figs. 2A and B). The number of DCX-immunoreactive neuroblasts and their dendrites were decreased in the dentate gyrus of the LFD-fed RSG-treated group compared to the LFD-fed vehicle-treated animals (Figs. 2C, D, and I). The number of DCX-positive neuroblasts was also markedly decreased in the HFD-fed vehicle-treated group compared to the LFD-fed vehicle-treated group. In addition, the abundance of DCX-immunoreactive dendrites was prominently decreased in the HFD-fed vehicle-treated group (Figs. 2E and F). However, the number of DCX-immunoreactive neuroblasts was increased in the dentate gyrus in the HFD-fed RSG-treated group compared to the HFD-fed vehicle-treated mice (Figs. 2G, H, and I).
Fig. 2.
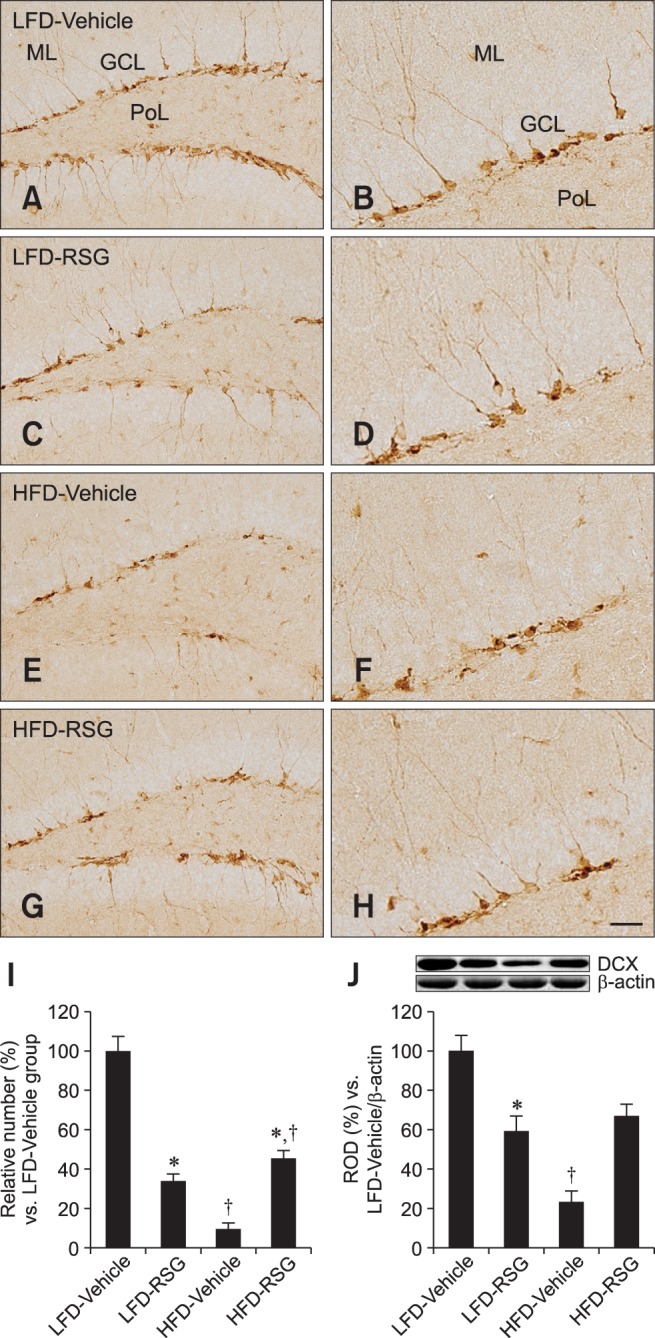
Immunohistochemistry specific for DCX in the dentate gyrus. DCX-positive neuroblasts were detected in the subgranular zone of the dentate gyrus. The number of DCX-immunoreactive neuroblasts was decreased in the LFD-RSG group compared to the LFD-Vehicle group. DCX-positive neuroblasts were rarely seen in the HFD-Vehicle group unlike the LFD-Vehicle group. The number of DCX-immunoreactive neuroblasts in the dentate gyrus was increased in the HFD-RSG group compared to the HFD-Vehicle group. (A and B) LFD-Vehicle. (C and D) LFD-RSG. (E and F) HFD-Vehicle. (G and H) HFD-RSG groups. (I) Relative number of DCX-immunoreactive cells in the LFD-Vehicle, LFD-RSG, HFD-Vehicle, and HFD-RSG groups (n = 7 per group; *p < 0.05, Vehicle vs. RSG groups; †p < 0.05, LFD vs. HFD groups). All data are expressed as the mean ± SEM. (J) Western blot analysis of DCX levels in the dentate gyrus of the LFD-Vehicle, LFD-RSG, HFD-Vehicle, and HFD-RSG groups. Relative optical density (ROD) of the bands is expressed as percentages (n = 5 per group; *p < 0.05, Vehicle vs. RSG groups; †p < 0.05, LFD vs. HFD groups). Data are presented as the mean ± SEM. Scale bars = 25 µm (B, D, F, and H) or 50 µm (A, C, E, and G).
Western blot analysis showed that DCX protein expression in the dentate gyrus was significantly lower in the LFD-fed RSG-treated mice than in the LFD-fed vehicle-treated group. Compared to the LFD-fed vehicle-treated group, the HFD-fed vehicle-treated group had significantly decreased levels of DCX protein. However, the expression of DCX protein in the HFD-fed RSG-treated group was markedly increased compared to that in the HFD-fed vehicle-treated group (Fig. 2J).
Effect of RSG on BDNF levels
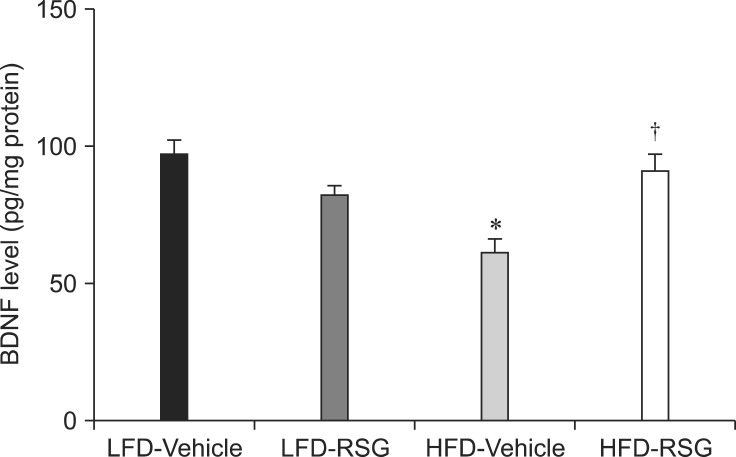
In the LFD-fed vehicle-treated group, the average level of BDNF in the hippocampal homogenates was 96.1 pg/mg of protein. BDNF protein levels were lower in the LFD-fed RSG-treated group than in the LFD-fed vehicle-treated mice. In contrast, BDNF levels in the HFD-fed RSG-treated animals were significantly increased compared to those in the HFD-fed vehicle-treated group (Fig. 3).
Fig. 3.
BDNF levels in the hippocampal homogenates from the LFD-Vehicle, LFD-RSG, HFD-Vehicle, and HFD-RSG groups (n = 5 per group; *p < 0.05, LFD vs. HFD groups; †p < 0.05, Vehicle vs. RSG groups). All data are expressed as the mean ± SEM.
Discussion
PPARγ is known to have a role in insulin sensitivity. However, it was recently reported that PPARγ and its signaling pathways are involved in regulating other cellular functions and homeostasis [11]. PPARs are also associated with chronic diseases such as diabetes, obesity, atherosclerosis, and cancer [23,24], and play an important role in various central nervous system disorders [11]. It was found that ciglitazone, a PPARγ agonist, can reduce excitotoxic neuronal damage [40] and PPARγ agonists can attenuate ischemic damage by reducing neuroinflammation [35]. In addition, the PPARγ agonist pioglitazone was shown to improve anatomical repair and locomotor function after spinal cord injury [28].
In the present investigation, we examined the effects of RSG, a PPARγ agonist, on cell proliferation and neuroblast differentiation (by measuring Ki67 and DCX expression, respectively) in adult LFD- and HFD-fed mice. In the LFD-fed group, RSG treatment decreased the number of DCX- and Ki67-positive cells. In contrast, RSG administration significantly increased the number of Ki67- and DCX-positive cells in the HFD-fed mice compared to that observed in the vehicle-treated HFD-fed group.
A previous study revealed that PPARγ plays an important role in controlling the proliferation and differentiation of neural stem cells mediated by the regulation of epidermal growth factor receptor and activation of extracellular signal-regulated kinase (ERK) as well as signal transducer and activator of transcription 3 (STAT-3) pathways [37]. In yet another investigation, RSG treatment decreased BDNF and glial cell line-derived neurotrophic factor levels in the dentate gyrus of normal adult mice [26]. BDNF is known to influence hippocampal neurogenesis, and BDNF-mediated neuronal precursor cell differentiation and survival are regulated by activation of the Akt, ERK1/2, and STAT-3 signaling pathways [14]. Therefore, changes in BDNF expression may be correlated with PPARγ pathway activity.
HFD-fed mice have been reported to have increased levels of PPARγ in adipose tissue, and PPARγ mRNA expression above a certain level helps regulate adipocyte development and function [36]. In the brain, PPARγ activation has been reported to promote neurogenesis as well as neurite outgrowth in mature neurons, which enhances neuronal connectivity [7,30]. However, excessive activation of PPARγ was shown to induce cell death and inhibit the differentiation of neural stem cells whereas optimal activation of the PPARγ pathway induces the neurogenesis of neural stem cells [37]. In addition, PPARγ deficiency decreases neural stem cell proliferation and subsequent apoptosis by activating the caspase pathway [37].
In the present study, a HFD significantly reduced BDNF levels in the hippocampus. BDNF expression reduced by HFD feeding can impair cell proliferation [27], progenitor survival [31], and neuronal differentiation [3] because BDNF has a potent impact on adult hippocampal neurogenesis [33]. In contrast, we found that the administration of RSG significantly rescued HFD-induced BDNF deficiency in the hippocampus. This result was consistent with findings from a previous study in which RSG administration rescued BDNF deficiency in the cerebral cortex of a model of Huntington's disease [16]. In addition, we previously observed that blocking the BDNF receptor reduces cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus of mice [38]. Thus, our present results indicate that modulation of BDNF levels can alter hippocampal neurogenesis in the dentate gyrus of LFD- and HFD-fed mice.
In conclusion, RSG administration decreased the number of Ki67- and DCX-positive cells in the dentate gyrus of LFD-fed mice. On the other hand, RSG increased the number of Ki67- and DCX-positive cells in the dentate gyrus of HFD-fed mice. These results demonstrate that RSG can affect cell proliferation and neuronal differentiation in the subgranular zone of the dentate gyrus by modulating BDNF levels in the hippocampus.
Acknowledgments
This work was supported by a National Research Foundation of Korea grant (2012R1A1B3001256) funded by the Korean government (MEST).
References
- 1.Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Layé S, Ferreira G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus. 2012;22:2095–2100. doi: 10.1002/hipo.22032. [DOI] [PubMed] [Google Scholar]
- 2.Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 3.Chan JP, Cordeira J, Calderon GA, Iyer LK, Rios M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008;39:372–383. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res. 2002;134:13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- 5.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 7.Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, Cipriano M, Carratù MR, Iuvone T, Steardo L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011;6:e28668. doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 9.Giaginis C, Tsourouflis G, Theocharis S. Peroxisome proliferator-activated receptor-γ (PPAR-γ) ligands: novel pharmacological agents in the treatment of ischemia reperfusion injury. Curr Mol Med. 2008;8:562–579. doi: 10.2174/156652408785748022. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood CE, Winocur G. High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging. 2005;26(Suppl 1):42–45. doi: 10.1016/j.neurobiolaging.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007;1771:1031–1045. doi: 10.1016/j.bbalip.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Hwang IK, Kim IY, Kim DW, Yoo KY, Kim YN, Yi SS, Won MH, Lee IS, Yoon YS, Seong JK. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res. 2008;1241:1–6. doi: 10.1016/j.brainres.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Hwang IK, Kim IY, Kim YN, Yi SS, Park IS, Min BH, Doo HK, Ahn SY, Kim YS, Lee IS, Yoon YS, Seong JK. Comparative study on high fat diet-induced 4-hydroxy-2E-nonenal adducts in the hippocampal CA1 region of C57BL/6N and C3H/HeN mice. Neurochem Res. 2009;34:964–972. doi: 10.1007/s11064-008-9846-y. [DOI] [PubMed] [Google Scholar]
- 14.Islam O, Loo TX, Heese K. Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res. 2009;6:42–53. doi: 10.2174/156720209787466028. [DOI] [PubMed] [Google Scholar]
- 15.Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 16.Jin J, Albertz J, Guo Z, Peng Q, Rudow G, Troncoso JC, Ross CA, Duan W. Neuroprotective effects of PPAR-γ agonist rosiglitazone in N171-82Q mouse model of Huntington's disease. J Neurochem. 2013;125:410–419. doi: 10.1111/jnc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2005;102:6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanakasabai S, Pestereva E, Chearwae W, Gupta SK, Ansari S, Bright JJ. PPARγ agonists promote oligodendrocyte differentiation of neural stem cells by modulating stemness and differentiation genes. PLoS One. 2012;7:e50500. doi: 10.1371/journal.pone.0050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaundal RK, Sharma SS. Peroxisome proliferator-activated receptor gamma agonists as neuroprotective agents. Drug News Perspect. 2010;23:241–256. doi: 10.1358/dnp.2010.23.4.1437710. [DOI] [PubMed] [Google Scholar]
- 21.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 23.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 24.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Nagai R, Tobe K, Kimura S, Kadowaki T. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 25.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 26.Lee CH, Choi JH, Yoo KY, Park OK, Moon JB, Sohn Y, Cho JH, Hwang IK, Won MH. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor γ, decreases immunoreactivity of markers for cell proliferation and neuronal differentiation in the mouse hippocampus. Brain Res. 2010;1329:30–35. doi: 10.1016/j.brainres.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 28.McTigue DM, Tripathi R, Wei P, Lash AT. The PPAR gamma agonist Pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp Neurol. 2007;205:396–406. doi: 10.1016/j.expneurol.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miglio G, Rattazzi L, Rosa AC, Fantozzi R. PPARγ stimulation promotes neurite outgrowth in SH-SY5Y human neuroblastoma cells. Neurosci Lett. 2009;454:134–138. doi: 10.1016/j.neulet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Park HR, Park M, Choi J, Park KY, Chung HY, Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett. 2010;482:235–239. doi: 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 31.Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur J Neurosci. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 34.Sodhi RK, Singh N, Jaggi AS. Neuroprotective mechanisms of peroxisome proliferator-activated receptor agonists in Alzheimer's disease. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:115–124. doi: 10.1007/s00210-011-0654-6. [DOI] [PubMed] [Google Scholar]
- 35.Sundararajan S, Landreth GE. Antiinflammatory properties of PPARgamma agonists following ischemia. Drug News Perspect. 2004;17:229–236. doi: 10.1358/dnp.2004.17.4.829049. [DOI] [PubMed] [Google Scholar]
- 36.Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR γ gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553–2561. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Sun Z, Guo Y, Yuan Y, Li L. PPARγ-mediated advanced glycation end products regulation of neural stem cells. Mol Cell Endocrinol. 2009;307:176–184. doi: 10.1016/j.mce.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Yoo DY, Kim W, Nam SM, Yoo KY, Lee CH, Choi JH, Won MH, Hwang IK, Yoon YS. Reduced cell proliferation and neuroblast differentiation in the dentate gyrus of high fat diet-fed mice are ameliorated by metformin and glimepiride treatment. Neurochem Res. 2011;36:2401–2408. doi: 10.1007/s11064-011-0566-3. [DOI] [PubMed] [Google Scholar]
- 39.Yoo DY, Shin BN, Kim IH, Kim W, Kim DW, Yoo KY, Choi JH, Lee CH, Yoon YS, Choi SY, Won MH, Hwang IK. Effects of Cu,Zn-superoxide dismutase on cell proliferation and neuroblast differentiation in the mouse dentate gyrus. Neurochem Res. 2012;37:261–267. doi: 10.1007/s11064-011-0605-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Ou Z, Grotta JC, Waxham N, Aronowski J. Peroxisome-proliferator-activated receptor-gamma (PPARγ) activation protects neurons from NMDA excitotoxicity. Brain Res. 2006;1073-1074:460–469. doi: 10.1016/j.brainres.2005.12.061. [DOI] [PubMed] [Google Scholar]