Abstract
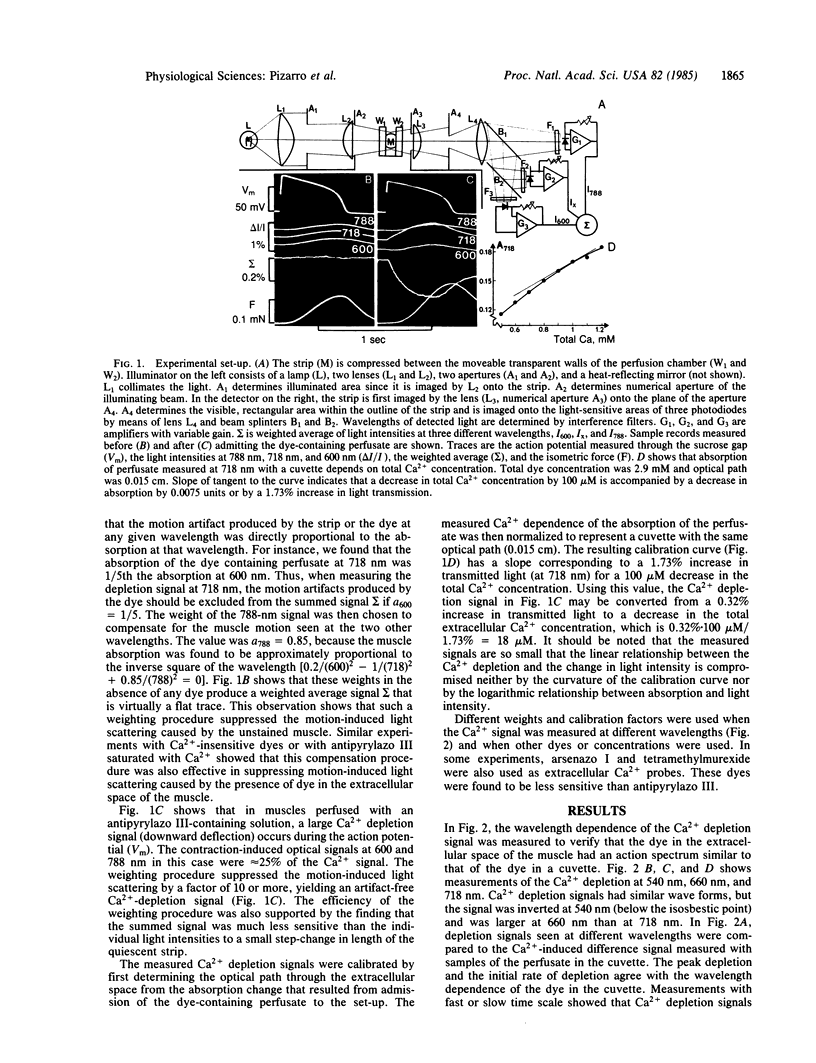
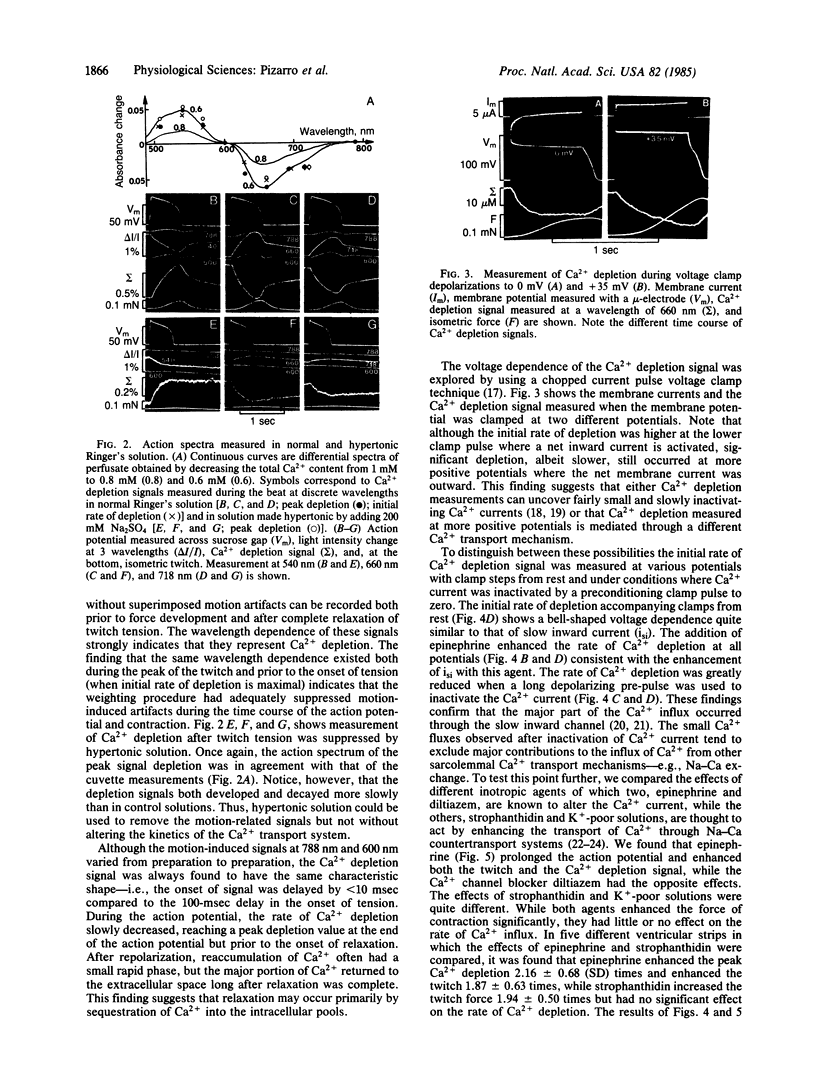
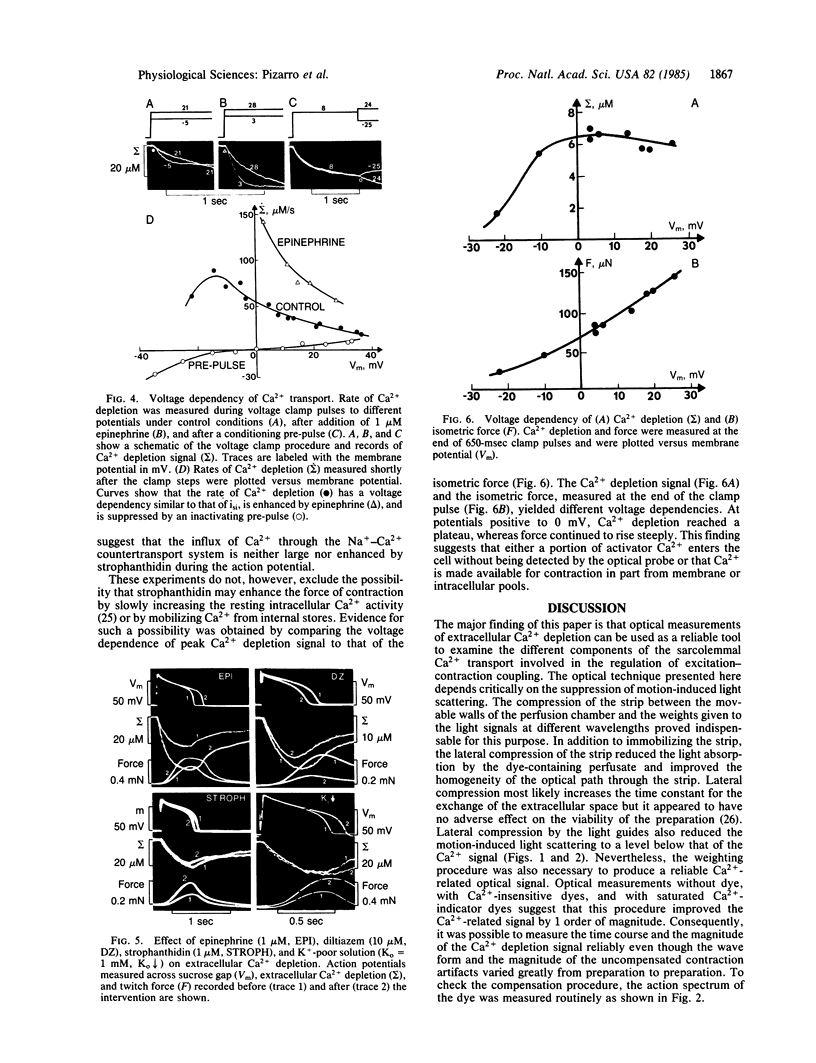
Sarcolemmal Ca2+ movements in frog ventricular strips were measured by monitoring Ca2+ depletion from the extracellular space with an impermeant Ca indicator dye, antipyrylazo III. Ca2+ depletion was measured as a weighted average of light signals recorded simultaneously at three different wavelengths. This weighting procedure was designed to reduce the motion-induced light scattering and to enhance the Ca2+-related optical signals. Comparison of the time course of Ca2+ depletion signal with that of contraction showed that the rate of Ca2+ depletion was maximal immediately after the upstroke of the action potential but prior to the onset of tension. Peak Ca2+ depletion was reached toward the end of the action potential and amounted to a 10-50 microM decrease in the total extracellular Ca2+ concentration. The reaccumulation of extracellular Ca2+ seen after the action potential was 2-5 sec slower than the relaxation of tension. The rate of Ca2+ depletion had a bell-shaped voltage dependence and was enhanced by epinephrine, suggesting that Ca2+ influx occurred primarily through a slowly inactivating ionic channel. Ca2+ transport through the Na+-Ca2+ exchange system was not significantly altered in the presence of strophanthidin or with decrease of extracellular K+ concentration despite marked potentiation of tension by these agents. Ca2+ depletion measured at the end of a 1-sec clamp pulse had a voltage dependence noticeably different from that of the developed tension. This finding may suggest that a fraction of activator Ca2+ is released from membrane-bound Ca2+ pools in a voltage-dependent manner. Our results show that Ca2+ indicator dyes can be used not only to measure rapid changes in the extracellular Ca2+ concentration during contraction, but also to quantify the contribution of various sarcolemmal Ca2+ transport systems to the generation of tension in cardiac muscle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeler G. W., Jr, Reuter H. The relation between membrane potential, membrane currents and activation of contraction in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):211–229. doi: 10.1113/jphysiol.1970.sp009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Chapman R. A. Excitation-contraction coupling in cardiac muscle. Prog Biophys Mol Biol. 1979;35(1):1–52. doi: 10.1016/0079-6107(80)90002-4. [DOI] [PubMed] [Google Scholar]
- Cleemann L., Morad M. Extracellular potassium accumulation in voltage-clamped frog ventricular muscle. J Physiol. 1979 Jan;286:83–111. doi: 10.1113/jphysiol.1979.sp012608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleemann L., Suenson M. Reduction of the sucrose-saline interdiffusion in the sucrose gap technique by controlled compression of the extracellular space in myocardial preparations. Acta Physiol Scand. 1984 Mar;120(3):417–427. doi: 10.1111/j.1748-1716.1984.tb07402.x. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann N Y Acad Sci. 1978 Apr 28;307:491–522. doi: 10.1111/j.1749-6632.1978.tb41979.x. [DOI] [PubMed] [Google Scholar]
- Goldman Y., Morad M. Measurement of transmembrane potential and current in cardiac muscle: a new voltage clamp method. J Physiol. 1977 Jul;268(3):613–654. doi: 10.1113/jphysiol.1977.sp011875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W., Delay M. J., Langer G. A. Activation-dependent cumulative depletions of extracellular free calcium in guinea pig atrium measured with antipyrylazo III and tetramethylmurexide. Circ Res. 1983 Dec;53(6):779–793. doi: 10.1161/01.res.53.6.779. [DOI] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Sodium-calcium exchange in regulation of cardiac contractility. Evidence for an electrogenic, voltage-dependent mechanism. J Gen Physiol. 1979 Apr;73(4):403–424. doi: 10.1085/jgp.73.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Scheuer T. Slow inactivation of calcium channels in the cardiac Purkinje fiber. J Mol Cell Cardiol. 1982 Oct;14(10):615–618. doi: 10.1016/0022-2828(82)90148-1. [DOI] [PubMed] [Google Scholar]
- Kline R., Morad M. Potassium efflux and accumulation in heart muscle. Evidence from K +/- electrode experiments. Biophys J. 1976 Apr;16(4):367–372. doi: 10.1016/S0006-3495(76)85694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzner T., Morad M. Excitation-contraction coupling in frog ventricle. Possible Ca2+ transport mechanisms. Pflugers Arch. 1983 Sep;398(4):274–283. doi: 10.1007/BF00657237. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Heart: excitation-contraction coupling. Annu Rev Physiol. 1973;35:55–86. doi: 10.1146/annurev.ph.35.030173.000415. [DOI] [PubMed] [Google Scholar]
- Langer G. A. The 'sodium pump lag' revisited. J Mol Cell Cardiol. 1983 Oct;15(10):647–651. doi: 10.1016/0022-2828(83)90254-7. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Uhm D. Y., Dresdner K. Sodium-calcium exchange in rabbit heart muscle cells: direct measurement of sarcoplasmic Ca2+ activity. Science. 1980 Aug 8;209(4457):699–701. doi: 10.1126/science.7394527. [DOI] [PubMed] [Google Scholar]
- Morad M., Goldman Y. E., Trentham D. R. Rapid photochemical inactivation of Ca2+-antagonists shows that Ca2+ entry directly activates contraction in frog heart. Nature. 1983 Aug 18;304(5927):635–638. doi: 10.1038/304635a0. [DOI] [PubMed] [Google Scholar]
- Morad M., Orkand R. K. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. J Physiol. 1971 Dec;219(1):167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Reeck S., Rao M. Potassium chloride versus voltage clamp contractures in ventricular muscle. Science. 1981 Jan 30;211(4481):485–487. doi: 10.1126/science.7455687. [DOI] [PubMed] [Google Scholar]
- Morad M., Tung L. Ionic events responsible for the cardiac resting and action potential. Am J Cardiol. 1982 Feb 18;49(3):584–594. doi: 10.1016/s0002-9149(82)80016-7. [DOI] [PubMed] [Google Scholar]
- Niedergerke R., Page S. Analysis of caffeine action in single trabeculae of the frog heart. Proc R Soc Lond B Biol Sci. 1981 Nov 13;213(1192):303–324. doi: 10.1098/rspb.1981.0068. [DOI] [PubMed] [Google Scholar]
- Page S. G., Niedergerke R. Structures of physiological interest in the frog heart ventricle. J Cell Sci. 1972 Jul;11(1):179–203. doi: 10.1242/jcs.11.1.179. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Brinley F. J., Jr, Dubyak G. Antipyrylazo III, a "middle range" Ca2+ metallochromic indicator. Biochemistry. 1978 Apr 18;17(8):1378–1386. doi: 10.1021/bi00601a004. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G., Rougier O. Membrane potential and slow inward current dependence of frog cardiac mechanical activity. Pflugers Arch. 1972;331(3):191–203. doi: 10.1007/BF00589126. [DOI] [PubMed] [Google Scholar]



